Abstract
Clinical and experimental studies show that inhibition of histone/protein deacetylases (HDAC) can have important anti-neoplastic effects through cytotoxic and proapoptotic mechanisms. There are also increasing data from nononcologic settings that HDAC inhibitors (HDACi) can exhibit useful anti-inflammatory effects in vitro and in vivo, unrelated to cytotoxicity or apoptosis. These effects can be cell-, tissue-, or context-dependent and can involve modulation of specific inflammatory signaling pathways as well as epigenetic mechanisms. We review recent advances in the understanding of how HDACi alter immune and inflammatory processes, with a particular focus on the effects of HDACi on T-cell biology, including the activation and functions of conventional T cells and the unique T-cell subset, composed of Foxp3+ T-regulatory cells. Although studies are still needed to tease out details of the various biologic roles of individual HDAC isoforms and their corresponding selective inhibitors, the anti-inflammatory effects of HDACi are already promising and may lead to new therapeutic avenues in transplantation and autoimmune diseases.
Introduction
Histone/protein deacetylase inhibitors (HDACi) were initially developed as anti-cancer agents, and much clinical and molecular data regarding their effects arose in the oncology field. However, nowadays HDACi are attracting interest as anti-inflammatory agents, independent of their known proapoptotic or cell cycle arrest actions on malignant cells. Although pan-HDACi enzymes have the potential to modulate the functions of all cells, their effects on T cells, and on CD4+ FOXP3+ T-regulatory (Treg) cells in particular, are of interest given the burgeoning role for Tregs in the area of cellular therapy. Treg-based therapies, involving ex vivo expansion and adoptive transfer to boost circulating Treg numbers, are proposed as therapies of neurodegenerative and autoimmune diseases, as well as GVHD and organ transplant rejection.1 However, in inflammatory conditions, conventional T cells can be resistant to suppression by Tregs,2 and pro-inflammatory cytokines (TNF-α, IL-1, IL-6, IFN-γ), lipopolysaccharides, and other agents have deleterious effects on Tregs, impairing their suppressive phenotype3 and promoting their conversion into Th174 or Th15 cells.6,7 Moreover, the extent to which patients with autoimmune diseases have decreased numbers of Treg cells remains unclear.8 An alternate therapeutic strategy to transfer of Treg cells is to use pharmacologic agents, such as rapamycin9 or HDACi, to curtail T-cell responses and facilitate Treg functions. We have shown that HDACi can enhance Treg suppressive function and promote their development in vitro and in vivo,10 with beneficial actions for transplantation11 and autoimmune diseases, including colitis12,13 and arthritis.14 This review summarizes some of the background and promise of HDACi therapy to modulate T cell–associated inflammatory and immunologic diseases. We apologize to those researchers whose work could not be cited because of limited space.
HDAC and HDACi
Histone/protein deacetylases (HDAC) remove acetyl groups (O = C-CH3) from ϵ-N-acetyl lysine amino acids and are classified into 4 main classes of enzymes15-17 : class I HDAC include HDAC1, 2, 3, and 8; class II HDAC include HDAC4, 5, 7, 9 (subclass IIa) and HDAC 6, 10 (subclass IIb); class III HDAC are homologs of yeast Sir2 proteins; and the sole class IV HDAC is HDAC11.
Class I HDAC enzymes are expressed in all cells, whereas class IIa HDAC enzymes have tissue-specific expression. Historically, class I HDAC were thought largely restricted to the nucleus, whereas class IIa HDAC enzymes shuttled between the nucleus and cytoplasm15,16 and even mitochondria (HDAC7).18 However, recent data show that class I HDAC can act in the cytoplasm as well as in the nucleus (eg, HDAC3 can associate with IκBα in the cytoplasm and translocate into the nucleus in response to TNF-α).19 Likewise, HDAC1,20 HDAC2,21 and HDAC822 can, on occasion, be identified in cytoplasmic complexes. Class I and IIa HDAC also differ with regard to their catalytic activities. Class I HDAC enzymes exhibit strong deacetylase activity, whereas class IIa HDAC proteins are enzymatically inactive and act primarily as scaffolding or recruiting proteins within large multimolecular complexes that include class I HDAC (especially HDAC323 ) and other regulatory elements. In addition to their well-established role in regulating acetylation of lysines within histone tails and thereby modulating chromatin compaction and remodeling, HDAC enzymes regulate the acetylation of nonhistone proteins. More than 1750 nonhistone proteins are now identified as HDAC substrates,24 including p53, GATA1-3, STAT3, STAT5, Foxp3, estrogen and androgen receptors, NF-κB, HSP90, and α-tubulin.25,26 Acetylation of nonhistone proteins has various effects, for instance, increasing (eg, Foxp327 ) or decreasing (eg, DNMT128 ) protein stability, disrupting protein-protein interaction (eg, NF-κB, HSP70), and increasing transcriptional activation (eg, p53).
HDACi have differing chemical structures and abilities to inhibit the various HDAC isoforms.29 The most-studied HDACi, trichostatin-A (TsA) and suberoylanilide hydroxamic acid (SAHA), are nonselective, but the development of isoform-selective HDACi to target certain processes and avoid unwanted side effects is an important aim of the ongoing research discussed in “Safety and side effects of HDACi and future directions.” HDACi may inhibit the functions of class IIa enzymes, despite their lack of significant catalytic activity, by binding to their residual catalytic sites and affecting their conformation and/or interactions with other molecules present within multimolecular complexes.30 Moreover, HDAC, like many other proteins, are subject to posttranslational modifications, including phosphorylation, acetylation, ubiquitylation, and sumoylation, and HDACi can modulate the acetylation of HDAC proteins themselves, causing alterations in stability or activity.31 Additional mechanisms of HDAC inhibition include disturbance of nuclear-cytoplasmic shuttling (eg, of HDAC732 ) and induction of HDAC proteasomal degradation.33,34 Lastly, several of the class IIa HDAC (HDAC4, HDAC5, and HDAC9) and the class III HDAC, Sirt 1, but not class I HDAC enzymes, are targets for multiple miRNA,35 and HDACi were shown to alter (mostly inhibit) miRNA levels,36 providing a novel and, as yet, largely unexplored mechanism for the regulation of class IIa HDAC enzymes.
HDACi and Tregs
Therapy with a pan-HDACi (eg, TsA or SAHA) can stimulate thymic production of murine Foxp3+ Tregs, promote the peripheral conversion of T cells into Tregs, and enhance Treg suppressive function in vitro and in vivo.11 Pan-HDACi use also enhances the suppressive function of human Tregs in vitro37 and promotes conversion of human T cells into suppressive Tregs.38 The transcription factor, Foxp3, has an indispensable role in Treg biology39 and is subject to epigenetic modifications that regulate its gene expression and protein function. Demethylation of certain regions in the Foxp3 gene locus are key to Foxp3 expression and Treg function,40 including the Treg-specific demethylated region, promoter, and enhancer region.41,42 HDACi can promote demethylation by inducing proteasomal degradation of DNMT128,43 and decrease levels of DNMT144 and DNMT3b45 mRNA. Because the Foxp3-associated Treg-specific demethylated region, promoter, and enhancer regions are normally largely demethylated in Tregs, this mechanism probably involves additional genes in Tregs other than Foxp3 itself. The most important effect of HDACi in Tregs appears to be an increase in Foxp3 acetylation11 and thereby protects it from proteasomal degradation.27 Biochemical studies show that Foxp3 occurs in a macromolecular complex that includes several HDAC and histone/protein acetyltransferases (HATs).3,46,47 Although the specific Foxp3 lysines that should be acetylated for optimal Treg function remain undetermined, acetylation of Foxp3 promotes its DNA binding and interaction with transcription factors that induce expression of genes associated with optimal Treg function or suppress expression of genes typical of non-Treg lineages.11
Given the dynamic association of Foxp3 with multiple HDAC and HAT enzymes, we used genetic and pharmacologic approaches to identify interactions that are essential for Treg cell viability and function versus those that may be targeted to enhance Treg function. We found that knockout of HDAC612 and HDAC9,13 as well as Treg-specific deletion of Sirt1,48 resulted in enhanced suppressive Treg functions in vitro and in vivo. These data were confirmed using isoform-specific HDACi applied to WT Tregs in the case of HDAC6 and Sirt1 but were not possible for HDAC9 given the lack of HDAC9-selective inhibitors. In the case of HDAC6, which is expressed primarily in the cell cytoplasm, genetic or pharmacologic targeting led to of heat shock protein 90 acetylation and induction of a potent heat shock response in Tregs.26 Indeed, targeting of both HDAC6 and HDAC9 induces HSP70 expression, which can complex with Foxp3 and act as a molecular chaperone.12,13 Targeting of Sirt1 also increased Foxp3 expression, but in contrast to our HDAC6 and HDAC9 data, was not associated with increased heat shock responses. Rather, Sirt1 targeting led to up-regulation of multiple genes involved in cholesterol metabolism and energy generation through the Krebs cycle and mitochondrial electron transport pathways.48
Although many HDAC remain to be evaluated, the available data emphasize the value of targeting particular class II or class III enzymes, whereas our pharmacologic targeting of class I HDAC had no obvious effects on murine Treg function in vitro or in vivo.49 However, this contrasts with our own37 and other data38 using human cells, as well as with data from rat models.50,51 Several explanations may apply. First, there are known interspecies differences in Foxp3 biology; for example, whereas murine T cells cannot express Foxp3 in the absence of significant levels of TGF-β, activated human Teffs can up-regulate Foxp3 under various stimulatory conditions.52 Differences in the epigenetic regulation of Foxp3 may apply because murine DNMT1−/− CD4+ T cells readily up-regulate Foxp3 on TCR stimulation, just as conventional human T cells do on activation.53 Unfortunately, the field lacks a marker to reliably discriminate naturally occurring and induced Tregs because even Helios, a transcription factor recently proposed of value in this regard,54 is up-regulated on activation of murine and human conventional T cells.55 Lastly, HDACi can have important effects on non-Treg cells, especially in vivo, as considered below.
HDACi and APC
HDACi (pan-, class I– and class IIb–specific) were shown to inhibit the production of multiple pro-inflammatory cytokines in antigen-presenting cells (APCs),56 promote conversion of inflammatory macrophages (M1) into tolerogenic M2 cells,50 decrease TLR signaling,56,57 disrupt antigen presentation,56 and diminish MHC class II58 and costimulatory molecule expression.56 Although the mechanisms of action of HDACi on APCs were often uncharacterized in the literature, a key action appears to be HDACi-mediated inhibition of the NF-κB pathway.59 The net result is to decrease the direct inhibitory signals of inflammation on Tregs, decrease APC stimulation of effector cells, and prevent resistance of Teff cells to Treg-mediated suppression.
Effects of HDACi on development of T-cell responses
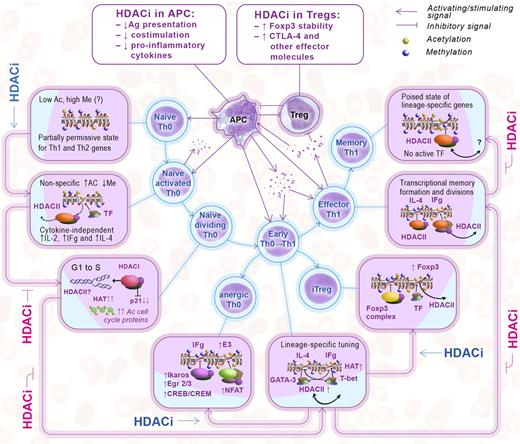
Key effects of HDACi on the development of host T-cell immune responses are illustrated in Figure 1 and summarized in the following sections. It should be noted, at the outset, that although HDACi obviously can act by inhibition of HDAC catalytic activity and directly affect acetylation of key transcription factors and other proteins, as reviewed for Tregs,26 there is only limited knowledge of the nonepigenetic effects of HDACi on conventional T cells. Although most published reports were interpreted by their authors as reflecting classic epigenetic effects, this assessment may well need to be revised as the field develops.
Effects of HDACi on the interactions of Tregs, APCs, and effector T cells. Epigenetic events are displayed according to each stage of T-cell maturation (boxes) and matched with corresponding HDACi effects as shown in the outer row of arrows. (Middle) Treg, APC, and T-cell interactions, cytokine production, reciprocal stimulatory and inhibitory signals, and effects of HDACi on Tregs and pro-inflammatory APC (top boxes); the suppressive effects of Tregs are not shown.
Effects of HDACi on the interactions of Tregs, APCs, and effector T cells. Epigenetic events are displayed according to each stage of T-cell maturation (boxes) and matched with corresponding HDACi effects as shown in the outer row of arrows. (Middle) Treg, APC, and T-cell interactions, cytokine production, reciprocal stimulatory and inhibitory signals, and effects of HDACi on Tregs and pro-inflammatory APC (top boxes); the suppressive effects of Tregs are not shown.
Stage 1: primary activation of resting naive T cells
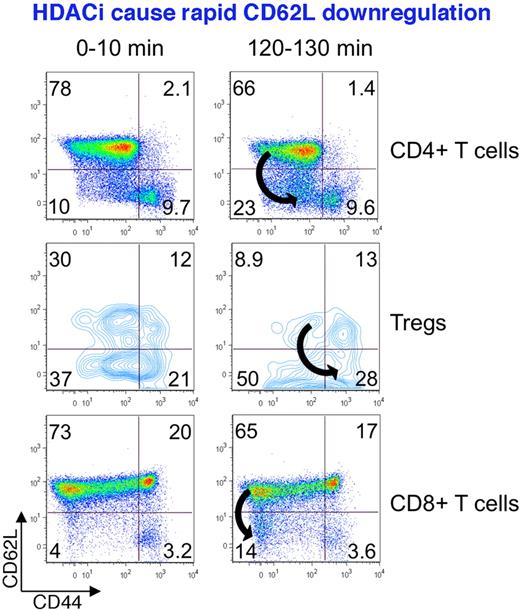
APCs deliver antigen complexed with MHC class II to CD4+ T cells, along with costimulatory signals, such as CD80/86, which bind to CD28 on T cells. Naive T cells produce low levels of IL-4 and IFN-γ (plus IL-2) as soon as 30 minutes of TCR stimulation. This is not dependent on the actions of cytokines or transcription factors, as it occurs under Th1 and Th2 polarizing or nonpolarizing conditions, and without T-bet or GATA-3 induction.60 The rapidity of these events that precede cell division suggests that silencing of lineage-specific cytokine genes in naive CD4+ cells is regulated by HDAC activity, rather than via the changes at heavily methylated promoters.61 Indeed, pan-HDACi (TsA) treatment of murine naive Th0 cells under nonpolarizing conditions led to acetylation of histone-4 at the IFN-γ gene locus, and enhanced IFN-γ production to a level comparable with Th1 polarization, in conjunction with inhibition of the Sin3A repressive complex that contains HDAC1 and/or HDAC2.62 On the other hand, TsA was shown to down-regulate CD28 expression in naive murine CD4+ cells within 4 hours.63 This effect may be attributable to the toxic effects of high concentrations, given a significant apoptotic rate observed. Our own flow cytometric studies involved use of naive murine cells stimulated with CD3 mAb alone or in the presence of fluorescently labeled pan-HDACi (Panobinostat); Panobinostat was added at a nontoxic concentration (1nM) that did not affect cell viability compared with untreated controls over the 3 hours of analysis. Consistent with data cited above, HDACi promoted down-regulation of a marker of naive T cells, CD62L, in Teffs (and in naive Tregs) within 2 hours of exposure (Figure 2), although the underlying mechanisms remain to be determined. Hence, HDACi compounds promote the early activation of CD4+ cells by enhancing histone deacetylation at gene loci responsible for production of Th1 and Th2 lineage cytokines and also promote cytokine release. Additional experiments are also required to identify the specific HDAC involved in this process.
HDACi promote the rapid down-regulation of CD62L, a marker of naive T cells. Freshly isolated murine CD4+ and CD8+ T cells, and Tregs, exposed to low, nontoxic concentrations of a pan-HDACi showed CD62L down-regulation within 2 hours of exposure, illustrating the potential for phenotypic modulation of T cells independently of their cell division.
HDACi promote the rapid down-regulation of CD62L, a marker of naive T cells. Freshly isolated murine CD4+ and CD8+ T cells, and Tregs, exposed to low, nontoxic concentrations of a pan-HDACi showed CD62L down-regulation within 2 hours of exposure, illustrating the potential for phenotypic modulation of T cells independently of their cell division.
Stage 2: division of activated naive T cells
Shortly after TCR stimulation, naive T cells begin proliferating, before their acquisition of effector functions.64 Mitosis is largely driven by posttranslational modification of histones and proteins, and knockout of both HDAC1 and HDAC2 abrogates cell division at the G1/S phase in primary mouse fibroblasts and in B cells.65 HDAC1 and HDAC2 are thought to promote cell division by inhibiting p21.65 HATs also affect the cell cycle; for example, p300/CBP are essential for the E2F activity that controls the G1/S transition,66 and TIP60 forms a complex with transformation/transactivation domain-associated protein, providing transcriptional activation of histone genes.67 Analysis of HeLa cells showed that mitotic cells are notable for their set of highly acetylated proteins, including RNA processing proteins, eIF4G, RNA helicase A, and several cell cycle proteins: APC1, anillin, and NudC.68 The addition of HDACi compounds typically causes cell cycle arrest and/or mitotic abnormalities, suggesting that class I HDAC enzymes are indispensable for cell division.68
HDACi ranging from pan-HDACi, such as TsA,69,70 SAHA,71 butyrate,72 scriptaid,70 trapoxin,69 to class I-specific HDACi, such as MS-275,73 and class II–specific HDACi, such as MC1575,74 were all shown to up-regulate p21 in tumor cells, but less is known of their effects in normal lymphocytes. Human PBMCs incubated for 2 days with a high proapoptotic concentration of TsA showed a moderate increase in p21 mRNA expression,75 but there were no data reported regarding the cell subsets involved. The finding that butyrate blocked the proliferation in primary cultures of both WT and p21-deficient cells76 raises doubts as to the role of p21 in inhibition of T-cell divisions by HDACi. Likewise, pan-HDACi and class I–specific HDACi (but not HDAC6-specific compounds12 ) showed anti-proliferative effects on T cells,77,78 probably by p21-independent actions. Thus, HDACi use inhibits production of IL-2, which is the major autocrine growth factor produced by stimulated T cells, as well as down-regulating expression of CD25 and CD154, and decreasing clustering of T cells with APCs as a result of down-regulation of CD11a and CD54.77,78
Stage 3: early differentiation of Th0 cells
The cellular environment, including the balance of cytokines and costimulatory signals, is key to T-cell activation and polarization. IL-12 and IL-4 drive differentiation of Th1 and Th2 cells, respectively, via transcription factors, such as STAT4 and STAT6. In addition, GATA-3 is expressed predominantly in Th2 cells, and T-bet is expressed in Th1 cells, and both act as “master regulators” of T helper cell lineage determination.79 Lineage-specific cytokines produced by adjacent cells stimulate expression (mostly by acetylation) of lineage-specific transcription factors and cytokines, and inhibit the expression of cytokines and transcription factors of other lineages: GATA3 inhibits IFN-γ expression, and T-bet inhibits Th2 cytokine expression.80,81 If naive Th0 cells are activated through the TCR in the presence of IFN-γ, signals activate STAT1 and induce the key Th1-specific transcription factor, T-bet, during the initial polarization phase. T-bet induces expression of the IL-12 receptor β2 subunit and increases the responsiveness of Th1 cells to IL-12. In turn, IL-12 activates the transcription factor, STAT4, that activates and triggers IFN-γ gene transcription. The production of IFN-γ also leads to the induction of T-bet, which induces the second wave of sustained T-bet expression. T-bet binds to regulatory elements of the IFN-γ gene that creates a positive-feedback loop in Th1 differentiating pathway.81 By contrast, IL-4 promotes the Th2 differentiation of naive Th0 cells and the production of Th2 cytokines. IL-4 induces transcription factor STAT6. STAT6 and TCR stimulation leads to subsequent activation of the Th2 master regulator, GATA-3. GATA-3 activates the IL-5 and IL-13 promoters and regulates Th2 lineage development. Differentiated cells are very sensitive to regulatory signals; for example, even trace quantities of IFN-γ caused significant up-regulation of T-bet mRNA in cells cultured under Th2 polarizing conditions, and inhibition of IFN-γ restored a pure Th2-like pattern.80 Moreover, acetylation of the IFN-γ gene preceded T-bet acetylation,80 suggesting that T-bet (or GATA-3) is responsible for stable lineage commitment, whereas cytokines are key to the early stages of cell differentiation. Thus, the initial acetylation of lineage-specific genes depends on cytokine and STAT signaling, whereas T-bet and GATA-3 contribute to the maintenance of appropriate chromatin remodeling, including a polarized acetylated state.
Little is known about the effects of HDACi use on these events. The T-bet and the IFN-γ genes are acetylated in histones H3 and H4 during Th1 differentiation, and histone acetylation of the T-bet gene was markedly suppressed by IL-4, whereas histones associated with the IL-4 gene are acetylated in Th2 cells. Thus, HDAC/HAT activity plays a key role in cytokine gene transcription and in maintaining the balance of cytokines during differentiation of T cells. Beyond the epigenetic effects, lineage-specific transcription factors themselves (GATA-3, STAT1, and STAT6), can be substrates for acetylation,25 and thereby their activity can be regulated by HDAC/HAT balance, but these effects need to be elucidated. Our murine data showed that HDAC6, HDAC9, and Sirt1 KO CD4+ and CD8+ cells displayed normal development, number, and phenotypes comparable with WT T cells. However, conditions of antigen challenge that stimulate Th1/Th2 differentiation can reveal differences (eg, SLE-prone MRL/lpr HDAC9−/− mice showed decreased cytokine production and inflammation compared with WT mice on the same background).82 HDACi can also impair the function of transcriptional repressors and thereby disturb lineage commitment regulation. Thus, butyrate can increase acetylation and chromatin remodeling adjacent to the IFN-γ and T-bet gene loci in Th2 differentiating cells.80 Likewise, HDACi can inhibit STAT183 and STAT684 activation, and inhibition of each STAT occurred only when it was up-regulated (ie, STAT6 in Th2-associated dermatitis), and STAT1 in Th1-associated GVHD.
Based on these considerations, a unifying model of the epigenetic effects of HDACi on T cells can be proposed. The balance of HAT and HDAC enzymes recruited by T-bet to the IFN-γ locus, or by GATA to the Th2 gene loci, determines whether or not the key events of histone acetylation and chromatin remodeling occur that regulate gene expression. Gene loci associated with acetylated histones undergo high levels of transcription that result in positive feedback loops required for appropriate Th1 or Th2 differentiation. However, HDACi can also disrupt the formation of dominant deacetylated repressive marks that need to be established at the loci of genes of other lineages. Such repressive marks actively prevent gene transcription and help promulgate the negative effects of Th2 factors on Th1 development and vice versa. As a result, HDACi use can lead to impaired or incomplete Th1 or Th2 differentiation. Because HDACi also suppress inflammatory cytokine production by T cells and APCs, and disturb stimulatory signals, we can conclude that the early differentiation of Th cells is sensitive to HDACi, especially under inflammatory conditions.
Alternate route: HDACi, T-cell anergy, and iTreg differentiation.
Early lineage differentiation is especially sensitive to external stimuli, and cells readily become anergic in response to a lack of costimulation, altered stability of immunologic synapses, and reduced cytokine levels. All of these conditions are enhanced by HDACi use. Indeed, butyrate, TsA, oxamflatin (class I), and scriptaid (class III) were each shown to induce antigen-specific anergy in naive murine CD4+ T cells by up-regulating p21 expression.70 The significance of p21 up-regulation for tolerance induction was shown using 2 different murine strains; DBA/2 cells had lower p21 expression than C57BL/6 mice, which correlated with impaired tolerance induction by an n-butyrate derivative, MEB. MEB-treated p21−/− cells did not induce tolerance, despite MEB inhibiting cell divisions in all cells to a comparable extent.85 Hence, induction of tolerance by HDACi does not appear to require inhibition of cell division. Overall, these data suggest that p21 up-regulation by HDACi is not responsible for the inhibitory effects of HDACi on T-cell division but is important for development of T-cell anergy, at least for murine T cells in vitro.
HDACi use can also promote the generation of iTregs from conventional T cells. Recently, Molinero et al showed that TCR-dependent NF-κB signaling, in conditions of high Ag dose and costimulatory signals, prevents Foxp3 up-regulation, despite iTreg-differentiating conditions, and indicating that NF-κB activation both promotes vigorous effector T-cell responses and prevents iTreg differentiation.86 NF-κB signal transduction pathway can be targeted by HDACi. Thus, HDACi exposure can inhibit proteasomal degradation and stabilize expression of the endogenous inhibitor of NF-κB, IκB. This leads to decreased nuclear translocation and DNA binding of NF-κB, and to down-regulation of NF-κB dependent pro-inflammatory genes.59 Because HDACi are suppressors of NF-κB–mediated pathway, this mechanism may be important to the development of iTregs in vitro and in vivo.11,38
Stage 4: epigenetic lineage memory formation and expansion of effector T cells
Once lineage differentiation is established, a rise in effector cell activity and cytokine release occurs in parallel with expansion, and usually gradually increases during cell divisions.64 At this stage, increases in the activity of DNMT enzymes lead to DNA methylation and specific and inheritable changes in daughter cells. Once activated, Th1 and Th2 effector cells become less sensitive to external signals, and permissive histone modifications and increased histone acetylation are observed at sites of lineage-specific genes.60 Differentiated Th1 and Th2 cells are less flexible than naive and early differentiating cells, as illustrated by the inability of retrovirally expressed GATA-3 and dominant-negative T-bet protein to induce normal levels of IL-4 or abrogate IFN-γ expression in Th1 clones.81 However, Th1 cells retain some flexibility because human Th1 and Th2 clones can produce both cytokines when cultured under opposing polarizing conditions.81 This stage is also associated with nuclear export of class IIa HDAC; HDAC787 was shown as regulator of cytokine production in T cells, and restricting HDAC localization to the nucleus inhibited cytokine production. HDACi use can affect Teff cell development and function at this stage. Thus, TsA and class I-specific FR901228 inhibited initial and ongoing proliferation of human CD4+ cells and caused CD154 down-regulation in freshly stimulated, preactivated and in 21-day expanded CD4+ cells.78 In addition, in vivo models in which HDACi treatment was started after development of disease showed that already enhanced cytokine production and T-cell activation could be suppressed by HDACi. This was shown, for example, in NOD mice with spontaneously developed diabetes,81 although the observed effects might reflect anti-inflammatory action of HDACi on pathogenic T cells and/or activated APCs or could encompass effects on Treg cells. In a model of colitis, we used Rag−/− mice with adoptively transferred CD4+CD25− T cells from scurfy mice (which cannot convert into Tregs). In this case, TsA treatment was ineffective, suggesting that the positive effects of HDACi therapy required functional Treg cells.13
Stage 5: memory T-cell differentiation
As inflammation resolves and cytokine levels decrease, activated effector cells die or become resting memory cells. Memory cells do not produce cytokines but are able to reactivate lineage-specific effector genes during the first hours after the antigen challenge, providing a fast and robust adaptive immune response.60,87 Memory cell formation involves epigenetic mechanisms, including methylation and acetylation. IL-2–promoter and IFN-γ enhancer are demethylated in CD8+ memory cells88 and have the permissive acetylated histone marks, H3K9Ac, characteristic of active genes. Similarly, CD4+ memory cells have increased acetylation at appropriate cytokine loci.89 Interestingly, when CD8+ memory formation was disrupted by an absence of the CD4+ help that is required for this process, the IFN-γ locus lost acetylation, and HDACi treatment restored acetylation and function of CD8+ memory cells.88 The absence of transcription in poised effectors genes, despite demethylated and acetylated events, suggests that transcriptional repressor complexes, which are known to be associated with HDAC, play an important role in gene silencing and may be of therapeutic significance. For instance, in HIV patients, latently infected memory CD4+ T cells have integrated HIV-1 proviral DNA, and given their resting condition and long life, form a viral reservoir capable of causing reinfection when activated and rendering antiretroviral therapy ineffective. However, reactivation of latent virus occurred when memory CD4+ cells were exposed to HDACi, cells were killed, and infection cleared by antiviral therapy.90
The integrated view: enhancing Treg survival and function and calming other cells
Inflammatory conditions recruit innate and adaptive immune cells and dictate them to activate, divide, and use an appropriate effector mechanism to reach pathogen clearance. Hence, the resistance of inflammatory cells to suppression by Tregs and active corruption of Treg phenotype by inflammatory environment, leading to impaired function or increased conversion of Tregs into Th1, Th2, and Th17 cells, are normal and indispensable processes of immune regulation. Normal Tregs should lose their phenotype and should be silenced when there is no need for them. But pro-inflammatory misbalances, such as degenerative disorders, chronic inflammation, autoimmunity, septic shock, or unwanted immune activity in allogeneic grafts, have the same inhibitory impact on Tregs as nonpathogenic inflammation has. The current anti-inflammatory therapies, such as nonsteroidal anti-inflammatory drugs, steroids, and calcineurin inhibitors (in transplantation), have many side effects but also they can harm Tregs as shown for calcineurin inhibitors.91 On the contrary, HDACi use is pathogenetically consistent. That can be illustrated by Bovenschen et al,92 who showed that ex vivo isolated Tregs from psoriatic patients had enhanced differentiation into IL-17+ cells, including CD4+ FOXP3+ IL-17+ cells within affected skin areas, and that TsA treatment inhibited IL-17 conversion of patients' Tregs. The integrated view of multiple inflammatory mechanisms known to impair Treg function and phenotype, and HDACi effects on these mechanisms are summarized in Figure 3.
Effects of inflammatory and/or immune stimuli on T-cell activation and their modulation by HDACi. (Left panel) How multiple mechanisms promote Teff cell resistance to Treg suppression, impair Treg function, and inhibit the development of iTregs. Intervention points for HDACi, as identified from the current literature, are indicated in red balls (inhibition) and green balls (stimulation). (Right panel) Mechanisms by which HDACi can promote resolution of inflammation, including through modulation of T-cell activation and enhancement of Treg suppressive functions.
Effects of inflammatory and/or immune stimuli on T-cell activation and their modulation by HDACi. (Left panel) How multiple mechanisms promote Teff cell resistance to Treg suppression, impair Treg function, and inhibit the development of iTregs. Intervention points for HDACi, as identified from the current literature, are indicated in red balls (inhibition) and green balls (stimulation). (Right panel) Mechanisms by which HDACi can promote resolution of inflammation, including through modulation of T-cell activation and enhancement of Treg suppressive functions.
Selective targeting of tumor and inflammatory, but not normal, cells in vivo
Our perspective, based on the work summarized above, is that HDACi compounds in nontumor immune cells have important cell-, tissue-, and condition-specific actions and thereby cannot be simply translated from HDACi effects on tumor cells. As malignant cells are the most sensitive to HDACi therapy, in situations when both types of cells are present (malignant and immune), anti-tumor effects of HDACi in vivo dominate over anti-inflammatory or immunosuppressive effects. Thus, in melanoma-bearing mice that were adoptively transferred with normal T cells, HDACi therapy led to enhanced anti-tumor effects, accompanied by improved, but not suppressed, functional activity of normal adoptively transferred T cells.93 In multiple solid tumor models, the combination of SAHA, pabinostat, and CD40 and CD137 antibodies lead to eradication of tumors and simultaneously stimulated APC and T cells, inducing not only the current anti-tumor response but also long-term antitumor immunologic memory.94 On the other hand, when tumor exist on highly inflammatory background, as inflammation-induced tumorigenesis in ulcerative colitis, the HDACi use showed anti-tumor activities along with local suppression of inflammation.95
As shown in the previous paragraph, highly activated inflammatory cells have increased sensitivity to HDACi, whereas resting immune cells are more resistant. Therefore, anti-inflammatory effects of HDACi in vivo tend to target pathologic inflammatory responses but preserve normal immune cell functions. Thus, in a fully MHC-mismatched model of GVHD, SAHA use reduced inflammation and GVHD-dependent mortality by targeting activated allo-specific cells but did not affect donor T-cell activation or expansion (ie, did not induce nonspecific immunosuppression).83 There are many other examples of the anti-inflammatory effects of HDACi.90,96-98 Collectively, these data indicate that HDACi use in vivo targets the most sensitive cells, especially tumor cells or activated immune cells, which might be beneficial compared with conventional anti-inflammatory therapies.
Safety and side effects of HDACi and future directions
Most of the current clinical data involve the use of pan-HDACi or class I HDACi at relatively high concentrations in oncology settings. Side effects include neutropenia or leukopenia, thrombocytopenia, intestinal problems (anorexia, nausea, vomiting, diarrhea), electrolyte disturbances, and profound fatigue.99 Except for neurocortical disturbances, such as confusion and tremor, which were reported mainly with butyrate and valproate, no marked differences in adverse effects or in anti-cancer activity were seen between pan-HDACi and class I HDACi. Overall, HDACi were better tolerated and had less toxicity than conventional chemotherapeutic agents, but in non–life-threatening conditions, even somewhat reduced levels of side effects may be unacceptable. It is anticipated that the HDACi concentrations capable of providing anti-inflammatory responses will be lower and involve less toxicity than the levels used in oncology trials, and the first clinical trials with nonmalignant diseases provide encouraging data.90 It may also be possible to develop methods to limit effects on nontarget cells using cell-specific drug delivery system, as shown for HDACi and human monocytes.100
Pan-HDACi target class I HDAC that are widely expressed and involve multiple pathways and substrates, whereas class II HDAC regulate a more discrete range of tissue and cell specific functions, including in Tregs. To our knowledge, no isoform-specific class II HDACi have been tested clinically, but ongoing developments suggest that this may be a promising goal. Additional questions to be addressed include the following: whether there is a single HDAC that is the best candidate for targeting in autoimmune or inflammatory conditions, which HDAC should be left unaffected, and whether therapy with HDACi can be usefully combined with other agents, such as the ability to develop allograft tolerance in transplant recipients by brief combined therapy with HDACi and rapamycin.11
Acknowledgments
This work was supported by the National Institutes of Health (1K08AI095353-01, U.H.B.; and P01AI073489 and R56AI095276, W.W.H.).
National Institutes of Health
Authorship
Contribution: T.A. performed research, analyzed data, prepared graphics, and wrote the paper; U.H.B., Y.L., and L.W. performed research and contributed ideas and editing skills; and W.W.H. designed the research and cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wayne W. Hancock, Division of Transplant Immunology, Pathology and Laboratory Medicine, 916B Abramson Research Center, Children's Hospital of Philadelphia, 3615 Civic Center Blvd, Philadelphia, PA 19104-4318; e-mail: whancock@mail.med.upenn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal