Abstract
We evaluated HLA-compatible donor leukocyte infusions (DLIs) and HLA-compatible or HLA-disparate EBV-specific T cells (EBV-CTLs) in 49 hematopoietic cell transplantation recipients with biopsy-proven EBV-lymphoproliferative disease (EBV-LPD). DLIs and EBV-CTLs each induced durable complete or partial remissions in 73% and 68% of treated patients including 74% and 72% of patients surviving ≥ 8 days after infusion, respectively. Reversible acute GVHD occurred in recipients of DLIs (17%) but not EBV-CTLs. The probability of complete response was significantly lower among patients with multiorgan involvement. In responders, DLIs and EBV-CTLs regularly induced exponential increases in EBV-specific CTL precursor (EBV-CTLp) frequencies within 7-14 days, with subsequent clearance of EBV viremia and resolution of disease. In nonresponders, EBV-CTLps did not increase and EBV viremia persisted. Treatment failures were correlated with impaired T-cell recognition of tumor targets. Either donor-derived EBV-CTLs that had been sensitized with autologous BLCLs transformed by EBV strain B95.8 could not lyse spontaneous donor-derived EBV-transformed BLCLs expanded from the patient's blood or biopsied tumor or they failed to lyse their targets because they were selectively restricted by HLA alleles not shared by the EBV-LPD. Therefore, either unselected DLIs or EBV-specific CTLs can eradicate both untreated and Rituxan-resistant lymphomatous EBV-LPD, with failures ascribable to impaired T-cell recognition of tumor-associated viral antigens or their presenting HLA alleles.
Introduction
EBV-induced lymphoproliferative diseases (EBV-LPDs) are a significant cause of morbidity and mortality for recipients of allogeneic hematopoietic cell transplantation (HCT), particularly in those who have received certain T-cell reactive Abs to prevent or treat GVHD,1,2 T cell–depleted HCT,3,4 or cord blood transplantation (CBT).5 EBV-LPDs in HCT recipients present as rapidly progressive, monoclonal, diffuse large B-cell lymphomas (DLBCLs).6 Combination chemotherapies can induce sustained remissions in 40%-50% of cases but there is a risk for suppression of the HCT.7,8 The CD20-specific mAb rituximab administered preemptively can induce sustained reversal of EBV viremia in up to 93% of patients.9 However, only 50%-60% of patients with clinically and radiologically established disease achieve durable remissions.10
In 1994, our group reported 5 patients with monoclonal EBV lymphomas who achieved durable complete remission (CR) after adoptive transfer of PBMCs containing unselected T cells from their EBV-seropositive transplantation donors (donor leukocyte infusions or DLIs).11 Other studies subsequently confirmed these results.12-14 However, a recent review of reported cases suggested that only 41% of patients with established disease achieve sustained CR.14 Rooney et al introduced the use of EBV-specific cytotoxic T cells (EBV-CTLs) generated in vitro to reconstitute EBV-specific immunity without GVHD.15 To date, they have administered EBV-CTLs as prophylaxis to 101 patients at risk, and none of the patients has developed EBV-LPD or GVHD. Rooney et al also successfully treated 11 of 13 patients with EBV viremia and clinical evidence of EBV-LPD. Of these patients, 8 were biopsy proven and of these, 6 achieved CR.16 Prior cumulative reported experience for treatment of established EBV-LPD (reviewed in Merlo et al17 ) comprises only 11 additional cases. Although this experience suggests that EBV-CTLs can induce regression of biopsy-proven EBV-LPD in 50%-70% of patients, investigators have still not adequately defined the clinical, virologic, and immunologic parameters associated with treatment response or failure. Furthermore, studies have neither compared the relative efficacy of DLIs and EBV-CTLs nor analyzed their activity in patients who have failed to respond to rituximab.
In the present study, we report a single-center experience with a cohort of 49 patients who were treated with DLI, EBV-CTL, or both for biopsy-proven EBV-LPD emerging after allogeneic HCT. We also analyze attributes of the disease, its prior treatment, and the T cells used for adoptive therapy that contribute to tumor response or continued progression of disease.
Methods
Patients
A total of 49 patients received either DLI or EBV-CTL or both between 1991 and 2009 as treatment for biopsy-proven EBV-LPD that developed after an allogeneic HCT. All patients and donors gave consent and were treated according to protocols approved by the Institutional Review/Privacy Board at Memorial Sloan Kettering Cancer Center and, for recipients of EBV-CTLs, the Food and Drug Administration and the National Marrow Donor Program. Of 49 patients, 17 received EBV-CTLs alone, 27 DLIs alone, 3 DLIs followed by EBV-CTLs, and 2 EBV-CTLs followed by DLIs. Twenty-one patients (46.7%) had received rituximab before T-cell therapies with no or short-lived responses followed by disease progression. Thirty-five patients (73%) were receiving no immunosuppressive drugs at the initiation of T-cell therapy; 14 were receiving cyclosporine (n = 3), sirolimus (n = 5), and/or steroids (n = 9) as treatment for GVHD or to reduce edema complicating a lymphoma of the CNS. In this analysis of our complete series, we have included the 5 patients treated with DLIs reported in 199411 (patients 38, 46, 47, 48, and 49) and 2 recently reported CBT recipients who received third party EBV-CTLs18 (patients 16 and 17).
Diagnosis and characterization of EBV-LPD
All patients had at least 1 diagnostic biopsy. Tumors were classified according to the histologic criteria of Knowles et al.19 Biopsy specimens were tested for EBV by immunohistochemical stains for Latent Membrane Protein-1 (LMP-1) and/or by chromogenic in situ hybridization for EBV-encoded early RNAs as described previously.20,21
Whenever possible, we examined the EBV+ tumor cells for clonality of the B cells, clonality of the EBV genome, and host or donor origin. DNA was extracted from snap-frozen tissue using an Oncor Probe-Tech vacuum blotting device (Oncor). The genetic origin of the lymphoma was analyzed by ascertainment of HLA type or definition of donor or host unique autosomal or sex chromatin polymorphisms by PCR-amplified restriction fragment length polymorphisms derived from a variable number of tandem repeats as described previously.22 B-cell and EBV clonality were determined as described previously.23,24
Treatment with DLIs or EBV-CTLs
Except as noted in Table 3, patients received 1 × 106 CD3+ EBV-CTLs/kg IV weekly for 3 weeks or a one-time infusion of 0.2-1 × 106 unselected CD3+ T cells/kg. DLIs and EBV-CTLs were obtained from HCT donors; EBV-CTLs were generated from HCT donors (n = 14) or third-party donors (n = 5) who specifically consented to donate for this purpose.
EBV-CTLs were generated under Good Manufacturing Practice conditions using a modification of methods described previously.3,25 Briefly, T cells were enriched from PBMCs by depletion of monocytes by adherence to plastic and natural killer (NK) cells by adsorption to anti-CD56 immunomagnetic beads (Miltenyi Biotec).25 T cells were sensitized in vitro at a 20:1 responder:stimulator ratio with irradiated autologous EBV transformed B cells (EBV-BLCLs) generated previously by transformation with the B95.8 strain of EBV26 (kindly provided by C. Rooney, Baylor College of Medicine). T cells were then cultured in Yssel medium (Gemini Bioproducts) supplemented with 5% heat-inactivated pooled normal human serum and restimulated with the same EBV-BLCLs weekly at a 4:1 responder:stimulator ratio.
Beginning on day 16, IL2 (Novartis) was added at 10-50 IU/mL 3 times/wk. After 28-35 days of culture, the T cells were characterized by flow cytometry using mAbs against CD3, CD4, CD8, CD56, CD19, TCRa/b, CD28, and CD45RA (BD Biosciences).25 The EBV-specific cytotoxicity, lack of alloreactivity, and HLA restrictions of the EBV-CTLs were identified by assessing their cytotoxicity against autologous donor- and patient-derived EBV+ BLCL and EBV− phytohemagglutinin (PHA) blasts and thereafter against a panel of allogeneic EBV-BLCLs, each matching one of the HLA alleles expressed by the T cells.27
The frequency of EBV-specific T cells in DLIs and EBV-CTLs was measured by limiting dilution analysis for EBV-specific CTL precursors (CTLps) and by FACS-based quantitation of T cells producing intracellular IFN-γ in response to secondary stimulation with autologous EBV-BLCLs, compared with autologous PHA blasts.3,27,28
Monitoring of patients
All patients were monitored sequentially for response by clinical assessments; imaging with CT, PET/CT, and/or MRI if clinically indicated; quantitation of peripheral blood EBV DNA copy numbers; and measurements of EBV-specific T-cell frequencies before and at intervals after the EBV-CTL and/or DLI therapy. Patients were also monitored closely for toxicities using the National Cancer Institute common toxicity grading criteria30 and for acute GVHD as graded by the Glucksberg criteria.31
Statistical methods
The Fisher exact test and the Wilcoxon rank sum test were used to examine covariate differences between treatment groups and between responses and in subgroup analyses to examine associations between clinical factors and response outcomes according to treatment groups. The cumulative incidence function was used to estimate the probabilities for time from primary T-cell therapy to death because of EBV, development of acute GVHD, and or development of chronic GVHD.34 Deaths from other causes were considered as competing events. The Gray test was used to determine whether the cumulative incidence curves differed by therapy administered.35 The software packages SAS Version 9.1 and R Version 2.3.1 were used to compute the test statistics.
Results
Patient characteristics
Forty-nine patients were treated primarily with DLIs (n = 30) or EBV-CTLs (n = 19). Patients who received both cell therapies were included in the treatment group of the initial cell therapy that they received for comparisons of the 2 therapy modalities. The characteristics of the patients and the clinical and radiologic presentation of EBV-LPD for all patients in each treatment group are summarized in Table 1. These groups differed significantly in 2 respects: (1) the median age at time of treatment for the DLI group was 33 years versus 13 years for the EBV-CTL group (P = .01), and (2) the DLI group consisted predominantly of recipients of HLA-matched transplantations (90%), whereas the EBV-CTL group mainly comprised patients receiving HLA-disparate grafts (68%; P < .03), who would have been at high risk of severe GVHD if treated with unselected DLIs. Patients treated with DLIs were somewhat less likely to have received rituximab before cell therapy (P = .08). There was no difference in sex (P = .13) or number of sites involved with disease (P = .08) between the 2 treatment groups. All but 6 patients had received T cell–depleted HCT. One patient in each group had received an unmodified PBSC graft; 3 patients in the EBV-CTL group had received an umbilical cord blood (UCB) transplantation.
Patient general characteristics and clinical features
| Variable . | All (n = 49) . | DLI (n = 30) . | EBV-CTL (n = 19) . | P . |
|---|---|---|---|---|
| Median age at T-cell treatment, y (IQR) | 29 (16-47) | 33 (24-52) | 13 (8-44) | < .01 |
| Sex | ||||
| Female | 18 (36) | 14 (47) | 4 (21) | .13 |
| Male | 31 (64) | 16 (53) | 15 (79) | |
| HLA match | ||||
| MMREL or MMUREL | 16 (33) | 3 (10) | 13 (68) | < .01 |
| MREL | 21 (43) | 18 (60) | 3 (16) | |
| MUR | 12 (24) | 9 (30) | 3 (16) | |
| BMT type | ||||
| T-cell depleted | 43 (88) | 29 (97) | 14 (74) | .03 |
| Others | 6 (12) | 1 (3) | 5 (26) | |
| Immunosuppressive medication* | 14 (28.6) | 6 (20) | 8 (42) | .32 |
| Presenting symptoms and/or PE findings | ||||
| Fever | 30 (61) | 18 (60) | 12 (63) | |
| Lymphoadenopathy | 21 (43) | 15 (50) | 6 (31) | |
| Enlarged tonsils/sore throat | 15 (31) | 11 (37) | 4 (21) | |
| EBV PCR positivity | 12 (24) | 6 (20) | 6 (31) | |
| Abdominal pain | 8 (16) | 5 (16) | 3 (16) | |
| Nausea/vomiting | 6 (12) | 4 (13) | 2 (11) | |
| Neurologic symptoms† | 4 (9) | 2 (6) | 2 (11) | |
| Hepatomegaly and/or splenomegaly | 4 (9) | 1 (3) | 3 (16) | |
| Jaundice | 3 (6) | 3 (10) | 0 (0) | |
| Diarrhea | 3 (6) | 1 (3) | 2 (11) | |
| None | 2 (4) | 1 (3) | 1 (5) | |
| Sites involved in disease (clinical and/or radiologic) | ||||
| Lymph nodes | 38 (78) | 23 (77) | 15 (79) | |
| Waldeyer ring | 21 (43) | 16 (53) | 5 (26) | |
| Liver | 16 (33) | 9 (30) | 7 (37) | |
| Lung | 18 (36) | 14 (47) | 4 (21) | |
| Gastrointestinal tract | 14 (29) | 8 (26) | 6 (31) | |
| Spleen | 7 (14) | 4 (13) | 3 (16) | |
| CNS/spine | 6 (12) | 4 (13) | 2 (11) | |
| Pancreas | 1 (2) | 1 (3) | 0 (0) | |
| Thyroid | 1 (2) | 1 (3) | 0 (0) | |
| Larynx | 1 (2) | 1 (3) | 0 (0) | |
| Median time to EBV-LPD after HSCT, d (IQR) | 102.5 (64-247) | 113 (84-138) | 91 (64-247) | |
| Variable . | All (n = 49) . | DLI (n = 30) . | EBV-CTL (n = 19) . | P . |
|---|---|---|---|---|
| Median age at T-cell treatment, y (IQR) | 29 (16-47) | 33 (24-52) | 13 (8-44) | < .01 |
| Sex | ||||
| Female | 18 (36) | 14 (47) | 4 (21) | .13 |
| Male | 31 (64) | 16 (53) | 15 (79) | |
| HLA match | ||||
| MMREL or MMUREL | 16 (33) | 3 (10) | 13 (68) | < .01 |
| MREL | 21 (43) | 18 (60) | 3 (16) | |
| MUR | 12 (24) | 9 (30) | 3 (16) | |
| BMT type | ||||
| T-cell depleted | 43 (88) | 29 (97) | 14 (74) | .03 |
| Others | 6 (12) | 1 (3) | 5 (26) | |
| Immunosuppressive medication* | 14 (28.6) | 6 (20) | 8 (42) | .32 |
| Presenting symptoms and/or PE findings | ||||
| Fever | 30 (61) | 18 (60) | 12 (63) | |
| Lymphoadenopathy | 21 (43) | 15 (50) | 6 (31) | |
| Enlarged tonsils/sore throat | 15 (31) | 11 (37) | 4 (21) | |
| EBV PCR positivity | 12 (24) | 6 (20) | 6 (31) | |
| Abdominal pain | 8 (16) | 5 (16) | 3 (16) | |
| Nausea/vomiting | 6 (12) | 4 (13) | 2 (11) | |
| Neurologic symptoms† | 4 (9) | 2 (6) | 2 (11) | |
| Hepatomegaly and/or splenomegaly | 4 (9) | 1 (3) | 3 (16) | |
| Jaundice | 3 (6) | 3 (10) | 0 (0) | |
| Diarrhea | 3 (6) | 1 (3) | 2 (11) | |
| None | 2 (4) | 1 (3) | 1 (5) | |
| Sites involved in disease (clinical and/or radiologic) | ||||
| Lymph nodes | 38 (78) | 23 (77) | 15 (79) | |
| Waldeyer ring | 21 (43) | 16 (53) | 5 (26) | |
| Liver | 16 (33) | 9 (30) | 7 (37) | |
| Lung | 18 (36) | 14 (47) | 4 (21) | |
| Gastrointestinal tract | 14 (29) | 8 (26) | 6 (31) | |
| Spleen | 7 (14) | 4 (13) | 3 (16) | |
| CNS/spine | 6 (12) | 4 (13) | 2 (11) | |
| Pancreas | 1 (2) | 1 (3) | 0 (0) | |
| Thyroid | 1 (2) | 1 (3) | 0 (0) | |
| Larynx | 1 (2) | 1 (3) | 0 (0) | |
| Median time to EBV-LPD after HSCT, d (IQR) | 102.5 (64-247) | 113 (84-138) | 91 (64-247) | |
Values in parenthesis are in raw percentages unless stated otherwise.
MMREL indicates mismatch related; MMUREL, mismatch unrelated; MREL, match related; and MUR, match unrelated.
Calcineurin inhibitor and/or steroids.
Headache/hemiparesis/lethargy/dysphasia.
Clinical features at diagnosis of EBV-LPD
As summarized in Table 1, the EBV-LPD emerged between 2 and 8 months after transplantation; the median time to diagnosis was 113 days for the DLI group and 91 days for the EBV-CTL group (P = NS). The median time from diagnosis of posttransplantation EBV-LPD to initial cell therapy was 9 days for patients treated with DLIs and 20 days for those treated with EBV-CTLs, reflecting the more immediate accessibility of the DLIs (P = nonsignificant).
Fever was the most common presenting symptom, occurring in 61% of the patients. Lymphadenopathy or enlarged tonsils/sore throat were presenting features in 43% and 31% of all patients, respectively. Other presenting symptoms summarized in Table 1 were observed in 4%-16% of cases. However, 4% of all patients were asymptomatic at the time of diagnosis. In these patients, the EBV-LPD was discovered while monitoring for increased levels of EBV DNA in the blood. Overall, an elevated level of EBV DNA was detected in the blood in 29 of 30 (97%) patients tested between diagnosis and initiation of any therapy for EBV-LPD.
Cervical lymph nodes and the lymphoid tissue in the Waldeyer ring were the sites most frequently involved by disease at diagnosis, followed by liver, lung parenchyma, GI tract, spleen, CNS/spine, pancreas, and larynx (Table 1). Sites of involvement were grouped into 5 categories for statistical analysis: CNS, liver, lung, abdomen/intestine, and Waldeyer ring. We included in the abdomen/intestine group patients with intraabdominal and pelvic lymphadenopathy with or without involvement of the spleen or the gastrointestinal tract. The Waldeyer group included patients with involvement of Waldeyer ring as well as cervical, post-/pre-auricular, supraclavicular, or intrathoracic lymph nodes without involvement of the lung parenchyma. Parenchymal involvement of the liver and lung were each grouped separately.
EBV-LPDs involving the CNS included those affecting the brain and/or the spinal cord.
Pathologic characteristics of posttransplantation EBV-LPD
Histopathologic properties of the EBV+ lymphomas are described in Table 2 and were similar in both treatment groups. Of the 49 patients, 45 (92%) presented with DLBCL. In the EBV-CTL group, 1 patient each had focal lymphoid hyperplasia, anaplastic large cell lymphoma, or EBV-LPD of the Hodgkin type. In the DLI group, 1 patient had polymorphic lymphoplasmacytic–type lymphoma. The lymphoma was monoclonal in 30 of 33 patients adequately tested and was composed of cells exclusively of donor origin in 24 of 30 patients (80%) or a mixture of cells of mostly donor origin in 4 of 30 patients (13%) tested; only 2 of 30 were of host origin (Table 2). The EBV genome was clonal in all but 2 of the 25 tumors adequately evaluated.
Histopathologic characteristics of EBV-LPD
| Characteristic . | EBV-CTL recipients (n = 19) . | DLI recipients (n = 30) . |
|---|---|---|
| Time to EBV-LPD after HSCT, d, median (IQR) | 91 (64-247) | 114 (84-138) |
| Histology | ||
| DLBL | 16 | 29 |
| FLH | 1 | 0 |
| PLPL | 0 | 1 |
| NHL | 1 | 0 |
| HD | 1 | 0 |
| Origin | ||
| Host | 1 | 1 |
| Donor | 10 | 14 |
| Mixed | 1 | 3 |
| Unknown | 7 | 12 |
| Clonality | ||
| EBV clonal | 7 of 7 tested | 16 of 18 tested |
| Ig clonal | 11 of 11 tested | 19 of 22 tested |
| Characteristic . | EBV-CTL recipients (n = 19) . | DLI recipients (n = 30) . |
|---|---|---|
| Time to EBV-LPD after HSCT, d, median (IQR) | 91 (64-247) | 114 (84-138) |
| Histology | ||
| DLBL | 16 | 29 |
| FLH | 1 | 0 |
| PLPL | 0 | 1 |
| NHL | 1 | 0 |
| HD | 1 | 0 |
| Origin | ||
| Host | 1 | 1 |
| Donor | 10 | 14 |
| Mixed | 1 | 3 |
| Unknown | 7 | 12 |
| Clonality | ||
| EBV clonal | 7 of 7 tested | 16 of 18 tested |
| Ig clonal | 11 of 11 tested | 19 of 22 tested |
FLH indicates focal lymphoid hyperplasia; PLPC, polymorphic lymphoplasmocytic lymphoma; NHL, nonHodgkin lymphoma; and HD, Hodgkin disease.
Characterization of EBV-CTLs and DLIs infused
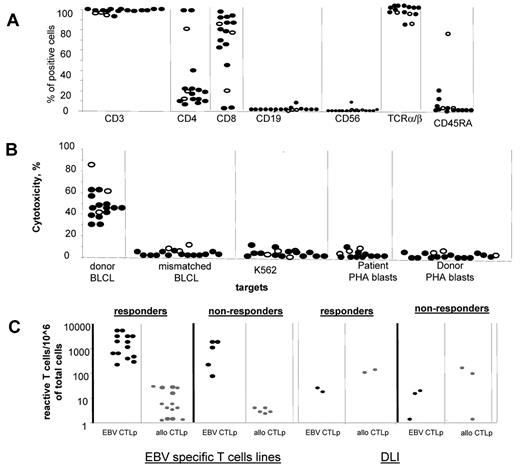
The populations of EBV-CTLs generated in vitro were 90% CD3+ T cells and contained < 5% CD3−CD56+ NK cells and < 1% CD19+ B cells (Figure 1A). Although the majority of EBV-CTL lines contained more than 90% CD8+ T cells, 3 CTL lines comprised mostly CD4+ T cells (> 90% of the cell population). All T-cell lines, including those predominantly containing CD4+ T cells, demonstrated EBV-specific cytotoxic activity against autologous EBV-BLCLs and did not kill NK cell–sensitive targets (K562), EBV-negative autologous or recipient-derived PHA blasts, or HLA-mismatched EBV-BLCLs (Figure 1B). In limiting dilution assays, the EBV-CTLs generated in vitro for 28-35 days contained high frequencies of EBV-CTLps (median, 1156 EBV-CTLps/106 cells; range, 66-6578 EBV-CTLps/106 cells), but low or undetectable alloreactive CTLps (median, 5.6; range, < 1.28-29 allo CTLps/106 cells; Figure 1C). In contrast, DLIs contained higher frequencies of major alloantigen–reactive CTLps (median, 97; range, 2.8-223 allo CTLps/106 cells) and much lower frequencies of the EBV-CTLps (median, 17; range, 5.7-33 EBV-CTLps/106 cells).
Characterization of the EBV specific T-cell lines. (A) Phenotype of the EBV specific T-cell lines used for the treatment of EBV-LPD in patients who responded (closed symbols) and did not respond (open symbols) to the T-cell therapy. All EBV-CTL lines infused contained more than 90% of CD3+ cells with predominance of CD8+ T cells. However, 3 CTL lines contained predominantly CD4+ T cells. Infusion of the CD4+ T cell–predominant lines achieved CR in 2 of the 3 patients infused. All T-cell lines were equally depleted of NK and B cells. (B) Cytotoxic activity of the EBV-CTL lines used for the treatment of EBV-LPD of those patients who responded (closed symbols) and those who did not respond (open symbols) to the T-cell therapy. (C) Frequencies of EBV-specific T cells (black symbols) and alloreactive (gray symbols) detected by limiting dilution analysis in EBV-specific T-cell lines and DLI products before their use for the treatment of EBV-LPD demonstrate higher frequencies of EBV-specific T cells and lower frequencies of alloreactive T cells in the EBV-specific T-cell lines than in the unstimulated donor leukocytes. There were no differences in the frequencies of EBV-specific and alloreactive cells between the cell products used in responders and nonresponders. (D) All EBV-CTL lines infused exhibited exclusively EBV-specific cytotoxicity without any activity against recipient PHA-activated blasts, mismatched EBV-BLCLs, and K562 (a target for NK cells).
Characterization of the EBV specific T-cell lines. (A) Phenotype of the EBV specific T-cell lines used for the treatment of EBV-LPD in patients who responded (closed symbols) and did not respond (open symbols) to the T-cell therapy. All EBV-CTL lines infused contained more than 90% of CD3+ cells with predominance of CD8+ T cells. However, 3 CTL lines contained predominantly CD4+ T cells. Infusion of the CD4+ T cell–predominant lines achieved CR in 2 of the 3 patients infused. All T-cell lines were equally depleted of NK and B cells. (B) Cytotoxic activity of the EBV-CTL lines used for the treatment of EBV-LPD of those patients who responded (closed symbols) and those who did not respond (open symbols) to the T-cell therapy. (C) Frequencies of EBV-specific T cells (black symbols) and alloreactive (gray symbols) detected by limiting dilution analysis in EBV-specific T-cell lines and DLI products before their use for the treatment of EBV-LPD demonstrate higher frequencies of EBV-specific T cells and lower frequencies of alloreactive T cells in the EBV-specific T-cell lines than in the unstimulated donor leukocytes. There were no differences in the frequencies of EBV-specific and alloreactive cells between the cell products used in responders and nonresponders. (D) All EBV-CTL lines infused exhibited exclusively EBV-specific cytotoxicity without any activity against recipient PHA-activated blasts, mismatched EBV-BLCLs, and K562 (a target for NK cells).
Clinical effects of EBV-CTLs and DLIs
The individual patients, their treatment before cell therapy, and the primary cell therapy given, together with their clinical outcomes and survival, are summarized in Table 3. Response to therapy was classified as CR, partial remission (PR), stable disease (SD), or progressive disease (PD) using the International Workshop Criteria for assessing response to treatment in nonHodgkin lymphoma.36 Of 30 patients primarily treated with DLIs, 17 achieved a CR and 1 a stable PR (73% CR or PR). This includes 2 patients in our original series (patients 47 and 48) who died of interstitial pneumonia antedating their treatment with DLIs 7 and 17 days after treatment. In both cases, at autopsy, no residual lymphoma was detected.11 An additional patient (patient 44) had stabilization of CNS disease. Of 19 patients primarily treated with EBV-CTLs, 13 (68%) achieved CR. Therefore, the rate of response (CR + PR) for each group were equivalent. Four patients with multiorgan involvement died soon after initial cell infusion before the cells could exert any effects. These included 1 patient in the EBV-CTL group who died of disease progression 8 days after EBV-CTL infusion and 3 patients in the DLI group who died of disease progression, GVHD predating DLIs, or complications of a lung biopsy at 4, 5, and 7 days after cell infusion. Among those patients surviving ≥ 8 days, the rates of response were 74% for those treated with DLIs and 72% for those treated with EBV-CTLs (P = NS).
Patient, treatment, and outcome specifics
| Patient no. . | Donor . | Primary diagnosis . | Sites involved in EBV-LPD . | Histology of EBV-LPD . | Origin of LPD . | Tumor clonal . | Rituximab prior to primary T-cell therapy . | Response to rituximab . | Time to primary T-cell therapy, d . | Primary T-cell therapy . | T-cell origin . | T-cell dose, CD3+ × 106/kg . | Infusions, no. . | Response to primary T-cell therapy . | Survival after T-cell therapy, d . | Status . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10/10MREL | ALL | WR,A/I | FLH | N/A | N/A | Y | PR | 16 | CTL | 3rd | 1.0 | 3 | CR | 357 | Alive | N/A |
| 2 | 6/10MMUR | AA | Li,Lu | DLBL | N/A | N/A | Y | Failed | 163 | CTL | SC | 1.0 | 1 | CR | 127 | Dead | GVHD predated CTL |
| 3 | 10/10MREL | ALPS type IA,HD | Li,A/I | HD | M | Y | N | N/A | 5 | CTL | SC | 1.0 | 1 | NE | 8 | Dead | Relapsed HD |
| 4 | 8/10MMREL | ALL | Li,A/I | ALCL | D | N/A | N | N/A | 4 | CTL | SC | 0.1 | 2 | PD | 16 | Dead | Sepsis |
| 0.4 | 1 | ||||||||||||||||
| 5 | 10/10MUR | AML | WR,A/I | DLBL | D | Y | Y | PR | 39 | CTL | SC | 1.0 | 3 | CR | 187 | Alive | N/A |
| 6 | 4/6MMUR | AML | WR, LI, Lu, CNS, A/I | DLBL | D | N/A | Y | Failed | 110 | CTL | 3rd | 1.0 | 9 | CR | 203 | Alive | N/A |
| 7 | 9/10MMUR | AML | WR | DLBL | D | Y | Y | GIR | 69 | CTL | SC | 1.0 | 3 | CR | 290 | Dead | Relapsed AML |
| 8 | 5/10MMREL | EBV+ HLH | A/I | DLBL | H | Y | Y | Failed | 1 (89)* | CTL | 3rd | 1.0 | 3 | CR | 217 | Alive | N/A |
| 9 | 3/6MMREL | AML | WR,A/I | DLBL | N/A | Y | Y | GIR | 312 | CTL | SC | 1.0 | 1 | CR | 2066 | Alive | N/A |
| 10 | 6/6MUR | CML | CNS | DLBL | N/A | N/A | N | N/A | 22 | CTL | SC | 1.0 | 1 | CR | 4848 | Alive | N/A |
| 11 | 7/10MMREL | FA | WR | DLBL | D | N/A | Y | Failed | 1 | CTL | SC | 1.0 | 3 | CR | 3569 | Alive | N/A |
| 12 | 8/10MMUR | AML | WR, A/I | DLBL | N/A | Y | Y | Failed | 17 | CTL | SC | 0.7 | 1 | PD | 17 | Dead | EBV-LPD |
| 0.96 | 2 | ||||||||||||||||
| 13 | 5/6MMUR | MLD | A/I,WR | DLBL | D | Y | Y | Failed | 161 | CTL | 3rd | 1.0 | 3 | PD | 24 | Dead | EBV-LPD |
| 14 | 7/10MMREL | ALL | WR, Li, Lu | DLBL | D | Y | Y | GIR | 26 | CTL | SC | 0.5 | 1 | PD | 29 | Dead | EBV-LPD |
| 1.0;1.0;1.0 | 3 | ||||||||||||||||
| 15 | 4/4MUR | AA | WR | DLBL | N/A | N/A | N | N/A | 9 | CTL | SC | 1.0 | 3 | CR | 719 | Dead | Primary Graft Failure |
| 16 | 8/10MMUR | AML | WR | DLBL | N/A | Y | N | N/A | 6 | CTL | SC | 1.0 | 3 | CR | 2006 | Alive | N/A |
| 17 | 8/10MMUR | T-cell NHL | WR, A/I, Lu | DLBL | D | N/A | Y | Failed | 29 | CTL | 3rd | 0.85 | 3 | CR | 476 | Alive | N/A |
| 1.0 | 2 | ||||||||||||||||
| 18 | 10/10MREL | MDS/AML | WR,A/I | DLBL | D | Y | Y | Failed | 70 | DLI | SC | 0.5 | 1 | PD | 17 | Dead | EBV-LPD |
| 19 | 10/10MREL | CML | WR,A/I | DLBL | M | Y | N | N/A | 7 | DLI | SC | 1.0 | 1 | CR | 4970 | Alive | N/A |
| 20 | 8/10MMUR | AML | Li,Lu | DLBL | D | Y | Y | Failed | 28 | DLI | SC | 0.095 | 1 | NE | 5 | Dead | GVHD |
| 21 | 8/8MREL | ABL | WR,A/I | DLBL | N/A | Y | N | N/A | 9 | DLI | SC | 1.0 | 1 | CR | 188 | Dead | Interstitial pneumonia |
| 22 | 5/6MMREL | CML | WR, Lu | DLBL | D | N/A | N | N/A | 16 | DLI | SC | 0.5 | 1 | CR | 2742 | Alive | N/A |
| 23 | 6/6MUR | AML | WR | DLBL | D | Y | N | N/A | 4 | DLI | SC | 0.5 | 1 | CR | 4573 | Alive | N/A |
| 24 | 10/10MREL | HD | WR, Lu | DLBL | D | Y | N | N/A | 3 | DLI | SC | 1.0 | 1 | CR | 2143 | Dead | Relapsed HD |
| 25 | 10/10MREL | AML | Li,A/I | DLBL | M | Y | N | N/A | 8 | DLI | SC | 0.5 | 1 | CR | 3045 | Alive | N/A |
| 26 | 10/10MREL | CML | WR, A/I | DLBL | D | Y | N | N/A | 5 | DLI | SC | 1.0 | 1 | CR | 3310 | Dead | Relapsed CML |
| 27 | 6/6MUR | ALL | Li, A/I, Lu | DLBL | D | N/A | N | N/A | 3 | DLI | SC | 0.5 | 1 | CR | 487 | Dead | Relapsed ALL |
| 28 | 8/8MREL | AML | WR,Li,Lu | DLBL | N/A | Y | N | N/A | 46 | DLI | SC | 1.0 | 1 | CR | 4203 | Alive | N/A |
| 29 | 10/10MREL | SecAML | WR, A/I | PLPL | D | N | Y | GIR | 8 | DLI | SC | 0.1 | 1 | CR | 887 | Alive | N/A |
| 30 | 6/6MUR | CML | A/I | DLBL | N/A | N/A | N | N/A | 14 | DLI | SC | 0.5 | 1 | CR | 801 | Dead | GVHD |
| 31 | 6/6MREL | CML | WR, Lu | DLBL | N/A | Y | N | N/A | 1 | DLI | SC | 0.5 | 1 | NE | 4 | Dead | Hemothorax post lung biopsy |
| 32 | 6/6MUR | CML | WR, A/I, Li | DLBL | D | Y | N | N/A | 40 | DLI | SC | 0.5 | 1 | PD | 15 | Dead | Sepsis |
| 33 | 6/6MUR | CML | CNS, Lu | DLBL | N/A | N/A | N | N/A | 1 | DLI | SC | 0.21 | 1 | CR | 407 | Dead | Relapsed CML |
| 0.37 | 1 | ||||||||||||||||
| 34 | 10/10MREL | MDS/RAEB | WR | DLBL | N/A | N/A | Y | Failed | 31 | DLI | SC | 0.5 | 1 | CR | 60 | Dead | Fungemia |
| 35 | 10/10MUR | AML | WR, Li, A/I, Lu | DLBL | N/A | Y | Y | Failed | 70 | DLI | SC | 0.5 | 1 | PD | 14 | Dead | EBV-LPD |
| 36 | 6/6MUR | ALL | Lu | DLBL | N/A | Y | Y | Failed | 13 | DLI | SC | 0.5 | 1 | CR | 183 | Dead | Sepsis |
| 37 | 6/6MREL | AML | WR | DLBL | N/A | N/A | N | N/A | 8 | DLI | SC | 0.5 | 1 | CR | 5007 | Alive | N/A |
| 38 | 10/10MREL | CML | A/I | DLBL | N/A | N/A | N | N/A | 9 | DLI | SC | 0.79 | 1 | CR | 21 | Alive | N/A |
| 39 | 10/10MREL | AML | Li, A/I | DLBL | D | Y | N | N/A | 4 | DLI | SC | 0.5 | 1 | NE | 7 | Dead | EBV-LPD |
| 1.0 | 1 | ||||||||||||||||
| 40 | 6/6MUR | CML | WR | DLBL | M | Y | N | N/A | 3 | DLI | SC | 0.33 | 1 | CR | 606 | Dead | Relapsed CML |
| 41 | 10/10MREL | XLPD | WR, A/I, Li | DLBL | D | Y | N | N/A | 4 | CTL | SC | 1.0 | 3 | PD | 47 | Dead | EBV-LPD |
| 42 | 9/10MMREL | NHL | Li, A/I | DLBL | D | Y | Y | N/A | 20 | CTL | SC | 1.0 | 3 | CR | 3190 | Alive | N/A |
| 43 | 8/10MMREL | ALL | WR, CNS | DLBL | D | Y | Y | Failed | 32 | DLI | SC | 0.5 | 1 | PD | 593 | Dead | Sepsis |
| 44 | 10/10MREL | AML | WR, CNS | DLBL | N/A | Y | Y | GIR | 63 | DLI | SC | 0.5 | 2 | SD | 85 | Dead | EBV-LPD |
| 45 | 10/10MUR | ALL | WR, A/I, CNS | DLBL | H | Y | Y | Failed | 17 | DLI | SC | 0.1 | 1 | PD | 144 | Dead | EBV-LPD |
| 46 | 10/10MREL | ALL | WR, Lu, Li, A | DLBL | N/A | N/A | N | N/A | 15 | DLI | SC | 0.55 | 1 | CR | 6149 | Alive | N/A |
| 0.35 | 1 | ||||||||||||||||
| 0.12 | 1 | ||||||||||||||||
| 47 | 10/10MREL | CML | Lu,WR,A | DLBL | D | Y | N | N/A | 13 | DLI | SC | 1.0 | 2 | CR | 7 | Dead | Interstitial pneumonia |
| 48 | 10/10MREL | ANLL | WR,Lu | DLBL | D | N | N | N/A | 6 | DLI | SC | 2.0 | 1 | CR | 17 | Dead | Interstitial pneumonia |
| 49 | 10/10MREL | ANLL | WR | DLBL | D | N/A | N | N/A | 13 | DLI | SC | 1.0 | 1 | CR |
| Patient no. . | Donor . | Primary diagnosis . | Sites involved in EBV-LPD . | Histology of EBV-LPD . | Origin of LPD . | Tumor clonal . | Rituximab prior to primary T-cell therapy . | Response to rituximab . | Time to primary T-cell therapy, d . | Primary T-cell therapy . | T-cell origin . | T-cell dose, CD3+ × 106/kg . | Infusions, no. . | Response to primary T-cell therapy . | Survival after T-cell therapy, d . | Status . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10/10MREL | ALL | WR,A/I | FLH | N/A | N/A | Y | PR | 16 | CTL | 3rd | 1.0 | 3 | CR | 357 | Alive | N/A |
| 2 | 6/10MMUR | AA | Li,Lu | DLBL | N/A | N/A | Y | Failed | 163 | CTL | SC | 1.0 | 1 | CR | 127 | Dead | GVHD predated CTL |
| 3 | 10/10MREL | ALPS type IA,HD | Li,A/I | HD | M | Y | N | N/A | 5 | CTL | SC | 1.0 | 1 | NE | 8 | Dead | Relapsed HD |
| 4 | 8/10MMREL | ALL | Li,A/I | ALCL | D | N/A | N | N/A | 4 | CTL | SC | 0.1 | 2 | PD | 16 | Dead | Sepsis |
| 0.4 | 1 | ||||||||||||||||
| 5 | 10/10MUR | AML | WR,A/I | DLBL | D | Y | Y | PR | 39 | CTL | SC | 1.0 | 3 | CR | 187 | Alive | N/A |
| 6 | 4/6MMUR | AML | WR, LI, Lu, CNS, A/I | DLBL | D | N/A | Y | Failed | 110 | CTL | 3rd | 1.0 | 9 | CR | 203 | Alive | N/A |
| 7 | 9/10MMUR | AML | WR | DLBL | D | Y | Y | GIR | 69 | CTL | SC | 1.0 | 3 | CR | 290 | Dead | Relapsed AML |
| 8 | 5/10MMREL | EBV+ HLH | A/I | DLBL | H | Y | Y | Failed | 1 (89)* | CTL | 3rd | 1.0 | 3 | CR | 217 | Alive | N/A |
| 9 | 3/6MMREL | AML | WR,A/I | DLBL | N/A | Y | Y | GIR | 312 | CTL | SC | 1.0 | 1 | CR | 2066 | Alive | N/A |
| 10 | 6/6MUR | CML | CNS | DLBL | N/A | N/A | N | N/A | 22 | CTL | SC | 1.0 | 1 | CR | 4848 | Alive | N/A |
| 11 | 7/10MMREL | FA | WR | DLBL | D | N/A | Y | Failed | 1 | CTL | SC | 1.0 | 3 | CR | 3569 | Alive | N/A |
| 12 | 8/10MMUR | AML | WR, A/I | DLBL | N/A | Y | Y | Failed | 17 | CTL | SC | 0.7 | 1 | PD | 17 | Dead | EBV-LPD |
| 0.96 | 2 | ||||||||||||||||
| 13 | 5/6MMUR | MLD | A/I,WR | DLBL | D | Y | Y | Failed | 161 | CTL | 3rd | 1.0 | 3 | PD | 24 | Dead | EBV-LPD |
| 14 | 7/10MMREL | ALL | WR, Li, Lu | DLBL | D | Y | Y | GIR | 26 | CTL | SC | 0.5 | 1 | PD | 29 | Dead | EBV-LPD |
| 1.0;1.0;1.0 | 3 | ||||||||||||||||
| 15 | 4/4MUR | AA | WR | DLBL | N/A | N/A | N | N/A | 9 | CTL | SC | 1.0 | 3 | CR | 719 | Dead | Primary Graft Failure |
| 16 | 8/10MMUR | AML | WR | DLBL | N/A | Y | N | N/A | 6 | CTL | SC | 1.0 | 3 | CR | 2006 | Alive | N/A |
| 17 | 8/10MMUR | T-cell NHL | WR, A/I, Lu | DLBL | D | N/A | Y | Failed | 29 | CTL | 3rd | 0.85 | 3 | CR | 476 | Alive | N/A |
| 1.0 | 2 | ||||||||||||||||
| 18 | 10/10MREL | MDS/AML | WR,A/I | DLBL | D | Y | Y | Failed | 70 | DLI | SC | 0.5 | 1 | PD | 17 | Dead | EBV-LPD |
| 19 | 10/10MREL | CML | WR,A/I | DLBL | M | Y | N | N/A | 7 | DLI | SC | 1.0 | 1 | CR | 4970 | Alive | N/A |
| 20 | 8/10MMUR | AML | Li,Lu | DLBL | D | Y | Y | Failed | 28 | DLI | SC | 0.095 | 1 | NE | 5 | Dead | GVHD |
| 21 | 8/8MREL | ABL | WR,A/I | DLBL | N/A | Y | N | N/A | 9 | DLI | SC | 1.0 | 1 | CR | 188 | Dead | Interstitial pneumonia |
| 22 | 5/6MMREL | CML | WR, Lu | DLBL | D | N/A | N | N/A | 16 | DLI | SC | 0.5 | 1 | CR | 2742 | Alive | N/A |
| 23 | 6/6MUR | AML | WR | DLBL | D | Y | N | N/A | 4 | DLI | SC | 0.5 | 1 | CR | 4573 | Alive | N/A |
| 24 | 10/10MREL | HD | WR, Lu | DLBL | D | Y | N | N/A | 3 | DLI | SC | 1.0 | 1 | CR | 2143 | Dead | Relapsed HD |
| 25 | 10/10MREL | AML | Li,A/I | DLBL | M | Y | N | N/A | 8 | DLI | SC | 0.5 | 1 | CR | 3045 | Alive | N/A |
| 26 | 10/10MREL | CML | WR, A/I | DLBL | D | Y | N | N/A | 5 | DLI | SC | 1.0 | 1 | CR | 3310 | Dead | Relapsed CML |
| 27 | 6/6MUR | ALL | Li, A/I, Lu | DLBL | D | N/A | N | N/A | 3 | DLI | SC | 0.5 | 1 | CR | 487 | Dead | Relapsed ALL |
| 28 | 8/8MREL | AML | WR,Li,Lu | DLBL | N/A | Y | N | N/A | 46 | DLI | SC | 1.0 | 1 | CR | 4203 | Alive | N/A |
| 29 | 10/10MREL | SecAML | WR, A/I | PLPL | D | N | Y | GIR | 8 | DLI | SC | 0.1 | 1 | CR | 887 | Alive | N/A |
| 30 | 6/6MUR | CML | A/I | DLBL | N/A | N/A | N | N/A | 14 | DLI | SC | 0.5 | 1 | CR | 801 | Dead | GVHD |
| 31 | 6/6MREL | CML | WR, Lu | DLBL | N/A | Y | N | N/A | 1 | DLI | SC | 0.5 | 1 | NE | 4 | Dead | Hemothorax post lung biopsy |
| 32 | 6/6MUR | CML | WR, A/I, Li | DLBL | D | Y | N | N/A | 40 | DLI | SC | 0.5 | 1 | PD | 15 | Dead | Sepsis |
| 33 | 6/6MUR | CML | CNS, Lu | DLBL | N/A | N/A | N | N/A | 1 | DLI | SC | 0.21 | 1 | CR | 407 | Dead | Relapsed CML |
| 0.37 | 1 | ||||||||||||||||
| 34 | 10/10MREL | MDS/RAEB | WR | DLBL | N/A | N/A | Y | Failed | 31 | DLI | SC | 0.5 | 1 | CR | 60 | Dead | Fungemia |
| 35 | 10/10MUR | AML | WR, Li, A/I, Lu | DLBL | N/A | Y | Y | Failed | 70 | DLI | SC | 0.5 | 1 | PD | 14 | Dead | EBV-LPD |
| 36 | 6/6MUR | ALL | Lu | DLBL | N/A | Y | Y | Failed | 13 | DLI | SC | 0.5 | 1 | CR | 183 | Dead | Sepsis |
| 37 | 6/6MREL | AML | WR | DLBL | N/A | N/A | N | N/A | 8 | DLI | SC | 0.5 | 1 | CR | 5007 | Alive | N/A |
| 38 | 10/10MREL | CML | A/I | DLBL | N/A | N/A | N | N/A | 9 | DLI | SC | 0.79 | 1 | CR | 21 | Alive | N/A |
| 39 | 10/10MREL | AML | Li, A/I | DLBL | D | Y | N | N/A | 4 | DLI | SC | 0.5 | 1 | NE | 7 | Dead | EBV-LPD |
| 1.0 | 1 | ||||||||||||||||
| 40 | 6/6MUR | CML | WR | DLBL | M | Y | N | N/A | 3 | DLI | SC | 0.33 | 1 | CR | 606 | Dead | Relapsed CML |
| 41 | 10/10MREL | XLPD | WR, A/I, Li | DLBL | D | Y | N | N/A | 4 | CTL | SC | 1.0 | 3 | PD | 47 | Dead | EBV-LPD |
| 42 | 9/10MMREL | NHL | Li, A/I | DLBL | D | Y | Y | N/A | 20 | CTL | SC | 1.0 | 3 | CR | 3190 | Alive | N/A |
| 43 | 8/10MMREL | ALL | WR, CNS | DLBL | D | Y | Y | Failed | 32 | DLI | SC | 0.5 | 1 | PD | 593 | Dead | Sepsis |
| 44 | 10/10MREL | AML | WR, CNS | DLBL | N/A | Y | Y | GIR | 63 | DLI | SC | 0.5 | 2 | SD | 85 | Dead | EBV-LPD |
| 45 | 10/10MUR | ALL | WR, A/I, CNS | DLBL | H | Y | Y | Failed | 17 | DLI | SC | 0.1 | 1 | PD | 144 | Dead | EBV-LPD |
| 46 | 10/10MREL | ALL | WR, Lu, Li, A | DLBL | N/A | N/A | N | N/A | 15 | DLI | SC | 0.55 | 1 | CR | 6149 | Alive | N/A |
| 0.35 | 1 | ||||||||||||||||
| 0.12 | 1 | ||||||||||||||||
| 47 | 10/10MREL | CML | Lu,WR,A | DLBL | D | Y | N | N/A | 13 | DLI | SC | 1.0 | 2 | CR | 7 | Dead | Interstitial pneumonia |
| 48 | 10/10MREL | ANLL | WR,Lu | DLBL | D | N | N | N/A | 6 | DLI | SC | 2.0 | 1 | CR | 17 | Dead | Interstitial pneumonia |
| 49 | 10/10MREL | ANLL | WR | DLBL | D | N/A | N | N/A | 13 | DLI | SC | 1.0 | 1 | CR |
Survival after T-cell therapy is the duration between the primary T-cell therapy and day of last visit or day of death.
Sec indicates secondary; MREL, matched related; MMREL, mismatched related; MUR, matched unrelated; MMUR, mismatched unrelated; HD, Hodgkin disease; NHL, nonHodgkin lymphoma; ALPS, autoimmune lymphoproliferative syndrome; AA, aplastic anemia; FA, Fanconi anemia; HLH, hemophagocytic lymphohistiocytosis; MLD, metachromatic leukodystrophy; MDS, myelodysplastic syndrome; RAEB, refractory anemia with excess blasts; WR, Waldeyer ring; A/I, abdomen/intestine; Li, liver; Lu, lung; NE, not evaluable; GIR, good initial response but short lived; N/A, not applicable; FLH, focal lymphoid hyperplasia; ALCL, anaplastic large cell lymphoma; PLPL, polymorphic lymphoplasmocytic lymphoma; Y, yes; D, donor; H, host; M, mixed (host and donor); N/A, not available; SC, stem cell donor; and 3rd, third-party donor.
This patient received EBV-CTLs from 2 different donors. The first set of cells was infused 1 day after the EBV-LPD diagnosis, and the patient failed to respond. The second set of cells was infused 89 days after failure. The patient achieved a CR with the second EBV-CTL therapy.
Five patients who initially received DLIs or EBV-CTLs subsequently received the alternative cell therapy 27-91 days thereafter in an attempt to halt disease progression. Of 2 patients who received EBV-CTLs followed by DLIs, 1 (patient 41) died of PD, and the other (patient 42) achieved CR. This patient was given DLIs 20 days after 3 infusions of predominantly CD4+ EBV-CTLs because imaging studies suggested that the disease was not responding to the EBV-CTLs. Testing of blood samples drawn immediately before infusion of the DLIs, however, demonstrated an increase in the frequency of EBV-CTLps together with a sharp reduction in EBV DNA copy number. Three patients received DLIs followed by EBV-CTLs. One patient (patient 43) achieved CR and a second (patient 45) died of PD. The third patient (patient 44) initially achieved SD after DLIs for an EBV-LPD involving the basal ganglia of the brain. Treatment with EBV-CTLs did not induce further improvement, and this patient ultimately died of neurologic complications of the EBV-LPD without evidence of progression.
Despite the higher initial doses of EBV-CTLps administered, the times to achieve clinical responses to EBV-CTL infusions did not differ significantly from those after DLIs. Fever resolved within 5-14 days after cell therapy if there were no other intercurrent infections. EBV DNA levels decreased significantly within 3-10 days. Clinical improvements, including shrinkage of palpable lymph nodes, reduction of organomegaly, and resolution of pain or intestinal bleeding, were first detected 8-15 days after infusion. Improvements in radiologic/endoscopic findings were documented by approximately 3 weeks after therapy with complete resolution of radiologic findings in patients who achieved CR by 3-6 months after therapy.
In contrast, patients who failed to respond showed persistence of fever with continued clinical deterioration and/or worsening of radiologic findings. In patients with PD who had not been previously treated with rituximab, circulating EBV DNA levels were unchanged or higher when tested 7 days or more after cell infusions. However, EBV DNA levels in the blood were undetectable before and after T-cell infusions in 6 of 17 patients previously treated with rituximab despite clinical and/or radiologic evidence of progression of disease.
Median followup has reached 80 months. All deaths attributable to EBV-LPD occurred within 4.3 months of initiation of T-cell therapy (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The cumulative incidences of EBV-specific mortality at 12 months were 24% and 21% for patients treated with DLIs and EBV-CTLs, respectively (supplemental Figure 1A; P = .93). No patient exhibited a late recurrence of disease.
Immunologic effects of the EBV-CTLs and DLIs
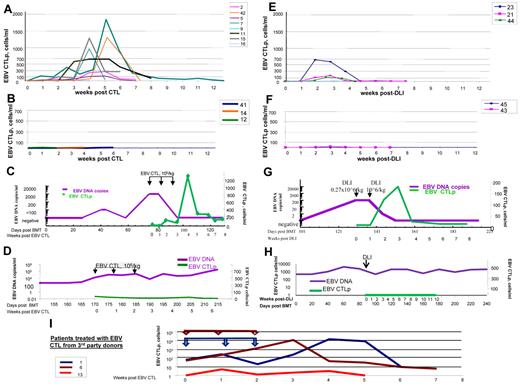
EBV-CTL and DLI infusions resulted in increased blood levels of EBV-CTLps only in those patients who subsequently responded to adoptive cell therapy (Figure 2A-D). Infusions of EBV-CTLs resulted in much higher EBV-CTLp levels (up to 2200 cells/mL), which were sustained in the circulation for longer periods of time (2-3 months) than those detected after DLIs (up to 600 cells/mL for ∼ 2-3 weeks, respectively). The overall expansion of EBV-CTLps after infusions of EBV-CTL therapy was up to 6 log10-fold (from < 0.001-12 EBV-CTLps/mL to 120-2200 EBV-CTLps/mL) and after DLIs up to 2 log10-fold (from 1.3-690 EBV-CTLps/mL). EBV-CTLp frequencies initially increased by 7-14 days after the initial infusion and coincided with decreases in EBV DNA levels, (Figure 2C,G). In contrast, in patients who failed to respond to DLIs or EBV-CTLs, EBV-CTLp frequencies did not increase (Figure 2D,H).
Monitoring of EBV-specific T cells in the circulation of patients with EBV-LPD treated with EBV-specific T cells or unstimulated DLIs. (A) Frequencies of EBV-specific T cells observed over a period of 12 weeks after initiation of T-cell therapy in the peripheral blood of patients who responded to treatment with EBV-specific T cells obtained from their stem cell donors (n = 8 patients evaluated). (B) Frequencies of EBV-specific T cells observed in peripheral blood of the patients with EBV-LPD who did not respond to treatment with EBV-specific T cells (n = 3) generated from PBMCs of their stem cell donors. (C) Monitoring of the EBV DNA levels (purple line) and EBV-CTLps (green line) of patient 15, who responded to treatment with EBV-specific T cells derived from the stem cell donor. EBV-CTLs were infused weekly for 3 consecutive weeks at doses of 1 × 106 cells/kg/infusion. After infusions of T cells, EBV-CTLps increased in frequency. Concurrently, EBV DNA levels in the peripheral blood of the patient decreased. (D) Monitoring of the EBV DNA levels (purple line) and EBV-CTLps (green line) of patient 41 who did not respond to treatment with EBV-specific T cells derived from the stem cell donor. EBV-CTLs were infused weekly for 3 consecutive weeks at doses of 1 × 106 cells/kg/infusion. No increase in EBV-CTLp frequency was observed over the period of 6 weeks of observation and the levels of EBV DNA remained high. (E) EBV-CTLps in patients who responded to therapy with DLIs from their HLA-matched stem cell donors (n = 3 patients evaluated). (F) EBV-CTLps in patients who did not respond to the therapy with DLIs (patients 43 and 45). (G-H) EBV DNA levels (purple line) and EBV-specific CTLps (green line) in the peripheral blood of patient 21, who responded to DLI treatment (G) and patient 45, who did not respond to the infusion of DLIs (H). (J) Monitoring of T-cell responses in the peripheral blood of patients with EBV-LPD who received EBV-specific T cells generated from third-party healthy donors (n = 5). The arrows indicate the times of the T-cell infusions for each of 3 patients treated and are presented in the same color as the line demonstrating the CTLps frequencies. Patients 1 and 6 achieved CR; patient 13 did not respond and died of PD.
Monitoring of EBV-specific T cells in the circulation of patients with EBV-LPD treated with EBV-specific T cells or unstimulated DLIs. (A) Frequencies of EBV-specific T cells observed over a period of 12 weeks after initiation of T-cell therapy in the peripheral blood of patients who responded to treatment with EBV-specific T cells obtained from their stem cell donors (n = 8 patients evaluated). (B) Frequencies of EBV-specific T cells observed in peripheral blood of the patients with EBV-LPD who did not respond to treatment with EBV-specific T cells (n = 3) generated from PBMCs of their stem cell donors. (C) Monitoring of the EBV DNA levels (purple line) and EBV-CTLps (green line) of patient 15, who responded to treatment with EBV-specific T cells derived from the stem cell donor. EBV-CTLs were infused weekly for 3 consecutive weeks at doses of 1 × 106 cells/kg/infusion. After infusions of T cells, EBV-CTLps increased in frequency. Concurrently, EBV DNA levels in the peripheral blood of the patient decreased. (D) Monitoring of the EBV DNA levels (purple line) and EBV-CTLps (green line) of patient 41 who did not respond to treatment with EBV-specific T cells derived from the stem cell donor. EBV-CTLs were infused weekly for 3 consecutive weeks at doses of 1 × 106 cells/kg/infusion. No increase in EBV-CTLp frequency was observed over the period of 6 weeks of observation and the levels of EBV DNA remained high. (E) EBV-CTLps in patients who responded to therapy with DLIs from their HLA-matched stem cell donors (n = 3 patients evaluated). (F) EBV-CTLps in patients who did not respond to the therapy with DLIs (patients 43 and 45). (G-H) EBV DNA levels (purple line) and EBV-specific CTLps (green line) in the peripheral blood of patient 21, who responded to DLI treatment (G) and patient 45, who did not respond to the infusion of DLIs (H). (J) Monitoring of T-cell responses in the peripheral blood of patients with EBV-LPD who received EBV-specific T cells generated from third-party healthy donors (n = 5). The arrows indicate the times of the T-cell infusions for each of 3 patients treated and are presented in the same color as the line demonstrating the CTLps frequencies. Patients 1 and 6 achieved CR; patient 13 did not respond and died of PD.
Clinical variables affecting outcome
As shown in Table 4, time of EBV-LPD onset after transplantation did not affect the probability of achieving CR after DLI or EBV-CTL therapy. The extent of disease at presentation, however, was inversely correlated with response rate. All patients with disease limited to 1 site achieved CR, compared with 60% for those with 2 sites and 50% for those with 3 or more involved sites (P < .01). This was true for patients treated with either DLIs or EBV-CTLs. However, all sites of involvement responded to treatment with either DLIs or EBV-CTLs, including the CNS. Indeed, of the 6 patients with clinical and radiologic evidence of CNS involvement, 4 achieved CR and 1 a prolonged stabilization of disease.
Variables associated with response to cell therapy
| Variable . | Response . | P . | |||
|---|---|---|---|---|---|
| Patients, n . | CR/SD/PR . | PD . | NE . | ||
| Overall association of number of sites involved and response (n = 49) | |||||
| 1 site | 14 | 14 (100%) | 0 (0%) | 0 (0%) | .01, .01* |
| 2 sites | 21 | 13 (60%) | 4 (20%) | 4 (20%) | |
| 3+ sites | 14 | 7 (50%) | 6 (50%) | 1 (0%) | |
| Overall association of rituximab use and response to cell therapy (n = 49) | |||||
| Patients failing Rituxan | 19 | 11 (58%) | 7 (37%) | 1 (5%) | .07, .06* |
| Patient w/o prior Rituxan or in PR after Rituxan | 30 | 23 (77%) | 3 (11%) | 4 (11%) | |
| Overall association of rituximab use and response (n = 49) | |||||
| Patients failing Rituxan by treatment | 19 | 11 | 7 | 1 | |
| DLI | 9 | 4 (44%) | 4 (44%) | 1 (11%) | .47, .63* |
| EBV-CTL | 10 | 7 (70%) | 3 (30%) | 0 (0%) | |
| Patients without prior Rituxan or in PR after Rituxan by treatment | 30 | 23 | 3 | 4 | |
| DLI | 21 | 17 (82%) | 1 (6%) | 3 (12%) | .32, .22* |
| EBV CTL | 9 | 6 (67%) | 2 (22%) | 1 (11%) | |
| Overall association of steroid use and response (n = 45) | |||||
| No steroid use | 40 | 29 (72%) | 8 (20%) | 3 (7%) | .85, .99* |
| Steroid use | 9 | 6 (66%) | 2 (22%) | 1 (11%) | |
| Overall association of use of steroids and/or cyclosporine or sirolimus (n = 49) | |||||
| No steroid use | 35 | 26 | 6 | 3 | .76, .44* |
| Steroid use | 14 | 9 | 4 | 1 | |
| Variable . | Response . | P . | |||
|---|---|---|---|---|---|
| Patients, n . | CR/SD/PR . | PD . | NE . | ||
| Overall association of number of sites involved and response (n = 49) | |||||
| 1 site | 14 | 14 (100%) | 0 (0%) | 0 (0%) | .01, .01* |
| 2 sites | 21 | 13 (60%) | 4 (20%) | 4 (20%) | |
| 3+ sites | 14 | 7 (50%) | 6 (50%) | 1 (0%) | |
| Overall association of rituximab use and response to cell therapy (n = 49) | |||||
| Patients failing Rituxan | 19 | 11 (58%) | 7 (37%) | 1 (5%) | .07, .06* |
| Patient w/o prior Rituxan or in PR after Rituxan | 30 | 23 (77%) | 3 (11%) | 4 (11%) | |
| Overall association of rituximab use and response (n = 49) | |||||
| Patients failing Rituxan by treatment | 19 | 11 | 7 | 1 | |
| DLI | 9 | 4 (44%) | 4 (44%) | 1 (11%) | .47, .63* |
| EBV-CTL | 10 | 7 (70%) | 3 (30%) | 0 (0%) | |
| Patients without prior Rituxan or in PR after Rituxan by treatment | 30 | 23 | 3 | 4 | |
| DLI | 21 | 17 (82%) | 1 (6%) | 3 (12%) | .32, .22* |
| EBV CTL | 9 | 6 (67%) | 2 (22%) | 1 (11%) | |
| Overall association of steroid use and response (n = 45) | |||||
| No steroid use | 40 | 29 (72%) | 8 (20%) | 3 (7%) | .85, .99* |
| Steroid use | 9 | 6 (66%) | 2 (22%) | 1 (11%) | |
| Overall association of use of steroids and/or cyclosporine or sirolimus (n = 49) | |||||
| No steroid use | 35 | 26 | 6 | 3 | .76, .44* |
| Steroid use | 14 | 9 | 4 | 1 | |
NE indicates not evaluated (patients dying within 8 days of initiated T-cell infusion who were not evaluated for response to T-cell therapy).
P excluding patients who were not evaluable; numbers in parentheses are raw percentages.
Response rates to DLIs and EBV-CTLs did not differ significantly among patients initially treated with cells or those initially treated with rituximab. However, patients who failed to respond to rituximab or relapsed after an initial response were somewhat less likely to respond to treatment with either DLIs or EBV-CTLs (58% vs 77%, P = .07).
We also examined responses in 14 patients receiving immunosuppressive drugs at the time of cell therapy, including steroids alone (n = 6) or steroids in combination with therapeutic levels of cyclosporine or tacrolimus (n = 3), sirolimus alone (n = 4), or cyclosporine alone (n = 1). Of these 14 patients, 9 achieved CR (64%) compared with 26 of 35 (74%) patients not receiving immunosuppressives (P = .76). Specific analysis of those who were or were not receiving systemic steroids also revealed no significant difference in response rate (P = .85). However, by the time cell infusions began, daily doses had been reduced to 0.07-0.0375 mg/kg of prednisone (median 0.14 mg/kg) or its equivalent. When we analyzed T-cell responses in these patients, we could not ascribe any clear effect of these agents on the expansion of EBV-CTLps in vivo. Therefore, among the responders analyzed in Figure 2, patients 2, 6, 16, 21, and 23, who were receiving sirolimus or cyclosporine, responses were comparable to the other patients who were not.
Because the EBV lymphomas were predominantly of donor origin, it is not surprising that the overall probabilities of achieving a CR after treatment with donor-derived DLIs or EBV-CTLs were equivalent for recipients of HLA-matched related, HLA-matched unrelated, or HLA-disparate related or unrelated transplantations. As detailed in the next section, however, in those instances where the donor of cells used for adoptive therapy differed from the origin of the EBV-LPD, HLA disparities between the cell donor and the EBV-LPD likely contributed to treatment failures.
Viral and immunologic variables affecting outcome
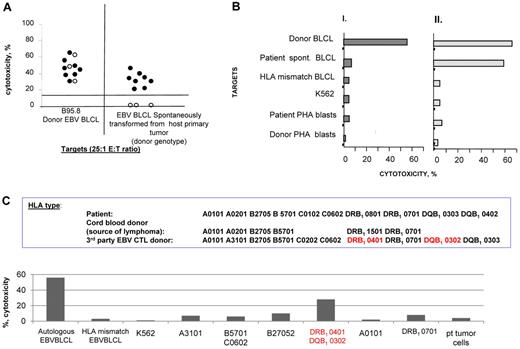
Because treatment failures were consistently correlated with a lack of EBV-CTL proliferation in vivo, we investigated whether the EBV-CTLs generated in vitro were able to recognize the EBV lymphomas in vivo. We succeeded in growing spontaneous EBV-transformed B cells from the blood or biopsied tumors of 11 recipients of HLA-matched HCT, who were subsequently treated with EBV-CTLs from the same donor. Each of these spontaneous transformants was of donor origin. In 8 patients who achieved CR, EBV-CTLp frequencies increased after T-cell infusion. The other 3 patients, whose CTLps frequencies remained low or undetectable, failed to respond and died of progressive disease. In all 11 cases, the donor's EBV-CTLs, sensitized with autologous B cells transformed with the B95.8 strain of EBV, lysed EBV B95.8 strain–transformed autologous EBV-BLCLs. As shown in Figure 3A, the EBV-CTLs from the 8 treatment responders also lysed B-cell lines transformed with the patient's endogenous EBV. In contrast, the EBV-CTLs administered to the 3 patients who failed to respond did not lyse the donor-type EBV-BLCLs transformed with endogenous virus. However, when T cells from these 3 donors were sensitized with spontaneous EBV-BLCL transformants grown from the patient's blood or tumor, they were able to lyse both the donor-derived B cells bearing endogenous EBV and the transformed B95.8 strain of EBV (Figure 3B).
Basis for nonresponse: donor-derived EBV-specific T cells that failed to induce a clinical response did not exhibit cytotoxic activity against the spontaneously transformed EBV-BLCLs generated from the patient's tissues. (A) Cytotoxicity of donor EBV–specific CTLs. There were 8 responders (closed symbols) and 3 nonresponders (open symbols; n = 11) sensitized with autologous B95.8-transformed B cells against EBV B95.8–transformed donor–derived autologous BLCL and against donor-type spontaneous EBV-transformed BLCLs cultured from the patient blood or EBV-LPD. The B95.8-sensitized EBV-specific CTLs used in nonresponding patients lysed EBV B95.8 transformed BLCL but did not lyse the spontaneous EBV transformants of donor origin cultured from the patient. (B) Cytotoxicity of donor-derived EBV-CTLs stimulated with spontaneously transformed EBV-BLCLs of donor origin cultured from the patient. The same T cells as in panel A were stimulated in vitro with the spontaneously transformed EBV-BLCLs and were able to kill both the stimulating B-cell line and the donor-derived B95.8 transformant. These data confirm that the EBV-LPD is sensitive to lysis by donor-derived EBV-CTLs if the T cells are sensitized with the endogenous strain of EBV. (C) HLA restriction of EBV-CTLs generated from an HLA haplotype–matched donor for patient 13, who developed an EBV lymphoma in cord blood–derived B cells; these EBV-CTLs were selectively restricted by an HLA DRB, 0401, not shared by the cord blood cells and did not lyse cord blood donor-derived spontaneous EBV-BLCL generated from the tumor.
Basis for nonresponse: donor-derived EBV-specific T cells that failed to induce a clinical response did not exhibit cytotoxic activity against the spontaneously transformed EBV-BLCLs generated from the patient's tissues. (A) Cytotoxicity of donor EBV–specific CTLs. There were 8 responders (closed symbols) and 3 nonresponders (open symbols; n = 11) sensitized with autologous B95.8-transformed B cells against EBV B95.8–transformed donor–derived autologous BLCL and against donor-type spontaneous EBV-transformed BLCLs cultured from the patient blood or EBV-LPD. The B95.8-sensitized EBV-specific CTLs used in nonresponding patients lysed EBV B95.8 transformed BLCL but did not lyse the spontaneous EBV transformants of donor origin cultured from the patient. (B) Cytotoxicity of donor-derived EBV-CTLs stimulated with spontaneously transformed EBV-BLCLs of donor origin cultured from the patient. The same T cells as in panel A were stimulated in vitro with the spontaneously transformed EBV-BLCLs and were able to kill both the stimulating B-cell line and the donor-derived B95.8 transformant. These data confirm that the EBV-LPD is sensitive to lysis by donor-derived EBV-CTLs if the T cells are sensitized with the endogenous strain of EBV. (C) HLA restriction of EBV-CTLs generated from an HLA haplotype–matched donor for patient 13, who developed an EBV lymphoma in cord blood–derived B cells; these EBV-CTLs were selectively restricted by an HLA DRB, 0401, not shared by the cord blood cells and did not lyse cord blood donor-derived spontaneous EBV-BLCL generated from the tumor.
The importance of ascertaining the origin of the EBV-LPD and the HLA restriction of the EBV-CTLs generated in vitro was demonstrated in 2 other patients. Patient 13, with a rituximab-unresponsive EBV+ lymphoma of UCB origin, was treated with EBV-CTLs from an HLA-haplotype–matched related donor. Subsequent analysis demonstrated that these EBV-CTLs were selectively restricted by a class II HLA allele not shared by the UCB donor and were unable to lyse spontaneously EBV-transformed EBV-BLCLs of UCB origin generated from the patient's tumor (Figure 3C).
The second patient, (patient 8) had received a 7 of 10 HLA allele–matched related TCD-HCT for chemotherapy-resistant EBV+ hemophagocytic lymphohistiocytosis. Shortly after engraftment, EBV reactivation was documented by rising levels of EBV DNA in the blood. The patient was immediately treated with EBV-CTLs previously generated from the donor. Despite this treatment, he continued to have fever, high EBV DNA levels, and worsening abdominal pain. EBV-CTLp frequencies did not increase. Subsequent PET/CT scans revealed progressively enlarging masses involving the gastric wall and mesenteric nodes. Biopsy of the gastric lesion revealed an EBV+ DLBCL of host rather than donor origin. Analysis of the donor's EBV-CTLs revealed that they were selectively restricted by HLA*A1101 (Figure 4B), an allele not shared with the patient (Figure 4A). Based on this finding, we treated the patient with EBV-CTLs from a partially mismatched third-party donor, which were restricted by an HLA*A2601 allele shared by the EBV-LPD. Ten days thereafter, EBV-CTLp frequencies increased (Figure 4C). By 14 days, EBV DNA levels and fever began to decline and abdominal pain lessened. By day 25, radiologic evidence of disease had markedly improved (Figure 4D). This patient subsequently achieved a CR, and remains in CR for both EBV-LPD and EBV+ HLH > 18 months later.
Basis for nonresponse: donor-derived EBV-specific T cells did not recognize host origin EBV-LPD (patient 8). (A) HLA type of the stem cell donor (mother), third-party EBV-CTLs donor 1, and third-party EBV-CTL donor 2. (B) HLA-restriction analysis of EBV-specific T cells generated from the HSCT donor (mother; blue bars) or from the third-party donor 1 (brown bars) or third-party donor 2 (yellow bars) tested in a Cr51-release assay against a panel of allogeneic EBV-BLCLs, each matching 1 HLA allele of each of the T-cell donors' HLA types. (C) Monitoring of the EBV DNA levels (red line) and EBV-specific T cells (green line) after infusions of EBV-CTLs derived from the stem cell donor (blue arrows), third-party donor 1 (purple arrow), and third-party donor 2 (red arrows) at the doses of 1 × 106 cells/kg/infusion. Treatment with Rituxan (375mg/m2; orange arrows), as well as injections of EBV-CTLs derived from the HSCT donor that were restricted by the donor-unique HLA A1101 allele, did not affect high levels EBV DNA, whereas the administration of the EBV-specific T cells from the third-party donors 1 and 2, both restricted by the A2601 HLA allele presented on the EBV+ tumor cells of patient origin, resulted in a rapid decrease of the EBV DNA in the circulation. (D) Sequential PET scans demonstrating no response to the transplant donor's T cells with rapid development of an EBV lymphoma of the gastric wall and adjacent lymph nodes. By 3 weeks after the first infusion of third-party T cells (d97), gastric lymphoma was no longer detected.
Basis for nonresponse: donor-derived EBV-specific T cells did not recognize host origin EBV-LPD (patient 8). (A) HLA type of the stem cell donor (mother), third-party EBV-CTLs donor 1, and third-party EBV-CTL donor 2. (B) HLA-restriction analysis of EBV-specific T cells generated from the HSCT donor (mother; blue bars) or from the third-party donor 1 (brown bars) or third-party donor 2 (yellow bars) tested in a Cr51-release assay against a panel of allogeneic EBV-BLCLs, each matching 1 HLA allele of each of the T-cell donors' HLA types. (C) Monitoring of the EBV DNA levels (red line) and EBV-specific T cells (green line) after infusions of EBV-CTLs derived from the stem cell donor (blue arrows), third-party donor 1 (purple arrow), and third-party donor 2 (red arrows) at the doses of 1 × 106 cells/kg/infusion. Treatment with Rituxan (375mg/m2; orange arrows), as well as injections of EBV-CTLs derived from the HSCT donor that were restricted by the donor-unique HLA A1101 allele, did not affect high levels EBV DNA, whereas the administration of the EBV-specific T cells from the third-party donors 1 and 2, both restricted by the A2601 HLA allele presented on the EBV+ tumor cells of patient origin, resulted in a rapid decrease of the EBV DNA in the circulation. (D) Sequential PET scans demonstrating no response to the transplant donor's T cells with rapid development of an EBV lymphoma of the gastric wall and adjacent lymph nodes. By 3 weeks after the first infusion of third-party T cells (d97), gastric lymphoma was no longer detected.
Complications of the EBV-CTL and DLI therapies
There were no immediate adverse reactions observed due to either of the cell therapies. Seven of 26 patients (27%) in the DLI group and 7 of 19 patients (36%) in the EBV-CTL group had documented GVHD before infusion. In the DLI group, 3 patients developed acute grade 2-3 GVHD de novo, 1 patient had a grade 2-3 flare of acute skin GVHD, and 3 patients developed chronic GVHD de novo (supplemental Figure 2A-B). Although most EBV-CTL donors were not HLA matched with the recipient, no patient in the EBV-CTL group developed de novo acute or chronic GVHD or a flare of preexisting GVHD. All patients with acute and all but 1 of the patients with chronic GVHD de novo cleared with therapy. The cumulative incidences of acute and chronic GVHD attributable to DLIs at 1 year after transplantation are 14% and 14%, respectively.
Discussion
This study details the effects of donor-derived DLIs or EBV-CTLs in the treatment of 49 allogeneic HCT recipients with pathologically confirmed EBV-LPD. These EBV-LPD cases presented as rapidly growing DLBCL in 45 of 49 patients, which were clonal in 95% and exclusively of donor origin in 24 of 30 (80%) tumors tested. Four other tumors containing predominantly donor cells were likely also of donor origin, but were infiltrated with residual host lymphocytes or stromal cell elements. These characteristics are consistent with prior published descriptions of EBV-LPD after allogeneic HCT.6,14
In this series, 73% of the patients series treated with DLIs and 68% of those treated with EBV-CTLs achieved a sustained CR, including 76% in the DLI group and 72% in the CTL group, who survived ≥ 8 days after initiation of adoptive immunotherapy, the minimum time required for expansion of EBV-CTLs in vivo. The doses of EBV-CTLs required to induce remissions of bulky multiorgan disease were modest. In patients treated with unselected DLIs, the administered doses of EBV-CTLps ranged from 2.8-223 EBV-CTLps/kg, or 196-15 610 total CTLps for a 70-kg adult. These doses are consistent with our previous study documenting a CR in a patient who received a total dose of approximately 800 EBV-CTLps.3 The total doses of EBV-CTLps provided by the 3 infusions of EBV-CTLs were approximately 90-fold higher. We previously found that the frequency of EBV-specific IFNγ+ T cells may be 100-fold greater than that of EBV-CTLps25 ; however, given that a 1-cm3 tumor nodule is estimated to contain 108-109 tumor cells,37 the EBV-specific T-cell doses administered were still extremely small compared with tumor burdens in the patients.
Because of the numeric disparity between the effector T cells administered and resident populations of growing EBV lymphoma cells, we hypothesized that: (1) expansion of adoptively transferred EBV-specific T cells in vivo would be essential to the induction of tumor regressions and the reestablishment of a normal equilibrium between virus-specific T cells and residual EBV transformed cells in the host; and (2) patients with more extensive disease at the initiation of cellular therapy might be less likely to achieve a CR. Extent of disease did in fact adversely affect outcome. Furthermore, if the DLIs or EBV-CTLs infused failed to expand in vivo, the patient did not respond. In contrast, in those patients responding to DLIs or EBV-CTLs, EBV-CTLp frequencies consistently increased by 2-3 log10. In patients responding to cells derived from their transplantation donor, increased frequencies of EBV-CTLps were usually sustained for periods of at least 4-8 weeks after infusion, by which time clinical and radiologic regression of disease was evident. Even among those patients responding to third party–derived EBV-CTLs, which only transiently engraft, such increments in circulating levels of EBV-CTLps were observed for at least 14-21 days after each infusion. In contrast, expansion of the EBV-CTLp populations was never observed in the patients whose disease progressed despite adoptive cell therapy.
The consistent failure of transferred EBV-CTLs to proliferate in nonresponding patients suggested at least 2 possibilities: (1) the transferred T cells were unable to recognize or effectively respond to the EBV+ lymphoma cells; or (2) they were actively prevented from doing so either by the tumor and its products or by the host environment. Consistent with the first possibility are our findings in 3 patients who failed treatment with HLA-matched, donor-derived EBV-CTLs. These EBV-CTLs, which had been sensitized with autologous BLCLs transformed with EBV strain B95.8, did not lyse spontaneous EBV-BLCLs of donor type isolated from the patient's blood or biopsied EBV lymphoma. Gottshalk et al have described a similar case in which the donor's EBV-CTLs, sensitized with EBV-B95.8–transformed autologous BLCLs, failed to lyse spontaneous tranformants derived from the patient.38 In that patient, the endogenous EBV strain had a deletion in the EBNA 3b protein that resulted in the elimination of 2 epitopes presented by HLA A11 that were specifically targeted by the transferred T cells. Whether such mutations in endogenous strains of EBV are also responsible for such treatment failures in our patient cohort is currently under study. Donor T cells, sensitized with these donor-type spontaneous BLCL transformants, could nevertheless lyse autologous B cells transformed with either the endogenous or the B95.8 strain. This indicates that the failure of the B95.8-sensitized T cells to lyse the endogenous transformants is not based on the transformants' intrinsic resistance to T-cell effector activity. In patients like these, treatment with EBV-CTLs from a second donor restricted by a different shared HLA allele should be considered, because such T cells would be expected to target a different peptide epitope that might not be mutated in the endogenous EBV strain.
In 2 other patients who failed to respond, the transferred donor-derived EBV-CTLs were selectively reactive against immunodominant epitopes of EBV presented by HLA alleles not expressed by an EBV+ lymphoma of host origin. One of these patients was later treated successfully with partially HLA-matched third-party EBV-CTLs restricted by an HLA allele expressed by the patient's EBV-LPD, again suggesting that an inability of the T cells to recognize the tumor rather than an intrinsic tumor resistance contributed to the initial treatment failure.
Our studies also indicate that both DLIs and EBV-CTLs can induce durable CRs in patients failing rituximab therapy. Such patients responded less consistently, however, than patients treated initially with cellular immunotherapy, even though the differences were not significant. The possibility that prior treatment with rituximab or the interval of time that rituximab is administered permits selection of more resistant EBV lymphoma cells must nevertheless be considered.
Because of the rapid progression of EBV lymphomas emerging after HCT and the protracted time required to generate EBV-CTLs (60 days), the equivalent therapeutic activity and immediate accessibility of DLIs constitute major advantages. However, DLIs also incurs the risk of GVHD, particularly if the transplantation donor is not HLA matched. To reduce this risk, we limited DLIs to patients with EBV-LPD arising after an HLA-matched or a single, donor-unique HLA allele–disparate HCT. The highest dose of T cells administered, 1 × 106 CD3+ T cells/kg, is 10-fold higher than the 105 CD3+ T-cell/kg reported as the threshold for GVHD at the time of HCT using BM stem cells,39,40 but 10-fold lower than the 107 CD3+ T-cell/kg doses that we have shown can induce molecular remissions of chronic myeloid leukemia with low risk of acute or chronic GVHD when administered ≥ 9 months after HCT.41 In our series, cumulative risks for acute and chronic GVHD attributable to DLIs administered from 2-9 months after HCT were 14% and 14% at 12 months, respectively. In all but 1 case, GVHD cleared with treatment.
In contrast to DLIs, no patient developed either acute or chronic GVHD or a flare of preexisting GVHD after infusion of HCT donor–derived EBV-CTLs, irrespective of their level of HLA disparity. Heslop et al have documented a similarly low risk of GVHD in more than 100 patients given EBV-CTLs to prevent EBV-LPD after HCT.16 These findings reflect the depletion of alloresponsive T cells achieved by extended in vitro sensitization with autologous EBV-BLCLs (Figure 1B).
Until recently, the time required for generating such EBV-CTLs had necessitated their production for all high-risk patients before disease onset to be able to rapidly treat the 4%-6% who develop EBV-LPD. However, new techniques permitting rapid selection of virus-specific T cells may accelerate access to HCT-donor–derived EBV-CTLs.42,43 In addition, as recently reported by Haque et al44 and our group18 and further demonstrated in the present study, EBV-CTLs selected from preestablished banks of normal third-party donor–derived EBV-CTLs on the basis of partial HLA matching and appropriate HLA restriction can provide an immediately accessible source of effector cells for the treatment of EBV-LPD after transplantation.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Institutes of Health (grants CA23766, CA59350, and CA08748), the Clair L. Tow Foundation, the Larry H. Smead Fund, the Aubrey Fund for Pediatric Cancer Research, the Ryan E. McGeough Charitable Gift Fund, the Major Family Fund for Cancer Research, and the Laura Rosenberg Foundation.
National Institutes of Health
Authorship
Contribution: E.D., B.O.-S., and R.J.O. performed the research, analyzed the clinical, virologic, and immune responses, interpreted the data, and wrote the manuscript; J.T.-F., C.H., and D.G. analyzed the pathologic, immunohistologic, and molecular features of each EBV-LPD; S.A. analyzed and interpreted all radiologic responses; G.H. and J.F.C. assisted with the study design and performed the biostatistical analyses; R.K. assisted in data management and analysis; and S.E.P., N.A.K., J.N.B., F.B., H.C.-M., A.J., G.K, E.B.P., A.S., T.N.S., and J.W.Y. managed the patients clinically, performed their sequential evaluations, and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard J. O'Reilly, MD, Department of Pediatrics and Medicine, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: oreillyr@mskcc.org.
References
Author notes
E.D. and B.O.-S. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal