Conventional assays evaluating antitumor activity of immune effector cells have limitations that preclude their high-throughput application. We adapted the recently developed Compartment-Specific Bioluminescence Imaging (CS-BLI) technique to perform high-throughput quantification of innate antitumor activity and to show how pharmacologic agents (eg, lenalidomide, pomalidomide, bortezomib, and dexamethasone) and autologous BM stromal cells modulate that activity. CS-BLI–based screening allowed us to identify agents that enhance or inhibit innate antitumor cytotoxicity. Specifically, we identified compounds that stimulate immune effector cells against some tumor targets but suppressed their activity against other tumor cells. CS-BLI offers rapid, simplified, and specific evaluation of multiple conditions, including drug treatments and/or cocultures with stromal cells and highlights that immunomodulatory pharmacologic responses can be heterogeneous across different types of tumor cells. This study provides a framework to identify novel immunomodulatory agents and to prioritize compounds for clinical development on the basis of their effect on antitumor immunity.
Introduction
Tumor immunotherapy is an attractive approach for the treatment of various hematologic malignancies. Cell-based immunotherapy strategies in diverse cancers have examined the use of different modalities, including CD40L gene therapy,1 targeting of tumor Ags,2,–4 ex vivo growth and stimulation of immune cells5 for adoptive cell transfer,6 and immunomodulation with cytokines,7,–9 individually or in combination. Recent literature indicates that conventional cytotoxic agents may exert at least part of their antitumor effects through modulation of cellular antitumor immunity.10,–12 Furthermore, an intact immune system is required for the induction of cellular senescence and tumor regression in a T-cell acute lymphoblastic leukemia mouse model of MYC oncogene addiction.13 In recent years, pharmacologic agents with immunomodulating properties have become key therapeutics for the management of certain cancers, including multiple myeloma (MM).14,–16 All these developments underscore the clinical relevance and therapeutic potential of cell-based antitumor immunity and its pharmacologically based immunostimulation.17 However, there is room for improvement, and a barrier to further progress of cell-based immunotherapies is the lack of high-throughput platforms to evaluate strategies to enhance cellular antitumor immunity.
Unlike Ab-based screens, conventional assays of antitumor activity of immune effector cells have limited scalability for high-throughput applications. This is because of limitations of these assays, such as the use of radioactivity (eg, Cr release), the need for complex normalization calculations (eg, lactate dehydrogenase release assay, Cr release assay), or the measurement of markers that indirectly reflect the killing of tumor cells by immune effector cells (eg, IFN-γ levels). In addition, conventional cell viability assays (eg, MTT, Alamar Blue, CellTiterGlo) are not suitable for quantification of immune effector cell antitumor activity, because the viability signal from immune cells interferes with the specific and selective readout of tumor cell viability. Other assays that distinguish tumor from nontumor cells measure cell proliferation rather than viability (eg, 3H-thymidine incorporation) or have limited scalability for high-throughput applications (eg, flow cytometry). Given the heterogeneity of cancers, high-throughput platforms are vital for screening the many drug libraries and cell types necessary to comprehensively interrogate the cytotoxicity of immune effector cells and to identify immunomodulatory agents.
Recently, we developed the Compartment-Specific Bioluminescence Imaging (CS-BLI) technique to specifically assess the cytotoxicity of anticancer agents in the context of tumor-stromal interactions.18 Here, we show that CS-BLI can be used to quantify antitumor cytotoxicity of immune effector cells by selectively measuring tumor cell viability and can detect both immunostimulating and immunosuppressive agents. We further evaluated how this activity can be influenced by the presence of other cell types, drugs, or combinations thereof. Specifically, we observed that coculture with autologous BM stromal cells attenuates innate antitumor activity. We also confirmed the immunostimulatory effects of lenalidomide (Len) and pomalidomide (Pom), as well as the effects of dexamethasone (Dex) and bortezomib (Bort) on IL-2–stimulated PBMCs. The clinical success of thalidomide, and its immunomodulatory drug analogs (Len, Pom), underscores the importance of identifying other agents that can enhance the antitumor immune response.19 We therefore screened a library of compounds in an open-ended manner to identify novel agents with immunostimulatory properties. This high-throughput scalable strategy provides the framework to identify novel immune-based anticancer therapies and to determine whether their activities modulated in the presence of stroma or other nonmalignant accessory cells.
Methods
Cell lines and reagents
The luciferase-expressing human MM cell lines MM.1S-GFP/luc, MM.1S-mCherry/luc, RPMI8226-mCherry/luc, KMS34-mCherry/luc, and Dox40-mCherry/luc; the lymphoma cell lines HT-mCherry/luc, Oci-Ly1-mCherry/luc, and Farage-mCherry/luc; and the leukemia cell line KU812F-mCherry/luc were grown in RPMI 1640 medium (BioWhittaker) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% FBS (Gibco/BRL). Cocultures of tumor cells with PBMCs were grown in RPMI 1640 medium with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and IL-2 (10 ng/mL; R&D Systems). PBMCs were isolated from different donors for each figure and panel, unless otherwise stated. Samples from healthy donors or from patients were obtained under protocol approved by the Institutional Review Board of the Dana-Farber Cancer Institute. Len and Pom were obtained from Celgene, Bort was obtained from Millennium Pharmaceuticals, and Dex was obtained from Sigma. IL-2 was obtained from R&D Systems. Luciferin was obtained from Xenogen Corp. The NKG2D blocking Ab was obtained from R&D Systems and used at a final concentration of 1 μg/mL.
Correlation of bioluminescence signal with number of viable cells in vitro
MM.1S-GFP/luc cells were plated in optical 384-well plates (Corning) at 312-10 000 cells/well in triplicate at a volume of 50 μL/well both in the presence and absence of PBMCs (10 000/well). Luciferin (2.5 mg/mL stock; Xenogen Corp) was added and incubated for 30 minutes at 37°C; the bioluminescence signal was immediately measured with a Luminoskan (Labsystems) or Envision (Perkin Elmer) luminometer.
Cytotoxicity evaluation with CS-BLI and Cr release
PBMCs from different healthy donor(s), as specifically indicated in each experiment, were isolated by Ficoll-Paque (GE Healthcare) gradient separation and were stimulated for 24 hours with IL-2 (10 ng/mL). Cultures were treated with Len, Pom, Bort, or Dex alone or in combination. For pretreatment experiments, PBMCs were stimulated with IL-2 (10 ng/mL) for 24 hours in the presence of Len, Pom, Bort, and Dex. MM cells were similarly grown in the presence or absence of these drugs for 24 hours before cytotoxicity assays. PBMCs and/or tumor cells were washed with PBS and cultured with luc+ MM.1S target cells at various effector-to-target ratios, as indicated. Results were analyzed by 2-way ANOVA or unpaired 2-tailed t tests. For Cr release experiments, MM.1S-GFP/luc target cells were assayed with a standard 51Cr protocol.20 Briefly, MM.1S-GFP/luc cells were labeled with 100 μCi (3.7 × 106 Bq) Na251CrO4 for 1 hour at 37°C. Cells were then washed and resuspended in RPMI media with 10% serum and then plated (5000 cells/well) with isolated PBMCs at various effector-to-target ratios in quadruplicate. Cultures were incubated for 4 hours at 37°C, at which point aliquots of supernatant fluid were analyzed for 51Cr release. Maximum and spontaneous release was determined by adding 0.1% Triton X-100 and media, respectively, and the percentage of killing was determined by subtracting the percentage of lysis from 100%. Specific lysis was calculated as follows: Specific lysis (%) = (experimental counts − spontaneous counts)/(maximum counts − spontaneous counts).
Depletion of CD4+, CD8+, and CD56+ cells
Healthy donor PBMCs were depleted of CD4+, CD8+, or CD56+ cells by 2 rounds of depletion with the use of Miltenyi microbeads (1 × 107 cells/mL per 100 μL of beads) and LD columns (Miltenyi Biotec). After depletion, cells were stained with anti-CD4–FITC (BD Biosciences), anti-CD8–FITC (BD Biosciences), or anti-CD56–PE (Immunotech) Abs to determine purity; samples were used when purity > 95%.
High-throughput screening
MM.1S-GFP/luc cells (5000/well) were plated in 384-well plates in the presence or absence of IL-2–stimulated PBMCs at 1:40 target-to-effector ratios. The drug library for our open-ended screening of immunostimulatory agents was obtained from the Developmental Therapeutics Program of the National Cancer Institute (NCI). Compounds from this library were then added to assay plates with the use of the pin transfer system (1μM final concentration). Tumor cell viability was evaluated in the presence and absence of PBMCs and/or drugs. Hits were identified as drugs enhancing immune cytotoxicity (activity = tumor cell viability after culture in the presence of PBMCs and presence of drug divided by tumor cell viability in the presence of PBMCs but absence of drug). As a counter screen, drugs were evaluated for their direct antitumor activity (activity = tumor cell viability after culture in the presence of drug treatment but absence of PBMCs divided by tumor cell viability in the absence of both drug and PBMCs).
Results
Correlation of in vitro bioluminescence signal with number of viable tumor cells
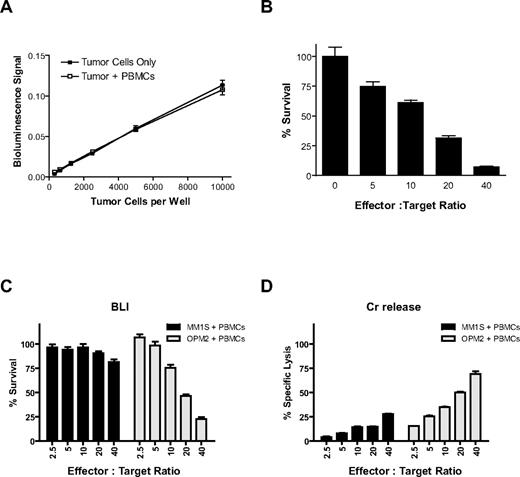
Luciferase (Luc)–expressing MM.1S (Figure 1) and KU812F (data not shown) cells were plated (312-10 000/well) in the presence and absence of healthy donor PBMCs (10 000/well). The counts of viable cells before their plating were established by Trypan blue exclusion assay. After the addition of PBMCs and luciferin substrate, bioluminescence signal was immediately measured. We observed a statistically significant linear correlation between bioluminescent signal and viable tumor cell number both in the presence (r = 0.9996, P < .01) and absence (r = 0.9994, P = .01) of luciferase-negative PBMCs in MM.1S (Figure 1A) and KU812F (data not shown) cells.
CS-BLI for quantitative analysis of antitumor immune effector cell function. Luciferase-expressing MM.1S tumor cells (312-10 000/well) were plated with and without PBMCs (10 000/well). Luciferin was added and incubated for 30 minutes at 37°C, and bioluminescence was read on a Luminoscan luminometer. Signal for luc+ MM.1S cells remained linear across a range of cell numbers and was equal both in the presence or absence of PBMCs (A). PBMCs were isolated from a healthy donor, stimulated for 24 hours with 10 ng/mL IL-2, and cocultured with 5000 luc+ MM.1S target cells/well at increasing effector-to-target ratios. Tumor cell viability was assessed by CS-BLI after 4 hours of coculture. Decreased MM.1S viability was observed with increasing effector PBMC-to-tumor cell ratios (B). In addition, CS-BLI was compared with the traditional Cr release. Identical IL-2–simulated PBMCs and luc+ MM1S and OPM2 target cells were cocultured and evaluated with the CS-BLI method (C) and the Cr release (D) system (n = 4 for each condition). The 2 techniques led to consistent results, in terms of the effect of PBMCs on tumor cells. PBMCs for panels C and D were from the same donor but different from PBMCs used for panel B.
CS-BLI for quantitative analysis of antitumor immune effector cell function. Luciferase-expressing MM.1S tumor cells (312-10 000/well) were plated with and without PBMCs (10 000/well). Luciferin was added and incubated for 30 minutes at 37°C, and bioluminescence was read on a Luminoscan luminometer. Signal for luc+ MM.1S cells remained linear across a range of cell numbers and was equal both in the presence or absence of PBMCs (A). PBMCs were isolated from a healthy donor, stimulated for 24 hours with 10 ng/mL IL-2, and cocultured with 5000 luc+ MM.1S target cells/well at increasing effector-to-target ratios. Tumor cell viability was assessed by CS-BLI after 4 hours of coculture. Decreased MM.1S viability was observed with increasing effector PBMC-to-tumor cell ratios (B). In addition, CS-BLI was compared with the traditional Cr release. Identical IL-2–simulated PBMCs and luc+ MM1S and OPM2 target cells were cocultured and evaluated with the CS-BLI method (C) and the Cr release (D) system (n = 4 for each condition). The 2 techniques led to consistent results, in terms of the effect of PBMCs on tumor cells. PBMCs for panels C and D were from the same donor but different from PBMCs used for panel B.
CS-BLI–based quantification of cytotoxic killing of myeloma cells
Luc+ MM.1S target cells were cultured for 4 hours with IL-2 prestimulated PBMCs at increasing PBMC-to-target cell ratios. After 4 hours of culture, luciferin substrate was added, and tumor cell viability was measured by bioluminescence. The percentage of viable MM.1S cells was compared in cultures in the presence versus absence of PBMCs (Figure 1B). Decreased tumor viability of MM.1S (Figure 1B) or KU812F (data not shown) cells was observed with increasing PBMC-to-tumor ratios. Luc+ MM.1S or OPM2 target cells were cocultured for 4 hours with IL-2 prestimulated PBMCs from another donor at increasing PBMC-to-target cell ratios. After 4 hours of culture, luciferin substrate was added, and tumor cell viability was measured by bioluminescence (Figure 1C) and compared with the same cells assayed by Cr release (Figure 1D). Results obtained with the 2 techniques were comparable for both MM.1S and OPM2 cells.
Depletion of CD56+ cells reduces anti-MM activity of IL-2–stimulated PBMCs
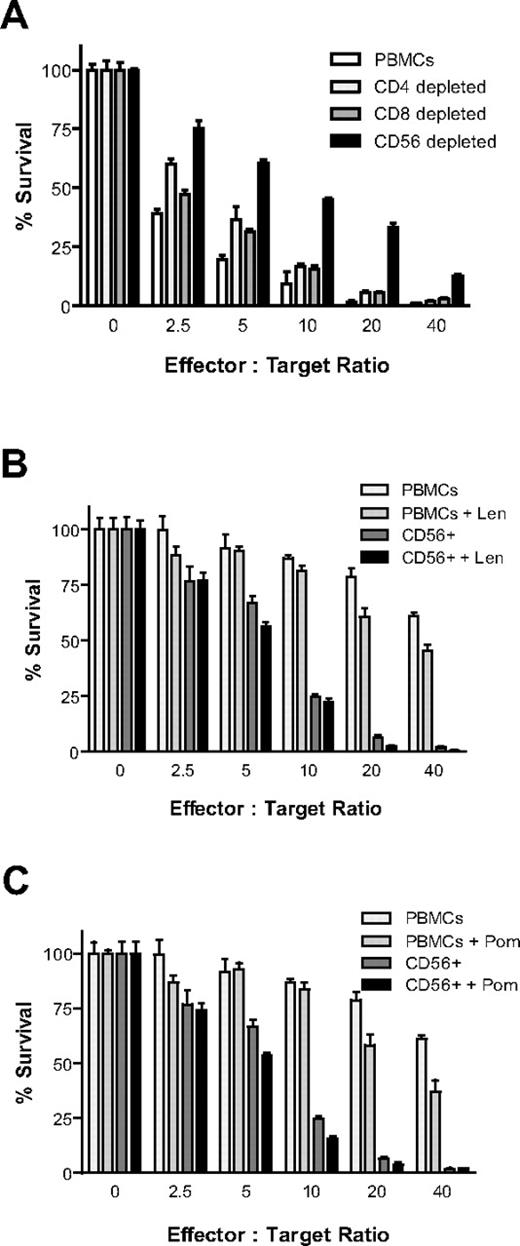
Healthy donor PBMCs were isolated, Ficoll gradient-separated, and depleted of CD4+, CD8+, or CD56+ cells by 2 rounds of depletion with the use of Miltenyi microbeads with LD columns. Cells were then cultured overnight in the presence of IL-2 (10 ng/mL). Luc+ MM.1S target cells (5000/well) were then plated at increasing effector-to-target ratios in the presence of IL-2 (10 ng/mL). Luc+ MM.1S cell viability was measured with CS-BLI at 4 hours. MM cytotoxicity was decreased in CD4-depleted cells (P < .0001), CD8-depleted (P < .0001) cells, and CD56-depleted fractions (P < .0001), but the decrease was more pronounced after CD56-depletion (Figure 2A). In addition, selection of CD56+ cells versus unselected PBMCs was compared in the presence and absence of Len and Pom. CD56+ cells had significantly higher antitumor activity than unselected PBMCs (P < .0001); importantly, both Len (Figure 2B; P = .0118) and Pom (Figure 2C; P = .002) moderately enhanced CD56+ MM cell killing, as determined by 2-way ANOVA analysis.
CS-BLI–based evaluation of antitumor activity of immune effector subsets. Healthy donor PBMCs were depleted of CD4+, CD8+, and CD56+ cells with the use of Miltenyi microbeads with LD columns (2 rounds of depletion). Cells were then cultured overnight in the presence of IL-2 (10 ng/mL). The next day luc+ MM.1S cells (5000/well) were plated at increasing effector-to-target ratios in the presence of IL-2 (10 ng/mL). luc+ MM.1S cell viability was measured 4 hours after the initiation of coculture. Cell viability was increased in fractions depleted of CD4+ (P < .0001), CD8+ (P < .0001), and CD56+ (P < .0001) cells; CD56 depletion had a more pronounced effect than CD4 or CD8 depletion (A). In addition, selection of CD56+ cells was compared with unselected PBMCs in the presence and absence of Len and Pom. Len (B; P = .0118) and Pom (C; P = .002) enhanced the antimyeloma cytotoxicity by CD56-enriched fractions (n = 4 for each condition). All experiments in this figure were conducted with PBMCs derived from the same donor, distinct from PBMCs for other figures.
CS-BLI–based evaluation of antitumor activity of immune effector subsets. Healthy donor PBMCs were depleted of CD4+, CD8+, and CD56+ cells with the use of Miltenyi microbeads with LD columns (2 rounds of depletion). Cells were then cultured overnight in the presence of IL-2 (10 ng/mL). The next day luc+ MM.1S cells (5000/well) were plated at increasing effector-to-target ratios in the presence of IL-2 (10 ng/mL). luc+ MM.1S cell viability was measured 4 hours after the initiation of coculture. Cell viability was increased in fractions depleted of CD4+ (P < .0001), CD8+ (P < .0001), and CD56+ (P < .0001) cells; CD56 depletion had a more pronounced effect than CD4 or CD8 depletion (A). In addition, selection of CD56+ cells was compared with unselected PBMCs in the presence and absence of Len and Pom. Len (B; P = .0118) and Pom (C; P = .002) enhanced the antimyeloma cytotoxicity by CD56-enriched fractions (n = 4 for each condition). All experiments in this figure were conducted with PBMCs derived from the same donor, distinct from PBMCs for other figures.
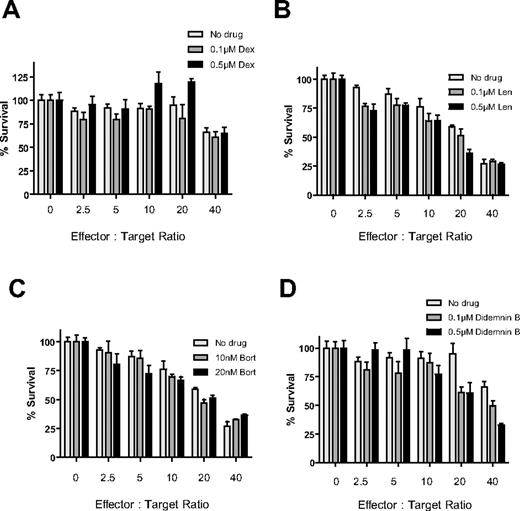
Effects of pretreatment with Len, Pom, Bort, or Dex on MM cytotoxicity of immune effector cells
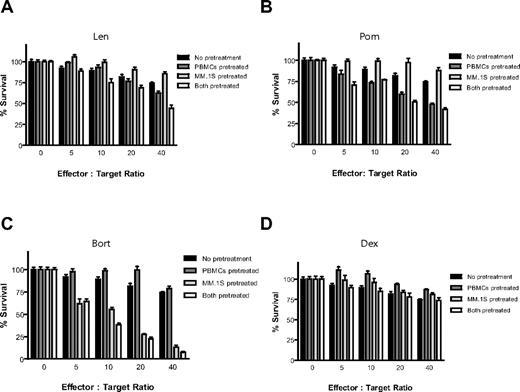
PBMCs isolated from a different healthy donor were stimulated for 24 hours in the presence of IL-2 (10 ng/mL), with or without Len (1μM), Pom (1μM), Bort (10nM), or Dex (50nM) for the final 24 hours (Figure 3). PBMCs were then washed with PBS and cultured with luc+ MM.1S target cells at increasing effector-to-tumor ratios. The anti-MM cytotoxicity of IL-2–stimulated PBMCs was enhanced by pretreatment of PBMCs with Len (Figure 3A) or Pom (Figure 3B; P < .0001 for both compounds, 2-way ANOVA), as well as by independent pretreatment of both PBMCs and MM cells with either compound (P < .0001 for both compounds). In contrast, pretreatment of only tumor cells without pretreatment of PBMCs with Len or Pom had no effect (P = NS). Bort pretreatment of only MM cells (P < .0001) or of both PBMCs and MM cells (Figure 3C; P < .0001) similarly resulted in significant increase in cytotoxicity of PBMCs against MM cells, whereas pretreatment of only PBMCs with Bort significantly decreased MM cytotoxicity (Figure 3C; unpaired 2-tailed t test; P = .229). Dex pretreatment of PBMCs attenuated their anti-MM activity (Figure 3D; P = .018), whereas Dex pretreatment of only MM cells or of both MM cells and PBMCs did not significantly affect the anti-MM effect of PBMCs (Figure 3D; P = NS).
Effect of drug pretreatment of effector or myeloma cells on antimyeloma immunity. PBMCs were isolated from a healthy donor and stimulated with IL-2 (10 ng/mL) for 24 hours, in the presence or absence of 1μM Len (A), 1μM Pom (B), 10nM Bort (C), or 50nM Dex (D). Luc+ MM.1S cells were similarly pretreated with Len, Pom, Bort, or Dex. PBMCs and MM cells were washed and counted, and viable luc+ MM.1S target cells were counted and cultured at increasing ratios of viable PBMCs for 4 hours in the absence of drug. Data were normalized to each respective drug- and PBMC-free culture of MM.1S cells. Len and Pom pretreated PBMCs alone or both PBMCs and MM cells significantly increased cytotoxicity (A-B; 2-way ANOVA; P < .0001 for each comparison). Bort pretreatment of MM cells alone or both PBMCs and MM cells also enhanced myeloma cytotoxicity (C; P < .0001). In contrast, Dex pretreatment of only MMs, or only PBMCs, or both did not augment myeloma cytotoxicity (D; n = 4 for each condition).
Effect of drug pretreatment of effector or myeloma cells on antimyeloma immunity. PBMCs were isolated from a healthy donor and stimulated with IL-2 (10 ng/mL) for 24 hours, in the presence or absence of 1μM Len (A), 1μM Pom (B), 10nM Bort (C), or 50nM Dex (D). Luc+ MM.1S cells were similarly pretreated with Len, Pom, Bort, or Dex. PBMCs and MM cells were washed and counted, and viable luc+ MM.1S target cells were counted and cultured at increasing ratios of viable PBMCs for 4 hours in the absence of drug. Data were normalized to each respective drug- and PBMC-free culture of MM.1S cells. Len and Pom pretreated PBMCs alone or both PBMCs and MM cells significantly increased cytotoxicity (A-B; 2-way ANOVA; P < .0001 for each comparison). Bort pretreatment of MM cells alone or both PBMCs and MM cells also enhanced myeloma cytotoxicity (C; P < .0001). In contrast, Dex pretreatment of only MMs, or only PBMCs, or both did not augment myeloma cytotoxicity (D; n = 4 for each condition).
Effect of combining Len, Bort, and/or Dex on immune effector cell cytotoxicity
To assess the triple combination of Len, Bort, and Dex, luc+ MM.1S cells were cocultured for 4 hours with increasing ratios of IL-2–stimulated PBMCs, in the presence and absence of each drug as well as combinations. Dex alone, or combined with Len, decreased immune function. Of note, combination of Bort and Len abrogated the suppressive effects of Dex on immune effector cell function (supplemental Figure 1, see the Supplemental Materials link at the top of the article).
Effect of stromal cells on the antitumor activity of NK cells in the presence of Len, Pom, Bort, and Dex
We next evaluated the effect of stromal cells on the anti-MM activity of natural killer (NK) cells in the presence of the immunomodulatory drugs Len and Pom, as well as the effects in the presence of Bort and Dex. BM stromal cells were collected from patients with MM in remission and cultured for > 3 weeks at which time PBMCs were collected from the same patients. Matched BM stromal cells and PBMCs were used to avoid any effects because of MHC-mismatched stroma and effector cells. Stroma were plated and allowed to adhere for 24 hours during which time PBMCs were isolated by Ficoll gradient separation and stimulated with IL-2 (10 ng/mL). The next day luc+ MM.1S cells were combined with increasing ratios of stimulated PBMCs in the presence and absence of stromal cells, with or without Len (supplemental Figure 2A), Pom (supplemental Figure 4B), Bort (supplemental Figure 2C), or Dex (supplemental Figure 2D). The presence of stromal cells significantly decreased the antitumor activity over 4 hours. In addition, Len (supplemental Figure 2A) and Pom, depending on the specific target-to-effector ratio (supplemental Figure 2B), slightly increased the antitumor activity in the presence of stromal cells; Bort has modest effect in the presence of stromal cells (supplemental Figure 2C), whereas Dex decreases the antitumor effect in the presence of stromal cells (supplemental Figure 2D).
CS-BLI–based high-throughput screening for identification of immunomodulatory agents
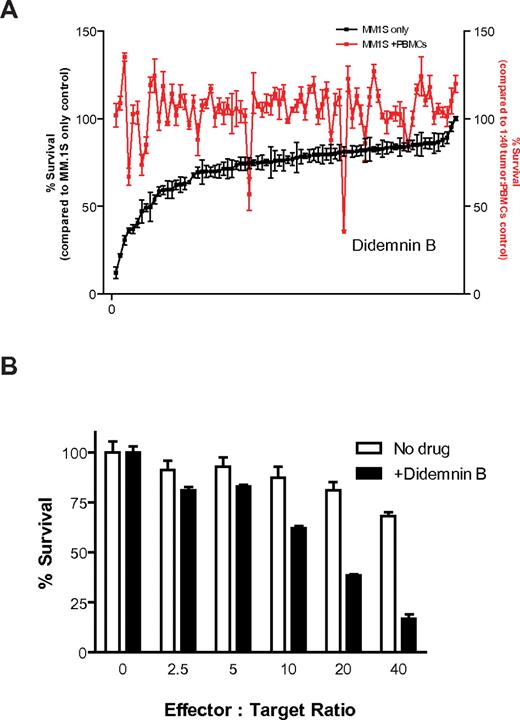
We next used the CS-BLI platform to screen, in an open-ended manner, a library of compounds for agents with immunomodulatory properties against MM cells. Luc+ MM.1S cells were plated in the presence or absence of PBMCs. With the use of pin transfer system (1μM final concentration), an NCI drug library was then added for 4 hours to plates with MM cells cultured either alone or together with PBMCs (stimulated with IL-2 as in previous experiments) and compared with untreated controls. CS-BLI measurements were performed to compare MM.1S cell viability as follows: after treatment with each compound, with IL-2–stimulated PBMCs, with IL-2–stimulated PBMCs in the presence of each compound of the library, and in the absence of either PBMCs or compounds. We evaluated each drug's ability to enhance antitumor cytotoxicity compared with the absence of drug. As a counter screen, we evaluated each drug's direct antitumor activity. One compound of this library, didemnin B, showed direct tumor activity in the absence of PBMCs, but it also enhanced the anti-MM cytotoxicity of PBMCs (Figure 4A). Similar results were obtained when the same compound library was tested against the same MM.1S target cells, but using NK cell line NKL at various effector-to-target ratios (supplemental Figure 3A). Didemnin B was further analyzed for its immunomodulatory activity. Indeed, enhanced tumor killing was observed at increasing PBMC-to-tumor cell ratios in the presence versus absence of drug (Figure 4B). In addition, brief exposure of luc+ MM.1S cells to didemnin B (1μM; 15 minutes) did not affect the bioluminescence signal, indicating that didemnin B was not having a spurious effect on luc enzymatic activity (supplemental Figure 3B).
High-throughput screening to identify enhancers of anti-MM immune cytotoxicity. Luc+ MM.1S cells were plated in the presence or absence of prestimulated PBMCs (IL-2 for 24 hours) in 96-well plates. Compounds from an NCI-derived chemical library were then added with the use of a pin transfer system (1μM final concentration) to plates with continued IL-2 stimulation (10 ng/mL) during coculture. After culture for 4 hours, we evaluated each drug's ability to enhance antitumor cytotoxicity compared with the absence of drug (A; red). As a counter screen, we evaluated each drug's direct antitumor activity (A; black) and ranked in descending order of direct anti-MM activity (n = 4 for each condition). One drug, didemnin B, which showed enhanced activity in the presence versus absence of PBMCs in the high-throughput screen (A), was further evaluated for its immunostimulatory activity. Luc+ MM.1S were cultured for 4 hours at increasing PBMC-to-tumor cell ratios in the presence and absence of 250nM didemnin B, and enhanced immune myeloma cytotoxicity was detected in the presence of drug (B).
High-throughput screening to identify enhancers of anti-MM immune cytotoxicity. Luc+ MM.1S cells were plated in the presence or absence of prestimulated PBMCs (IL-2 for 24 hours) in 96-well plates. Compounds from an NCI-derived chemical library were then added with the use of a pin transfer system (1μM final concentration) to plates with continued IL-2 stimulation (10 ng/mL) during coculture. After culture for 4 hours, we evaluated each drug's ability to enhance antitumor cytotoxicity compared with the absence of drug (A; red). As a counter screen, we evaluated each drug's direct antitumor activity (A; black) and ranked in descending order of direct anti-MM activity (n = 4 for each condition). One drug, didemnin B, which showed enhanced activity in the presence versus absence of PBMCs in the high-throughput screen (A), was further evaluated for its immunostimulatory activity. Luc+ MM.1S were cultured for 4 hours at increasing PBMC-to-tumor cell ratios in the presence and absence of 250nM didemnin B, and enhanced immune myeloma cytotoxicity was detected in the presence of drug (B).
Direct comparison of immunomodulating activity of didemnin B versus established anti-MM drugs
To further compare the immune-stimulatory effects of didemnin B in MM cells, isolated PBMCs from another healthy donor were stimulated with IL-2 (10 ng/mL) for 24 hours. PBMCs were then cocultured with viable luc+ MM.1S target cells at increasing ratios of PBMCs for 4 hours in the presence or absence of Dex, Len, Bort, or didemnin B (Figure 5). We observed, as in previous experiments, the enhanced activity of effector cell killing in the presence of didemnin B. In addition, we observed that this magnitude of enhanced antitumor killing was greater than what was observed with Len and Bort treatment with the use of the same PBMC effector cells and MM.1S tumor targets (Figure 5).
Direct comparison of immunomodulatory effects of established anti-MM drugs compared with didemnin B. PBMCs were isolated from a healthy donor and stimulated with IL-2 (10 ng/mL) for 24 hours. PBMCs were then cocultured with viable luc+ MM.1S target cells at increasing ratios of PBMCs for 4 hours in the presence or absence of Dex (A), Len (B), Bort (C), or didemnin B (D). Tumor cell viability was quantified by CS-BLI. Data were normalized to each respective drug- and PBMC-free culture of MM.1S cells (n = 4 for each condition).
Direct comparison of immunomodulatory effects of established anti-MM drugs compared with didemnin B. PBMCs were isolated from a healthy donor and stimulated with IL-2 (10 ng/mL) for 24 hours. PBMCs were then cocultured with viable luc+ MM.1S target cells at increasing ratios of PBMCs for 4 hours in the presence or absence of Dex (A), Len (B), Bort (C), or didemnin B (D). Tumor cell viability was quantified by CS-BLI. Data were normalized to each respective drug- and PBMC-free culture of MM.1S cells (n = 4 for each condition).
Differential immunomodulatory activity of didemnin B across multiple cell lines
In addition, another donor PBMC sample was used to validate these immunomodulatory effects of didemnin B (0.1μM, 0.5μM, or 1μM) against MM.1S and K562 targets. Didemnin B again enhanced anti-MM activity of IL-2–stimulated PBMCs, but it attenuated the cytotoxicty of these same cells against K562 targets. In both cases, the effect of didemnin B was dose dependent (supplemental Figure 4). We then evaluated the immunomodulatory effect of didemnin B across a panel of MM, leukemia, and lymphoma cell lines. PBMC isolated from another healthy donor were stimulated with IL-2 (10 ng/mL) for 24 hours. PBMCs were then cocultured with viable luc+ tumor target cells at increasing ratios of PBMCs for 4 hours in the presence or absence of didemnin B (Figure 6). We observed that 2 of the MM lines tested (MM.1S and RPMI8226) were more sensitive to PBMC killing in the presence of didemnin B. In contrast, 2 other MM cell lines (KMS34 and Dox40), the lymphoma lines HT, OCILY1, and Farage, and the leukemia line KU812F were less sensitive to PBMC killing in the presence of didemnin B (Figure 6C-H). To elucidate the mechanism of didemnin B–mediated simulation, PBMCs were cocultured with viable luc+ MM.1S or RPMI8226 target cells in the presence or absence of the anti-NKG2D blocking Ab and/or didemnin B. Tumor cell viability was quantified by CS-BLI after 4 hours of culture and was higher in the presence of the anti-NKG2D blocking Ab, which attenuated the stimulatory effect of didemnin B against the target tumor cells (supplemental Figure 5A-B).
Differential immune-modulatory effect of didemnin B against different myeloma, leukemia, and lymphoma cell lines. PBMCs were isolated from a healthy donor and stimulated with IL-2 (10 ng/mL) for 24 hours. PBMCs were then cultured at increasing effector-to-target ratios for 4 hours in the presence or absence of didemnin B with the luc+ myeloma lines MM.1S (A), RPMI8226 (B), KMS34 (C), and Dox40 (D); the luc+ lymphoma lines HT (E) OCILY1 (F) Farage (G); and the leukemia line KU812F (H). Tumor cell viability was quantified by CS-BLI. Data were normalized to each respective drug- and PBMC-free culture of MM.1S cells (n = 4 for each condition).
Differential immune-modulatory effect of didemnin B against different myeloma, leukemia, and lymphoma cell lines. PBMCs were isolated from a healthy donor and stimulated with IL-2 (10 ng/mL) for 24 hours. PBMCs were then cultured at increasing effector-to-target ratios for 4 hours in the presence or absence of didemnin B with the luc+ myeloma lines MM.1S (A), RPMI8226 (B), KMS34 (C), and Dox40 (D); the luc+ lymphoma lines HT (E) OCILY1 (F) Farage (G); and the leukemia line KU812F (H). Tumor cell viability was quantified by CS-BLI. Data were normalized to each respective drug- and PBMC-free culture of MM.1S cells (n = 4 for each condition).
Having observed that the immunomodulatory effects of didemnin B are different, depending on the tumor target, even when using the same donor effector cells, we rescreened the same NCI compound library against a different target cell line. We specifically tested the HT lymphoma cell line, as a representative line against which didemnin B was immunosuppressive. In contrast to results from the MM.1S screen (Figure 4) and consistent with the prior testing of didemnin B against HT cells (Figure 6E), we observed that didemnin B decreased the immune effector cell activity against HT cells (supplemental Figure 6), indicating that the assay can distinguish between the suppressive versus stimulatory effects against different target cells. This rescreening of the NCI compound library identified immunostimulatory compounds that previously had immunosuppressive activity against MM.1S cells, suggesting that the specific interplay of tumor cells with given pharmacologic agents is critical to understanding how to improve immunotherapy response. From a mechanistic standpoint, the surface expression of cell surface ligands was evaluated in MM tumor cells treated with didemnin B. Flow cytometric analysis, however, showed no changes in the patterns of surface expression of NKG2D ligands (anti-MICA and hNKG2D/mFc fusion protein), DNAM-1 ligands (CD112 and CD155), and NKp44 ligands (hNKp44/Fc fusion protein; supplemental Figure 7).
Comparative high-throughput screening to identify enhancers of antitumor immune cytotoxicity
Having observed the differential immunomodulatory effects of didemnin B against multiple cell lines, we screened a larger panel of drugs to identify the overlapping stimulatory and suppressive effects against luc+ MM.1S myeloma (supplemental Figure 8A) and HT lymphoma (supplemental Figure 8B) cells. Compounds (n = 800) from an NCI-derived chemical library were added to cocultures for 4 hours. Only 2.1% of compounds enhanced antitumor killing in both MM.1S and HT, and 7.5% had opposite effects in each tumor type (supplemental Figure 8C). Surprisingly, the number of drugs with discordant immunomodulatory effects against each cell line was higher than the number with concordant immunostimulatory activity against both tumor targets. High-throughput evaluation of many tumor targets is therefore necessary for the development of drugs with broad immunostimulatory properties.
Activity of didemnin B on PBMCs and macrophage anti-MM immunity
To further identify the applicability of the CS-BLI approach to different cells of the immune system, we compared the activity of didemnin B at enhancing PBMC antitumor activity with macrophages. PBMCs were isolated from a healthy donor and stimulated with IL-2 (10 ng/mL) and compared with macrophages established by IFN-γ (50 U/mL) stimulation of the adherent fraction from isolated PBMCs. Luc+ MM.1S and RPMI8226 cells were cocultured at increasing ratios of viable effector cells for 4 hours in the presence and absence of didemnin B. PBMCs showed increased activity in the presence of drug as well as increased activity at higher effector-to-target ratios, whereas macrophages exhibited only minimal cytotoxicity against MM targets and only slightly enhanced in the presence of didemnin B (supplemental Figure 9).
Discussion
Immune effector cells represent an important component of an antitumor immune response, and clinically applicable strategies to enhance their function are critical for development of more effective antitumor immunotherapies. To accomplish this goal, preclinical assays that facilitate the study of immune effector cells will play an important role. In this study, we show that CS-BLI can selectively measure tumor cell viability in the context of tumor cell interaction with immune effectors cells.
CS-BLI offers several advantages in detecting the antitumor activity of immune effector cells and the immunomodulatory effects of various compounds. Specifically, CS-BLI directly and selectively measures tumor cell viability, without interference from other cell compartments. Moreover, CS-BLI does not require radioactivity, and its bioluminescent readout allows for sensitive quantification of cell viability. These features facilitate the scalability of CS-BLI for high-throughput applications, thereby allowing for screening of large drug libraries for their immune-modulating properties.
In the process of anticancer drug development, compounds are evaluated primarily for their ability to perturb postulated molecular targets and have direct cytotoxic effects on tumor cells. However, other studies have shown that various agents can have both direct and indirect effects against targeting the tumor.21 Indeed, the successful clinical development of anticancer therapeutics with potent immunomodulatory properties, such as thalidomide, Len, and Pom,22,23 highlights the relevance of preclinical evaluation of novel agents for their immunomodulating properties. To date, such evaluation of anticancer agent's indirect effects has been hampered by the lack of robust, sensitive, and high-throughput scalable assays. The application of the CS-BLI platform in the context of immune effector cell-tumor assays addresses this void.
Our results provide new insight into the interaction between tumor cells, the immune system, and the tumor microenvironment. Specifically, our results show that nonmalignant cells present in the tumor microenvironment are capable of blunting the antitumor cytotoxicity of the immune effector cells. This finding is not only compatible with our previous work on stroma-induced resistance to small molecule inhibitors,18 but could explain, at least in part, why potent in vitro tumor cell responses to immune effector cells may not translate into clinical successes. CS-BLI, therefore, allows for the early identification of stromal cell influence on antitumor immunity, which may significantly affect clinical applications.
Our primary goal was to identify new immunostimulators with more potent effects compared with available agents. Importantly, however, we were able to also identify immuno-suppressors and, surprisingly, agents with dual activity, depending on the cell target. Indeed, didemnin B, in our studies, exhibited immunostimulatory features against some cell line targets but was immunosuppressive against others. This novel finding can have implications for the treatment of various cancers. Studying a limited number of models may lead to biased interpretation of results. Evaluating the effects of didemnin B only on K562 or HT cells would have led to a different conclusion about the compound's immunomodulatory effects compared with testing only MM.1S and RPMI8226 cells. In addition, we observed that not all cell lines representing the same disease have concordant immunomodulatory responses to a given compound. For example, didemnin B was immunostimulatory toward MM.1S and RPMI8226 cells, but it was immunosuppressive against other cell lines of the same disease. These results are not a rare phenomenon. Indeed, screening of a larger library of compounds against MM.1S and HT cells showed a surprisingly high number of compounds that stimulated immune responses against one, but suppressed against the other cell line. These observations further highlight the need for high-throughput systems to probe the effect of immunomodulatory effects across a broad spectrum of tumor cells, drugs, and conditions.
Large-scale screening allows for the evaluation of not only different types of tumor cells but also other arms of the immune system, such as adaptive immunity, as well as other classes of candidate immunomodulators. The application of CS-BLI is not simply restricted to NK-mediated innate immunity but can be extended to probe the role of other cellular subsets, including CD4, CD8, and regulatory T cells, dendritic cells, and others, as well as identify compounds that can selectively modulate the function of these distinct populations. This platform can be used to screen libraries of peptides, shRNA constructs, and Abs, similarly to our functional studies that used the anti-NKG2D Ab, to probe the activity of these various immune cell subsets.
The evaluation of novel drugs and their prioritization for further clinical development depends on informative preclinical models and screening technologies. We have shown that CS-BLI can be used to evaluate libraries of compounds for their immunomodulatory effects to identify novel therapeutics that stimulate patients' own antitumor immunity. With the use of the high-throughput nature of the CS-BLI technique, it is possible to identify compounds not only on the basis of direct cytotoxic activity in mixed cell cultures but also on the basis of their ability to enhance antitumor immune responses. Importantly, new agents with immunomodulatory properties can be evaluated with CS-BLI not only in oncology but also in other settings in which agents with immunostimulatory (ie, infection) or immunosuppressive (ie, inflammation/autoimmunity and transplant immunology) properties are important. Our results therefore provide the framework for identification and validation of novel pharmacologically and/or biologically active agents on the basis of their ability to modulate the activity of immune effector cells against their targets.
This article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Chambers Medical Foundation (C.S.M. and P.G.R.), the Steven Cobb Foundation (D.W.M. and C.S.M.), the R. J. Corman Fund for Myeloma Research (P.G.R. and C.S.M.), the Stepanian Fund for Myeloma Research (P.G.R. and C.S.M.), the Department of Veterans Affairs (Merit Review Award I01-BX001584 to N.C.M.), and from the National Institutes of Health (grant RO1-50947 to C.S.M. and K.C.A.; grants RO1-124929 and PO1-155258 to NCM; and grant PO-1-78378 to G.D. and K.C.A.). C.S.M. is supported by the “Dunkin Donuts Rising Stars” Program at the Dana-Farber Cancer Institute. Compounds libraries for screening were provided by the National Cancer Institute Developmental Therapeutics Program (http://dtp.cancer.gov/).
National Institutes of Health
Authorship
Contribution: D.W.M. designed and performed research experiments, conducted data analyses, and wrote the paper; J.D., J.M.N., and M.V. performed research experiments; R.L.S., N.C.M., J.L., and P.G.R. generated vital research reagents; G.D. contributed to design of experiments and writing of manuscript; K.C.A. generated vital reagents and contributed to the writing of the manuscript; and C.S.M. designed research experiments and wrote the paper.
Conflict-of-interest disclosure: D.W.M. is an equity holder in Axios Biosciences. P.G.R. received honoraria from Millennium and Celgene. K.C.A. has received research grants and honoraria from Millennium and Celgene. P.G.R. has participated in advisory boards for Celgene, Millennium, and Johnson and Johnson. C.S.M. has received in the past consultant honoraria from Millennium Pharmaceuticals, Novartis Pharmaceuticals, Bristol-Myers Squibb, Merck & Co, Kosan Pharmaceuticals, Pharmion, and Celgene; licensing royalties from PharmaMar; and research funding from Amgen Pharmaceuticals, AVEO Pharma, EMD Serono, Sunesis, Gloucester Pharmaceuticals, and Johnson & Johnson. The remaining authors declare no competing financial interests.
Correspondence: Constantine S. Mitsiades, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Ave, HIM Bldg, Rm 346, Boston, MA 02215; e-mail: constantine_mitsiades@dfci.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal