Abstract
Essential thrombocythemia (ET) is characterized by enhanced platelet generation and thrombotic complications. Once-daily low-dose aspirin incompletely inhibits platelet thromboxane A2 (TXA2) in the majority of ET patients. In the present study, we investigated the determinants of aspirin-insensitive platelet TXA2 biosynthesis and whether it could be further suppressed by changing the aspirin dose, formulation, or dosing interval. In 41 aspirin-treated ET patients, the immature platelet count predicted serum TXB2 independently of platelet count, age, JAK-2 V617F mutation, or cytoreduction (β = 3.53, P = .001). Twenty-one aspirin-treated patients with serum TXB2 ≥ 4 ng/mL at 24 hours after dosing were randomized to the following 7-day regimens in a crossover design: enteric-coated aspirin 100 mg twice daily, enteric-coated aspirin 200 mg once daily, or plain aspirin 100 mg once daily. A twice-daily regimen caused a further 88% median (IQR, 78%-92%, P < .001) TXB2 reduction and normalized the functional platelet response to aspirin, as assessed by urinary 11-dehydro-TXB2 excretion and the VerifyNow Aspirin assay. Doubling the aspirin dose reduced serum TXB2 only partially by 39% median (IQR, 29%-54%, P < .05). We conclude that the abnormal megakaryopoiesis characterizing ET accounts for a shorter-lasting antiplatelet effect of low-dose aspirin through faster renewal of platelet cyclooxygenase-1, and impaired platelet inhibition can be rescued by modulating the aspirin dosing interval rather than the dose.
Introduction
Essential thrombocythemia (ET) is a myeloproliferative neoplasm characterized by enhanced platelet generation. Its prevalence is calculated to be approximately 20 of 100 000 people,1 with estimates likely to increase in the near future because of the continuous rise of occasional asymptomatic diagnoses.2–4 ET usually occurs in 50- to 60-year-old patients and has a longer life expectancy and a lower leukemic transformation rate compared with other myeloproliferative neoplasms.2–6 However, up to 60% of ET patients experience a thrombotic event in their lifetimes, such as a transient ischemic attack,2–8 myocardial infarction, or stroke.2–8
Annual thrombotic event rates range from 2%-7% in ET patients treated with cytoreductive agents with or without coadministration of antiplatelet drugs,2,4,9–11 with estimates up to 13% in the absence of cytoreduction.9 In the United Kingdom Medical Research Council Primary Thrombocythemia 1 study, the rate of a composite cardiovascular end point was approximately 2.8% per year in high-risk ET patients treated with hydroxyurea (HU) on a background of aspirin (98% of enrolled patients were on aspirin 75 mg daily).10 Moreover, after a first thrombotic event, the annual recurrence rate of thrombosis is estimated to be approximately 6%-8% on antiplatelet drugs.11 In 1995, Cortelazzo et al reported the results of a placebo-controlled trial demonstrating that cytoreduction with HU has a major impact on thrombosis reduction.9 More recently, the presence of the Janus Activated Kinase (JAK)–2 V617F mutation was associated with a higher thrombotic risk in a large, retrospective study.8 Hemorrhagic complications are less frequent in ET, estimated at 0.3%-0.7% per year, possibly because of an acquired von Willebrand factor–like defect, and are often associated with the highest platelet counts.2–5,12
Several studies have reported evidence of platelet activation in ET.13,14 In particular, we reported previously enhanced urinary excretion of thromboxane (TX) metabolites in untreated ET patients.15,16 TX-metabolite excretion is a validated index of in vivo platelet activation that has been found to be consistently increased in different clinical settings characterized by enhanced cardiovascular risk or low antiplatelet drug response (reviewed in Davì and Patrono17 ).
On the basis of the thrombotic diathesis and evidence of persistent platelet activation in ET, low-dose aspirin (75-100 mg once daily) is currently recommended for both secondary and primary cardiovascular prevention independently of the level of risk, as variously defined on the basis of age, leukocytosis, JAK-2 V617F mutation, platelet count, cytoreduction, and/or other established risk factors (eg, hypertension, smoking, or diabetes).2–5,8 This recommendation is mainly based on retrospective, observational analyses2–5 and on extrapolation from an aspirin trial in polycythemia vera.18 However, aside from cohort analyses, controlled trials formally assessing the efficacy and safety of low-dose aspirin in ET are lacking.
Aspirin achieves its antithrombotic effect by permanently inactivating platelet cyclooxygenase-1 (COX-1), thus blocking TXA2 biosynthesis.19 Whereas low-dose aspirin given once daily is known to inhibit platelet TXA2 biosynthesis by approximately 97%-99% in healthy subjects,20,21 the same aspirin regimen is unable to fully inhibit platelet TXA2 production in approximately 80% of ET patients.16 The residual platelet COX (both COX-1 and COX-2) activity was shown to be fully suppressed to levels comparable to controls by adding aspirin to whole blood in vitro.16 Whether incomplete suppression of platelet COX activity in ET is because of disease-related changes in the pharmacokinetics or pharmacodynamics of aspirin is currently unknown. Whereas changes in aspirin pharmacokinetics seem unlikely, faster renewal of the drug target because of enhanced platelet turnover is both biologically and pharmacologically plausible. Accelerated platelet turnover might generate unacetylated COX-1 and/or COX-2 during the 24-hour dosing interval, which would account for partial recovery of TX-dependent platelet function. Another potential mechanism of altered aspirin pharmacodynamics in ET would be an oxidative modification of the drug target because of a higher formation of platelet hydroperoxides22 and/or increased oxidative tone in vivo,23 which may reduce the ability of aspirin to acetylate COX-1 and COX-2.24
The present study was designed to evaluate: (1) the determinants of residual platelet TXA2 production in aspirin-treated ET patients, and (2) whether aspirin-insensitive platelet TXA2 production can be further suppressed by changing the daily aspirin dose, the drug formulation, or the dosing interval.
Methods
Design of the studies
The present investigation included 2 sequential studies: (1) an intervention-randomized study and (2) a cross-sectional study.
Intervention study.
We first screened 32 patients with a diagnosis of ET according to World Health Organization criteria25 who were on ongoing treatment with aspirin 100 mg (an enteric-coated formulation marketed by Bayer Italy as Cardioaspirina) once daily. Exclusion criteria were: a recent (< 6 months) major vascular event (myocardial infarction or stroke); bleeding history; gastroduodenal ulcer; aspirin intolerance; obesity defined as a BMI ≥ 30 kg/m2 according to the World Health Organization; diagnosis of diabetes according to the American Diabetes Association26 ; hypertension or hypercholesterolemia not controlled adequately by pharmacologic treatment; pregnancy; use of anticoagulants, other antiplatelet agents, or nonsteroidal anti-inflammatory drugs (NSAIDs); platelet count > 1 100 000/μL on at least 2 consecutive occasions; major kidney or liver disease; and age > 70 years. Vitamin supplements, NSAIDs, other antiplatelet drugs, and anticoagulants were not allowed during the study. Paracetamol (up to 500 mg daily) was allowed as an analgesic. Two screening visits were performed within a 2- to 3-week interval and patients were instructed to take their aspirin tablet at breakfast (between 7 and 8 am). To enhance compliance, patients were contacted by telephone 10 and 3 days before each visit. The screening phase was aimed at identifying patients with serum TXB2 levels ≥ 4 ng/mL at 24 hours after dosing on at least 2 occasions. This threshold was calculated on the basis of the mean ± 2 SD of serum TXB2 values measured previously in 48 healthy subjects on repeated occasions over several weeks of treatment with the same aspirin regimen.21
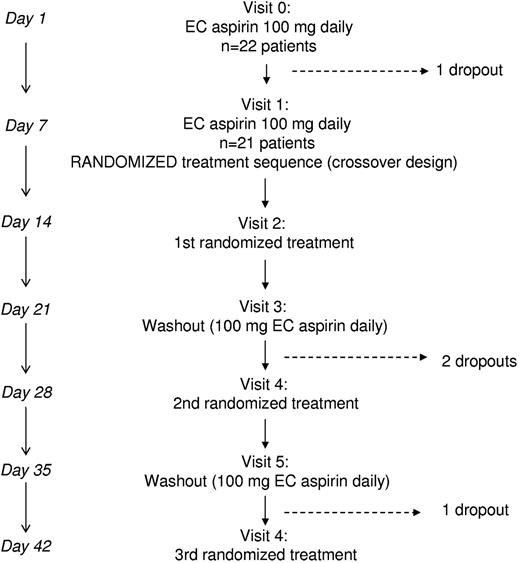
Patients who were eligible according to the serum TXB2 threshold entered a run-in phase before randomization. A schematic representation of the run-in phase (V0 and V1 visits) and of the randomized, crossover study (V2-V6 visits) is depicted in Figure 1. At day −7, patients were given aspirin tablets for the next 7 (+1) days at the same 100-mg daily dose; the first run-in visit (V0) of the intervention study was planned after 1 week (+1 day if needed). At V0, patients were given again aspirin tablets, and another visit was planned after 1 week (V1). At V1, 21 patients were randomized to computer-generated sequences of the following 7-day treatments: (1) enteric-coated aspirin 100 mg twice daily (8 am and 8 pm) or (2) enteric-coated aspirin 200 mg once daily (see Figure 1). After randomizing the first 8 subjects to 2 treatment sequences, we obtained approval for a third treatment and the next 13 patients were randomized to sequences including the above treatments (1 and 2) and plain aspirin 100 mg once daily (Aspirinetta; Bayer Italy). Between each treatment, there was a 1-week washout during which time patients resumed their standard enteric-coated aspirin 100 mg daily (see Figure 1). Patients were seen by a study investigator at the end of each study week and aspirin was provided at each visit for the following 7 days. Compliance was assessed by: (1) pill counts at each visit, (2) telephone reminders 3 days before the next visit, and (3) written personal diaries in which patients were asked to record daily aspirin intake from day −7 to the last visit.
Cross-sectional study.
The cross-sectional study was performed after the intervention study to further evaluate the value of reticulated platelets, JAK-2 V617F mutation, cytoreduction with HU, and of an index of platelet peripheral destruction, that is, the glycocalicin index (GCI), in predicting the adequacy of aspirin response in a larger group of ET patients. Nineteen additional patients who were already on aspirin (enteric coated 100 mg once daily) and had synchronized their aspirin intake at 7-8 am before being studied for at least 2 weeks before study visit were studied. Therefore, 41 patients were evaluated in the cross-sectional study. To assess reference values of some platelet related indexes (glycocalicin, GCI, mean platelet volume, platelet distribution width, immature platelet fraction and counts, and their correlations), 38 healthy subjects who were age and gender matched with ET patients were also studied.
The intervention study was performed between January and December 2010 and the cross-sectional study was performed between September and October 2011.
The protocol was approved by the local Ethics Committee (approval no. 314, 15-12-2009, Civil Hospital of Pescara, Italy, and subsequent amendments of April 2010 for the addition of a plain aspirin regimen and September 2011 for the cross-sectional study). Written informed consent was obtained from each participant. The study was conducted in accordance with the Declaration of Helsinki.
Biochemical and hematological measurements and platelet function assays
Blood and urine samples were collected at each visit both in patients and controls after an overnight fast. Complete blood counts, including the measurement of the immature platelet fraction (IPF, expressed as a percentage), were performed at each visit using a Sysmex XE 2100 hemocytometer (TOA Medical Electronics).27 The immature platelet count was calculated as follows: %IPF × (platelet count/μL)/100, and is expressed per microliter of whole blood. Complete blood chemistry was performed at screening to assess inclusion criteria and at V0. C-reactive protein (CRP), IL-6, and glycocalicin were measured by commercial kits (high-sensitivity CRP ELISA Kit, Cyclex; IL-6 EIA Kit, Cayman Chemicals; and glycocalicin EIA kit, Takara Biomedical, respectively) according to the manufacturers' instructions. The GCI, a known marker of peripheral platelet destruction, was calculated as follows28,29 : [glycocalicin concentration (ng/mL) × 250 × 109]/individual platelet count/L.
To compare functional versus biochemical assays of platelet inhibition and their sensitivity to different levels of platelet COX-1 inactivation, we also evaluated platelet function by standard light-transmittance aggregometry (LTA) in response to 1.3mM arachidonic acid21 and by the VerifyNow Aspirin whole-blood assay (Accumetrics) from V1 to V6 in a subgroup of 15 randomly selected ET patients. LTA was expressed as a percentage of maximal light transmittance (Tmax) of the aggregometric trace.21 The VerifyNow Aspirin results were expressed in aspirin reaction units, as indicated by the manufacturer. Analysis of the JAK-2 V617F mutation was performed as described previously.16
Serum TXB2 and PGE2 and urinary 11-dehydro-TXB2 and 8-iso-PGF2α measurements
To assess the adequacy of platelet COX-1 inactivation by aspirin ex vivo, we measured TXB2 produced during whole-blood clotting in vitro, which is a validated index of the maximal COX-dependent biosynthetic activity of circulating platelets.30 Briefly, a 2-mL sample of peripheral venous blood without anticoagulant was transferred into a glass tube, clotted at 37°C for 1 hour, and centrifuged at 1200g for 10 minutes. The supernatant serum was stored at −20°C and then assayed for TXB2 using an enzyme-immunoassay, as described previously.31 Serum PGE2, the other major end product of COX activity from activated platelets, was also measured.32,33
To investigate the rate of TXA2 biosynthesis in vivo, we measured the urinary excretion of its major enzymatic metabolite, 11-dehydro-TXB2.30,31 Lipid peroxidation in vivo was assessed by measuring the urinary excretion of an F2-isoprostane, 8-iso-PGF2α.31,34 Urinary 11-dehydro-TXB2 and 8-iso-PGF2α were measured by immunoassays described previously.31,34,35
Both serum and urinary metabolite measurements were performed blindly with respect to the randomized treatment.
Statistical analyses
The sample size of the randomized, crossover, intervention study was calculated to evaluate the impact of aspirin 100 mg twice daily compared with 100 mg once daily on serum TXB2 as the primary end point. Based on enhanced platelet turnover in ET and consequent faster platelet COX-1 renewal during the 24-hour dosing interval, the primary hypothesis was that the same dose of aspirin administered twice daily would cause more profound platelet COX-1 suppression than a once-daily regimen. We estimated that 14 patients randomized to a crossover design would be required to have 95% power to detect a difference of at least 70% between the serum TXB2 measured on aspirin 100 mg once daily (assuming a median value of serum TXB2 at 24 hours after intake of ≥ 8 ng/mL, as reported previously16 ) and the serum TXB2 on aspirin 100 mg twice daily, with a 2-sided α of 0.05. Assuming a dropout rate of approximately 30% because of the intrinsic complexity of the crossover design, we decided to enroll a minimum of 21 patients. Our secondary aims were: (1) to characterize the determinants of residual serum TXB2 at baseline, (2) to compare platelet function assays and serum TXB2, (3) to measure changes in urinary TX metabolite excretion as a function of different aspirin regimens, and (4) to assess whether in vivo lipid peroxidation would affect aspirin responsiveness.
The results were analyzed by parametric or nonparametric methods according to their distribution. Comparisons were made using ANOVA or the Kruskal-Wallis test. Correlations were assessed by the Pearson or Spearman rank test as appropriate. Differences between treatments were analyzed by ANOVA for repeated measurements. Data are reported as means ± SD or median and interquartile range (IQR) according to their distribution. Multiple linear regression analysis was performed to characterize independent predictors of different biomarkers. The relationship between serum TXB2 and VerifyNow Aspirin values was evaluated by fitting the experimental data with different equations (linear, logarithmic, and exponential), and the highest R2 was considered to indicate the best-fitting equation and experimental model. Significance was defined as P < .05. All tests were 2-tailed, and analyses were performed using SigmaStat Version 3.1 software (Systat Software).
Results
Screening and run-in phases of the intervention study
The intervention study was preceded by 2 screening visits aimed at identifying ET patients with serum TXB2 levels consistently ≥ 4 ng/mL at 24 hours after aspirin intake. Of 32 screened ET patients, 22 patients were enrolled (7 were excluded for serum TXB2 < 4 ng/mL and 3 eligible patients refused to participate) and entered the run-in phase, which included 2 visits (V0 and V1) 1 week apart (Figure1). The characteristics of the enrolled patients are detailed in Table 1. One patient dropped out after V0 because of a flu requiring NSAID therapy. Therefore, 21 patients at V1 were randomized to computer-generated sequences of different aspirin regimens. Three more patients dropped out for reasons not related to their willingness to participate in the study.
Design of the randomized crossover study. Before randomization, ET patients on enteric-coated aspirin 100 mg once daily were visited twice and randomized at visit 1 to computer-generated sequences of 7-day treatments that included: enteric-coated aspirin 200 mg daily, enteric-coated aspirin 100 mg twice daily, and plain aspirin 100 mg once daily, with 7-day washouts between each treatment. Blood and urine collections were performed on the last day of treatment or washout visits. EC indicates enteric-coated.
Design of the randomized crossover study. Before randomization, ET patients on enteric-coated aspirin 100 mg once daily were visited twice and randomized at visit 1 to computer-generated sequences of 7-day treatments that included: enteric-coated aspirin 200 mg daily, enteric-coated aspirin 100 mg twice daily, and plain aspirin 100 mg once daily, with 7-day washouts between each treatment. Blood and urine collections were performed on the last day of treatment or washout visits. EC indicates enteric-coated.
Baseline characteristics of 22 patients with ET recruited for the randomized study of different aspirin regimens
| Age, y | 51 (29-67) |
| Female sex, n (%) | 13 (59) |
| Time from diagnosis, y | 5 (1-10) |
| Previous vascular events, n | Vein thrombosis (2), ischemic stroke (2) |
| HU, n (%) | 9 (41) |
| JAK-2 mutation, n (%) | 14 (64) |
| Platelet count at diagnosis, × 103/μL | 604 (403-963) |
| BMI at study entry, kg/m2 | 27.5 (25-29) |
| Duration of aspirin therapy, mo | 54 (24-72) |
| Comorbidities, n | Controlled hypercholesterolemia (4); controlled hypertension (3); and controlled hypercholesterolemia and hypertension (1) |
| LDH, units/mL | 380 (371-428) |
| BM fibrosis, n (%) | 3 (14) |
| Age, y | 51 (29-67) |
| Female sex, n (%) | 13 (59) |
| Time from diagnosis, y | 5 (1-10) |
| Previous vascular events, n | Vein thrombosis (2), ischemic stroke (2) |
| HU, n (%) | 9 (41) |
| JAK-2 mutation, n (%) | 14 (64) |
| Platelet count at diagnosis, × 103/μL | 604 (403-963) |
| BMI at study entry, kg/m2 | 27.5 (25-29) |
| Duration of aspirin therapy, mo | 54 (24-72) |
| Comorbidities, n | Controlled hypercholesterolemia (4); controlled hypertension (3); and controlled hypercholesterolemia and hypertension (1) |
| LDH, units/mL | 380 (371-428) |
| BM fibrosis, n (%) | 3 (14) |
Values are medians and IQRs unless otherwise indicated.
BMI indicates body mass index; HU, hydroxyurea; and LDH, lactic dehydrogenase.
Median values of serum TXB2 and PGE2, urinary 11-dehydro-TXB2, 8-iso-PGF2α, and other hematological and biochemical parameters at the pre-randomization V0 and V1 visits are detailed in Table 2. The 2 consecutive measurements (V0 and V1) indicated a stable biochemical phenotype for maximal platelet TXA2-biosynthetic capacity30 as assessed by serum TXB2 (ρ = 0.89, P < .0001 between V0 and V1) and for the actual rate of in vivo TXA2 biosynthesis30,31 as assessed by urinary 11-dehydro-TXB2 excretion (ρ = 0.75, P < .0001). Similarly to TXB2, serum PGE2 was also stable at V0 and V1 (ρ = 0.73, P < .0001). At the run-in phase, in addition to blood counts, CRP and IL-6 were also measured based on a recent retrospective study associating inflammatory biomarkers and thromboses in ET,36 and glycocalicin and the GCI were measured to investigate whether peripheral platelet destruction may affect aspirin responsiveness. All hematologic and clinical parameters were measured weekly and were stable during the entire study (Table 2 and data not shown). Based on 4 determinations (ie, the 2 screening visits plus V0 and V1) obtained in each patient before randomization, the intra-subject coefficient of variation of serum TXB2 averaged 20% ± 13%.
Hematological and biochemical parameters measured at prerandomization visits
| . | V0 . | V1 . |
|---|---|---|
| Serum TXB2, ng/mL | 16.3 (10.5-35) | 22.6 (13.2-34.8) |
| Serum PGE2, ng/mL | 0.72 (0.53-1.4) | 1.1 (0.53-1.9) |
| 11-dehydro TXB2, pg/mg creatinine | 262.2 (177-330) | 306.1 (212-467) |
| 8-iso PGF2α, pg/mg creatinine | 467.1 (397-521) | 482.5 (427-647) |
| Platelet count, × 103/μL | 502 (331-655) | 474 (335-668) |
| IPF, % | 2.5 (2-3.1) | 2.45 (2.2-3.2) |
| Immature platelets, × 103/μL | 11.07 (8.61-15.57) | 12.42 (8.57-16.39) |
| MPV, fL | 10.1 (9.6-10.3) | 10.1 (9.6-10.5) |
| PDW, fL | 11.7 (10.9-12.2) | 11.9 (11-12.3) |
| Hct, % | 42.3 (41-44.9) | 42.2 (40.4-45.2) |
| WBCs, /μL | 7,040 (5,620-8,480) | 7,310 (5,510-8,710) |
| Glycocalicin (μg/mL) | 10.62 (5.1-14.6) | 10.0 (5.98-14.6)* |
| GCI | 5.1 (3.4-7.3) | 4.5 (3.6-6.3) |
| CRP, mg/L | 1.37 (0.59-8) | NA |
| IL-6, pg/mL | 8.7 (4.9-19) | NA |
| . | V0 . | V1 . |
|---|---|---|
| Serum TXB2, ng/mL | 16.3 (10.5-35) | 22.6 (13.2-34.8) |
| Serum PGE2, ng/mL | 0.72 (0.53-1.4) | 1.1 (0.53-1.9) |
| 11-dehydro TXB2, pg/mg creatinine | 262.2 (177-330) | 306.1 (212-467) |
| 8-iso PGF2α, pg/mg creatinine | 467.1 (397-521) | 482.5 (427-647) |
| Platelet count, × 103/μL | 502 (331-655) | 474 (335-668) |
| IPF, % | 2.5 (2-3.1) | 2.45 (2.2-3.2) |
| Immature platelets, × 103/μL | 11.07 (8.61-15.57) | 12.42 (8.57-16.39) |
| MPV, fL | 10.1 (9.6-10.3) | 10.1 (9.6-10.5) |
| PDW, fL | 11.7 (10.9-12.2) | 11.9 (11-12.3) |
| Hct, % | 42.3 (41-44.9) | 42.2 (40.4-45.2) |
| WBCs, /μL | 7,040 (5,620-8,480) | 7,310 (5,510-8,710) |
| Glycocalicin (μg/mL) | 10.62 (5.1-14.6) | 10.0 (5.98-14.6)* |
| GCI | 5.1 (3.4-7.3) | 4.5 (3.6-6.3) |
| CRP, mg/L | 1.37 (0.59-8) | NA |
| IL-6, pg/mL | 8.7 (4.9-19) | NA |
Values are medians and IQR.
MPV indicates mean platelet volume; PDW, platelet distribution width; Hct, hematocrit; and NA, not available (CRP and IL-6 were measured on 1 occasion only).
The second glycocalicin measurement was performed at V3.
We performed analyses, including all pre-randomization measurements, to identify clinical, biochemical, or hematologic determinants of residual serum TXB2 in ET patients while on daily, enteric-coated 100-mg aspirin. The analyses of pre-randomization data were performed by averaging the measurements obtained at V0 and V1.
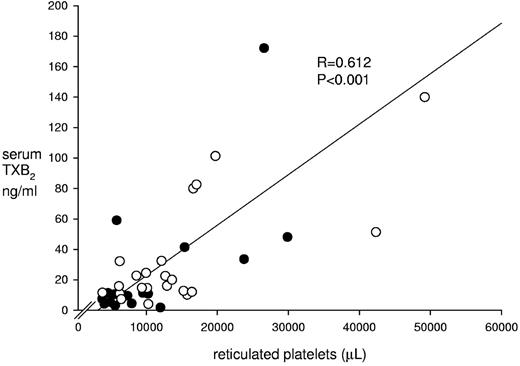
A simple correlation analysis that included all hemochromocytometric, clinical, and biochemical variables showed that the residual level of serum TXB2 at baseline was significantly associated with age (ρ = −0.47, P = .02), platelet count (ρ = 0.66, P < .001), JAK-2 V617F mutation (ρ = 0.48, P = .02), RBC count (ρ = 0.46, P = .03), immature platelet count (ρ = 0.51, P = .01), and glycocalicin level (ρ = 0.4, P = .03; also see supplemental Results, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). On multivariate analysis, the immature platelet count remained the only independent predictor of serum TXB2 (β = 2.6, P = .013; Figure 2). Serum PGE2 showed the same pattern of association in univariate and multivariate analyses as TXB2. The GCI was not correlated with serum TXB2 (ρ = 0.08, P = .75).
Baseline serum TXB2 values and reticulated platelet counts. The figure depicts individual serum TXB2 levels as a function of reticulated platelet counts measured in 41 ET patients treated with 100 mg enteric-coated aspirin. The 22 patients enrolled in the randomized intervention study are indicated by open circles.
Baseline serum TXB2 values and reticulated platelet counts. The figure depicts individual serum TXB2 levels as a function of reticulated platelet counts measured in 41 ET patients treated with 100 mg enteric-coated aspirin. The 22 patients enrolled in the randomized intervention study are indicated by open circles.
Baseline urinary 11-dehydro-TXB2 excretion was significantly correlated with IL-6 (ρ = −0.62, P = .02) and 8-iso-PGF2α (ρ = 0.55, P = .008). On multivariate analysis, the sole independent predictor of urinary 11-dehydro-TXB2 excretion was urinary 8-iso-PGF2α (β = 2.82, P = .044).
Cross-sectional study in ET patients and controls
We recruited 19 additional aspirin-treated ET patients, regardless of serum TXB2 level, to enhance the statistical power of the cross-sectional analyses. Therefore, 41 ET patients (age 57 years [IQR, 46-66] including 23 female patients and 8 with a previous thrombosis; for main characteristics, see Table 3) were evaluated to explore the determinants of aspirin-insensitive TXB2 production. Univariate analysis showed a significant association between serum TXB2 and age (ρ = −0.46, P = .003), platelet count (ρ = 0.60, P < .001), JAK-2 V617F mutation (ρ = 0.34, P = .03), HU treatment (ρ = −0.49, P = .001), immature platelets expressed both as %IPF (ρ = 0.34, P = .03) and absolute counts (ρ = 0.61, P = .0001 and Figure 2), glycocalicin (ρ = 0.41, P = .01), and erythrocytes (ρ = 0.5, P = .01; also see supplemental Results). On multivariate analysis, the absolute count of immature platelets remained the sole predictor of the level of aspirin-insensitive serum TXB2 (P = .001, β = 3.53, adjusted R2 = 0.47 for the entire model). Despite lack of a predictive value of the JAK-2 V617F mutation on multivariate analysis, ET patients bearing this mutation had significantly higher serum TXB2, a marginally significantly higher IPF, and a trend toward increased immature platelet counts compared with JAK-2 V617F–negative patients (supplemental Table 1), in part confirming recent data from another study.37 Serum PGE2 showed a pattern similar to TXB2. In addition, the GCI was unrelated to serum prostanoids in a larger patient group.
Characteristics of 41 ET patients and 38 matched controls of the cross-sectional study
| . | Patients . | Controls . |
|---|---|---|
| Age, y | 57 (46-66) | 57.5 (46-65) |
| Females, n (%) | 23 (56) | 26 (68) |
| Platelets, × 103/μL | 399 (335-649) | 212 (192-245)* |
| WBCs, μL | 6760 (5545-8652) | 6310 (4637-7280) |
| Hb, g/dL | 14 (13.4-15) | 13.4 (13-15) |
| MPV, fL | 10.1 (9.7-10.5) | 10.8 (10.4-11.3)* |
| PDW, fL | 11.9 (11-12.4) | 13.1 (12-14)* |
| IPF, % | 2.3 (1.9-3.1) | 2.6 (1.8-4.1) |
| Immature platelets, × 103/μL | 10.3 (6.3-16.4) | 5.7 (4.1-9.4)* |
| Glycocalicin, μg/mL | 8.1 (5.2-12.9) | 2.4 (1.3-3.3)* |
| GCI | 4.6 (3.5-6) | 2.6 (1.5-4)* |
| HU, n (%) | 23 (56) | NA |
| JAK-2 mutation, n (%) | 28 (68) | NA |
| Duration of aspirin therapy, mo | 42 (12-72) | NA |
| Serum TXB2, ng/mL | 13.6 (9.9-33) | ND |
| Serum PGE2, ng/mL | 0.68 (0.48-1.44) | ND |
| . | Patients . | Controls . |
|---|---|---|
| Age, y | 57 (46-66) | 57.5 (46-65) |
| Females, n (%) | 23 (56) | 26 (68) |
| Platelets, × 103/μL | 399 (335-649) | 212 (192-245)* |
| WBCs, μL | 6760 (5545-8652) | 6310 (4637-7280) |
| Hb, g/dL | 14 (13.4-15) | 13.4 (13-15) |
| MPV, fL | 10.1 (9.7-10.5) | 10.8 (10.4-11.3)* |
| PDW, fL | 11.9 (11-12.4) | 13.1 (12-14)* |
| IPF, % | 2.3 (1.9-3.1) | 2.6 (1.8-4.1) |
| Immature platelets, × 103/μL | 10.3 (6.3-16.4) | 5.7 (4.1-9.4)* |
| Glycocalicin, μg/mL | 8.1 (5.2-12.9) | 2.4 (1.3-3.3)* |
| GCI | 4.6 (3.5-6) | 2.6 (1.5-4)* |
| HU, n (%) | 23 (56) | NA |
| JAK-2 mutation, n (%) | 28 (68) | NA |
| Duration of aspirin therapy, mo | 42 (12-72) | NA |
| Serum TXB2, ng/mL | 13.6 (9.9-33) | ND |
| Serum PGE2, ng/mL | 0.68 (0.48-1.44) | ND |
Values are medians and IQR.
IPF indicates immature platelet fraction; MPV, mean platelet volume; PDW, platelet distribution width; Hb, hemoglobin; NA, not applicable; and ND, not determined (healthy controls were not on aspirin).
P < .001 versus patients.
Because of the apparent role of the immature platelet compartment in predicting poor aspirin response, we enrolled 38 sex- and age-matched healthy subjects (age, 57.5 years [IQR, 46-65], P = .93 vs ET; for main characteristics, see Table 3) to compare platelet-related indexes, including glycocalicin with ET patients. Compared with matched controls, ET patients had approximately double the reticulated platelet counts (P = .001, Table 3), confirming a significantly higher number of immature platelets in ET. Interestingly, the platelet count and IPF were significantly and inversely correlated in controls but not in patients, whereas the immature platelet count was directly correlated with the platelet count in patients but not in controls (supplemental Table 2).
Randomized crossover study
In the randomized crossover study, each experimental regimen was separated by a 7-day washout period when patients resumed their standard enteric-coated aspirin 100 mg once daily. To explore whether there was a carryover or rebound effect after each experimental aspirin regimen, we compared serum TXB2 levels at the end of the corresponding washout period. There were no statistically significant differences between pre-randomization serum TXB2 measurements averaged at V0 and V1 and values measured at the end of each washout period. Serum TXB2 averaged 20.3 ng/mL (IQR, 6-37), 20.3 ng/mL (IQR, 15.5-34.8), and 17 ng/mL (IQR, 6.7-35) at washout after 200 mg once daily, plain aspirin 100 mg once daily, and 100 mg twice daily, respectively, and none of these medians was different from each other or from pre-randomization values (20 ng/mL [IQR, 12.4-37.1], P = .61), thus ruling out carryover effects. Moreover, median serum TXB2 values measured at the end of each experimental regimen were not influenced by the randomized sequence (data not shown).
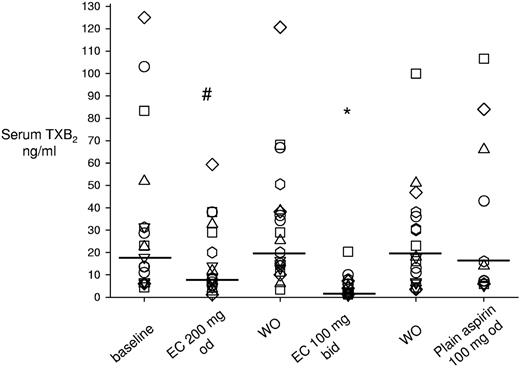
The twice-daily administration of 100 mg enteric-coated aspirin reduced serum TXB2 levels significantly by 88% (IQR, 78%-92%) compared with enteric-coated 100 mg once daily, to a median value of 2.1 ng/mL (IQR, 1.3-5.9, P < .001; Figure 3). Enteric-coated aspirin 200 mg once daily significantly reduced serum TXB2 by 39% (IQR, 29%-54%) compared with enteric-coated 100 mg once daily (Figure 3), to 8.6 ng/mL (IQR, 5.2-32.6, P < .05). In contrast, plain aspirin 100 mg once daily did not modify serum TXB2 values to any statistically significant extent (Figure 3).
Effect of different aspirin regimens on serum TXB2. The figure depicts individual data of serum TXB2 levels measured in the different phases of the study, including baseline, washout, and randomized aspirin regimens. During washout, patients took enteric-coated aspirin 100 mg once daily. Horizontal lines indicate medians. Each symbol indicates 1 patient across different treatments. *P < .001 versus washouts and plain aspirin, and #P < .05 versus baseline, washout, and enteric-coated 100 mg twice daily. WO indicates washout; od, once daily; bid, twice daily; and EC, enteric-coated.
Effect of different aspirin regimens on serum TXB2. The figure depicts individual data of serum TXB2 levels measured in the different phases of the study, including baseline, washout, and randomized aspirin regimens. During washout, patients took enteric-coated aspirin 100 mg once daily. Horizontal lines indicate medians. Each symbol indicates 1 patient across different treatments. *P < .001 versus washouts and plain aspirin, and #P < .05 versus baseline, washout, and enteric-coated 100 mg twice daily. WO indicates washout; od, once daily; bid, twice daily; and EC, enteric-coated.
The residual value of serum TXB2 after enteric-coated aspirin 100 mg twice daily was significantly correlated with the immature platelet count (ρ = 0.55, P = .01), baseline level of serum TXB2 (ρ = 0.53, P = .01), JAK-2 V617F mutation (ρ = 0.51, P = .02), and HU treatment (ρ = −0.55, P = .01). On multivariate analysis, the immature platelet count remained the sole predictor (β = 3.4, P = .004) of serum TXB2 after enteric-coated aspirin 100 mg twice daily.
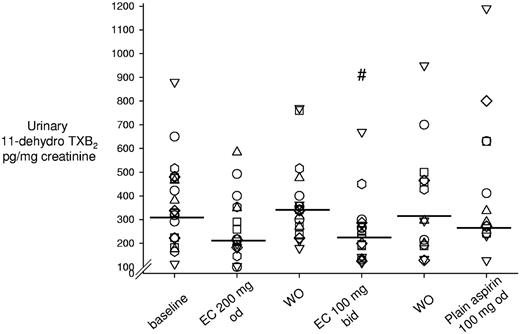
Compared with the standard regimen of enteric-coated aspirin 100 mg once daily, urinary 11-dehydro-TXB2 excretion was significantly lowered only by enteric-coated aspirin 100 mg twice daily by 23% (IQR, 1%-44%, P < .05; Figure 4). Urinary 8-iso-PGF2α excretion was not significantly modified by any experimental aspirin regimen (supplemental Figure 1), which is consistent with the largely nonenzymatic mechanism of its formation.38
Effect of different aspirin regimens on urinary excretion of 11-dehydro-TXB2. Shown are individual data on urinary 11-dehydro-TXB2 excretion rates measured in the different phases of the study, including baseline, washout, and randomized aspirin regimens. During washout, patients took enteric-coated aspirin 100 mg once daily. Horizontal lines indicate medians. Each symbol indicates 1 patient across different treatments. #P < .05 versus baseline and washout. WO indicates washout; od, once daily; bid, twice daily; and EC, enteric-coated.
Effect of different aspirin regimens on urinary excretion of 11-dehydro-TXB2. Shown are individual data on urinary 11-dehydro-TXB2 excretion rates measured in the different phases of the study, including baseline, washout, and randomized aspirin regimens. During washout, patients took enteric-coated aspirin 100 mg once daily. Horizontal lines indicate medians. Each symbol indicates 1 patient across different treatments. #P < .05 versus baseline and washout. WO indicates washout; od, once daily; bid, twice daily; and EC, enteric-coated.
To compare the sensitivity to aspirin inhibition of different platelet function assays versus serum TXB2, we measured the traditional arachidonic acid–induced LTA and the functional response recorded by the VerifyNow Aspirin whole-blood assay in a subgroup of 15 randomly selected ET patients. We could not detect any statistically significant difference in the maximal aggregation between different aspirin regimens as assessed by LTA (supplemental Figures 2-3). Conversely, there were statistically significant differences between the functional response to enteric-coated aspirin 100 mg twice daily or 200 mg once daily and other aspirin regimens, as recorded by the VerifyNow Aspirin assay (Figure 5). On a simple correlation analysis including all measurements from the LTA and VerifyNow Aspirin assays, serum TXB2 was significantly correlated with the VerifyNow Aspirin measurements (ρ = 0.62; P < .001, n = 79; Figure 6), but not with LTA. However, the relationship between average serum TXB2 and VerifyNow Aspirin measurements obtained after different aspirin regimens was best described by a nonlinear equation, as shown in Figure 6.
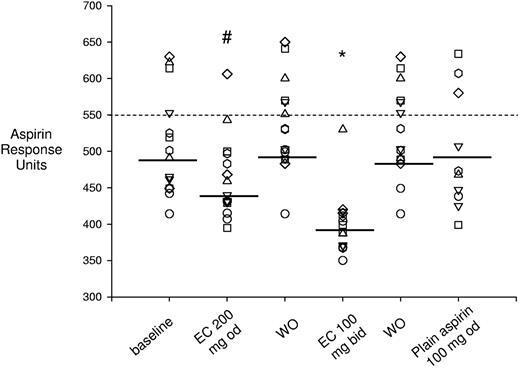
Effect of different aspirin regimens on the VerifyNow Aspirin assay. Shown are individual data on platelet function measured by the VerifyNow Aspirin assay and expressed in arbitrary response units measured in the different phases of the study, including baseline, washout, and randomized aspirin regimens. During washout, patients took enteric-coated aspirin 100 mg once daily. Horizontal lines indicate medians. Each symbol indicates 1 patient across different treatments. *P < .001 versus baseline, washout, and plain aspirin; #P < .05 versus baseline, washout, plain aspirin, and 100 mg enteric-coated aspirin twice daily. WO indicates washout; od, once daily; bid, twice daily; and EC, enteric-coated.
Effect of different aspirin regimens on the VerifyNow Aspirin assay. Shown are individual data on platelet function measured by the VerifyNow Aspirin assay and expressed in arbitrary response units measured in the different phases of the study, including baseline, washout, and randomized aspirin regimens. During washout, patients took enteric-coated aspirin 100 mg once daily. Horizontal lines indicate medians. Each symbol indicates 1 patient across different treatments. *P < .001 versus baseline, washout, and plain aspirin; #P < .05 versus baseline, washout, plain aspirin, and 100 mg enteric-coated aspirin twice daily. WO indicates washout; od, once daily; bid, twice daily; and EC, enteric-coated.
Relationship between serum TXB2 and VerifyNow Aspirin measurements during different aspirin regimens. The figure depicts the relationship between serum TXB2 and VerifyNow Aspirin measurements performed in 15 patients during different aspirin regimens. The dotted line depicts the best fitting of the experimental data. The logarithmic relation between the 2 variables is as follows: y = 40ln(x) + 378 (R2 = 0.43, n = 75). Each point in the graph represents the median value for each aspirin regimen, the horizontal and vertical bars indicate the corresponding IQR (25-75th percentile). EC indicates enteric-coated aspirin; od, once daily; and bid, twice daily.
Relationship between serum TXB2 and VerifyNow Aspirin measurements during different aspirin regimens. The figure depicts the relationship between serum TXB2 and VerifyNow Aspirin measurements performed in 15 patients during different aspirin regimens. The dotted line depicts the best fitting of the experimental data. The logarithmic relation between the 2 variables is as follows: y = 40ln(x) + 378 (R2 = 0.43, n = 75). Each point in the graph represents the median value for each aspirin regimen, the horizontal and vertical bars indicate the corresponding IQR (25-75th percentile). EC indicates enteric-coated aspirin; od, once daily; and bid, twice daily.
Discussion
Low-dose aspirin taken once daily is an effective antithrombotic regimen in many clinical settings characterized by high cardiovascular risk39 because it causes permanent inactivation of platelet COX-1 through selective acetylation of a critically located serine residue at the apex of the COX-1 channel.40 Acetylation of the platelet enzyme, inhibition of platelet TXA2 production, and reduced risk of major vascular events by aspirin can be demonstrated with once-daily dosing and display saturability at low doses.39,41,42
Whereas acetylation of peripheral platelet COX-1, largely occurring in the presystemic circulation,43 is a major determinant of the extent of TXA2 suppression shortly after oral aspirin dosing,20 persistency of complete TXA2 suppression throughout the 24-hour dosing interval is critically dependent on acetylation of megakaryocyte COX-1 (and COX-2).39,41 In the absence of COX-1 acetylation in megakaryocytes, 10%-12% recovery of platelet COX-1 activity would be expected 24 hours after aspirin dosing, with TXA2 levels sufficient to sustain platelet aggregation.20
We reported previously that approximately 80% of low-dose aspirin–treated ET patients display inadequate suppression of platelet TXA2 production.16 The present study was designed to test the hypothesis that altered megakaryopoiesis resulting in accelerated renewal of the drug target might account for the incomplete response to the conventional regimen of once-daily low-dose aspirin administration. We selected ET patients with aspirin-insensitive platelet TXA2 biosynthesis and compared the effects of different aspirin doses, dosing intervals, and drug formulations. We selected a serum TXB2 value of 4.0 ng/mL, which corresponds to the upper limit of levels measured in 48 healthy subjects treated with the same aspirin regimen for at least 2 weeks.21 A serum TXB2 > 3.1 ng/mL was recently characterized as a cutoff value predicting major cardiovascular events in 700 aspirin-treated high-risk patients presenting for cardiac catheterization.44
Whereas doubling the dose of aspirin (from 100 to 200 mg) given once daily would be expected to increase systemic drug levels and the extent of COX-isozyme acetylation in BM megakaryocytes, this strategy would not be expected to influence the rate of de novo synthesis of the enzyme(s) over the 24-hour dosing interval, given the short half-life of aspirin (approximately 20 minutes).39 In contrast, shortening the dosing interval to 12 hours would be expected to produce more sustained, possibly cumulative, acetylation of the megakaryocyte COX-isozymes and to address the time-dependent renewal of the drug target as a function of de novo protein synthesis in the rapidly proliferating ET megakaryocytes.3 Further, a plain aspirin formulation of the same dose as the commonly used enteric-coated 100-mg formulation would address the potentially reduced systemic bioavailability of the latter.45
The main finding of the present study is that twice-daily dosing of the conventional enteric-coated 100-mg aspirin formulation suppressed aspirin-insensitive platelet TXA2 biosynthesis by approximately 90%, down to levels closer to serum TXB2 measured in low-dose aspirin–treated healthy volunteers.20,21 Interestingly, the only predictor of residual platelet COX-1 activity in 41 ET patients treated with aspirin 100 mg once daily was the number of immature platelets (Figure 2). Therefore, patients with the highest platelet turnover had the worst response to aspirin independently of the absolute platelet number. In our population, both the lack of cytoreduction by HU and the JAK-2 V617F mutation were associated with a lower response to aspirin, as reflected by significantly higher levels of serum TXB2 compared with cytoreduced or JAK-2 V617F–negative patients, respectively. Interestingly, both lack of HU treatment and the JAK-2 V617F mutation were associated with a significantly higher absolute number or percentage of immature platelets, respectively (supplemental Table 1), in part confirming recent data.37 However, once these variables were included in a multivariate analysis, only the immature platelet count remained as an independent predictor of poor platelet response, suggesting that the variability of drug response associated with a concomitant treatment or genotype is likely indirect and mediated by changes in the immature platelet compartment. Interestingly, immature platelets have also been shown to be associated with a higher cardiovascular risk46 and poor aspirin response47 in other clinical settings.
It is worth noting that both the level of serum TXB2 and platelet responsiveness to arachidonic acid in ET patients 24 hours after enteric-coated aspirin 100 mg was comparable to the levels observed in healthy subjects between 44 and 66 hours after aspirin withdrawal,21 at a time when approximately 25%-30% of the acetylated platelet COX-1 pool has been replaced by physiologic platelet turnover and functional responsiveness to arachidonic acid has been almost completely restored.21 This finding is consistent with the hypothesis that the abnormal biochemical and functional platelet phenotype that we have characterized in aspirin-treated ET patients most likely reflects accelerated renewal of the peripheral drug target. Another mechanism potentially underlying aspirin-insensitive TXA2 biosynthesis might involve enhanced formation of lipid hydroperoxides, limiting COX-1 acetylation by aspirin24 in both megakaryocytes and platelets. However, the finding of virtually normal levels of serum TXB2 in most ET patients treated with low-dose aspirin every 12 hours regardless of the excretion rate of a product of lipid peroxidation (ie, 8-iso-PGF2α) argues against this mechanism being operative in the setting of ET.
Profound suppression of serum TXB2 in patients given enteric-coated aspirin 100 mg twice daily was associated with normalization of platelet responsiveness in all ET patients when using the conventional platelet response threshold of 550 aspirin reaction units of the VerifyNow Aspirin assay (Figure 5).21,48 Conversely, doubling the once-daily dose of aspirin only partially reduced platelet TXA2 biosynthetic capacity (Figure 3) and did not significantly affect in vivo platelet activation (Figure 4), but was able to restore the functional responsiveness to aspirin in all but 2 patients (Figure 5). This apparent discrepancy highlights the limitations of using a largely arbitrary threshold of functional responsiveness to aspirin as the basis for dichotomizing patients as being “resistant” or a “responder” to the antiplatelet effect of the drug independently of the population under study.21,49
Switching from an enteric-coated to a plain aspirin formulation of the same strength given once daily did not affect the biochemical or functional platelet response in ET patients. This finding makes it unlikely that reduced systemic bioavailability of the enteric-coated formulation45 is responsible for aspirin-insensitive platelet TXA2 biosynthesis in ET. Finally, the lack of correlation between the GCI and aspirin response rules out the existence of a possible feedback among peripheral platelet destruction, BM platelet regeneration, and drug response.
Low-dose aspirin is currently recommended for the primary or secondary prevention of atherothrombosis in ET2,4,5,7 despite the lack of direct, randomized evidence for its efficacy and safety in this setting. The results of the present study argue against the adequacy of a conventional aspirin regimen for a substantial number of ET patients and suggest the need for a properly sized, randomized trial testing the efficacy and safety of a twice-daily regimen of antiplatelet prophylaxis. Although twice-daily dosing may reduce compliance compared with a once-daily regimen, such an approach has been used successfully for stroke prevention in patients with cerebrovascular disease.50
We conclude that the abnormal megakaryopoiesis that characterizes ET is responsible for shorter-lasting antiplatelet effects of low-dose aspirin through faster renewal of platelet COX-1, as modeled in Figure 7. This abnormal biochemical and functional phenotype can be reverted to a normal pattern of platelet response by modulating the aspirin dosing interval but not the dose.
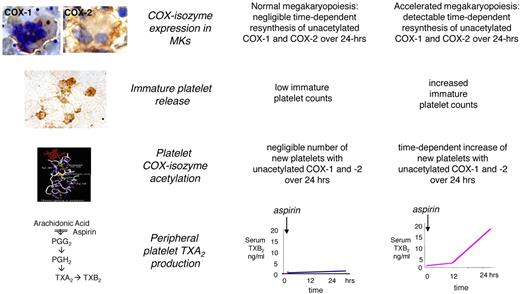
Model of altered aspirin pharmacodynamics in ET. Under conditions of normal megakaryopoiesis, low-dose aspirin acetylates COX isozymes in both circulating platelets and BM megakaryocytes, but negligible amounts of unacetylated enzymes are resynthesized within the 24-hour dosing interval. This pharmacodynamic pattern is associated with virtually complete suppression of platelet TXA2 production in the peripheral blood throughout the dosing interval. Under conditions of abnormal megakaryopoiesis, an accelerated rate of COX-isozyme resynthesis is biologically plausible in BM megakaryocytes, accompanied by faster release of immature platelets with unacetylated enzyme(s) during the aspirin dosing interval, and in particular between 12 and 24 hours after dosing. This pharmacodynamic pattern is associated with incomplete suppression of platelet TXA2 production in the peripheral blood and time-dependent recovery of TXA2-dependent platelet function during the 24-hour dosing interval. Immunohistochemistry panels depict megakaryocytes from an ET patient stained for COX-1 and from a normal subject stained for COX-2 and peripheral washed platelets from an ET patient stained for COX-2 (from Dragani et al16 and Rocca et al33 and unpublished data). Immunohistochemistry samples in the inserts were analyzed with an Axioskop light microscope (Zeiss) at a numeric aperture of 1.3 in oil. Original magnification ×1000. Images were photographed with a CoolPix 950 digitial camera (Nikon).
Model of altered aspirin pharmacodynamics in ET. Under conditions of normal megakaryopoiesis, low-dose aspirin acetylates COX isozymes in both circulating platelets and BM megakaryocytes, but negligible amounts of unacetylated enzymes are resynthesized within the 24-hour dosing interval. This pharmacodynamic pattern is associated with virtually complete suppression of platelet TXA2 production in the peripheral blood throughout the dosing interval. Under conditions of abnormal megakaryopoiesis, an accelerated rate of COX-isozyme resynthesis is biologically plausible in BM megakaryocytes, accompanied by faster release of immature platelets with unacetylated enzyme(s) during the aspirin dosing interval, and in particular between 12 and 24 hours after dosing. This pharmacodynamic pattern is associated with incomplete suppression of platelet TXA2 production in the peripheral blood and time-dependent recovery of TXA2-dependent platelet function during the 24-hour dosing interval. Immunohistochemistry panels depict megakaryocytes from an ET patient stained for COX-1 and from a normal subject stained for COX-2 and peripheral washed platelets from an ET patient stained for COX-2 (from Dragani et al16 and Rocca et al33 and unpublished data). Immunohistochemistry samples in the inserts were analyzed with an Axioskop light microscope (Zeiss) at a numeric aperture of 1.3 in oil. Original magnification ×1000. Images were photographed with a CoolPix 950 digitial camera (Nikon).
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms Daniela Basilico for expert editorial assistance and Prof Stefania Basili (Sapienza University of Rome) and Prof Raimondo De Cristofaro (Catholic University of Rome) for valuable statistical advice.
This study was supported by a grant from the European Commission FP6 Program (EICOSANOX, LSHMCT-2004-005033).
Authorship
Contribution: S.P. performed the clinical research (patient recruitment into clinical trials), pre-analytical work, and measurements of platelet response by Verify Now; G.P. performed all measurements of IL-6, serum TXB2, and CRP and wrote the manuscript; A.D. coordinated and performed the clinical research (patient recruitment into clinical trials) and collected patient clinical and hematologic data; A.H. contributed vital noncommercial reagents and analytical tools; F.Z. analyzed the data and wrote the manuscript; F.P. performed PGE2 and glycocalicin measurements; D.P. performed the urinary metabolite measurements; E.R. performed the urinary extractions; G.R. performed the optical aggregation studies; and B.R. and C.P. designed the study, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: B.R. received speaker fees from Shire and Astra Zeneca. C.P. received consultant and speaker fees from AstraZeneca, Bayer, Eli Lilly, Merck, NicOx, Novartis, Sanofi-Aventis, and Servier, and received grant support for investigator-initiated research from the European Commission, FP6 and FP7 Programmes, Bayer, and Servier. The remaining authors declare no competing financial interests.
Correspondence: Bianca Rocca, MD, PhD, Department of Pharmacology, Catholic University School of Medicine, Largo F. Vito 1, 00168 Rome, Italy; e-mail: b.rocca@tiscali.it.
References
Author notes
S.P., G.P., and A.D. contributed equally to this work.