Abstract
During mouse cytomegalovirus (CMV) infection, a population of Ly49H+ natural killer (NK) cells expands and is responsible for disease clearance through the induction of a “memory NK-cell response.” Whether similar events occur in human CMV infection is unknown. In the present study, we characterized the kinetics of the NK-cell response to CMV reactivation in human recipients after hematopoietic cell transplantation. During acute infection, NKG2C+ NK cells expanded and were potent producers of IFNγ. NKG2C+ NK cells predominately expressed killer cell immunoglobulin–like receptor, and self-killer cell immunoglobulin–like receptors were required for robust IFNγ production. During the first year after transplantation, CMV reactivation induced a more mature phenotype characterized by an increase in CD56dim NK cells. Strikingly, increased frequencies of NKG2C+ NK cells persisted and continued to increase in recipients who reactivated CMV, whereas these cells remained at low frequency in recipients without CMV reactivation. Persisting NKG2C+ NK cells lacked NKG2A, expressed CD158b, preferentially acquired CD57, and were potent producers of IFNγ during the first year after transplantation. Recipients who reactivated CMV also expressed higher amounts of IFNγ, T-bet, and IL-15Rα mRNA transcripts. Our findings support the emerging concept that CMV-induced innate memory-cell populations may contribute to malignant disease relapse protection and infectious disease control long after transplantation.
Introduction
Natural killer (NK) cells are important effectors during the host innate immune response to viral infections. They can recognize and eliminate virally infected cells, interact with dendritic cells, and produce a range of cytokines and chemokines (eg, IFNγ, TNF-α, MIP-1α, MIP-1β, and RANTES) that recruit and modulate the adaptive immune response.
Under normal homeostatic conditions, a balance of activating and inhibitory signals tightly controls NK-cell function.1,2 The best-characterized inhibitory receptors are the inhibitory killer cell immunoglobulin–like receptors (KIRs) that recognize allelic epitopes present on certain class I human leukocyte antigens (HLAs) and the C-type lectin-like receptor NKG2A, which recognizes the nonclassic class I HLA, HLA-E.3,4 Activating signals are mediated by receptor families, including activating KIR, NKG2C, NKG2D, the natural cytotoxicity receptors (NKp30, NKp44, and NKp46), CD16, and CD244.1 When self HLA is down-regulated, cells are susceptible to NK-cell lysis because of the lack of ligands for the inhibitory receptors, a phenomenon known as the “missing self” hypothesis.5,6
Human cytomegalovirus (CMV), a member of the Herpesviridae family, causes asymptomatic or mild illness in healthy people.7 CMV remains latent in infected hosts and, by adulthood, approximately 60% of people in the United States are seropositive for CMV.8 However, for patients immunosuppressed due to HIV infection or solid organ or hematopoietic call transplantation (HCT), CMV is a potentially life-threatening complication. CMV has evolved escape mechanisms to evade both the innate (NK cells) and adaptive (CD8+ T cells) immune responses.9 Down-regulation of class I HLA expression and interference with Ag presentation by virus-encoded genes diminishes T-cell recognition but renders infected cells susceptible to NK-cell lysis.10 Therefore, CMV encodes viral glycoproteins that mimic class I HLAs (eg, UL-18) and that interfere with the expression of ligands for the activating receptors NKG2D and DNAM-1.11-13 Furthermore, HLA-E appears to be resistant to down-regulation, presumably to inhibit NK-cell function through NKG2A.14,15 In mice, NK cells expressing the activating receptor Ly49H preferentially expand and persist in high numbers after CMV infection, and this response is driven by the interaction of Ly49H with the viral protein m157.16 A memory-like response was also observed after rechallenge with CMV. In humans, NK cells expressing the activating receptor NKG2C preferentially expand after coculture with CMV-infected fibroblasts and are highly enriched in those who are seropositive.17,18 Expansion of NKG2C+ NK cells was also observed after infections with Hantavirus19 and Chikungunya20 and in patients with HIV,21 but only in patients co-infected with CMV. In CMV-seronegative people, the frequency of NK cells expressing NKG2C is low.17 NKG2C recognizes HLA-E, although with a lower affinity then NKG2A.22 It remains unclear whether the expansion of NKG2C+ NK cells is driven though interaction with HLA-E, with HLA-E loaded with viral peptides, or with an unknown ligand of either host or viral origin.
Evidence that NK cells play a critical role in the host response against viral infections is supported by the fact that people with NK-cell deficiencies are particularly susceptible to infections with certain viruses such as herpesviruses.23,24 Much of our understanding of how NK cells respond to acute viral infections has come from experimental animal models; much less is known in humans. However, 2 recent studies have characterized the NK-cell response during acute infection with Hantavirus19 and CMV reactivation in solid-organ transplantation recipients.25 Both studies demonstrated the expansion and persistence of NKG2C-expressing NK cells in response to infection. As the first lymphocytes to reconstitute in the host after HCT, NK cells may play a pivotal role in the control of viral infections acquired after HCT; therefore, CMV reactivation in post-HCT recipients provides a unique model with which to study acute infection in humans with developing immune systems in which weekly CMV monitoring is standard-of-care. In the present study, we used multicolor flow cytometry to investigate the kinetics of the NK-cell response during acute CMV reactivation in post-HCT recipients and the effect of CMV reactivation on the receptor repertoire and function of reconstituting NK cells in the first year after HCT.
Methods
Patients and samples
Two cohorts comprising 33 allogeneic HCT patients at the University of Minnesota, 32 umbilical cord blood (UCB) and 1 HLA-matched sibling transplantation, were studied with a total of 18 CMV reactivation events. A majority received conditioning with cyclophosphamide, fludarabine, and total body irradiation without (n = 23) or with antithymocyte globulin (n = 7) and GVHD prophylaxis with cyclosporine and mycophenolate mofetil (n = 30). There was no difference in conditioning or GVHD prophylaxis between patients who reactivated CMV and those who did not. Patients were monitored weekly for CMV reactivation by quantitative PCR performed in the clinical virology laboratory. CMV viremia (> 100 copies) was treated with an 8-week course of ganciclovir. For a first cohort of 10 patients (9 receiving UCB and 1 receiving an HLA-matched sibling graft), PBMCs were collected before viral reactivation (at either day 28 or day 60 after HCT), at viral diagnosis, and 2, 4, and 8 weeks after antiviral therapy. PBMCs were also collected from a second cohort of 23 patients receiving UCB transplantations at day 28, day 60, day 100, 6 months, and 1 year after HCT. Of the 23 patients, 10 patients were CMV-seronegative and 13 were CMV-seropositive. Eight patients developed detectable CMV in the blood 22-82 days after transplantation. Samples were obtained after informed consent and approval from the University of Minnesota Institutional Review Board according to the declaration of Helsinki. High-resolution HLA typing was performed and NK ligand status was assigned to Bw4, HLA-C1, and HLA-C2 group ligands.
PBMCs were isolated from each sample by density centrifugation and cryopreserved. Before analysis with the CD107a and IFNγ assay, the thawed cells were incubated overnight at 37°C in complete DMEM without exogenous cytokines (Cellgro) supplemented with 20% human AB serum (Valley Biomedical), 30% Ham F-12 medium (Cellgro), 100 U/mL of penicillin (Invitrogen), 100 U/mL of streptomycin (Invitrogen), 24μM 2-β–mercaptoethanol, 50μM ethanolamine, 20 mg/L of ascorbic acid, and 50 μg/L of sodium selenate.
Target cells
The human erythroleukemia cell line K562 was maintained in IMDM (Cellgro) supplemented with 10% FBS (Invitrogen), 100 U/mL of penicillin, and 100 U/mL of streptomycin (both Invitrogen).
CD107a and IFNγ assay
Expression of CD107a and production of IFNγ were measured as described previously.26 Briefly, PBMCs were incubated in medium alone or with K562 cells at an effector-to-target ratio of 2:1 for 5 hours. Brefeldin A and monensin (both BD Biosciences) were added after 1 hour. The following Abs were used: PeCy5.5-conjugated anti-CD158a (clone EB6; Beckman Coulter), APC-conjugated anti-CD158b (clone GL183; Beckman Coulter), FITC-conjugated anti-CD158b (clone CH-L; BD Biosciences), Alexa Fluor 700–conjugated anti-CD158e (clone DX9; BioLegend), PE-conjugated anti-NKG2C (clone 134591; R&D Systems), APC-conjugated anti-NKG2A (clone z199; Beckman Coulter), PeCy7-conjugated anti-CD56 (clone HCD56; BioLegend), FITC-conjugated anti-CD57 (clone HNK-1; BD Biosciences), Pacific blue–conjugated anti-CD57 (clone HCD57; BioLegend), ECD-conjugated anti-CD3 (clone UCHT1; Beckman Coulter), APC-Cy7–conjugated anti-CD16 (clone 3G8; BioLegend), PerCP-Cy5.5–conjugated anti-CD107a (clone H4A3; BioLegend), and Pacific blue-conjugated anti-IFNγ (clone 4S.B3; BioLegend). Data were analyzed on an LSRII 11-color flow cytometer (BD Biosciences) using FlowJo Version 9.3.2 software (TreeStar).
Real-time quantitative PCR
Total RNA was extracted with the RNeasy Mini Kit (QIAGEN) and digested with RNase-free DNase (Invitrogen). The quantity of RNA was determined with an AlphaSpec Spectrophotometer (Alpha Innotech). First-strand cDNA synthesis was performed with SuperScript III (Invitrogen); reverse transcription was at 50°C for 50 minutes followed by heat inactivation at 70°C for 15 minutes; RNA was removed by the addition of 1 μL of Escherichia coli RNase H (Invitrogen) and 20 minutes of incubation at 37°C. KIR2DL2, KIR2DL3, and KIR2DS2 primers and TaqMan probes were designed and synthesized so that the annealing temperature of the probes was 8-10°C higher than their respective primers using Primer Express Version 2.0 software (Applied Biosystems).27 IL-15Rα, IFNγ, T-box 21 (T-bet), and 18S primers and probes were obtained from Applied Biosystems. All reactions were performed using the TaqMan Universal PCR Master Mix (Applied Biosystems) in a total reaction volume of 25 μL and the 7500 Q-PCR System (Applied Biosystems). Thermal cycling conditions were performed using the default conditions of the 7500 System SDS Software.
Statistical analysis
The Student t test was used for comparisons between independent samples; the paired t test was used for comparisons between matched samples. The linear mixed-effects model was used for comparisons of time trends among the different groups; the fixed effects included group indicator(s), time, and group-time interaction(s) and the random effects included the random intercept and the random slope for time. The Wald test was used to determine the difference in the slope of time between groups. Variables are summarized as means ± SEM. Statistical analyses were performed using SAS Version 9.2 software.
Results
IFN-γ–producing NKG2C+ NK cells increase during acute CMV reactivation
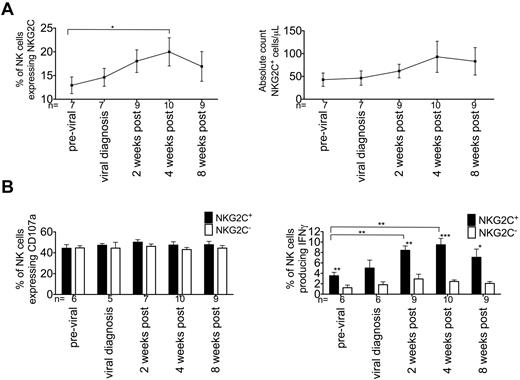
NK cells expressing NKG2C have been shown to expand after coculture with CMV-infected fibroblasts.18 Using multicolor flow cytometry, we measured receptor expression and NK-cell function on a cohort of 10 HCT recipients of allogeneic grafts who reactivated CMV after transplantation. Recipients were monitored for CMV reactivation weekly by PCR and placed on antiviral therapy when CMV viremia was detected. After 2 weeks of antiviral therapy, CMV viremia was virtually undetectable in the blood (6 of 10 patients, data not shown), and the rest was cleared by 4 weeks. This clearance is limited by the testing in blood and does not reflect low-level latent infection that invariably exists in tissues. The number of NK cells expressing NKG2C increased after the detection of CMV viremia (at viral diagnosis, 14.6% ± 1.9% vs at 2 weeks after therapy, 18.0% ± 2.4%), peaking at 4 weeks after therapy (20.0% ± 3%; Figure 1A). The absolute count of NKG2C+ NK cells also increased during acute CMV viremia, confirming the expansion of these cells. To determine whether expanding NKG2C+ NK cells were functional, CD107a expression and IFNγ production were measured after incubation in either medium alone or medium plus the HLA class I–negative cell line K562. CD107a expression and IFNγ production were minimal when the NK cells were incubated in medium alone at any time point (data not shown). After exposure to K562 cells, there was no difference between NKG2C+ and NKG2C− NK cells expressing CD107a before or after the detection of CMV viremia (Figure 1B left panel). However, NKG2C+ NK cells produced significantly more IFNγ compared with NKG2C− NK cells both before and after detection of CMV viremia (Figure 1B right panel). In addition, NKG2C+ NK cells producing IFNγ after therapy were significantly increased (previral, 3.6% ± 0.7% vs 4 weeks after therapy, 9.5% ± 1.2%, P = .0034).
NKG2C+ NK cells increase after CMV reactivation after HCT and produce high levels of IFNγ. PBMCs from HCT recipients were studied before viral infection, at diagnosis of viral reactivation, and after 2, 4, and 8 weeks of antiviral therapy. Cells were incubated in either medium alone (not shown) or with K562 cells for 5 hours. (A) After incubation, NKG2C expression was measured on CD56+CD3− NK cells and the absolute number of NKG2C+ NK cells/μL was calculated. Aggregate data from recipients in the indicated groups is shown. Points represent the means ± SEM. Previral samples were compared with samples after infection using the Student t test. *P ≤ .05. (B) CD107a expression (left panel) and IFNγ production (right panel) was measured on NKG2C+ (black bars) or NKG2C− (white bars) NK cells. Bars represent the means ± SEM. Previral samples were compared with samples after infection using the Student t test. NKG2C+ NK cells were compared with NKG2C− NK cells using the paired t test. *P ≤ .05, **P < .01, and ***P < .001.
NKG2C+ NK cells increase after CMV reactivation after HCT and produce high levels of IFNγ. PBMCs from HCT recipients were studied before viral infection, at diagnosis of viral reactivation, and after 2, 4, and 8 weeks of antiviral therapy. Cells were incubated in either medium alone (not shown) or with K562 cells for 5 hours. (A) After incubation, NKG2C expression was measured on CD56+CD3− NK cells and the absolute number of NKG2C+ NK cells/μL was calculated. Aggregate data from recipients in the indicated groups is shown. Points represent the means ± SEM. Previral samples were compared with samples after infection using the Student t test. *P ≤ .05. (B) CD107a expression (left panel) and IFNγ production (right panel) was measured on NKG2C+ (black bars) or NKG2C− (white bars) NK cells. Bars represent the means ± SEM. Previral samples were compared with samples after infection using the Student t test. NKG2C+ NK cells were compared with NKG2C− NK cells using the paired t test. *P ≤ .05, **P < .01, and ***P < .001.
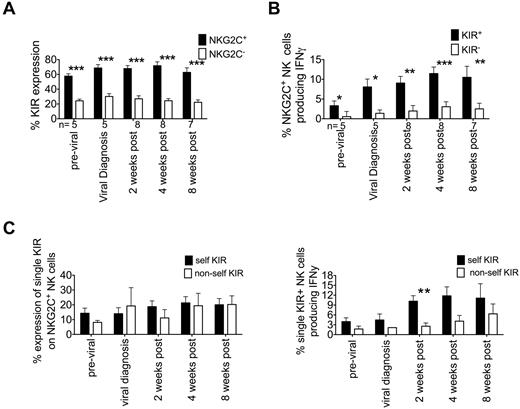
We demonstrated previously that KIR expression is required for NK cells to be educated to produce IFNγ after allogeneic HCT.26 In the present study, we investigated whether these same rules hold true for NKG2C+ NK cells that expand after acute CMV viremia. NK cells were determined to be KIR+ if they expressed at least one of the following KIRs: CD158a/h (KIR2DL1, KIR2DS1), CD158b/j (KIR2DL2, KIR2DL3, KIR2DS2), or CD158e (KIR3DL1). NKG2C+ NK cells expressed significantly more KIR than did NKG2C− cells both before and after detection of CMV viremia, suggesting that NKG2C− NK cells are more immature (Figure 2A). Furthermore, only NKG2C+ NK cells that also expressed KIR were potent producers of IFNγ (Figure 2B). We further designated NKG2C+ single KIR+ NK cells as expressing either self-KIR (ie, the ligands for their inhibitory KIR were present in the recipient) or non-self-KIR (ie, the ligands for their inhibitory KIR were not present in the recipient; Figure 2C). There was no detectable difference between the frequency of single self-KIR- or single non-self-KIR–expressing NKG2C+ NK cells before or after antiviral therapy. In marked contrast, NKG2C+ single self-KIR+ NK cells were significantly more potent at producing IFNγ than NKG2C+ single non-self-KIR+ NK cells after therapy (2 weeks, 10.18% ± 1.7% vs 2.56% ± 1%, P = .0020). These results demonstrate that during acute CMV reactivation in patients undergoing HCT, there is an expansion of NKG2C+ NK cells educated through KIRs, which are potent producers of IFNγ.
NKG2C+ NK cells are predominantly KIR+ and education through self-KIR are required for robust IFNγ production. (A) NKG2C+ (black bars) and NKG2C− (white bars) NK cells were gated as being KIR+ based on staining with a cocktail of Abs against CD158a/h, CD158b/j, and CD158e. Bars represent the means ± SEM. NKG2C+ cells were compared with NKG2C− samples using the paired t test. *P ≤ .05, **P < .01, and ***P < .001. (B) NKG2C+ NK cells were gated as being either KIR+ or KIR− and IFNγ production was measured after incubation in either medium alone (not shown) or with K562 cells for 5 hours. Bars represent the means ± SEM. KIR+ cells were compared with KIR− samples using the paired t test. *P ≤ .05, **P < .01, and ***P < .001. (C) Single KIR+ NKG2C+ NK cells were grouped as expressing either self-KIR (black bars) or nonself KIR (white bars) based on the recipient KIR ligand status (left panel). IFNγ production was measured after incubation in either medium alone (not shown) or with K562 cells for 5 hours. Bars represent the means ± SEM. Self-KIR+ cells were compared with non-self-KIR+ samples using the Student t test. Bars represent the means ± SEM. **P < .01.
NKG2C+ NK cells are predominantly KIR+ and education through self-KIR are required for robust IFNγ production. (A) NKG2C+ (black bars) and NKG2C− (white bars) NK cells were gated as being KIR+ based on staining with a cocktail of Abs against CD158a/h, CD158b/j, and CD158e. Bars represent the means ± SEM. NKG2C+ cells were compared with NKG2C− samples using the paired t test. *P ≤ .05, **P < .01, and ***P < .001. (B) NKG2C+ NK cells were gated as being either KIR+ or KIR− and IFNγ production was measured after incubation in either medium alone (not shown) or with K562 cells for 5 hours. Bars represent the means ± SEM. KIR+ cells were compared with KIR− samples using the paired t test. *P ≤ .05, **P < .01, and ***P < .001. (C) Single KIR+ NKG2C+ NK cells were grouped as expressing either self-KIR (black bars) or nonself KIR (white bars) based on the recipient KIR ligand status (left panel). IFNγ production was measured after incubation in either medium alone (not shown) or with K562 cells for 5 hours. Bars represent the means ± SEM. Self-KIR+ cells were compared with non-self-KIR+ samples using the Student t test. Bars represent the means ± SEM. **P < .01.
CMV reactivation induces a more mature phenotype in the first year after transplantation
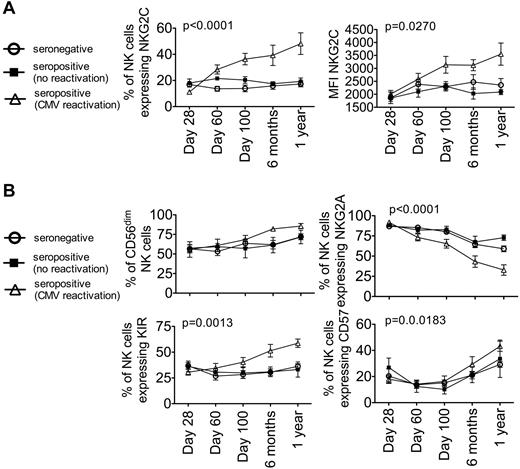
We observed that during acute CMV viremia, NKG2C+ NK cells initially expand and then persist in a manner similar to the expansion and persistence of Ly49H+ NK cells in mouse CMV.16 We examined the maintenance of this population of NK cells after clearance of the virus. In a separate cohort, we assessed the influence of CMV viremia on reconstituting NK cells during the first year after transplantation to validate our findings and to assess memory of the response. We chose UCB as a transplantation source because these cells are CMV naive (and thus all are considered to be CMV-negative donors) to avoid confounding effects from the adult donors who may have encountered CMV previously. Eight of 23 UCB transplantation recipients (10 CMV-seronegative and 13 CMV-seropositive) reactivated CMV between days 22 and 82 after HCT. PBMCs were collected from each patient at day 28, day 60, day 100, 6 months, and 1 year after HCT. A linear mixed model was used to compare different groups' slope of time to detect overall changes in NK-cell receptor repertoire during the first year after HCT. NKG2C expression was measured at each time point (Figure 3A). NKG2C expression remained unchanged in patients without CMV viremia during the first year after HCT. However, in patients who reactivated CMV between days 22 and 82, NKG2C expression increased significantly during the acute phase (day 28, 11.2% ± 1% vs day 100, 36.4% ± 4.4%, P = .0010). This increase in NKG2C was similar to that observed in the 10 patient cohort triggered by CMV reactivation, supporting the premise that this expansion is due to CMV reactivation. Interestingly, NK cells expressing NKG2C continued to persist and, by 1 year after HCT, accounted for 48% ± 8.3% of the reconstituting NK-cell population; this was significantly higher compared with recipients who did not reactivate CMV (P = .02). This was also reflected in a significant increase in the absolute count of NKG2C+ NK cells by 1 year in recipients who reactivated CMV (158.2 ± 62/μL, n = 5 vs 51.1 ± 10/μL, n = 11 in those without CMV reactivation, P = .025). Furthermore, cell-surface density of NKG2C increased over time and was significantly higher at 1 year after HCT than patients without CMV reactivation (mean fluorescence intensity, 3552 ± 428 vs 2296 ± 198, P = .0098).
CMV reactivation induces an increase of NKG2C+ NK cells and a more mature NK-cell phenotype in the first year after transplantation. NKG2C expression (left panel), mean fluorescence intensity (MFI, right panel, A), CD56dim expression (B, left top panel), NKG2A expression (B, right top panel), and KIR expression based on a cocktail of Abs against CD158a/h, CD158b/j, and CD158e (B, left bottom panel) and CD57 expression (B, right bottom panel) were measured on CD56+ CD3− NK cells from seronegative (○, n = 10), seropositive (no CMV reactivation; ■, n = 5) and seropositive (CMV reactivation; ▵, n = 8) recipients at day 28, day 60, day 100, 6 months, and 1 year after transplantation. Points represent the means ± SEM. The slope of time was compared between recipients who reactivated CMV and combined recipients who did not reactivate CMV using a linear mixed model.
CMV reactivation induces an increase of NKG2C+ NK cells and a more mature NK-cell phenotype in the first year after transplantation. NKG2C expression (left panel), mean fluorescence intensity (MFI, right panel, A), CD56dim expression (B, left top panel), NKG2A expression (B, right top panel), and KIR expression based on a cocktail of Abs against CD158a/h, CD158b/j, and CD158e (B, left bottom panel) and CD57 expression (B, right bottom panel) were measured on CD56+ CD3− NK cells from seronegative (○, n = 10), seropositive (no CMV reactivation; ■, n = 5) and seropositive (CMV reactivation; ▵, n = 8) recipients at day 28, day 60, day 100, 6 months, and 1 year after transplantation. Points represent the means ± SEM. The slope of time was compared between recipients who reactivated CMV and combined recipients who did not reactivate CMV using a linear mixed model.
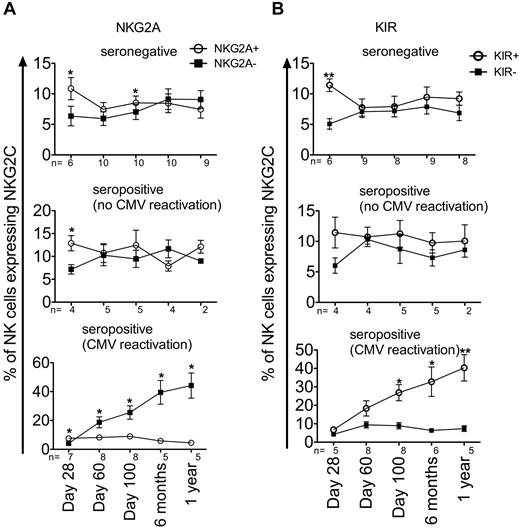
Reconstituting NK cells from patients who reactivated CMV also had a more mature phenotype, a higher number of CD56dim NK cells, a significantly more rapid decrease in NKG2A− NK cells (test of slope difference, P < .0001), and a significantly more rapid increase in KIR+ NK cells (test of slope difference, P = .0013) by 1 year after HCT (Figure 3B). In addition, KIR expression remained fairly constant across all time points in the recipients who did not reactivate CMV. In contrast, KIR expression increased consistently during the first year after HCT in recipients who reactivated CMV. Expression of CD57, a marker typically thought to be expressed on more mature NK cells,28,29 increased similarly among all groups, although recipients who reactivated CMV had higher CD57 expression over time (test of slope difference, P = .0183). NKG2A and KIR coexpression with NKG2C was determined for all 3 groups across all time points (Figure 4). Recipients without CMV reactivation had similar numbers of NKG2C+ NK cells that expressed NKG2A than those who did not (Figure 4A). This was also true for KIR expression (Figure 4B). However, for recipients who did reactivate CMV, there was an expansion of NKG2C+ NK cells that coexpressed KIR and lacked NKG2A (test of slope difference for NKG2A expression, P < .0001; KIR expression, P = .0007). The numbers of NKG2A+ and KIR− NKG2C+ NK cells remained consistently low during the first year after HCT. These data demonstrate that CMV reactivation after HCT results in a more mature phenotype, characterized by an increase in NKG2C+, KIR+, CD57+, and NKG2A− NK cells.
Expanding NKG2C+ NK cells are more mature (KIR+ and NKG2A−) in the first year after CMV reactivation. NKG2C+ NK cells from seronegative (top panel), seropositive (no CMV reactivation, middle panel), and seropositive (CMV reactivation, bottom panel) recipients at the indicated time points were gated as being either NKG2A+ (○) or NKG2A− (■, A) and either KIR+ (○) or KIR− (■, B) based on a cocktail of Abs against CD158a/h, CD158b/j, and CD158e. Points represent the means ± SEM. NKG2A+ and NKG2A− NK cells (or KIR+ and KIR− NK cells) were compared for the same group of recipients at each time point using the paired t test. *P ≤ .05 and **P < .01.
Expanding NKG2C+ NK cells are more mature (KIR+ and NKG2A−) in the first year after CMV reactivation. NKG2C+ NK cells from seronegative (top panel), seropositive (no CMV reactivation, middle panel), and seropositive (CMV reactivation, bottom panel) recipients at the indicated time points were gated as being either NKG2A+ (○) or NKG2A− (■, A) and either KIR+ (○) or KIR− (■, B) based on a cocktail of Abs against CD158a/h, CD158b/j, and CD158e. Points represent the means ± SEM. NKG2A+ and NKG2A− NK cells (or KIR+ and KIR− NK cells) were compared for the same group of recipients at each time point using the paired t test. *P ≤ .05 and **P < .01.
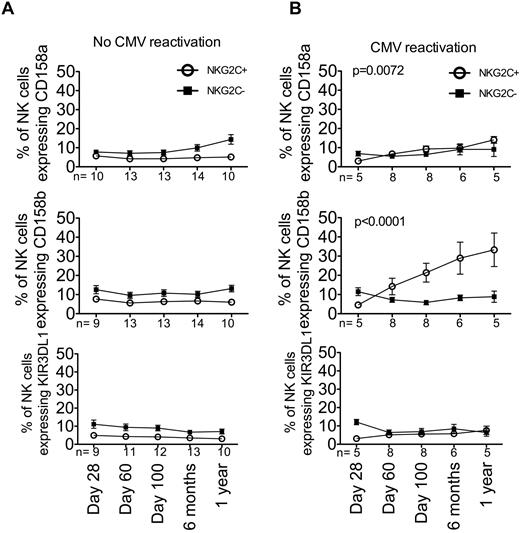
CD158b is preferentially expressed on expanding NKG2C+ NK cells during CMV reactivation
Having shown that expanding NKG2C+ NK cells are KIR+, we were interested in determining whether individual KIRs were equally represented. Recipient PBMCs from all groups and at all time points were stained with anti-CD158a, anti-CD158b, and anti-KIR3DL1, and expression was measured on NKG2C+ and NKG2C− NK cells (Figure 5). As expected, the number of NKG2C+ NK cells expressing CD158a, CD158b, or KIR3DL1 from recipients without CMV reactivation (combined seronegative and seropositive recipients) remained unchanged during the first year after HCT. Whereas there was a slight increase in NKG2C+ NK cells expressing CD158a over time (test of slope difference, P = .0072), the expansion of NKG2C+ NK cells expressing CD158b was far greater (test of slope difference, P < .0001). This preferential expansion was specific to NKG2C+ NK cells, because there was no change in the number of CD158b+ NK cells that lacked NKG2C. Of the 8 recipients who reactivated CMV, 6 expressed the ligands for both CD158a (C2) and CD158b (C1), and 5 of these patients also expressed the ligand for KIR3DL1 (Bw4; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article); however, there was a preferential expansion of NK cells bearing CD158b. Because anti-CD158b recognizes 3 different KIRs (KIR2DL2, KIR2DL3, and KIR2DS2), we determined KIR expression by quantitative PCR to determine whether one KIR was up-regulated preferentially. Of the 8 recipients who reactivated CMV, 6 (all expressing C1) were KIR2DL3 homozygotes and did not express KIR2DS2 (data not shown). Therefore, the preferential expansion of CD158b is reflected predominately by an increase in KIR2DL3.
CD158b+ is preferentially expressed on expanding NKG2C+ NK cells after CMV reactivation. NKG2C+ (○) and NKG2C− (■) NK cells from recipients without CMV reactivation (A) and with CMV reactivation (B) at the indicated time points were further gated as being CD158a+ (top panel), CD158b+ (middle panel), or KIR3DL1+ (bottom panel). Points represent the means ± SEM. The slope of time for NKG2C+ NK cells was compared using a linear mixed model.
CD158b+ is preferentially expressed on expanding NKG2C+ NK cells after CMV reactivation. NKG2C+ (○) and NKG2C− (■) NK cells from recipients without CMV reactivation (A) and with CMV reactivation (B) at the indicated time points were further gated as being CD158a+ (top panel), CD158b+ (middle panel), or KIR3DL1+ (bottom panel). Points represent the means ± SEM. The slope of time for NKG2C+ NK cells was compared using a linear mixed model.
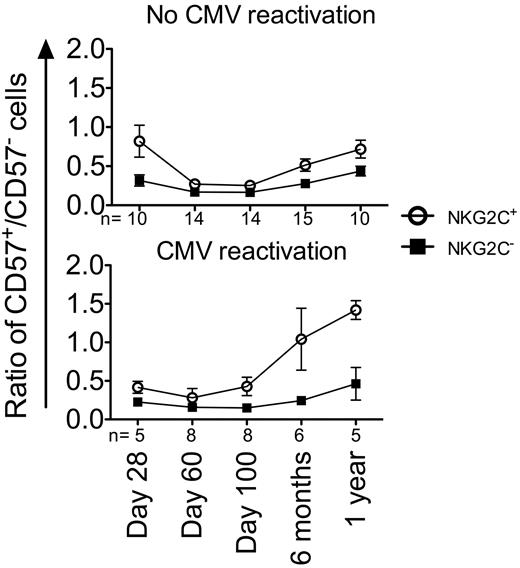
NKG2C+ NK cells preferentially acquire CD57 during the first year after HCT
Recently, expanding NKG2C+ NK cells were shown to progressively acquire CD57 after CMV reactivation in solid-organ transplantation recipients, potentially representing a memory-like population of NK cells.25 As shown in Figure 3B, the number of CD57+ NK cells increased similarly during the first year after HCT in all recipients. Therefore, we measured changes in the ratio of CD57+/CD57− NKG2C+ NK cells and NKG2C− NK cells to determine whether NKG2C+ NK cells preferentially acquired CD57 after CMV reactivation (Figure 6). The ratio of CD57+/CD57− NKG2C+ NK cells increased more rapidly in recipients who reactivated CMV compared with recipients who did not reactivate CMV (combined seronegative and seropositive with no CMV reactivation; test of slope difference for NKG2C+, P = .0015), suggesting that after CMV reactivation, expanding NKG2C+ NK cells preferentially acquire CD57.
CD57 is preferentially acquired on NKG2C+ cells in the first year after CMV reactivation. CD57 co-expression was measured on NKG2C+ (○) and NKG2C− (■) NK cells from recipients without CMV infection (top panel) and with CMV infection (bottom panel). The ratio of CD57+/CD57− was plotted at the indicated time points. Points represent the means ± SEM. The slope of time for NKG2C+ NK cells was compared between recipients who reactivated CMV and those who did not using a linear mixed model, P = .0015.
CD57 is preferentially acquired on NKG2C+ cells in the first year after CMV reactivation. CD57 co-expression was measured on NKG2C+ (○) and NKG2C− (■) NK cells from recipients without CMV infection (top panel) and with CMV infection (bottom panel). The ratio of CD57+/CD57− was plotted at the indicated time points. Points represent the means ± SEM. The slope of time for NKG2C+ NK cells was compared between recipients who reactivated CMV and those who did not using a linear mixed model, P = .0015.
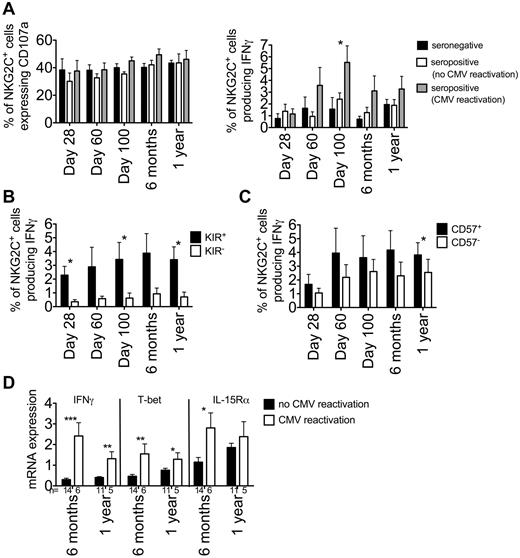
NKG2C+ NK cells are functional long-term after UCB HCT
In a recent analysis on the recovery of function and NK-cell education after transplantation, we demonstrated that NK cells from UCB transplantation recipients were very poor producers of target cell–induced IFNγ, possibly because of a more immature phenotype.26 Because CMV reactivation after HCT results in a more mature NK-cell phenotype, we investigated the effect of CMV viremia on the function of reconstituting NK cells. We measured CD107a expression and IFNγ production on NKG2C+ NK cells after incubation with K562 cells. There was no detectable difference in CD107a expression between the 3 recipient groups, and the number of CD107a-expressing NKG2C+ NK cells remained consistent during the first year after transplantation (Figure 7A). NKG2C+ NK cells from seropositive recipients who reactivated CMV before day 100 produced significantly more IFNγ compared with patients without CMV viremia (day 100 seropositive CMV reactivation, 5.5% ± 1.4% vs day 100 combined seronegative and seropositive with no CMV reactivation, 1.8% ± 0.7%, P = .0137), confirming the findings in our acute cohort that an increase in IFNγ production by NKG2C+ NK cells is because of CMV reactivation (Figure 1B). This increase in IFNγ production in recipients with CMV reactivation was maintained at 6 months after HCT. However, by 1 year after HCT, an interval when NK-cell function is found to recover, IFNγ production was found to increase in patients even without CMV reactivation. NKG2C+ NK cells producing IFNγ were predominately KIR+, as we have shown herein, and this was maintained during the first year after HCT (Figure 7B). Because CD57 was preferentially acquired on expanding NKG2C+ NK cells over time and could potentially represent a memory-like population of NK cells, we investigated whether these NK cells were more robust at producing IFNγ compared with NKG2C+ NK cells that lacked CD57 (Figure 7C). Whereas NKG2C+CD57+ NK cells produced more IFNγ at each time point, this was only significant at 1 year after HCT (3.8% ± 0.9% vs 2.6% ± 1.0%, P = .02).
IFNγ production, but not CD107a expression, is increased after CMV reactivation. PBMCs from seronegative (black bars, n = 10), seropositive (no CMV reactivation, white bars, n = 5), and seropositive (CMV reactivation, gray bars, n = 8) recipients at the indicated time points were incubated in either medium alone (not shown) or with K562 cells for 5 hours. (A) CD107a expression (left panel) and IFNγ production (right panel) was measured on NKG2C+ NK cells. Bars represent the means ± SEM. NKG2C+ cells from different groups of recipients were compared at each time point using the Student t test. *P ≤ .05. (B) NKG2C+ NK cells from seropositive (CMV reactivation) recipients were further gated as being KIR+ (black bars) or KIR− (white bars) based on staining with a cocktail of Abs against CD158a/h, CD158b/j, and CD158e, and IFNγ production was measured for each subset. Bars represent the means ± SEM. KIR+ and KIR− NK cells were compared at each time point using the paired t test. *P ≤ .05. (C) NKG2C+ NK cells from seropositive (CMV reactivation) recipients were further gated as being CD57+ (black bars) or CD57− (white bars) and IFNγ production was measured for each subset. Bars represent the means ± SEM. CD57+ and CD57− NK cells were compared at each time point using the paired t test. *P ≤ .05. (D) IFNγ, T-bet, and IL-15Rα mRNA expression were measured on resting PBMCs at 6 months and 1 year after HCT. Samples were normalized to 18S RNA. Bars represent the means ± SEM. Recipients who reactivated CMV and those who did not were compared at each time point using the t test. *P ≤ .05, **P < .01, and ***P < .001.
IFNγ production, but not CD107a expression, is increased after CMV reactivation. PBMCs from seronegative (black bars, n = 10), seropositive (no CMV reactivation, white bars, n = 5), and seropositive (CMV reactivation, gray bars, n = 8) recipients at the indicated time points were incubated in either medium alone (not shown) or with K562 cells for 5 hours. (A) CD107a expression (left panel) and IFNγ production (right panel) was measured on NKG2C+ NK cells. Bars represent the means ± SEM. NKG2C+ cells from different groups of recipients were compared at each time point using the Student t test. *P ≤ .05. (B) NKG2C+ NK cells from seropositive (CMV reactivation) recipients were further gated as being KIR+ (black bars) or KIR− (white bars) based on staining with a cocktail of Abs against CD158a/h, CD158b/j, and CD158e, and IFNγ production was measured for each subset. Bars represent the means ± SEM. KIR+ and KIR− NK cells were compared at each time point using the paired t test. *P ≤ .05. (C) NKG2C+ NK cells from seropositive (CMV reactivation) recipients were further gated as being CD57+ (black bars) or CD57− (white bars) and IFNγ production was measured for each subset. Bars represent the means ± SEM. CD57+ and CD57− NK cells were compared at each time point using the paired t test. *P ≤ .05. (D) IFNγ, T-bet, and IL-15Rα mRNA expression were measured on resting PBMCs at 6 months and 1 year after HCT. Samples were normalized to 18S RNA. Bars represent the means ± SEM. Recipients who reactivated CMV and those who did not were compared at each time point using the t test. *P ≤ .05, **P < .01, and ***P < .001.
Because NKG2C+ NK cells from recipients who reactivated CMV had heightened IFNγ responses to K562 compared with recipients who did not reactivate CMV (Figure 7A), we investigated whether this would be correlated with higher resting levels of IFNγ mRNA. Transcript expression was measured on resting PBMCs from recipients at 6 months and 1 year after HCT. In addition to IFNγ, we also examined mRNA expression of the transcription factor T-bet, which is involved in the regulation of IFNγ,30 and IL-15Rα to determine whether CMV reactivation induced cells to be more responsive to IL-15 (Figure 7D). Both at 6 months and 1 year, recipients who reactivated CMV had significantly higher IFNγ and T-bet mRNA expression, and IL-15Rα expression was significantly increased in recipients who reactivated CMV at 6 months. These results suggest that after HCT, CMV reactivation promotes a more rapid reconstitution of mature, functional NK cells with potential memory-like characteristics.
Discussion
In the present study, we describe the expansion and persistence of NKG2C+ self-KIR+–educated NK cells with potent IFNγ production after CMV reactivation in patients receiving allogeneic HCT. During the acute phase of CMV reactivation, there was an initial and sustained increase in the percentage of NK cells expressing NKG2C, which was correlated with an increase in IFNγ production. The NKG2C+ NK cells expanding after viral reactivation, which were predominately KIR+, required expression of self-KIR for the development of robust IFNγ production. In a longitudinal cohort of 23 patients, we confirmed that the expansion of NKG2C+ NK cells was CMV specific and demonstrated that the expansion of these cells continued long after viral clearance. We have also demonstrated that CMV reactivation after HCT induces a more mature phenotype, characterized by an increase in the percentage of CD56dim NK cells, an increase in KIR expression, and a decrease in NKG2A expression.
Several studies have found a relationship between human CMV and the expression of NKG2C.17,18,25,31 Normal immunocompetent individuals who are CMV-seropositive have an increased number of NK cells expressing NKG2C, and this percentage remains high years after the initial infection with CMV. In recipients who reactivate CMV after solid-organ transplantation, there is a substantial expansion of NKG2C+ NK cells.25 Coculture of NK cells with CMV-infected fibroblasts results in the expansion of NKG2C+ cells.18 This effect is enhanced by the presence of IL-15 and diminished with the addition of an Ab directed against CD94, which is expressed as a dimer with NKG2C molecules. It is believed that NKG2C expansion is driven by the recognition of CMV-infected cells, but the exact nature of the ligand remains unknown. We studied NK cells developing from hematopoietic stem cells from allogeneic (UCB) grafts. CMV reactivation triggered a profound expansion of NK cells preferentially expressing NKG2C that persisted long-term after viral clearance. No increase in NKG2C-expressing NK cells was observed in either seronegative or seropositive recipients who did not reactivate CMV, a finding that supports the hypothesis that NKG2C is most likely directly involved in the response to human CMV. The NKG2C+ NK cells that expanded after CMV reactivation expressed KIR, but lacked NKG2A, which also recognizes HLA-E. This result is consistent with the finding that the NKG2C+ cells expanding after coculture with CMV-infected fibroblasts rarely co-express NKG2A.18 Furthermore, in CMV-seropositive individuals co-infected with either Hantavirus or HIV or in solid-organ transplantation recipients experiencing CMV reactivation, only the NKG2A− NKG2C+ NK cells expanded.19,21,25
The NKG2C+ NK cells expanding after CMV reactivation also expressed KIR. Whereas the NKG2C+ KIR+ NK cells were potent producers of IFNγ both during the acute phase and long after viral clearance, the response was more robust when the KIR was specific for a self-ligand. In our longitudinal cohort, the NKG2C+ KIR+ NK cells preferentially expressed CD158b (predominately KIR2DL3). In our cohort, all but one patient who reactivated CMV expressed the ligand for CD158b (C1), supporting our conclusion that NKG2C+ NK cells are licensed through self-HLA. Interestingly, whereas none of the patients who reactivated CMV expressed HLA-C1 as their only self-ligand, and 5 recipients expressed all 3 ligands (C1, C2, and Bw4), it was only the CD158b+ cells that expanded. In a cohort of patients with Hantavirus, expanded NKG2C+ NK cells uniformly expressed one inhibitory KIR specific for self (both KIR2DL1 and KIR2DL2/3 were represented).19 Similarly, in a cohort of patients infected with Chikungunya virus, expanding NKG2C+ NK cells preferentially expressed KIR2DL2/3.20 These data suggest that long-lived expanding NKG2C+ NK cells are educated and that there is some degree of clonal skewing based on the predominance of a single inhibitory KIR. These findings support the hypothesis that CMV reactivation may promote education of NK cells engrafting after HCT. We reported recently that IFNγ production is impaired early after UCB transplantation and proposed that this may be because of the immature phenotype of reconstituting NK cells.26 Furthermore, whereas both KIR and NKG2A were able to educate NK cells for cytotoxic function, only KIRs were able to educate NK cells to produce IFNγ. In the present study, we report data showing that CMV reactivation induces a more mature NK-cell phenotype, including increased KIR expression. In these cohorts of post-HCT patients reactivating CMV, we did observe an increase in IFNγ production in the early post-transplantation setting. Further studies will be needed to determine how CMV drives NK-cell education.
These results are especially interesting given the recent report that early CMV reactivation is associated with a reduced risk of relapse in AML patients undergoing allogeneic HCT from HLA-matched sibling or unrelated donors.32 All transplantations were unmanipulated. The risk of leukemic relapse was 9% at 10 years after HCT compared with 42% in patients who did not reactivate CMV, and CMV reactivation was not detrimental to overall survival. It is also possible that the lower risk of relapse reported in UCB transplantation33 is associated with CMV reactivation.34 CMV infection may induce expression of a ligand that activates either CD8+ T cells and/or NK cells. Our data support the possibility that CMV infection may induce NK cells with increased potential to eliminate residual leukemic blasts. Like virus-infected cells, tumor cells can also down-regulate class I HLA but may retain expression of HLA-E,35,36 and this is true for AML blasts.37 Because CMV viremia induces a population of NK cells that express NKG2C and lack NKG2A, activation of NKG2C through HLA-E may increase elimination of residual leukemic cells, producing the graft-versus-leukemia effect in patients who reactivate CMV. Future experiments investigating NK-cell recognition of CMV-infected AML blasts and comparing the ability of NK cells from patients who reactivated CMV and those who did not to eliminate leukemic blasts are certainly warranted. Furthermore, these data suggest that CMV reactivation is not as detrimental as was once believed.
Our data are consistent with observations of the response of mouse NK cells to CMV infection. The expansion and sustained persistence of NKG2C+ NK cells we observed after CMV reactivation in post-HCT patients mimics the response of memory-like Ly49H+ NK cells produced during mouse CMV infection.16 Ly49H recognition of the viral protein m157 drives expansion of the cells, which persist in lymphoid and nonlymphoid tissues for months after the initial viral infection. After adoptive transfer of Ly49H+ NK cells from CMV-exposed mice into naive mice challenged with CMV, the transferred Ly49H+ NK cells expanded and were more protective against CMV infection then were the naive Ly49H+ NK cells. The expanded Ly49H+ NK cells had heightened IFNγ responses and degranulation (CD107a expression) to plate-bound anti-NK1.1 and anti-Ly49H and expressed more IFNγ transcripts compared with Ly49H+ NK cells from naive mice. Ly49H+ NK cells share many of the same characteristics as memory CD8+ T cells.38 The response of human NKG2C+ NK cells to CMV is reminiscent of the response of Ly49H+ NK cells to mouse CMV. Our data support the premise that NKG2C+ NK cells may represent memory NK cells in humans. However, without looking at the NK-cell response to subsequent CMV exposure, this conclusion is not precise and the term “memory” must be used carefully. It is possible that our results are consistent with the viral clearance seen in all patients, but are also a result of low-level chronic stimulation that invariably exists with latent herpesvirus infections. Nevertheless, NKG2C+ NK cells in patients who reactivated CMV had higher resting levels of IFNγ mRNA transcripts at 6 months and 1 year after HCT, suggesting the presence of a pool of cells that could respond more rapidly to a second stimulus. The transcription factor T-bet, an important regulator of IFNγ,30 and IL-15Rα transcripts were also expressed at higher levels in recipients who reactivated CMV. IL-15 is critical for NK-cell development, homeostasis, and survival, and memory CD8+ T cells depend on IL-15 for survival.39 An increase in IL-15Rα would allow more transpresentation of IL-15 by monocytes and dendritic cells to NK cells.40
In solid-organ transplantation recipients with CMV viremia, a preferential expansion of NKG2C+ NK cells that acquire CD57 has been observed.25 In that setting, the cell-surface density of NKG2C also increased over time, similar to the increase in the cell-surface density of Ly49H seen after mouse CMV infection. In our cohorts of HCT recipients, the cell-surface density of NKG2C was higher in patients who reactivated CMV compared with those patients who remained free of CMV viremia. In normal adult stem cell donors, these NKG2Chi CD57+ NK cells are only detected in CMV-seropositive individuals long after primary CMV infection. In the present study, we observed that NKG2C+ NK cells progressively acquired CD57 over the first year after transplantation, although the magnitude of the increase in CD57 was less pronounced that that reported in solid-organ transplantation recipients. These data suggest that memory NK cells may be present in humans after CMV viremia and may be exploited for therapeutic purposes. Because CMV reactivation induces a more rapid re-constitution of fully functional, educated NK cells with in-creased survival capacity and the ability to respond rapidly with cytokines, carefully monitored CMV reactivation may actually be beneficial.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge sample procurement and cell processing services from the Translational Therapy Core supported from the National Cancer Institute (P30-CA77598) and Minnesota Masonic Charities. The authors also thank the dedicated research staff in the Cancer Center clinical trials office for tracking CMV reactivation and sample procurement.
This work was supported in part by the National Institutes of Health (grant P01-CA65493 to J.S.M., S.C., and M.R.V.; and grant P01-CA111412-01 to J.S.M., S.C., M.R.V., and D.J.W.).
National Institutes of Health
Authorship
Contribution: B.F. designed and performed the research, analyzed the data, and wrote the manuscript; S.C, M.R.V, S.L-V, L.L.L, and D.W. helped to interpret data and helped with manuscript preparation; M.P. performed quantitative PCR and data analysis; J.C. contributed to sample procurement and manuscript preparation; X.L performed the statistical analysis and helped with manuscript preparation; and J.S.M. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey S. Miller, MD, Professor of Medicine, University of Minnesota Cancer Center, MMC 806, Division of Hematology, Oncology, and Transplantation, Harvard St at E River Rd, Minneapolis, MN 55455; e-mail: mille011@umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal