Abstract
Strategies that augment a GVL effect without increasing the risk of GVHD are required to improve the outcome after allogeneic stem cell transplantation (SCT). Azacitidine (AZA) up-regulates the expression of tumor Ags on leukemic blasts in vitro and expands the numbers of immunomodulatory T regulatory cells (Tregs) in animal models. Reasoning that AZA might selectively augment a GVL effect, we studied the immunologic sequelae of AZA administration after allogeneic SCT. Twenty-seven patients who had undergone a reduced intensity allogeneic transplantation for acute myeloid leukemia were treated with monthly courses of AZA, and CD8+ T-cell responses to candidate tumor Ags and circulating Tregs were measured. AZA after transplantation was well tolerated, and its administration was associated with a low incidence of GVHD. Administration of AZA increased the number of Tregs within the first 3 months after transplantation compared with a control population (P = .0127). AZA administration also induced a cytotoxic CD8+ T-cell response to several tumor Ags, including melanoma-associated Ag 1, B melanoma antigen 1, and Wilm tumor Ag 1. These data support the further examination of AZA after transplantation as a mechanism of augmenting a GVL effect without a concomitant increase in GVHD. The trial was registered at http://isrctn.org as #ISRCTN36825171.
Introduction
Disease relapse and GVHD remain the main causes of treatment failure after allogeneic stem cell transplantation (SCT) in adults with acute myeloid leukemia (AML).1,2 Recurrent disease represents a particular challenge in patients who received an allograft with a reduced intensity conditioning (RIC) regimen,3 and several approaches that augment an immunologically mediated GVL effect without a concomitant increase in GVHD have been explored. Because most patients who are destined to relapse do so within the first 12 months after transplantation, it is important such interventions are both well tolerated and delivered early.4 To date most attention has been paid to either minimizing immunosuppression after transplantation or the early use of donor lymphocyte infusion (DLI).4,5 The clinical utility of both approaches has been limited by the attendant risk of acute and chronic GVHD. This has represented a particular challenge to the early administration of DLI because the likelihood of inducing GVHD is highest when it is administered within the first year after transplantation. Therefore, interest is growing in the administration of targeted antitumor therapy after transplantation with the aim of either reducing the risk of disease recurrence or manipulating the kinetics of disease relapse so that the requirement for DLI can be postponed to a time when the risk of inducing GVHD is lower. Encouraging preliminary data have been reported in patients who received an allograft for chronic myeloid leukemia and lymphoma with imatinib and rituximab, respectively, raising the possibility of applying a similar strategy to patients who received an allograft for AML.6,7
The DNA methyltransferase inhibitor azacitidine (AZA) is clinically active in high-risk AML and, given its favorable toxicity profile, represents an important new treatment modality, particularly in older adults.8 Recent studies have also confirmed significant antitumor activity in patients with AML who have relapsed after an allogeneic transplantation with complete remission rates in the region of 20%-40%.9,10 Furthermore, AZA appears to be well tolerated after transplantation.11 The mechanism by which AZA exerts an antitumor effect in AML remains unknown. Recent data have shown that epigenetic therapies such as AZA and histone deacetylase inhibitors up-regulate the expression of epigenetically silenced putative tumor Ags such as MAGE-A1 (melanoma-associated Ag 1) and WT-1 (Wilm tumor Ag 1) and furthermore that AZA has the capacity to induce a CD8+ T-cell response to MAGE-A1 in vivo in patients with hematologic malignancies.12-15 It has therefore been hypothesized that the notable clinical activity of AZA in patients who have relapsed after an allogeneic transplantation may be because of augmentation of a GVL effect as a consequence of the increased expression of epigenetically silenced leukemia-associated Ags.9,16
CD4+ T regulatory cells (Tregs) play an important role in the establishment and maintenance of tolerance after allogeneic SCT. The main population of Tregs are generated in the thymus and are immunophenotypically characterized as CD4+CD25+FoxP3+CD127lo.17,18 Murine studies have established an important role for Tregs in the suppression of GVHD without compromising a GVL effect,8,19 and clinical studies of the feasibility and clinical consequences of adoptive transfer of this cellular population after allogeneic SCT are ongoing.19 FOXP3 is a forkhead transcription factor that plays a critical role in the differentiation of Tregs from CD4+CD25− cells in vivo.20 Recent studies have reported that the CpG island associated with the promoter of the FOXP3 gene is hypermethylated in CD4+CD25− cells and that administration of AZA up-regulates expression of FoxP3, resulting in Treg expansion in vitro.21,22 In murine transplantation models administration of AZA after transplantation results in expansion of Tregs and a reduction in the incidence of acute GVHD without apparent abrogation of a GVL effect.23,24
Taken together these studies provide a rationale for the administration of AZA after allogeneic transplantation in patients with AML at a high risk of relapse. Here, we report data about the immunologic sequelae of AZA with particular reference to its affect on circulating Tregs and tumor-specific immunity after transplantation.
Methods
Patients and methods
Patients with AML who had undergone a RIC allogeneic SCT were eligible for recruitment to this phase 1/2 study (RICAZA). All patients gave informed consent in accordance with the Declaration of Helsinki, and the clinical trial was approved by the National Research Ethics Services Committee. The trial was registered at http://isrctn.org as #ISRCTN36825171. The primary end point of this study was to assess safety and tolerability of AZA in patients after RIC allogeneic transplantation for AML. Patient demographics are summarized in Table 1.
All patients received an identical conditioning regimen that consisted of fludarabine (30 mg/m2 intravenously for 5 days), melphalan (140 mg/m2 intravenously), and alemtuzumab (10 mg intravenously for 5 days). GVHD prophylaxis consisted of cyclosporine commencing on day −1 at an adjusted dose to achieve therapeutic levels between 100 and 200 μg/L after transplantation with the aim of tapering immunosuppression between day 60 and day 90 after transplantation.
Subcutaneous AZA was commenced at a dose of 36 mg/m2 on day 42 after transplantation in patients with stable engraftment (neutrophil count ≥ 1 × 109/L and platelet count ≥ 50 × 109/L) and administered daily for 5 consecutive days every 28 days for up to a total of 10 cycles. Toxicity was graded with the use of the National Cancer Institute's Common Terminology Criteria for Adverse Events. (http://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm).
The dose of AZA was reduced to 24 mg/m2 in patients experiencing grade 3 or 4 hematologic toxicity that persisted for > 2 weeks. Clinical responses and transplantation outcome were assessed every 3 months by sequential bone marrow (BM) aspirates and peripheral blood lineage-specific chimerism analyses, as previously described,26 until 12 months after transplantation. As a control group we selected 19 patients who had undergone a RIC transplantation with the use of an identical conditioning regime who did not receive AZA after transplantation, either for logistical reasons or because they did not meet eligibility criteria (see supplemental Table 3, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). After transplantation immune reconstitution was measured in both cohorts according to the number of PBMCs recovered from patient or control.
Intracellular Treg Fox P3 analysis
The number of CD4+CD25+CD127loFoxP3+ Tregs per liter was measured at monthly intervals from all trial patients and compared with the same population of 16 RIC allograft controls used above. PBMCs were surface stained with CD127 FITC (Insight Ltd/eBioscience), CD25 PeCy5 (Beckman Coulter), CD4 PeCy7 (eBioscience), CD3 APC-Cy7 (Biolegend), and dead cell exclusion dye (red; Invitrogen) and washed twice in PBS/0.5% BSA (Sigma-Aldrich). After surface labeling, cells were fixed and permeabilized and stained for FoxP3 according to the manufacturer's instructions (eBioscience/Insight Ltd). The frequency of Tregs was calculated as the number of CD4+ T cells that were CD4+CD25hiCD127loFoxP3+ per liter (supplemental Figure 1). Samples were closely matched at time points after transplantation to perform comparative analysis.
Quantification of circulating tumor-specific CD8+ T cells
The number of circulating tumor-specific CTLs was measured in PBMCs prepared from 50 mL of fresh peripheral blood with the use of a CD137 expression and enrichment assay (Miltenyi Biotec). The procedure was conducted as previously described with some minor adjustments.15,27,28 The HLA type of each patient was known, and peptides that matched the HLA type were chosen from the list of previously described tumor-associated Ags selected from supplemental Table 2. (Alta Bioscience) The frequency of CD137+ Ag-specific T cells was calculated as a percentage of the total CD8+ T-cell pool from the estimates before enrichment estimates. The analysis after enrichment was used to validate the results obtained in samples before enrichment.
Memory phenotype and expansion of MAGE-specific CD8+ T cells in peptide lines
MAGE-specific dextramers (Immudex; Dako) were obtained and used to further characterize and confirm the detectable MAGE-specific CD8+ T-cell response. CD8+ T cells were enriched with MACS beads (Miltenyi Biotec) and then stained with the MAGE-specific dextramer or negative control for 20 minutes at room temperature in the dark. Cells were then counterstained with CD8 Amcyan (BD Biosciences); CD3 APC-Cy7 (Biolegend). The phenotype was characterized with Abs to CCR7-FITC (R&D Systems) and CD45RA-AF-700 (BD PharMingen). In patients with additional samples available, short-term cultures to expand MAGE-specific CD8 T cells were generated as previously described29 except dextramers (Immudex; Dako) were used to stain MAGE-specific CD8+ T cells.
CD107a and intracellular IL-2, TNF-α, and IFN-γ analysis of the reactive cells
Analysis of the reactive cells was conducted on cryopreserved PBMCs or BM cells as described previously.29 After 5 hours of incubation with peptide, cells were washed in PBS only and stained with surface Abs CD8 Amcyan (BD Biosciences), CD3 APC-Cy7 (Biolegend), dead cell exclusion dye (red; Invitrogen), CD19 ECD (Beckman Coulter), CD14 ECD (Beckman Coulter), and CD56 ECD (Invitrogen) for 20 minutes at 4°C. CD19, CD14, and CD56 were added to create a “dump channel” so that only the CD8+ cells were quantified. Samples were then fixed and permeabilized and stained for IL-2–PE (eBioscience, Insight Ltd), TNF-α–PE-Cy7 (eBioscience, Insight Ltd), and IFN-γ–AF-700 (Biolegend) according to the manufacturer's instructions. Samples were then washed and analyzed by flow cytometry.
Flow cytometric analysis
All flow cytometric analyses were performed with an LSR II flow cytometer (BD Biosciences). Single-color compensation beads (BD Biosciences) were included for each experiment, and offline automated compensation (FACS DIVA Version 6.1.3; BD Biosciences) was used.
Results
Patient accrual, treatment toxicity, and outcome
Twenty-seven patients who had undergone a RIC allograft for AML commenced AZA at a median time of 64 days (range, 40-194 days) after transplantation with a median follow-up of 7 months (range, 3-21 months; Table 1). At the time of this report 16 patients have completed ≥ 6 cycles of whom 7 have continued treatment until ≥ 1 year after transplantation.
Patient characteristics and percentage of tumor-specific CD8+ T cells detected in PBMCs of RICAZA trial patients
| PIN . | Age, y/sex . | Diagnosis . | Previous treatment . | Disease status at SCT . | Genetic risk* . | Donor type . | Acute GVHD (grade ≥ 2) . | Tumor-specific CD8+ T-cell response, % lymphocytes . | Antigen . | Status at last follow-up . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle 1 before tx . | Cycle 3 . | Cycle 6 . | Cycle 9 . | ||||||||||
| 1 | 54/M | AML | ADE, MACE, HD ara-C | First remission | Intermediate | MUD | No | 0 | 0 | 1.4 | 0 | BAGE-12-10, MAGE-A2157-166, MAGE-3112-120, MAGE-C2336-344, RAGE-132-40 | CR (21 mo after SCT) |
| 2 | 63/M | AML | DA × 2, FLAG × 1 | First remission | Poor | MUD | No | 0 | Relapsed after cycle 1 | ||||
| 3 | 52/M | AML | ADE, FLAG + IDA, HD cytarabine | First remission | Intermediate | MRD | No | 0 | 0.04 | 0.03 | 0.042 | MAGE-A2157-166, MAGE-3112-120 | CR (15 mo after SCT); DLI administered (13 mo after SCT) |
| 4 | 62/M | AML | DA | First remission | Intermediate | MUD | Yes (grade 2) | 0 | 0 | 0 | 0.21 | MAGE-A1161-169/MAGE-A1289-298 | Relapsed after cycle 10 and died |
| 5 | 55/F | AML with multilineage dysplasia after MDS | DA | First remission | Intermediate | MRD | No | 0.1 | 0.13 | 0.025 | 0.02 | MAGE-A2157-166/MAGE-C2336-344/RAGE-1352-360 | CR (18 mo after SCT) |
| 6 | 58/F | AML | DA + Mylotarg × 2, FLAG-IDA | First remission | Poor | MRD | No | 0 | 0 | Relapsed after cycle 3 | |||
| 7 | 71/M | AML | DA × 3, sodium valproate + AZA | Second remission | Intermediate | MUD | No | 0 | 0 | 0 | 0.1 | MAGE-A1161-169/MAGE-A3167-176/BAGE-12-10/MAGE-A3168-176 | CR (15 mo after SCT) |
| 8 | 64/M | AML with multilineage dysplasia | DCLo | First remission | Poor | MRD | No | 0 | 0 | 0 | Relapsed after cycle 6 | ||
| 9 | 70/M | AML with multilineage dysplasia | DA, FLAG | Second remission | Poor | MUD | Yes (grade 2) | 0 | 0 | 0 | Withdrawn after cycle 9 | ||
| 10 | 58/M | AML | ADE + Mylotarg, ADE | First remission | Poor | MRD | No | 0 | Withdrawn after cycle 1 | ||||
| 11 | 63/M | AML | DA, FLAG | Second remission | Intermediate | MUD | No | ND | 0 | 0.42 | MAGE-A1289-298/MAGE-A1161-169/MAGE-A3168-176 | Withdrawn after cycle 8; DLI administered (8 mo after SCT) | |
| 12 | 67/F | AML with multilineage dysplasia after MDS | RCVP. DAT 3 + 10 | First relapse | Intermediate | MUD | No | 0 | ND | 0.2 | 0.11 | MAGE-A2212-220 | CR (12 mo after SCT) |
| 13 | 66/F | AML | Daunorubicin, clofarabine, Mylotarg, FLAG/IDA | Second remission | Intermediate | MUD | No | 0 | 0 | Relapsed after cycle 4 and died | |||
| 14 | 58/F | Secondary AML in association with previous Jak2+ myelofibrosis | ADE, Mylotarg, ADE, DA, Clo | First remission | Intermediate | MUD | No | ND | 1.6 | 0.44 | 0.8 | MAGE-A2212-220/MAGE-A3112-120/MAGE-A2157-166/MAGE-A3167-176 | CR (12 mo after SCT) |
| 15 | 59/M | AML after MDS | FLAG, DA | First remission | Intermediate | MRD | No | 0 | 0.284 | 0.68 | 0.45 | MAGE-A3168-176 | Active treatment after cycle 9 |
| 16 | 68/M | AML | DA, FLAG, MACE, FLAG-IDA | Second remission | Intermediate | MUD | No | ND | Relapsed after cycle 2 | ||||
| 17 | 48/F | AML | DA, DA, MIDAC | First Remission | Intermediate | MRD | No | ND | 0 | 0 | 0.013 | MAGE-A2157-166/MAGE-A1161-169/RAGE-1352-360 | Active treatment after cycle 9 |
| 18 | 51/M | AML | DA, Etoposide, Clo | First remission | Intermediate | MUD | No | 0 | 0 | 0.9 | 0.09 | MAGE-A3112-120/MAGE- C2336-344/WT-1317-327 | Active treatment after cycle 6 |
| 19 | 54/F | AML | DA × 2, HD araC | First remission | Intermediate | MRD | No | 0 | 0.01 | MAGE-A3112-120 | Relapsed after cycle 5 | ||
| 20 | 63/M | AML | MIDAC × 4 | Second remission | Intermediate | MUD | No | 0 | 0 | 1.36 | MAGE-C2336-344/WT-1235-243/MAGE-A3112-120/MAGE-A397-105 | Active treatment after cycle 7 | |
| 21 | 60/M | AML with multilineage dysplasia | DA, Mylotarg, DCLo | First remission | Intermediate | MUD | No | ND | 0 | 0 | 0 | Active treatment after cycle 5 | |
| 22 | 40/F | AML | ADE + Mylotarg, DCLo, FLAG-IDA | First relapse | Intermediate | MRD | No | 0 | Active treatment after cycle 4 | ||||
| 23 | 53/F | AML | ADE × 2 | First remission | Intermediate | MUD | Yes (grade 2) | 0 | 0 | Withdrawn after cycle 3 | |||
| 24 | 66/M | AML | MIDAC | First remission | Intermediate | MRD | No | 0 | 0.04 | 0.01 | 0.17 | MAGE-A2157-166/MAGE-A1289-298 | Active treatment after cycle 6 |
| 25 | 55/M | Acute myelomonocytic leukemia | MIDAC × 4, FLAG-IDA | Second remission | Intermediate | MRD | No | 0 | 0 | 0† | 0† | MAGE-A397-105/WT-1235-243 | Active treatment after cycle 6 |
| 26 | 62/F | Therapy-related AML after previous exposure to a topoisomerase II inhibitor | DA × 2, HD araC | First remission | Poor | MUD | No | 0 | 0 | 0 | Active treatment after cycle 3 | ||
| 27 | 62/M | AML after MDS | DA × 3, FLAG-IDA, HD araC | First remission | Intermediate | MUD | No | ND | Active treatment after cycle 3 | ||||
| PIN . | Age, y/sex . | Diagnosis . | Previous treatment . | Disease status at SCT . | Genetic risk* . | Donor type . | Acute GVHD (grade ≥ 2) . | Tumor-specific CD8+ T-cell response, % lymphocytes . | Antigen . | Status at last follow-up . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle 1 before tx . | Cycle 3 . | Cycle 6 . | Cycle 9 . | ||||||||||
| 1 | 54/M | AML | ADE, MACE, HD ara-C | First remission | Intermediate | MUD | No | 0 | 0 | 1.4 | 0 | BAGE-12-10, MAGE-A2157-166, MAGE-3112-120, MAGE-C2336-344, RAGE-132-40 | CR (21 mo after SCT) |
| 2 | 63/M | AML | DA × 2, FLAG × 1 | First remission | Poor | MUD | No | 0 | Relapsed after cycle 1 | ||||
| 3 | 52/M | AML | ADE, FLAG + IDA, HD cytarabine | First remission | Intermediate | MRD | No | 0 | 0.04 | 0.03 | 0.042 | MAGE-A2157-166, MAGE-3112-120 | CR (15 mo after SCT); DLI administered (13 mo after SCT) |
| 4 | 62/M | AML | DA | First remission | Intermediate | MUD | Yes (grade 2) | 0 | 0 | 0 | 0.21 | MAGE-A1161-169/MAGE-A1289-298 | Relapsed after cycle 10 and died |
| 5 | 55/F | AML with multilineage dysplasia after MDS | DA | First remission | Intermediate | MRD | No | 0.1 | 0.13 | 0.025 | 0.02 | MAGE-A2157-166/MAGE-C2336-344/RAGE-1352-360 | CR (18 mo after SCT) |
| 6 | 58/F | AML | DA + Mylotarg × 2, FLAG-IDA | First remission | Poor | MRD | No | 0 | 0 | Relapsed after cycle 3 | |||
| 7 | 71/M | AML | DA × 3, sodium valproate + AZA | Second remission | Intermediate | MUD | No | 0 | 0 | 0 | 0.1 | MAGE-A1161-169/MAGE-A3167-176/BAGE-12-10/MAGE-A3168-176 | CR (15 mo after SCT) |
| 8 | 64/M | AML with multilineage dysplasia | DCLo | First remission | Poor | MRD | No | 0 | 0 | 0 | Relapsed after cycle 6 | ||
| 9 | 70/M | AML with multilineage dysplasia | DA, FLAG | Second remission | Poor | MUD | Yes (grade 2) | 0 | 0 | 0 | Withdrawn after cycle 9 | ||
| 10 | 58/M | AML | ADE + Mylotarg, ADE | First remission | Poor | MRD | No | 0 | Withdrawn after cycle 1 | ||||
| 11 | 63/M | AML | DA, FLAG | Second remission | Intermediate | MUD | No | ND | 0 | 0.42 | MAGE-A1289-298/MAGE-A1161-169/MAGE-A3168-176 | Withdrawn after cycle 8; DLI administered (8 mo after SCT) | |
| 12 | 67/F | AML with multilineage dysplasia after MDS | RCVP. DAT 3 + 10 | First relapse | Intermediate | MUD | No | 0 | ND | 0.2 | 0.11 | MAGE-A2212-220 | CR (12 mo after SCT) |
| 13 | 66/F | AML | Daunorubicin, clofarabine, Mylotarg, FLAG/IDA | Second remission | Intermediate | MUD | No | 0 | 0 | Relapsed after cycle 4 and died | |||
| 14 | 58/F | Secondary AML in association with previous Jak2+ myelofibrosis | ADE, Mylotarg, ADE, DA, Clo | First remission | Intermediate | MUD | No | ND | 1.6 | 0.44 | 0.8 | MAGE-A2212-220/MAGE-A3112-120/MAGE-A2157-166/MAGE-A3167-176 | CR (12 mo after SCT) |
| 15 | 59/M | AML after MDS | FLAG, DA | First remission | Intermediate | MRD | No | 0 | 0.284 | 0.68 | 0.45 | MAGE-A3168-176 | Active treatment after cycle 9 |
| 16 | 68/M | AML | DA, FLAG, MACE, FLAG-IDA | Second remission | Intermediate | MUD | No | ND | Relapsed after cycle 2 | ||||
| 17 | 48/F | AML | DA, DA, MIDAC | First Remission | Intermediate | MRD | No | ND | 0 | 0 | 0.013 | MAGE-A2157-166/MAGE-A1161-169/RAGE-1352-360 | Active treatment after cycle 9 |
| 18 | 51/M | AML | DA, Etoposide, Clo | First remission | Intermediate | MUD | No | 0 | 0 | 0.9 | 0.09 | MAGE-A3112-120/MAGE- C2336-344/WT-1317-327 | Active treatment after cycle 6 |
| 19 | 54/F | AML | DA × 2, HD araC | First remission | Intermediate | MRD | No | 0 | 0.01 | MAGE-A3112-120 | Relapsed after cycle 5 | ||
| 20 | 63/M | AML | MIDAC × 4 | Second remission | Intermediate | MUD | No | 0 | 0 | 1.36 | MAGE-C2336-344/WT-1235-243/MAGE-A3112-120/MAGE-A397-105 | Active treatment after cycle 7 | |
| 21 | 60/M | AML with multilineage dysplasia | DA, Mylotarg, DCLo | First remission | Intermediate | MUD | No | ND | 0 | 0 | 0 | Active treatment after cycle 5 | |
| 22 | 40/F | AML | ADE + Mylotarg, DCLo, FLAG-IDA | First relapse | Intermediate | MRD | No | 0 | Active treatment after cycle 4 | ||||
| 23 | 53/F | AML | ADE × 2 | First remission | Intermediate | MUD | Yes (grade 2) | 0 | 0 | Withdrawn after cycle 3 | |||
| 24 | 66/M | AML | MIDAC | First remission | Intermediate | MRD | No | 0 | 0.04 | 0.01 | 0.17 | MAGE-A2157-166/MAGE-A1289-298 | Active treatment after cycle 6 |
| 25 | 55/M | Acute myelomonocytic leukemia | MIDAC × 4, FLAG-IDA | Second remission | Intermediate | MRD | No | 0 | 0 | 0† | 0† | MAGE-A397-105/WT-1235-243 | Active treatment after cycle 6 |
| 26 | 62/F | Therapy-related AML after previous exposure to a topoisomerase II inhibitor | DA × 2, HD araC | First remission | Poor | MUD | No | 0 | 0 | 0 | Active treatment after cycle 3 | ||
| 27 | 62/M | AML after MDS | DA × 3, FLAG-IDA, HD araC | First remission | Intermediate | MUD | No | ND | Active treatment after cycle 3 | ||||
ADE indicates cytosine arabinoside/daunorubicin/etoposide; AML, acute myeloid leukemia; BAGE-1, B melanoma Ag 1; CR, complete remission; Clo, clofarabine; DA, daunorubicin/cytosine arabinoside; DAT, daunorubicin,/cytosine arabinoside/6-thioguanine; DClo, daunorubicin/clofarabine; DLI, donor lymphocyte infusion; HD araC, high-dose cytosine arabinoside; FLAG, fludarabine/cytosine arabinoside/GCSF; FLAG-IDA, G-CSF/fludarabine/cytosine arabinoside/idarubicin; MACE, amsacrine/cytosine arabinoside/etoposide; MAGE, melanoma-associated Ag; MDS, myelodysplastic syndrome; MIDAC, mitoxantrone/cytosine arabinoside; MRD, matched related donor; MUD, matched unrelated donor; ND, not done; PB, peripheral blood; PIN, personal identification number; RAGE-1, renal tumor Ag 1; RCVP, rituximab/cyclophosphamide/vincristine/prednisolone; RICAZA, reduced intensity conditioning with azacitidine; SCT, stem cell transplantation; and tx, treatment.
Karyotype risk stratification according to Grimwade et al.25
CTA-specific response detected in the BM (cycle 6 = 0.09%) and (cycle 9 = 0.042%) but below detection in PB.
AZA was well tolerated in most patients, and side effects were managed by appropriate supportive care or treatment delays. No patients required dose reduction of AZA. Ten patients discontinued AZA therapy because of disease relapse (n = 6), treatment toxicity (n = 3), or falling chimerism (n = 1). Adverse events are described in supplemental Table 1. Hematologic toxicity was modest, and only 2 patients experienced treatment delay because of neutropenia or thrombocytopenia. Three of 27 assessable patients developed grade 2 acute GVHD, and no patient developed > grade 2 acute GVHD. To date 2 patients have developed limited chronic GVHD of the skin, but no patient developed chronic extensive GVHD. In contrast 7 of 19 patients in the control population have developed grade 2 acute GVHD and 8 have developed chronic GVHD. At day 90 of the 24 patients with available informative chimerism analysis 19 showed full donor chimerism in whole blood and 6 of 11 patients showed full donor chimerism in the T-cell fraction. In the control population 19 of 19 patients had achieved full donor chimerism in whole blood at day 90 and 5 of 15 in whom data were available achieved full donor chimerism in the T-cell fraction. Seven patients have relapsed to date at a median of 6 months (range, 4-15 months) after transplantation.
Effect of AZA administration on Treg numbers after transplantation
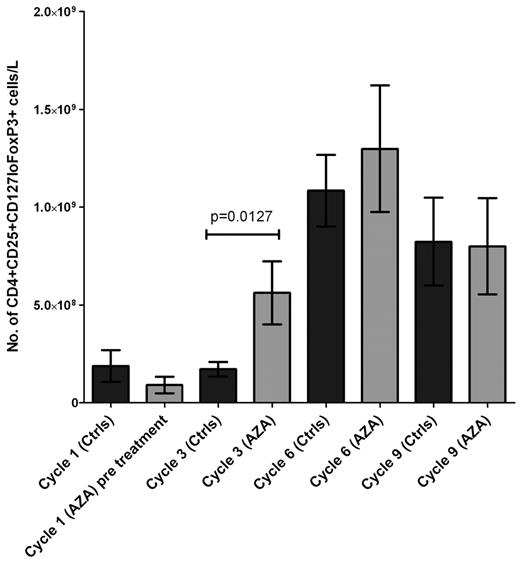
Increased numbers of circulating Tregs were present in patients who had received 3 courses of AZA after transplantation compared with a control group of patients studied at a similar time point after transplantation (P = .0127). This resulted in a > 3-fold increase in Treg numbers in patients treated with AZA compared with time-matched controls. In contrast, no difference was detected in the number of circulating Tregs after 6 or 9 courses of AZA with the use of similar time-matched controls. Thus, the expansion in Treg numbers induced by AZA was confined to the immediate period after transplantation (Figure 1). We also studied the effect of AZA administration on CD8+, CD4+, and absolute lymphocyte numbers but observed no difference compared with similar time-matched controls (data not shown).
Comparison of the number of CD4+CD25+CD127loFoxP3+ T cells per liter after transplantation in RICAZA patients compared with time-matched samples from RIC controls. (A) The number of Tregs per liter was quantified at sequential intervals after transplantation and plotted in a histogram that displayed the mean and the SEM. In total, 16 RIC controls and 19 RICAZA patients were analyzed at similar time points after transplantation. For the RICAZA patients, samples were collected at cycle 1 before treatment. A significant increase in the number per liter of CD4+CD25+CD127loFoxP3+ Tregs at cycle 3 was observed in AZA-treated patients (n = 17) compared with the control population (n = 11; P = .0127, Mann-Whitney t test). Dark gray columns indicate control patients; and light gray columns, RICAZA patients.
Comparison of the number of CD4+CD25+CD127loFoxP3+ T cells per liter after transplantation in RICAZA patients compared with time-matched samples from RIC controls. (A) The number of Tregs per liter was quantified at sequential intervals after transplantation and plotted in a histogram that displayed the mean and the SEM. In total, 16 RIC controls and 19 RICAZA patients were analyzed at similar time points after transplantation. For the RICAZA patients, samples were collected at cycle 1 before treatment. A significant increase in the number per liter of CD4+CD25+CD127loFoxP3+ Tregs at cycle 3 was observed in AZA-treated patients (n = 17) compared with the control population (n = 11; P = .0127, Mann-Whitney t test). Dark gray columns indicate control patients; and light gray columns, RICAZA patients.
Induction of a CD8+ T-cell response to putative tumor Ags after transplantation
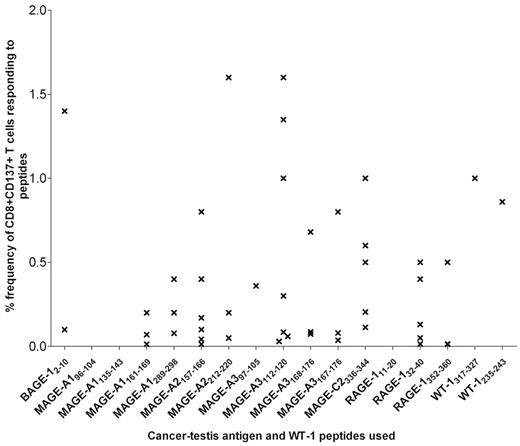
AZA induced a CD8+ T-cell response to ≥ 1 tumor-specific peptides in 15 of 22 patients who had received > 3 cycles of AZA after transplantation (Table 1). Of the 18 patients who had received ≥ 6 cycles of treatment, 14 showed a CD8+ CTL response (Table 1) to a range of tumor Ags, including MAGE-A1, MAGE-A2, MAGE-A3, BAGE-1 (B melanoma antigen 1), RAGE-1 (renal tumor antigen 1), and WT-1 (Figure 2). Before commencement of AZA, a CD8+ T-cell response to these tumor Ags was detectable in only 1 of 21 patients, consistent with previous reports that CD8+ T-cell responses to putative tumor Ags are only rarely observed in patients immediately after transplantation.30,31 The frequency of CD8+ T cells directed against the studied tumor Ags ranged from 0.01% to 1.6% (mean, 0.36%) of circulating CD8+ T cells, which is comparable to EBV-specific T-cell responses. Of note, no CD4+ T-cell responses to the studied tumor Ags were detectable before or after the commencement of AZA. Although technically very challenging, it has been possible to perform microsatellite marker chimerism analysis in one patient, personal identification number (PIN) 15, which showed that 72% of the CD8+CD137+ tumor-specific T-cell population was of donor origin. In contrast, no T-cell response was detectable to any of the candidate tumor peptides in 6 of 7 controls who were sequentially analyzed after transplantation. In one patient a T-cell response was detectable at 1 and 3 months after transplantation, but interestingly the frequency decreased over this time and then became undetectable 8 months after transplantation.
CD8+ T-cell responses were detected in most tumor Ag peptides used in the experimental procedure. The graph shows the maximal T-cell response detected to the different peptides. Values represent the percentage of CD137+CD8+ T cells specific to the different peptides and are calculated by subtracting the percentage of cells detected in the unstimulated population. Details of peptides studied are contained in supplemental Table 2.
CD8+ T-cell responses were detected in most tumor Ag peptides used in the experimental procedure. The graph shows the maximal T-cell response detected to the different peptides. Values represent the percentage of CD137+CD8+ T cells specific to the different peptides and are calculated by subtracting the percentage of cells detected in the unstimulated population. Details of peptides studied are contained in supplemental Table 2.
Elevated tumor Ag-specific CD8+ T-cell responses detected in BM compared with blood
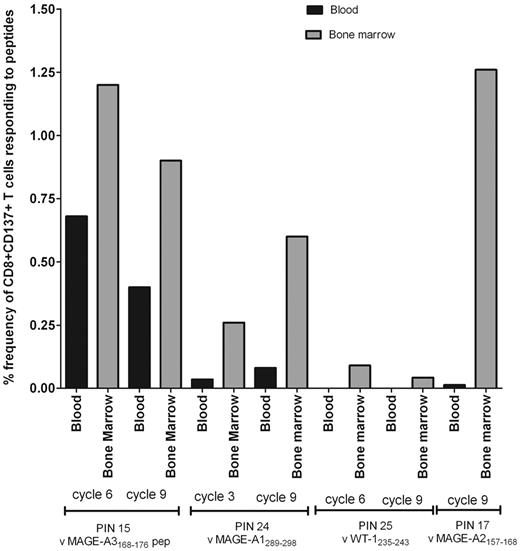
Paired blood and BM samples were studied in 4 patients, PIN 15 (MAGE-A3168-176), PIN 17 (MAGE-A2157-166), PIN 24 (MAGE-A1289-298 and MAGE-A2157-166), and PIN25 (WT-1235-243), and the frequency of tumor-specific CD8+ T cells was shown to be higher in BM, by up to 100-fold. For patients PIN 15, 24, and 25, further paired blood and BM samples were available for analysis at cycle 9 and, again, showed elevated tumor-specific CD8+ T cells in the BM (Figure 3). Our observations are consistent with a previous report that showed increased numbers of leukemia Ag-specific T cells in the BM.32
Frequency of tumor-specific CD8+ T cells in paired blood and BM samples from 4 patients during the course of AZA administration. The percentage of frequency of CD8+CD137+ T cells responding to the peptide is shown. Values are calculated by subtracting the percentage detected in the unstimulated from the test.
Frequency of tumor-specific CD8+ T cells in paired blood and BM samples from 4 patients during the course of AZA administration. The percentage of frequency of CD8+CD137+ T cells responding to the peptide is shown. Values are calculated by subtracting the percentage detected in the unstimulated from the test.
Functional analysis and characterization of the CD8+ tumor-specific responses
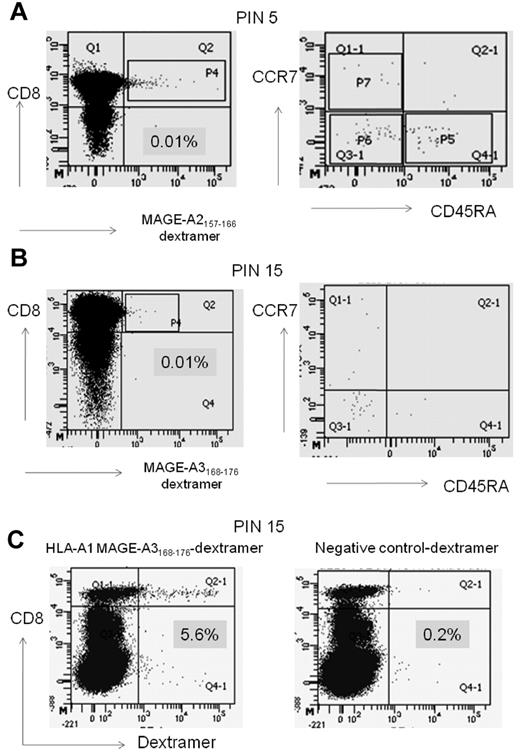
Ex vivo characterization of the MAGE T-cell response with the use of dextramers showed that the MAGE-specific CD8+ T cells were of effector memory (CD45RA−CCR7−) phenotype, indicating appropriate priming and activation (Figure 4A-B). Moreover, the detectable MAGE-specific CD8+ T cells showed expansion after 10 days of culture in vitro (Figure 4C). Dextramers specific to the HLA-A24–restricted peptide WT-1235-243 were not available; therefore, this response could not be further characterized in the same way. We did, however, show that the WT-1 response was functional and present in the BM (Table 2).
Ex vivo characterization of the MAGE T-cell response with the use of dextramers. (A-B) Ex vivo dextramer stain of patient PIN 5 (A) and PIN 15 (B) PBMCs counterstained with the MAGE-specific dextramer and anti-CCR7 and CD45RA Abs. (A) MAGE-A2157-166–specific dextramer stain from PIN 5, 12 months after transplantation. (B) MAGE-A3168-176–specific dextramer stain from PIN 15, 9 months after transplantation. (C) Expansion of the MAGE-A3168-176–specific CD8+ T-cell response from PIN 15 showing expansion by ∼ 500-fold (from 0.01% to 5.6% of CD8 T cells). The percentage of dextramer-positive T cells is displayed on the x-axis and CD8 is displayed on the y-axis. The other 2 patients studied, PIN 5 and PIN 11, showed 20-fold (0.2% of CD8+ T cells) and 30-fold (0.3% of CD8+ T cells) expansions, respectively (data not shown).
Ex vivo characterization of the MAGE T-cell response with the use of dextramers. (A-B) Ex vivo dextramer stain of patient PIN 5 (A) and PIN 15 (B) PBMCs counterstained with the MAGE-specific dextramer and anti-CCR7 and CD45RA Abs. (A) MAGE-A2157-166–specific dextramer stain from PIN 5, 12 months after transplantation. (B) MAGE-A3168-176–specific dextramer stain from PIN 15, 9 months after transplantation. (C) Expansion of the MAGE-A3168-176–specific CD8+ T-cell response from PIN 15 showing expansion by ∼ 500-fold (from 0.01% to 5.6% of CD8 T cells). The percentage of dextramer-positive T cells is displayed on the x-axis and CD8 is displayed on the y-axis. The other 2 patients studied, PIN 5 and PIN 11, showed 20-fold (0.2% of CD8+ T cells) and 30-fold (0.3% of CD8+ T cells) expansions, respectively (data not shown).
CD107a and intracellular cytokine staining of PBMCs and BM cells from PIN 5, 7, 12, 15, and 25
| PIN . | Tumor-antigen peptide . | % Expression of CD8+ T cells, minus the % in the unstimulated . | |||
|---|---|---|---|---|---|
| CD107α . | IL-2 . | TNFα . | IFNγ . | ||
| 5 (PBMCs) | MAGE-A2157-166 | 0.1 | 0.012 | 0.014 | 0.034 |
| 5 (PBMCs) | MAGE-A3112-120 | 0 | 0 | 0 | 0.042 |
| 12 (PBMCs) | MAGE-A1161-169 | 0.03 | 0.03 | 0 | 0 |
| 12 (PBMCs) | MAGE-A3168-176/MAGE-A2212-220 | 0.1 | 0 | 0.014 | 0 |
| 7 (PBMCs) | BAGE-12-10 | 0.013 | 0 | 0 | 0.03 |
| 7 (BM) | BAGE-12-10 | 0.01 | 0 | 0 | 0.05 |
| 15 (PBMCs) | MAGE-A3168-176/MAGE-A196-104 | ND | 0 | 0.02 | 0.09 |
| 15 (BM) | MAGE-A3168-176 | 0.044 | 0.09 | 0.5 | 0.251 |
| 15 (BM) | MAGE-A196-104 | 0.35 | 0.035 | 0.5 | 0.08 |
| 25 (BM) | MAGE-A397-105 | 0.06 | 0 | 0.8 | 0 |
| 25 (BM) | WT-1235-243 | 0.3 | 0.06 | 0.9 | 0 |
| PIN . | Tumor-antigen peptide . | % Expression of CD8+ T cells, minus the % in the unstimulated . | |||
|---|---|---|---|---|---|
| CD107α . | IL-2 . | TNFα . | IFNγ . | ||
| 5 (PBMCs) | MAGE-A2157-166 | 0.1 | 0.012 | 0.014 | 0.034 |
| 5 (PBMCs) | MAGE-A3112-120 | 0 | 0 | 0 | 0.042 |
| 12 (PBMCs) | MAGE-A1161-169 | 0.03 | 0.03 | 0 | 0 |
| 12 (PBMCs) | MAGE-A3168-176/MAGE-A2212-220 | 0.1 | 0 | 0.014 | 0 |
| 7 (PBMCs) | BAGE-12-10 | 0.013 | 0 | 0 | 0.03 |
| 7 (BM) | BAGE-12-10 | 0.01 | 0 | 0 | 0.05 |
| 15 (PBMCs) | MAGE-A3168-176/MAGE-A196-104 | ND | 0 | 0.02 | 0.09 |
| 15 (BM) | MAGE-A3168-176 | 0.044 | 0.09 | 0.5 | 0.251 |
| 15 (BM) | MAGE-A196-104 | 0.35 | 0.035 | 0.5 | 0.08 |
| 25 (BM) | MAGE-A397-105 | 0.06 | 0 | 0.8 | 0 |
| 25 (BM) | WT-1235-243 | 0.3 | 0.06 | 0.9 | 0 |
BAGE indicates B melanoma Ag; MAGE, melanoma-associated Ag; PIN, personal identification number; and WT-1, Wilm tumor Ag 1.
The detectable tumor-specific responses were also functional as defined by mobilization of CD107a and secretion of IL-2, IFN-γ, and TNF-α in response to peptide. Further samples were available for analysis from PIN 5, 7, 12, 15, and 25 (Table 2); data from PIN 5 are shown in Figure 5. Interestingly, this sample was obtained 6 months after finishing AZA treatment and shows that the MAGE-A2157-165–specific response was maintained after treatment. In patients PIN 7, 15, and 25 BM samples were available and showed that the detectable T cells present within the BM microenvironment were cytotoxic and secreted particularly high levels of TNF-α (Table 2).
CD107a and intracellular cytokine staining of PBMCs from PIN 5 12 months after transplantation. PBMCs were stimulated for 5 hours with the tumor-specific peptide, left unstimulated or stimulated with CMV peptides or PMA/ionomycin as positive controls. The data show percentage of CD107a mobilization and IL-2, TNF-α, and IFN-γ secretion after stimulation with the relevant peptide.
CD107a and intracellular cytokine staining of PBMCs from PIN 5 12 months after transplantation. PBMCs were stimulated for 5 hours with the tumor-specific peptide, left unstimulated or stimulated with CMV peptides or PMA/ionomycin as positive controls. The data show percentage of CD107a mobilization and IL-2, TNF-α, and IFN-γ secretion after stimulation with the relevant peptide.
Discussion
Our data showed that the adjunctive administration of AZA after allogeneic SCT is well tolerated and results in increased numbers of Tregs within the first few months after transplantation. This is, to our knowledge, the first report that AZA has the capacity to increase circulating Tregs after transplantation in humans and is consistent with previous data obtained in a murine transplantation model. Compelling data imply an important role for Tregs in the establishment of tolerance after allogeneic transplantation, and in murine models adoptive transfer of Tregs has been shown to reduce the risk of acute and chronic GVHD.33 As a consequence, several groups are exploring the possibility of adoptive transfer of Tregs after transplantation. Such studies are however hampered by the inefficiency of current isolation techniques, and the administration of AZA after transplantation therefore represents a potentially important alternative strategy by which Treg numbers might be increased in the immediate period after transplantation. It has previously been reported that AZA augments expansion of Tregs through hypomethylation of the CpG island associated with the promoter of the FOXP3 gene, but several other genes have also been identified as mediators of AZA-induced Treg expansion, and it will be important to explore their differential sensitivity to AZA in vivo.24 Of interest, AZA-induced augmentation of Treg expansion appeared confined to the immediate period after transplantation, and a more detailed analysis of the kinetics of AZA-induced Treg expansion will be important. Although there are no convincing reasons to explain the time-dependent effect of AZA on Treg numbers, one could speculate that this might be consequent on AZA to induce naive CD4 T cells to differentiate into CD4 FoxP3+ Tregs. Given the importance of early clinical intervention in the design of strategies aimed at improving outcome after allograft, it is of interest that AZA has the capacity to increase Treg numbers in the immediate period after transplantation. In this context it is relevant to note the low incidence of acute and chronic GVHD in patients treated with AZA in this study compared with control patients (Table 1; supplemental Table 3) and previous studies that used a similar preparative regimen. Larger studies with longer follow-up will be required to confirm the effect of AZA after transplantation on the incidence and severity of GVHD, particularly in the context of an alemtuzumab-based conditioning regimen. Although murine studies have reported no abrogation of a GVL effect consequent on AZA administration, extended follow-up of these patients will be important to exclude the possibility of an increased risk of disease relapse. Interestingly, administration of AZA had no effect on the numbers of circulating Tregs detected at later time points, and it may, therefore, be that administration of AZA should be limited to the first 6 months after transplantation with the possibility of administering DLI in patients deemed to be at high risk of disease relapse thereafter.
The ability of AZA to induce a cytotoxic CD8+ T-cell response to candidate tumor Ags such as MAGE-A1, MAGE-A2, MAGE-A3, BAGE-1, RAGE-1, and WT-1 identifies an additional mechanism by which its administration after transplantation could improve transplantation outcome (Figure 2). No studies to date have addressed whether epigenetic therapies have the capacity to induce CD8+ T-cell responses to tumor Ags after allogeneic SCT. Although the clinical relevance of such responses remains undetermined, it is of interest that only 2 of the 15 patients who showed a CD8+ T-cell response to the tumor Ags studied have relapsed to date. From first principles up-regulation of target Ag expression represents a potential mechanism by which a GVL effect might be augmented after transplantation, and, given the tumor specificity of aberrant hypermethylation, our data argue that the administration of AZA after transplantation may possess the ability to selectively augment a GVL reaction without increasing the risk of GVHD. In addition the possibility of using AZA to augment the antitumor effect of DLI requires further examination.9 These data also highlight the possibility that epigenetic therapies may induce a CD8+ T-cell response to novel tumor Ags, and prior exposure to epigenetic therapies might be usefully incorporated into strategies aimed at novel tumor Ag discovery.
Strategies after transplantation that augment a GVL effect without increasing the risk of GVHD have, to date, proved elusive. In this study we show that the administration of an antileukemic therapy, AZA, is well tolerated and appears to be associated with a low risk of GVHD. The observation that AZA induces a CD8+ T-cell response directed at potential tumor Ags at the same time as augmenting the expansion of Tregs identifies the adjunctive administration of epigenetic therapies after transplantation as a potential therapeutic approach by which a GVL effect may be epigenetically manipulated without a concomitant increase in the risk of GVHD.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Cancer Research UK Experimental Cancer Medicine Centre (ECMC) Network, the Central England Haemato-oncology Research Biobank, Leukaemia and Lymphoma Research, the QE Hospital Charity Fund, and Cure Leukaemia. P.V. and C.F.C. were supported by National Institute for Health Research (NIHR) funding to the Oxford Biomedical Research Center. Azacitidine was provided free of charge from Celgene Ltd.
Authorship
Contribution: O.C.G. designed research, performed experiments, analyzed data, and contributed to writing the paper; MD recruited patients, collected data, and contributed to writing the paper; N.Y.J. and J.L. collected and analyzed data and contributed to writing the paper; S.S. designed research, collected data, and contributed to writing the paper; G.R. collected and analyzed data and performed experiments; J.N. collected data and performed research; R.K. performed experiments; M.R., M.C., J.A.S., N.R., J.Y., C.C., and G.C. recruited patients and collected data; M.G. collected and analyzed data; P.V., and P.M. analyzed and interpreted data; R.M. recruited patients and interpreted data; and C.F.C. designed research, recruited patients, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: C.F.C., M.C., and P.V. have attended Celgene Advisory Boards and the Centre for Clinical Haematology; M.D. received an unrestricted education grant/research funding from Celgene. Birmingham has received an unrestricted educational grant from Celgene. The remaining authors declare no competing financial interests.
Correspondence: Charles Craddock, Centre for Clinical Haematology, Queen Elizabeth Hospital, Birmingham, B15 2TH United Kingdom; e-mail: charles.craddock@uhb.nhs.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal