Abstract
The circulating peptide hormone hepcidin maintains systemic iron homeostasis. Hepcidin production increases during inflammation and as a result of endoplasmic reticulum (ER) stress. Elevated hepcidin levels decrease dietary iron absorption and promote iron sequestration in reticuloendothelial macrophages. Furthermore, increased plasma hepcidin levels cause hypoferremia and the anemia associated with chronic diseases. The signal transduction pathways that regulate hepcidin during inflammation and ER stress include the IL-6–dependent STAT-3 pathway and the unfolded protein response–associated cyclic AMP response element-binding protein-H (CREBH) pathway, respectively. We show that carbon monoxide (CO) suppresses hepcidin expression elicited by IL-6– and ER-stress agents by inhibiting STAT-3 phosphorylation and CREBH maturation, respectively. The inhibitory effect of CO on IL-6–inducible hepcidin expression is dependent on the suppressor of cytokine signaling-3 (SOCS-3) protein. Induction of ER stress in mice resulted in increased hepatic and serum hepcidin. CO administration inhibited ER-stress–induced hepcidin expression in vivo. Furthermore, ER stress caused iron accumulation in splenic macrophages, which could be prevented by CO. Our findings suggest novel anti-inflammatory therapeutic applications for CO, as well as therapeutic targets for the amelioration of anemia in the hypoferremic condition associated with chronic inflammatory and metabolic diseases.
Introduction
Hepcidin, a circulating peptide hormone synthesized by the liver, functions as a master regulator of iron homeostasis.1-3 Elevated levels of hepcidin can block the release of iron from macrophages, impair intestinal iron absorption, and cause hypoferremia, leading to iron deficiency anemia.4-7 Hepcidin plays a major role in the anemia of inflammation observed in patients with a variety of disorders, including infection, arthritis, inflammatory bowel disease, trauma, cancer, and organ failure.1,8
IL-6 is the principal regulator of hepcidin expression associated with the anemia of inflammation.8 In humans, IL-6 can increase hepcidin levels and decrease serum iron levels. IL-6 treatment also induces hepcidin expression in vivo and in primary hepatocytes and hepatoma cell lines. The critical role of IL-6 in hepcidin expression is illustrated by experiments showing that anti–IL-6 Abs block hepcidin mRNA expression in vivo and in hepatocytes stimulated by lipopolysaccharide (LPS).9 Furthermore, IL-6–deficient mice display impaired hepcidin induction and do not display low serum iron in response to pro-inflammatory stimuli.9 These observations suggest a relationship between IL-6 and the expression of hepcidin in inflammation.
IL-6 plays a central role in the regulation of the acute-phase response (APR) in hepatocytes.10 After exposure to pro-inflammatory stimuli, IL-6 is released and binds to a complex of the IL-6 receptor-α and gp130.11 The IL-6 ligand–receptor interaction results in the activation of JAKs, which in turn activate STAT-3 by tyrosine (Y705) phosphorylation. Phosphorylated STAT-3 subsequently translocates into the nucleus, where it regulates the transcription of target genes including hepcidin.12 The transcriptional activity of STAT-3 is regulated by serine (S727) phosphorylation. Additional factors appear to regulate hepcidin expression, because IL-6–knockout mice maintain some hepcidin responsiveness to LPS. For example, IL-1 has been shown to stimulate hepcidin expression in both mouse hepatocytes and human Huh7 cells.13,14
Recent studies indicate that the unfolded protein response (UPR) associated with endoplasmic reticulum (ER) stress can induce APR proteins, including C-reactive protein (CRP), serum amyloid P component, and hepcidin, through the activation of the transcription factor cyclic AMP response element-binding protein-H (CREBH).15 CREBH binds and trans activates the hepcidin promoter. ER stress can induce hepcidin-dependent hypoferremia and splenic iron sequestration in mice.16 These studies suggest linkages among the ER-stress associated UPR, innate immunity, and iron homeostasis.
Carbon monoxide (CO) arises endogenously as the by-product of the cytoprotective heme oxygenase (HO) enzyme system, which also generates equimolar biliverdin-IXα and ferrous iron during the oxidative catabolism of heme. CO, when applied at low concentration, can exert anti-inflammatory, antiproliferative, and antiapoptotic effects in a variety of models of cellular injury.17 We have shown previously that exogenous CO can activate anti-inflammatory signaling through the UPR. Specifically, CO selectively activated 1 branch of the UPR, protein kinase R-like endoplasmic reticulum kinase (PERK), resulting in increased activation of Nrf-2, a master regulator of the stress response, and downstream expression of the cytoprotective protein HO-1.18 Concomitantly, CO suppressed the other 2 branches of the UPR involving inositol-requiring transmembrane kinase/endonuclease-1α (IRE1α) and activating transcription factor 6 (ATF-6).18,19 However, the precise mechanisms and signaling pathways involved in CO-dependent anti-inflammatory and cytoprotective effects remain to be fully elucidated.
Because both pro-inflammatory and ER-stress responses can induce hepcidin expression, in the present study, we investigated whether the modulation of cytokine- and UPR-dependent signaling pathways by therapeutic gases such as CO could attenuate overproduction of hepcidin. Specifically, we found that CO could inhibit both IL-6– and ER-stress–induced hepcidin expression. We therefore investigated the underlying mechanisms for this homeostatic function of CO on iron metabolism.
Methods
Reagents
The tricarbonyl dichlororuthenium (II) dimer (RuCO also known as CO-releasing molecule-2 or CORM-2), thapsigargin, tunicamycin (TM), homocysteine, 4-phenylbutyric acid, and ruthenium chloride were from Sigma-Aldrich. Tricarbonylchlor-(glycinate) ruthenium (II) (CORM-3) was kindly contributed by Dr Haksung Kim (Kangwon National University, Kangwon, Korea). CORM-3 was first synthesized by Motterlini et al and its properties are well documented.20 CORM-3 is a novel, water-soluble ruthenium-based carbonyl CO carrier that was developed for in vivo applications. CORM-3 is stable in water at 37°C and at acidic pH for more than 24 hours and liberates CO rapidly in physiologic solutions and biologic fluids. Moreover, in vivo studies have demonstrated that CORM-3 specifically delivers CO to cells and tissues, mimicking the biologic activities associated with CO gas inhalation.21
Recombinant human and mouse IL-6, and oncostatin M (OSM) were from R&D Systems. Recombinant leukemia inhibitory factor (LIF) and bone morphogenetic protein-2 (BMP-2) were from Sigma-Aldrich. The Ab to hepcidin was from Abcam. According to manufacturer's data sheet for hepcidin Ab (ab30760; Abcam), this Ab detects the mature, cleaved form of hepcidin. The immunogen is a synthetic peptide conjugated to keyhole limpet hemocyanin derived from within residues 50 to the C-terminus of human hepcidin-25. This Ab recognized a band with an approximate molecular size of 32 kDa when tested against human recombinant hepcidin. Abs to total STAT-1 and STAT-3, CREBH, phospho-ERK, and total ERK were from Santa Cruz Biotechnology. Abs to phospho–STAT-1 and phospho–STAT-3, phospho-SMAD 1/5/8, were purchased from Cell Signaling Technology. Abs to Flag and all other chemicals were from Sigma-Aldrich.
Animals
All experiments with mice were approved by the Animal Care Committee of the University of Ulsan. Seven-week-old male C57BL/6 wild-type mice and C3H mice were purchased from ORIENT. The mice were maintained under specific pathogen-free conditions at 22°C and given access to food and water ad libitum. CORM-3 was freshly dissolved in distilled water before each experiment and administered intravenously to C57BL/6 mice (10 mg/kg/d).22 The control mice received the same amounts of distilled water. Three hours after CORM-3 treatment, mice were IP injected with recombinant mouse IL-6 (mIL-6, 25 μg/kg) or TM (1.5 mg/kg) dissolved in 0.5% vol/vol DMSO/saline solution. The control mice received the same amounts of 0.5% DMSO/saline solution. After 24 hours, CORM-3 was administrated again. At 48 hours after injection, mice were killed by cervical dislocation and serum (from venous blood collected from the orbital sinus), spleen, and liver tissue were obtained for experiments.
Cell culture
Cell cultures were grown at 37°C in humidified incubators containing an atmosphere of 5% CO2. The human hepatocellular carcinoma cell line HepG2 was purchased from ATCC and maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin solution. Human hepatocytes (Huh7) were maintained in RPMI medium supplemented with 10% FBS and 1% penicillin/streptomycin solution.
Immunoblot analysis
After experimental treatments, cells were harvested and washed twice with ice-cold PBS. Cells were lysed with 1× RIPA buffer containing phosphatase and protease inhibitors. Serum samples obtained from mice were diluted in 1× PBS. Equal amounts of cell lysates and serum were measured with the BCA protein assay reagent (Pierce Biotechnology). The samples were diluted with 2× sample buffer containing β-mercaptoethanol, and then equal amounts of protein were separated on 6%-15% SDS-PAGE gels followed by transfer to polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat milk in PBS containing 0.1% Tween 20 (PBS-T) for 20 minutes, and incubated overnight with Abs to hepcidin (1:1000), CREBH (1:500), and suppressors of cytokine signaling (SOCS-3; 1:1000) in PBS-T containing 1% nonfat milk. The blots were developed with a peroxidase-conjugated secondary Ab and reacted proteins were visualized using the ECL Plus Western Blotting Detection System (GE Healthcare Life Sciences). The relative signal intensities of bands were determined and standardized using Scion Image Beta 4.0.3 Software.
RNA isolation and RT-PCR
Total cellular RNA was isolated from the HepG2 cells and liver tissue of mice using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Briefly, 2 μg of extracted RNA was reverse-transcribed into first-strand cDNA using M-MLV reverse transcriptase and the oligo (dT) 15 primer (Promega). cDNA was amplified using primers specific for human hepcidin, mouse hepcidin, and spliced XBP-1 using PCR.16 Gene expression data from RT-PCR was quantified relative to GAPDH. Primer sequences and reaction conditions are provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Real-time qRT-PCR
For real-time quantitative RT-PCR (qRT-PCR), total cellular RNA was extracted using TRIzol reagent according to the manufacturer's instructions (Invitrogen). cDNA was generated by reverse transcription. Real-time qRT-PCR was performed using SYBR Green qPCR Master Mix (2X; USB products; Affymetrix) on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems). Primer sequences are provided in supplemental Methods.
Plasmid, siRNA transfection
pCMV-Flag/wtSTAT3, pCMV-Flag/dnSTAT3(R382W), or pCMVT2B-Flag were kind gifts from Dr Jeong-Woo Park (University of Ulsan, Ulsan, Korea). Plasmids were purified by Hispeed Plasmid Maxi Kit (QIAGEN). Cells were transfected with plasmid (4-μg DNA concentration per well) for 12 hours using the lipofectamine method according to the manufacturer's protocol (Invitrogen), and then restored to fresh medium containing 10% FBS for 36 hours.
Predesigned siRNAs against human STAT-3, SOCS-3, and CREBH were purchased from Santa Cruz Biotechnology. Cells were transfected with siRNA (50 or 100nM) for 12 hours using the lipofectamine method according to the manufacturer's protocol (Invitrogen), and then restored to fresh medium containing 10% FBS for 36 hours. The interference of STAT-3, SOCS-3, and CREBH expression was confirmed by Western blotting using anti–STAT-3, SOCS-3, and CREBH Abs, respectively. Corresponding scrambled siRNAs were used as transfection controls.
Serum iron and hepcidin analysis
Serum iron was determined using a commercially available iron assay kit (BioVision). The mouse hepcidin ELISA kit was purchased from USCN Life Science. Serum iron and hepcidin levels were analyzed according to the manufacturer's instructions.
Iron staining of paraffin-embedded sections
To evaluate iron deposition in the liver and spleen, mouse tissues were immersion fixed in 4% formalin, embedded in paraffin wax, sectioned, and stained with Perl's Prussian blue for iron content using an iron staining kit (Sigma-Aldrich).
Tissue nonheme iron analysis
Spleen samples were dried at 65°C for 24 hours and weighed. Samples were digested in an acid-digestion mixture (3M hydrochloric acid/10% trichloroacetic acid; Sigma-Aldrich) at 65°C for 24 hours, and then 100 μL of each acid extract was then incubated with 300 μL of ferine (3mM; Sigma-Aldrich) chromogen reagent. The absorbance at 592 nm was measured in a Spetramax M2 spectrophotometer (Molecular Devices). A calibration curve was constructed using serial dilutions of a solution of 45mM ferrous ammonium sulfate (Sigma-Aldrich). Results were expressed in millimoles per gram dry weight of spleen tissue.
Statistical analysis
Data are expressed as means ± SD. Statistical analysis was performed using paired nonparametric t tests and calculated using a 1-tail P value in Prism Version 5 software (GraphPad). A P < .05 was considered to represent a statistically significant change.
Results
CO inhibits hepcidin expression by IL-6 and ER-stress inducers
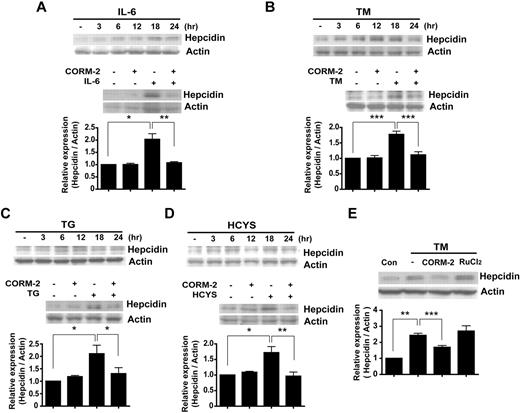
IL-6 acts as a major regulator of the APR in hepatocytes. In response to pro-inflammatory stimuli, macrophages secrete IL-6, which induces hepcidin expression in vitro and in vivo.9,23 Consistent with these observations, we found that IL-6 treatment induced the time-dependent expression of hepcidin in HepG2 cells beginning at 12 hours, with a maximum at 18 hours after treatment (Figure 1A). To investigate whether the anti-inflammatory potential of CO can suppress the induction of hepcidin by IL-6, HepG2 cells were treated with IL-6 in the absence or presence of CORM-2. CORM-2 treatment markedly inhibited the IL-6–dependent induction of hepcidin expression in these cells (Figure 1A and supplemental Figure 1A).
CO inhibits hepcidin expression by IL-6 and ER-stress inducers. HepG2 cells were treated with IL-6 (10 ng/mL; A), TM (10 mg/mL; B), thapsigargin (TG; 10mM; C), or homocysteine (HCYS; 1mM; D) for the indicated periods of time. The expression levels of hepcidin were analyzed by Western blotting (top panels). Cells were preincubated for 3 hours with CORM-2 (20μM) and were then exposed for 18 hours to IL-6, TM, TG, or HCYS (bottom panels). (E) HepG2 cells were treated with CORM-2 or RuCl2 and treated with TM for 18 hours. (A-E) β-actin served as the standard. Values are means ± SEM from 3 independent experiments. *P < .05; **P < .01; ***P < .001.
CO inhibits hepcidin expression by IL-6 and ER-stress inducers. HepG2 cells were treated with IL-6 (10 ng/mL; A), TM (10 mg/mL; B), thapsigargin (TG; 10mM; C), or homocysteine (HCYS; 1mM; D) for the indicated periods of time. The expression levels of hepcidin were analyzed by Western blotting (top panels). Cells were preincubated for 3 hours with CORM-2 (20μM) and were then exposed for 18 hours to IL-6, TM, TG, or HCYS (bottom panels). (E) HepG2 cells were treated with CORM-2 or RuCl2 and treated with TM for 18 hours. (A-E) β-actin served as the standard. Values are means ± SEM from 3 independent experiments. *P < .05; **P < .01; ***P < .001.
Previous studies have shown that agents that trigger ER stress can induce hepcidin expression in vivo, resulting in hypoferremia and splenic iron sequestration.15,16 We sought to confirm whether 3 well-characterized inducers of the ER-stress response (ie, TM, thapsigargin, and homocysteine) could induce hepcidin expression in vitro in our hepatoma cell line model. Treatment of HepG2 cells with these ER-stress activators markedly increased the level of hepcidin (Figure 1B-D). We also examined whether CO treatment could modify hepcidin expression induced by these ER-stress agents. Pretreatment with CORM-2 significantly inhibited ER-stress–induced expression of hepcidin protein (Figure 1B-D) and mRNA (supplemental Figure 1B). To confirm that the effect of CORM-2 on hepcidin expression was attributable to its CO-releasing effect rather that to an off-target effect, HepG2 cells were pretreated with CORM-2 or ruthenium chloride (RuCl2) and then treated with TM to induce hepcidin. Although both compounds contain ruthenium, RuCl2 did not have a suppressive effect on hepcidin expression induced by TM (Figure 1E). We also conducted similar experiments to confirm that the effect of CO on hepcidin expression induced by IL-6 or ER-stress inducers could also be observed in the hepatocyte cell line Huh7 (supplemental Figure 1C-D).
OSM and LIF, members of the IL-6–related cytokine family, play important roles in immune and inflammatory responses. OSM is known as an alternate cytokine that is able to regulate hepcidin gene expression24,25 and LIF can regulate hepcidin expression.24 In the anemia of multiple myeloma, increased levels of BMP-2 stimulate hepcidin expression.26 Therefore, we also investigated whether CO could down-regulate hepcidin expression elicited by these inflammatory mediators. Treatment of HepG2 cells with OSM, LIF, or BMP-2 resulted in a significant increase of hepcidin protein levels at 18 hours. Pretreatment with CO greatly attenuated the increased expression of hepcidin in response to these factors (supplemental Figure 1E). We investigated whether CORM-2 could inhibit SMAD signaling, because CORM-2 down-regulated BMP-–-induced hepcidin (supplemental Figure 1H). CORM-2 was not effective in reducing BMP-SMAD signaling when assessed with the p-SMAD 1/5/8 Ab (supplemental Figure 1F). We also investigated whether CO could down-regulate another BMP-2 target gene, Id2. Treatment with BMP-2 for 24 hours increased the gene expression of Id2, whereas CO pretreatment markedly down-regulated the expression of Id2 induced by BMP-2 (supplemental Figure 1G). These data suggest that CO could suppress BMP-2 signaling through a SMAD-independent pathway. We hypothesized that CO can inhibit BMP-2 signaling through inhibition of ERK1/2 activation. It is well documented that BMP-2 signaling activates ERK1/2 phosphorylation to modulate SMAD signaling.27 CO suppressed ERK1/2 activation under our experimental conditions (supplemental Figure 1H).
CO attenuates IL-6–induced STAT-3 and STAT-1 activation
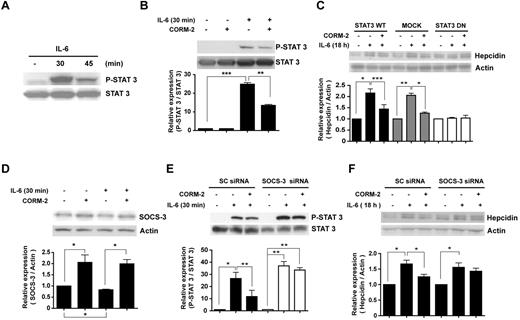
IL-6 can induce hepcidin though the activation of STAT-3.28 To determine whether the inhibition of hepcidin by CO is because of its regulation of the STAT-3 pathway, we examined STAT-3 tyrosine phosphorylation (Y705) in response to IL-6 treatment. Phospho-STAT-3 was significantly increased after IL-6 treatment, with an apparent maximum at 30 minutes after treatment (Figure 2A). Pretreatment with CORM-2 decreased the IL-6–dependent activation of STAT-3, as assessed by Y705 phosphorylation (Figure 2B) and serine phosphorylation (supplemental Figure 2A). Like STAT-3, STAT-1 activation was also inhibited by CO pretreatment (supplemental Figure 2B).
CO attenuates IL-6–induced STAT-3 activation. (A) HepG2 cells were treated with IL-6 (10 ng/mL) in a time-dependent manner, and then total cell lysates were probed with Ab for tyrosine phosphorylated or total STAT-3. Cells were preincubated for 2 hours with CORM-2 (20μM) and were then exposed for 30 minutes to IL-6 (B,D). (C) HepG2 cells were transiently transfected with STAT-3 DN vector, STAT-3 wild-type vector, or empty vector and exposed to 10 ng/mL of IL-6 for 18 hours in the presence or absence of CORM-2. Cells were transiently transfected with siRNA against human SOCS-3. After transfection, the cells were incubated with CORM-2 and then exposed to IL-6 for 30 minutes (E) or 18 hours (F). Values are means ± SEM from 3 independent experiments. *P < .05; **P < .01; ***P < .001.
CO attenuates IL-6–induced STAT-3 activation. (A) HepG2 cells were treated with IL-6 (10 ng/mL) in a time-dependent manner, and then total cell lysates were probed with Ab for tyrosine phosphorylated or total STAT-3. Cells were preincubated for 2 hours with CORM-2 (20μM) and were then exposed for 30 minutes to IL-6 (B,D). (C) HepG2 cells were transiently transfected with STAT-3 DN vector, STAT-3 wild-type vector, or empty vector and exposed to 10 ng/mL of IL-6 for 18 hours in the presence or absence of CORM-2. Cells were transiently transfected with siRNA against human SOCS-3. After transfection, the cells were incubated with CORM-2 and then exposed to IL-6 for 30 minutes (E) or 18 hours (F). Values are means ± SEM from 3 independent experiments. *P < .05; **P < .01; ***P < .001.
To examine the involvement of STAT-3 in hepcidin expression, we transfected cells with empty plasmid, expression constructs encoding wild-type STAT-3, or STAT-3 DN, which is mutated in the DNA-binding domain. In HepG2 cells transfected with wild-type STAT-3 or subjected to mock transfection, treatment with recombinant IL-6 induced hepcidin expression. In contrast, recombinant IL-6 treatment failed to induce hepcidin in STAT-3 DN–transfected cells. Furthermore, CO pretreatment could not effectively down-regulate hepcidin in STAT-3 DN–transfected cells (Figure 2C). To confirm the transfection efficiency of the STAT-3 DN mutant expression construct, we probed with Flag Ab because the vector was tagged with Flag (supplemental Figure 2C). Similar results were shown in cells transfected with STAT-3 siRNA (supplemental Figure 2D). The efficiency of STAT-3 siRNA was confirmed (supplemental Figure 2E). These results suggest that CO inhibits the IL-6–dependent induction of hepcidin through inhibition of STAT-3 activation.
The SOCS proteins act as negative regulators of cytokine-dependent signal transduction. Once expressed, SOCS can down-regulate JAK/STAT-dependent signaling pathways. SOCS proteins contain a variable amino-terminal region, a central Src-homology 2 (SH2) domain, and a novel conserved carboxy-terminal motif termed the SOCS box.29 We hypothesized that CO-mediated inhibition of IL-6–induced activation of the JAK/STAT signaling pathway involves the induction of SOCS-3. CORM-2 induced SOCS-3 protein expression in the absence or presence of IL-6 (Figure 2D). In addition, we confirmed that CORM-2 induced SOCS-3 gene expression (supplemental Figure 2F). To investigate the involvement of SOCS-3 in the inhibition of IL-6–induced STAT-3 activation, we transiently transfected HepG2 cells with SOCS-3 siRNA, and confirmed siRNA efficiency (supplemental Figure 2G). Cells transfected with SOCS-3 siRNA showed greater induction of phospho-STAT-3 in response to IL-6 relative to cells transfected with scrambled siRNA. Furthermore, CO was ineffective at inhibiting STAT-3 activation in SOCS-3 siRNA–infected cells (Figure 2E). Next, we investigated whether the reduced inhibitory effect of CO on STAT-3 activation under these conditions could affect hepcidin levels. In the SOCS-3 siRNA–transfected cells, a significant increase of hepcidin expression was observed after treatment with recombinant human IL-6. CORM-2 was ineffective at reducing hepcidin expression, which was slightly up-regulated by IL-6 (Figure 2F). These data demonstrate that SOCS-3 is required for the inhibitory effect of CO on the STAT-3 pathway.
CO inhibits hepcidin expression via suppression of CREBH activation
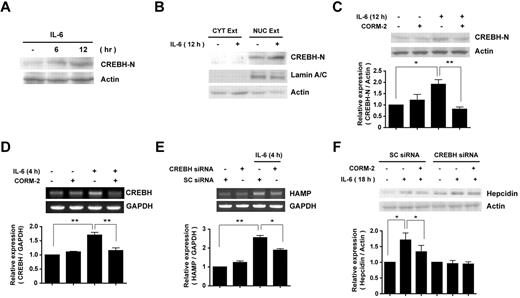
Several studies have reported that CREBH is a key transcription factor for constitutive and inducible expression of hepcidin in vitro and in vivo.15 The activation of CREBH during ER stress requires its cleavage to a truncated form, which mediates the expression of APR genes, including hepcidin.23,30 We therefore sought to determine whether the inhibition of hepcidin expression afforded by CO could be mediated in part by its modulation of CREBH activation. We first confirmed whether IL-6 could induce the cleavage of CREBH,19 resulting in the production of its active form (CREBH-N) in HepG2 cells. As shown in Figure 3A, IL-6 induced the time-dependent cleavage of CREBH to form the activated product CREBH-N, which began to appear at 6 hours after treatment (Figure 3A). The cleaved CREBH-N was translocated to the nucleus (Figure 3B). CREBH-N was exclusively detected in the nuclear fraction, marked with the nuclear protein Lamin A/C, after 12 hours of IL-6 treatment (Figure 3B).
CO inhibits IL-6–induced hepcidin expression via suppression of CREBH activation. (A) HepG2 cells were incubated with IL-6 for increasing time intervals. (B) To detect the cleavage and translocation of CREBH, cell lysates were divided into cytoplasmic and nuclear fractions, and each fraction was probed with CREBH-N Ab. Lamin A/C served as a marker for nuclear fractions. (C) Cells were preincubated for 3 hours with CORM-2 (20μM) and then treated with IL-6 for 12 hours. CREBH-N was analyzed by Western blotting. (D) CREBH mRNA levels were analyzed by RT-PCR. (E-F) HepG2 cells were transiently transfected with scramble siRNA or CREBH siRNA, preincubated for 3 hours with or without 20μM of CORM-2, and then treated with IL-6 for 18 hours to induce hepcidin expression (F). To analyze HAMP mRNA level, cells were incubated with IL-6 for 4 hours (E). β-actin served as the standard for Western blots. GAPDH served as the standard for mRNA. Values are means ± SEM from 3 independent experiments. *P < .05; **P < .01;
CO inhibits IL-6–induced hepcidin expression via suppression of CREBH activation. (A) HepG2 cells were incubated with IL-6 for increasing time intervals. (B) To detect the cleavage and translocation of CREBH, cell lysates were divided into cytoplasmic and nuclear fractions, and each fraction was probed with CREBH-N Ab. Lamin A/C served as a marker for nuclear fractions. (C) Cells were preincubated for 3 hours with CORM-2 (20μM) and then treated with IL-6 for 12 hours. CREBH-N was analyzed by Western blotting. (D) CREBH mRNA levels were analyzed by RT-PCR. (E-F) HepG2 cells were transiently transfected with scramble siRNA or CREBH siRNA, preincubated for 3 hours with or without 20μM of CORM-2, and then treated with IL-6 for 18 hours to induce hepcidin expression (F). To analyze HAMP mRNA level, cells were incubated with IL-6 for 4 hours (E). β-actin served as the standard for Western blots. GAPDH served as the standard for mRNA. Values are means ± SEM from 3 independent experiments. *P < .05; **P < .01;
Pretreatment of HepG2 cells with CORM-2 decreased IL-6–induced cleavage of CREBH (Figure 3C). We also analyzed the effect of CORM-2 on CREBH mRNA expression. The increased CREBH gene expression stimulated by IL-6 was decreased after pretreatment of HepG2 cells with CORM-2 (Figure 3D). To confirm the requirement for CREBH in IL-6–dependent hepcidin expression, we transiently transfected HepG2 cells with CREBH siRNA or the corresponding control (scramble) siRNA. The siRNA-dependent inhibition of CREBH was confirmed (supplemental Figure 2H) and resulted in the attenuated expression of hepcidin mRNA levels (Figure 3E) and protein levels (Figure 3F) compared with the control siRNA–transfected group. In CREBH siRNA–transfected cells, CORM-2 pretreatment did not significantly modulate hepcidin expression compared with the IL-6–treated group (Figure 3F).
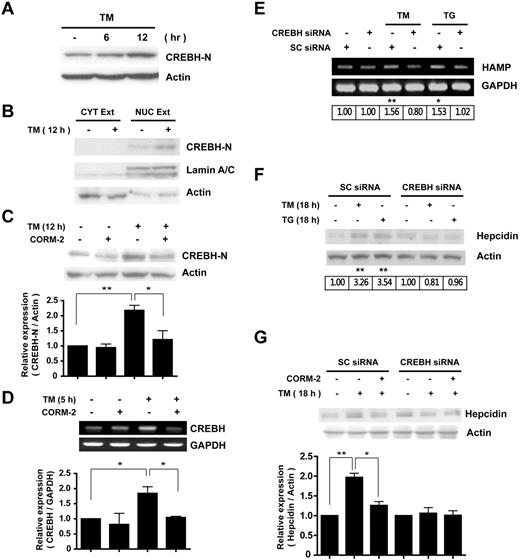
To further explore the mechanisms by which CO inhibits ER-stress–induced hepcidin expression, we examined the effects of CO on the activation of CREBH. As expected, ER stress by TM treatment induced the cleavage of CREBH and increased the amount of CREBH-N (Figure 4A). TM treatment promoted the translocation of CREBH-N to the nucleus (Figure 4B). The increased formation of CREBH-N by ER stress was markedly reduced by pretreatment with CORM-2 (Figure 4C). CORM-2 also inhibited CREBH gene expression in response to TM (Figure 4D).
CO inhibits ER-stress–induced hepcidin expression via suppression of CREBH activation. (A) HepG2 cells were treated with TM (10 mg/mL) for the indicated times. (B) To detect cleavage and translocation of CREBH, cell lysates were divided into cytoplasmic and nuclear fractions after 12 hours of TM treatment, and each fraction was probed with CREBH-N Ab. Lamin A/C served as a marker for nuclear fractions. (C) Cells were incubated in the absence or presence of CORM-2 (20μM) and treated with TM for 12 hours to detect CREBH cleavage. (D) CREBH mRNA levels were analyzed by RT-PCR. (E-G) HepG2 cells were transiently transfected with scramble (SC) siRNA or CREBH siRNA and treated with TM and thapsigargin (TG) for 5 hours (E) or 18 hours (F), and hepcidin expression was analyzed for mRNA (using RT-PCR) and protein levels. (G) Transfected cells were treated with CORM-2 or DMSO, and incubated for 18 hours to induce hepcidin expression. β-actin served as the standard for Western blots. GAPDH served as the standard for mRNA. Values are means ± SEM from 3 independent experiments. *P < .05; **P < .01.
CO inhibits ER-stress–induced hepcidin expression via suppression of CREBH activation. (A) HepG2 cells were treated with TM (10 mg/mL) for the indicated times. (B) To detect cleavage and translocation of CREBH, cell lysates were divided into cytoplasmic and nuclear fractions after 12 hours of TM treatment, and each fraction was probed with CREBH-N Ab. Lamin A/C served as a marker for nuclear fractions. (C) Cells were incubated in the absence or presence of CORM-2 (20μM) and treated with TM for 12 hours to detect CREBH cleavage. (D) CREBH mRNA levels were analyzed by RT-PCR. (E-G) HepG2 cells were transiently transfected with scramble (SC) siRNA or CREBH siRNA and treated with TM and thapsigargin (TG) for 5 hours (E) or 18 hours (F), and hepcidin expression was analyzed for mRNA (using RT-PCR) and protein levels. (G) Transfected cells were treated with CORM-2 or DMSO, and incubated for 18 hours to induce hepcidin expression. β-actin served as the standard for Western blots. GAPDH served as the standard for mRNA. Values are means ± SEM from 3 independent experiments. *P < .05; **P < .01.
Similar to the results observed with IL-6, transfection with CREBH siRNA attenuated TM-inducible expression of hepcidin mRNA (Figure 4E) and protein levels (Figure 4F-G) compared with the control siRNA–transfected group. In CREBH siRNA–transfected cells, CORM-2 pretreatment did not significantly modulate hepcidin expression compared with the TM-treated group (Figure 4G). These results suggest that CO can exert inhibitory effects on IL-6–dependent and ER-stress–induced hepcidin expression by IL-6 or ER-stress inducers through inhibition of CREBH cleavage and activation.
CO inhibits hepcidin expression and regulates iron homeostasis in ER-stressed mice
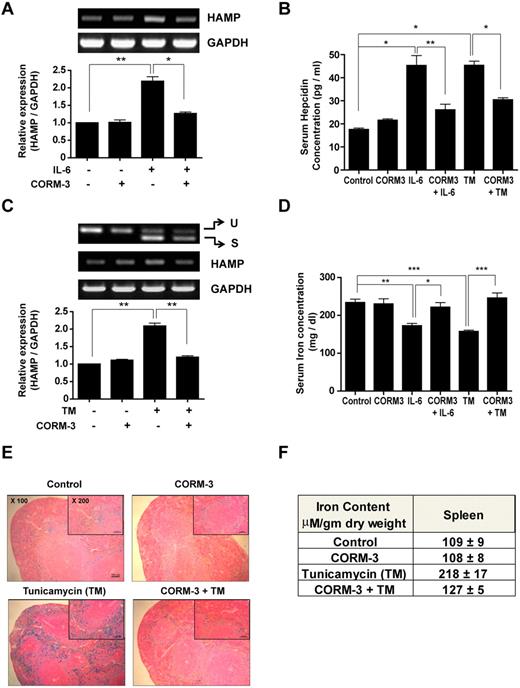
We next investigated whether the observed inhibitory effects of CO treatment on hepcidin expression in vitro could also be demonstrated in vivo. C57BL/6 and C3H mice were pretreated with CORM-3 and then injected 3 hours later with either recombinant mIL-6 or TM to cause inflammatory or ER stress, respectively. C57BL/6 mice were killed at the 48-hour time point. At that time, blood was collected for the analysis of serum iron and hepcidin and liver tissue was collected for the analysis of hepatic hepcidin mRNA levels. After the same experimental procedure, the spleen tissue of C3H mice was collected for iron staining and nonheme iron analysis. Whereas CORM-2 is soluble in organic solvents such as DMSO, CORM-3 dissolves in water. To eliminate potential off-target effects of DMSO, we used CORM-3 in mice. Mice injected with mIL-6 displayed enhanced hepcidin expression (Figure 5A and supplemental Figure 3A). CORM-3 pretreatment blocked the expression and release of hepcidin in both hepatic tissue (Figure 5A) and serum (Figure 5B and supplemental Figure 3B). We also investigated the effect of CORM-3 in TM-injected mice. We first confirmed the occurrence of ER stress in TM-injected mice by assessing the splicing of XBP-1 mRNA. TM injection caused the increased accumulation of the spliced form of XBP-1 mRNA in hepatic tissue. Under these conditions, CORM-3 significantly down-regulated the levels of serum hepcidin protein (Figure 5B and supplemental Figure 3C), and hepatic hepcidin mRNA (Figure 5C)
CO inhibits ER-stress–induced hepcidin expression and controls iron homeostasis in ER-stressed mice. C57BL/6 and C3H mice were preinjected with CORM-3 (10 mg/kg/d) and then injected 3 hours later with either recombinant mIL-6 (25 μg/kg) or TM (1.5 mg/kg). Mice were killed at the 48-hour time point. (A) Hepatic hepcidin gene expression was analyzed by RT-PCR in IL-6–injected C57BL/6 mice. (B) Hepcidin protein levels were detected from the serum of IL-6– or TM-injected C57BL/6 mice by mouse hepcidin ELISA. (C) Hepatic hepcidin gene expression was analyzed by RT-PCR in TM-injected C57BL/6 mice. (D) Serum iron levels of C57BL/6 mice were determined using an iron assay kit. (E) Iron accumulation in splenic macrophages of C3H mice were assessed by staining with Perl Prussian blue. (F) Nonheme iron content was assessed in the spleen tissue of C3H mice. Ten mice were used in each group. Values are means ± SEM from 3 independent experiments. *P < .05; **P < .01; ***P < .001. U indicates unspliced; and S, spliced.
CO inhibits ER-stress–induced hepcidin expression and controls iron homeostasis in ER-stressed mice. C57BL/6 and C3H mice were preinjected with CORM-3 (10 mg/kg/d) and then injected 3 hours later with either recombinant mIL-6 (25 μg/kg) or TM (1.5 mg/kg). Mice were killed at the 48-hour time point. (A) Hepatic hepcidin gene expression was analyzed by RT-PCR in IL-6–injected C57BL/6 mice. (B) Hepcidin protein levels were detected from the serum of IL-6– or TM-injected C57BL/6 mice by mouse hepcidin ELISA. (C) Hepatic hepcidin gene expression was analyzed by RT-PCR in TM-injected C57BL/6 mice. (D) Serum iron levels of C57BL/6 mice were determined using an iron assay kit. (E) Iron accumulation in splenic macrophages of C3H mice were assessed by staining with Perl Prussian blue. (F) Nonheme iron content was assessed in the spleen tissue of C3H mice. Ten mice were used in each group. Values are means ± SEM from 3 independent experiments. *P < .05; **P < .01; ***P < .001. U indicates unspliced; and S, spliced.
Because hepcidin is an iron-regulatory hormone that degrades the iron exporter ferroportin,3 we determined the serum iron level and intracellular iron accumulation in the spleen. Serum iron levels were significantly lower in ER-stressed mice, whereas CORM-3 pretreatment completely restored the levels of serum iron to control values (Figure 5D). Intracellular iron accumulation in the spleen was also assessed by Perl Prussian blue staining. Because staining capacity varies with mouse strain, we used the C3H strain, which is more susceptible to Prussian blue staining. Iron accumulation in splenic macrophages, as detected by blue staining, was increased in ER-stressed mice, whereas CORM-3 pretreatment blocked the accumulation of iron (Figure 5E). To quantify the nonheme iron content in spleen tissue, an acid extract of the spleen was reacted with ferine chromogen reagent, and the nonheme iron content was found to be increased approximately 2-fold compared with control mice. In contrast, CORM-3–pretreated mice displayed an attenuated increase in nonheme iron content (Figure 5F). These results suggest that CO inhibits the increase of hepcidin expression, and therefore could be used to regulate iron metabolism in ER-stressed mice.
Discussion
In the present study, we have demonstrated a novel anti-inflammatory function of CO involving the modulation of intracellular iron homeostasis through the down-regulation of hepcidin expression in hepatoma cell lines. The anemia of inflammation, a complication of common inflammatory disorders, is largely triggered by excess production of hepcidin by the liver.1,6,8 Hepcidin binds to the iron-export protein ferroportin, resulting in its endocytosis and degradation.3 As a consequence, the efflux of cellular iron is inhibited, resulting in iron accumulation in macrophages, enterocytes, and hepatocytes.1 The regulation of hepcidin during inflammation is primarily mediated by IL-6, although other pro-inflammatory mediators may play a role.9 Therefore, the regulation of hepcidin may represent an important therapeutic target for controlling the pathologic consequences of inflammation.31
The anti-inflammatory potential of CO has been characterized extensively in vitro and in vivo, but the signaling and effector pathways are not yet fully understood.17 In the present study, exogenous CO, when applied at low concentration, inhibited the LPS-dependent production of the pro-inflammatory cytokines IL-1β, TNFα, and macrophage inflammatory protein-1β in cultured RAW.264.7 macrophages, and also inhibited cytokine levels in the serum of endotoxin-challenged mice. These effects were found to be mediated by selective up-regulation of p38β MAPK.32 CO inhalation was also shown to inhibit IL-6 production in LPS-stimulated macrophages by modulating the c-Jun–NH2-terminal kinase pathway.33 In addition to MAPK pathways, recent studies have revealed additional molecules that function as downstream mediators of CO-dependent anti-inflammatory effects, including stress proteins such as HO-1 and peroxisome proliferator–activated receptor-γ (PPAR-γ).18,34
The endogenous production of CO is inextricably linked to intracellular and systemic iron homeostasis and metabolism. CO can bind to heme iron centers of hemoproteins, resulting in gain-of-function (ie, guanylate cyclase) or loss-of-function (ie, hemoglobin and cytochrome p-450).35 The evolution of CO during the enzymatic degradation of heme by heme oxygenases is stoichiometrically related to heme-iron release.36 Interestingly, genetic deletion of HO-1, the enzymatic source of CO, results in aberrant tissue iron deposition in experimental mice, although the role of hepcidin in this phenomenon has not been investigated.37 In the present study, we show for the first time that exogenously applied CO can also affect iron metabolism during pro-inflammatory states by inhibiting the expression of the circulating hormone hepcidin. We also show that inflammatory responses associated with ER stress may also be modulated by CO. In vivo, the protection afforded by CO translated to significant inhibition of splenic iron deposition.
We also found that the down-regulation of cytokine-induced hepcidin expression by CO involves the inhibition of STAT-3 phosphorylation. Activated STAT-3 binds to the hepcidin promoter at a target sequence (TTCTTGGAA) located −64 bp upstream of the transcriptional start site, and this represents a major mechanism for the IL-6–dependent regulation of hepcidin.38 Interestingly, STAT-3 was previously found to mediate the cytoprotective effects of CO in endothelial cells subjected to high oxygen stress.39
The ER is a cellular organelle responsible for the biosynthesis, folding, assembly, and modification of proteins.40 The aberrant accumulation of unfolded proteins, starvation, hypoxia, toxins, viral infections, and increased demand on the biosynthetic machinery can cause perturbations in the ER lumen commonly referred to as ER stress. Under these conditions, the ER activates a complex response system known as the UPR.41
In our previous study, we showed that CO inhibits ER-stress–induced CRP expression at the transcriptional and translational levels; CO induced phosphorylation of the PERK branch of the UPR, but inhibited activation of IRE1α, ATF-6, and CREBH.19 In the present study, we found that CO also can down-regulate hepcidin through impairment of CREBH activation during ER stress. CREBH is a regulated intramembrane proteolysis (RIP)–regulated liver-specific transcription factor that is cleaved by ER stress and required to activate expression of acute-phase proteins such as CRP and the serum amyloid P component.42 CREBH directly binds to at least 1 cyclic-AMP–responsive element consensus sequence on the hepcidin promoter.16 Hepcidin induction in response to the UPR during ER stress was found to be defective in CREBH-knockout mice.16 With respect to IL-6–dependent stimulation of hepcidin expression, both the STAT-3 and CREBH pathways are involved, which is consistent with the fact that IL-6 treatment also induces biochemical markers of the ER-stress response.19 These results point to additional therapeutic targets that are modulated by CO.
It is well known that tissue hypoxia affects hepcidin expression. It has been demonstrated that acute hypoxia can reduce the level of hepcidin mRNA in HepG2 cells cultured at 2% O2 and in vivo in mice housed in hypobaric hypoxia chambers simulating an altitude of 5500 m.7 It has been also demonstrated that chronic hypoxia reduced the expression of hepcidin mRNA in HepG2 cells cultured at 1% O2 and in the livers from rats placed in atmospheric chambers containing 10% O2.43 However, the molecular mechanisms by which hypoxia can reduce hepcidin expression remain unexplained. A major mechanism of CO toxicity is tissue hypoxia due to binding of CO to hemoglobin, myoglobin, and other hemoproteins, and inhibition of cytochrome-dependent electron transport. In this regard, CO might reduce hepcidin expression, at least in part, through its induction of hypoxia.
We also examined the effect of CO on alternative pathways to hepcidin expression. BMPs are cytokines belonging to the TGF-β superfamily. Binding of BMP to complexes of receptors causes phosphorylation of type I receptors. Activated BMP type I receptors subsequently phosphorylate the BMP-responsive SMAD 1/5/8. These proteins form complexes with a common mediator, SMAD-4, which translocates to the nucleus to regulate transcription of BMP-responsive genes. BMPs have been found to have an unexpected role in iron metabolism through inducing hepcidin expression in HepG2 and in primary murine hepatocytes.44-46 In addition to the SMAD pathway, non–SMAD-dependent pathways of BMP signaling include ERK1/2.27 A recent study demonstrating cross-talk between the BMP/hemojuvelin and ERK1/2 pathways showed that ERK1/2 activation by holotransferrin provoked increased levels of phospho-Smad1/5/8.47 Our present data suggest that CO regulates hepcidin expression in part via ERK1/2 regulation. A previous study reported that ERK1/2 activation was significantly attenuated in the presence of CO in human airway smooth muscle cells.48
In conclusion, the present study demonstrated that the down-regulation of cytokine-induced (ie, IL-6) hepcidin expression by CO involves 2 distinct and separable mechanisms: the inhibition of STAT-1/3 activation through a SOCS-3–dependent mechanism and the inhibition of CREBH maturation (see Figure 6 for diagram). Furthermore, we have shown that CO can exert a suppressive effect on ER-stress–induced hepcidin expression in vitro and in the mouse model. The results of this study support the notion that CO, one of the biologically important gases, could potentially be used in therapeutic strategies designed to control inflammation. This promise stems largely from animal-modeling studies highlighting the protective effects of inhaled CO in organ-injury models.17 The pharmacologic application of CO using CORMs and related compounds may provide a feasible alternative to the application of inhaled gas. The principle advantage of using CORMs involves the delivery of CO to tissues without excessive carboxyhemoglobinemia.49 Determination of the safety and feasibility of pharmacologic CO application in humans, however, awaits further experimentation and clinical trials.
Schematic diagram of proposed pathways. Down-regulation of cytokine-induced (ie, IL-6) hepcidin expression by CO involves 2 distinct and separable mechanisms: the inhibition of STAT-1/3 activation through a SOCS-3–dependent mechanism and the inhibition of CREBH maturation. CO can exert a suppressive effect on ER-stress–induced hepcidin expression in vitro and in the mouse model. CO also regulates hepcidin expression in part via ERK1/2 regulation induced by BMP signaling.
Schematic diagram of proposed pathways. Down-regulation of cytokine-induced (ie, IL-6) hepcidin expression by CO involves 2 distinct and separable mechanisms: the inhibition of STAT-1/3 activation through a SOCS-3–dependent mechanism and the inhibition of CREBH maturation. CO can exert a suppressive effect on ER-stress–induced hepcidin expression in vitro and in the mouse model. CO also regulates hepcidin expression in part via ERK1/2 regulation induced by BMP signaling.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by a Korea Research Foundation grant funded by the Korean government (MOEHRD, BRL-2011-0087350).
Authorship
Contribution: D.-Y.S. performed the research, analyzed the data, and wrote the manuscript; J.C., Y.J., H.-O.P., K.C.C., and G.J.C. designed and performed the research; and S.W.R. and H.-T. C. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hun-Taeg Chung, MD, PhD, School of Biological Sciences, University of Ulsan, Ulsan 680-749, Republic of Korea; e-mail: chung@ulsan.ac.kr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal