Abstract
The most common form of neurologic injury in sickle cell anemia (SCA) is silent cerebral infarction (SCI). In the Silent Cerebral Infarct Multi-Center Clinical Trial, we sought to identify risk factors associated with SCI. In this cross-sectional study, we evaluated the clinical history and baseline laboratory values and performed magnetic resonance imaging of the brain in participants with SCA (HbSS or HbSβ° thalassemia) between the ages of 5 and 15 years with no history of overt stroke or seizures. Neuroradiology and neurology committees adjudicated the presence of SCI. SCIs were diagnosed in 30.8% (251 of 814) participants who completed all evaluations and had valid data on all prespecified demographic and clinical covariates. The mean age of the participants was 9.1 years, with 413 males (50.7%). In a multivariable logistic regression analysis, lower baseline hemoglobin concentration (P < .001), higher baseline systolic blood pressure (P = .018), and male sex (P = .030) were statistically significantly associated with an increased risk of an SCI. Hemoglobin concentration and systolic blood pressure are risk factors for SCI in children with SCA and may be therapeutic targets for decreasing the risk of SCI. This study is registered at www.clinicaltrials.gov as #NCT00072761.
Introduction
Silent cerebral infarcts (SCIs) have been recognized by neuroimaging in neurologically normal older adult populations since 19811 and were documented in sickle cell anemia (SCA) soon afterward.2 As with overt stroke, SCIs represent a clinical finding that is common in older adults without SCD, but they appear during early childhood in persons with SCA. SCIs are defined as an MRI signal abnormality visible on 2 views on the T2-weighted images (axial and coronal) that must measure at least 3 mm in one dimension; further, the person deemed to have an SCI must have an absence of focal neurologic deficit compatible with the anatomic location of the brain lesion.3 SCI is the most common form of neurologic injury among children with SCA, occurring in at least 27% before 6 years of life4 and 37% by 14 years of life.5 SCIs in children with SCA are associated with increased risk of future overt strokes and new or progressive SCIs.6,7 In addition, children with SCA and SCI have been found to have poorer cognitive function than children with SCA with normal MRI of the brain8-10 or sibling controls.10,11
Clinical and laboratory risk factors for SCI have been evaluated only sparingly. In the most rigorous study to date, the investigators from the Cooperative Study for Sickle Cell Disease (CSSCD) described risk factors associated with SCI in 42 participants, comparing them with 188 controls with normal MRI.12 In the final multivariable analysis, the authors found that SCI was associated with history of seizure, a relatively low frequency of pain episodes, and an elevated white blood cell (WBC) count. Elevated systolic blood pressure (SBP) measurement was not associated with SCI; however, this analysis of CSSCD data had several limitations, including misclassifications of SCI that were corrected in a subsequent evaluation6 and a relatively small number of participants with SCI, compared with the current study. Neurologic examinations were neither routinely performed by a pediatric neurologist nor adjudicated by a central committee. Thus, a participant classified as having SCI could, indeed, have had an overt stroke. In addition, other risk factors have not been examined. Therefore, despite the observation that elevated SBP measurement is a risk factor for overt stroke in patients with SCA,13,14 the relationship between SBP measurements and SCI has not been established. The major objective of the present study was to assess previously identified risk factors and identify new risk factors for SCI, through analysis of data from the Silent Cerebral Infarct Multi-Center Clinical Trial (SIT Trial) clinical repository. Specifically, we tested the hypothesis that SBP measurement or other previously identified risk factors for SCI in SCA and the general population were associated with an increased risk of SCI in SCA.
Methods
The SIT Trial is a multicenter trial designed to determine the efficacy of blood transfusion therapy for prevention of recurrent SCI in participants with SCA. To be eligible for screening, participants must: have a confirmed diagnosis of HbSS or HbSβ° thalassemia, be between the ages of 5 and 15 years, have no history of focal neurologic deficits associated with an ischemic event or seizure disorder, have received no treatment with hydroxyurea in the previous 3 months, have not had a history of elevated transcranial Doppler scan measurement, and not be on chronic transfusions. A detailed description of the protocol and details of the study design can be found in Casella et al.15
The SIT Trial was approved by the institutional review boards of all participating institutions and is registered at www.clinicaltrials.gov (#NCT00072761) and www.ISRCTN.org (#ISRCTN52713285). During screening, parents of the participants completed a comprehensive questionnaire and medical records were reviewed.
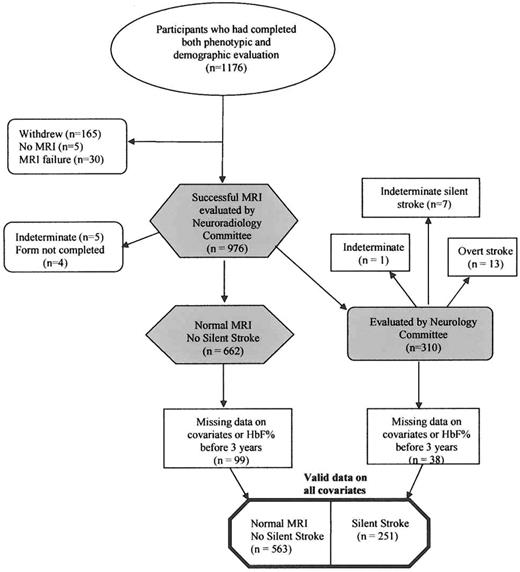
The current study used a subset of the registered participants in the SIT Trial. At the time the data were finalized for assessment and analysis (September 10, 2010), 1176 participants had completed phenotypic and demographic evaluation (Figure 1). The dataset used for this study is designated as the SIT Trial screening database, which includes cleaned data from these 1176 patients. After review and exclusion where silent infarct information was missing or hemoglobin F (HbF) percentage was obtained before 3 years of age, and where information was missing on the prespecified covariates, the final analysis dataset consisted of 814 participants. A priori, we wanted to determine the association between SBP and SCI. The SBP, as a single measurement, was performed as part of routine medical care during the most recent well visit.
A flow diagram of the participants in the SIT Trial who completed all clinical forms and whose MRIs were adjudicated by both the Neuroradiology and Neurology Committees and who were available for multivariable modeling (814 subjects).
A flow diagram of the participants in the SIT Trial who completed all clinical forms and whose MRIs were adjudicated by both the Neuroradiology and Neurology Committees and who were available for multivariable modeling (814 subjects).
SCIs
MRI of the brain was performed and read by the Neuroradiology Committee using a protocol detailed previously in Vendt et al.16 Between 2004 and 2010, 1091 participants underwent baseline MRI of the brain; 1047 were performed using a 1.5-Telsa scanner and the remaining were done using a 3.0 Telsa scanner.
An SCI-like lesion was defined by an MRI signal abnormality visible on two views on the T2-weighted images (axial and coronal) and at least 3 mm in one dimension, based on consensus of 2 of 3 neuroradiologists. Subsequently, each SCI-like lesion was adjudicated by the neurology committee as to whether it was truly silent, after review of the local site pediatric neurologist's examination. The local neurologist was unaware of the results of the MRI. If the neurologic examination was normal or there was an abnormality on the neurologic examination that was not explained by the location of the MRI lesion, the participant was classified as having an SCI.
Statistical methods
The relationship between the demographic and clinical covariates and the presence of SCI was assessed with a χ2 test or a t test for categorical or continuous covariates, respectively. A multivariable logistic model was constructed with covariates in their original scale, entering all covariates in one block. Before enrollment began in the trial, we elected not to include site location as a variable in the analyses. A second reduced model included only those covariates that were nominally significant predictors (P < .20) from the first model. Separate models were created with the statistically significant covariates in their original scales or grouped into tertiles, and the latter is presented here. Additional analysis was done to determine the effect, if any, of case selection because of eligibility criteria and missing data on the covariates, using a χ2 or t test.
Subject selection effects
A total of 225 participants of the original 1176 were not eligible for this analysis. The reasons for ineligibility were as follows: (1) The Neuroradiology Committee could not decide whether there was an SCI or not (n = 5); (2) the Neurology Committee considered that the participant had had an overt stroke (n = 13); (3) the Neurology Committee could not determine whether the stroke was overt or silent (n = 1); (4) subjects withdrew from the study before the MRI was completed (n = 165); (5) the MRI failed (n = 30); (6) MRI data were not available at the time of the Neurology Consensus Committee review (n = 5); or (7) the Neurology Examination form was not completed at the time of the Neurology Consensus Committee review (n = 4). An additional 137 participants could not be included in the multivariable models because they had missing data on one or more of the prespecified covariates (listwise deletion was used for all models) or had HbF percentage levels measured before 3 years of age.
Results
A total of 30.8% (251 of 814) of the participants were determined to have SCI. The demographic features of the participants with and without SCI are described in Table 1. The mean SBP was 108.0 mmHg (median, 108.0 mmHg; SD, 11.5 mmHg; range, 66-151 mmHg).
Potential clinical and laboratory risk factors among 814 participants with HbSS or HbSβ° thalassemia between 5 and 15 years of age, with and without SCI
| Variable . | No SCI (n = 563) . | SCI (n = 251) . | P* . |
|---|---|---|---|
| Age, y | 9.06 ± 2.43 | 9.35 ± 2.52 | .115 |
| Sex, % male | 47.4 | 58.2 | .005 |
| Frequent headaches, % | 34.8 | 40.2 | .156 |
| Pain event per y | 0.63 ± 0.84 | 0.58 ± 0.88 | .416 |
| Acute chest syndrome event rate per y | 0.13 ± 0.26 | 0.17 ± 0.34 | .134 |
| Hemoglobin, g/dL | 8.25 ± 1.10 | 7.95 ± 1.06 | < .001 |
| HbF, % | 12.53 ± 9.61 | 11.12 ± 9.86 | .055 |
| Baseline SBP, mmHg | 107.31 ± 11.55 | 109.41 ± 11.23 | .016 |
| Baseline DBP, mmHg | 60.36 ± 8.04 | 60.95 ± 7.78 | .328 |
| White blood cell count, × 109/L | 12 342 ± 4215 | 12 561 ± 6437 | .563 |
| Baseline oxygen saturation, % | 96.58 ± 2.92 | 96.12 ± 3.01 | .040 |
| Variable . | No SCI (n = 563) . | SCI (n = 251) . | P* . |
|---|---|---|---|
| Age, y | 9.06 ± 2.43 | 9.35 ± 2.52 | .115 |
| Sex, % male | 47.4 | 58.2 | .005 |
| Frequent headaches, % | 34.8 | 40.2 | .156 |
| Pain event per y | 0.63 ± 0.84 | 0.58 ± 0.88 | .416 |
| Acute chest syndrome event rate per y | 0.13 ± 0.26 | 0.17 ± 0.34 | .134 |
| Hemoglobin, g/dL | 8.25 ± 1.10 | 7.95 ± 1.06 | < .001 |
| HbF, % | 12.53 ± 9.61 | 11.12 ± 9.86 | .055 |
| Baseline SBP, mmHg | 107.31 ± 11.55 | 109.41 ± 11.23 | .016 |
| Baseline DBP, mmHg | 60.36 ± 8.04 | 60.95 ± 7.78 | .328 |
| White blood cell count, × 109/L | 12 342 ± 4215 | 12 561 ± 6437 | .563 |
| Baseline oxygen saturation, % | 96.58 ± 2.92 | 96.12 ± 3.01 | .040 |
Data are mean ± SD.
DBP indicates diastolic blood pressure.
P value from an independent-samples t test or χ2 test.
Baseline hemoglobin and SBP measurements are associated with SCI
When all of the clinical and laboratory covariates of interest were forced into the multivariate logistic regression model, the overall model was statistically significant (χ2 = 29.37, df = 11, P = .002). The model had a C statistic of 0.620. Three factors were significant at the level of P less than .20: hemoglobin concentration (P = .007), SBP (P = .112), and sex, with female sex as the reference (P = .046; Table 2).
Logistic regression model for SCI using all potential covariates
| . | Odds ratio . | 95.0% CI for odds ratio . | ||
|---|---|---|---|---|
| Lower . | Upper . | P . | ||
| Age, y | 1.04 | 0.97 | 1.11 | .28 |
| Sex (male reference) | 1.37 | 1.01 | 1.87 | .05 |
| Hemoglobin concentration | 0.80 | 0.67 | 0.94 | .01 |
| HbF % | 1.00 | 0.98 | 1.02 | .89 |
| Baseline SBP | 1.01 | 1.00 | 1.03 | .11 |
| Baseline DBP | 1.01 | 0.99 | 1.03 | .51 |
| WBC count | 1.00 | 1.00 | 1.00 | .99 |
| Baseline O2 saturation | 0.99 | 0.94 | 1.04 | .70 |
| Acute chest syndrome event rate | 1.36 | 0.81 | 2.30 | .25 |
| Pain event rate | 0.90 | 0.75 | 1.10 | .30 |
| Frequent headaches (yes or no) | 1.18 | 0.86 | 1.62 | .31 |
| Constant | 1.32 | NA | NA | .92 |
| . | Odds ratio . | 95.0% CI for odds ratio . | ||
|---|---|---|---|---|
| Lower . | Upper . | P . | ||
| Age, y | 1.04 | 0.97 | 1.11 | .28 |
| Sex (male reference) | 1.37 | 1.01 | 1.87 | .05 |
| Hemoglobin concentration | 0.80 | 0.67 | 0.94 | .01 |
| HbF % | 1.00 | 0.98 | 1.02 | .89 |
| Baseline SBP | 1.01 | 1.00 | 1.03 | .11 |
| Baseline DBP | 1.01 | 0.99 | 1.03 | .51 |
| WBC count | 1.00 | 1.00 | 1.00 | .99 |
| Baseline O2 saturation | 0.99 | 0.94 | 1.04 | .70 |
| Acute chest syndrome event rate | 1.36 | 0.81 | 2.30 | .25 |
| Pain event rate | 0.90 | 0.75 | 1.10 | .30 |
| Frequent headaches (yes or no) | 1.18 | 0.86 | 1.62 | .31 |
| Constant | 1.32 | NA | NA | .92 |
The same population of participants was used as in Table 1.
DBP indicates diastolic blood pressure; and NA, not applicable.
The reduced model with only these 3 covariates was statistically significant overall (χ2 = 24.41, df = 3, P < .001), with a C statistic of 0.610. Lower hemoglobin concentrations were associated with increasing odds of SCI: (P = .001); higher SBP was associated with increasing odds of SCI (P = .018). Patient sex remained statistically significant (P = .030), with males having increased odds of SCI. In the final model, no variance inflation factor value was more than 1.34, indicating very little multicollinearity.
As an exploratory analysis, we evaluated separately indirect measures of hemolysis, such as absolute reticulocyte and total bilirubin. We added one of the two measures to the preexisting covariates and followed the previously determined model. Absolute reticulocyte count (P = .063) was included in the reduced model, with only the variables significant at less than P = .20, which were again sex, hemoglobin level, and SBP measurement; however, reticulocyte count was less statistically significant (P = .065). When total bilirubin was added to the model with all of the covariates, there was no significant association between the total bilirubin and risk of SCI (P = .990). We did not obtain lactate dehyrogenase levels in the study.
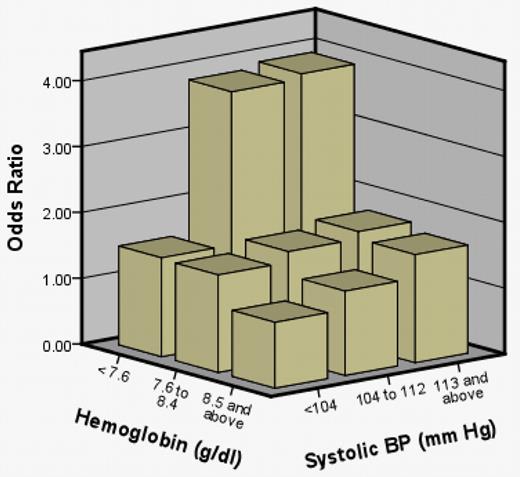
A model with both clinical covariates grouped into tertiles was constructed. In this model, the highest tertile for SBP and lowest tertile of hemoglobin had the greatest odds of SCI (Table 3). The model had a C statistic of .615. The third tertile was the reference category for hemoglobin (> 8.5 g/dL); the first tertile was the reference category for SBP (< 104 mmHg). Odds ratios for SCI in the first tertile of hemoglobin (< 7.6 g/dL) were 2.12 (95% CI, 1.45-3.10, P < .001). Odds ratios in the second tertile (7.6-8.5 g/dL) were 1.19 (95% CI, 0.81-1.76, P = .371). Odds ratios for SCI in the second tertile for SBP (104-112 mmHg) were 1.55 (95% CI, 1.06-2.27, P = .025); for the third tertile (> 112 mmHg), odds ratios were 1.73 (95% CI, 1.18-2.54; P = .005). No interaction was detected between baseline hemoglobin and SBP (P = .90). For further model understanding, we constructed a chart (Figure 2) to show the joint effect of hemoglobin and SBP measurements. The reference category was based on persons in the highest hemoglobin and lowest blood pressure group, holding sex constant. Persons in the lowest hemoglobin and middle and upper tertile of SBP had the highest odds of an SCI compared with their respective reference groups.
Logistic regression model for SCI with reduced set of covariates, grouped into tertiles
| Covariate . | Odds ratio . | 95.0% CI for odds ratio . | P . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| Hemoglobin < 7.6 g/dL | 2.12 | 1.45 | 3.10 | < .001 |
| Hemoglobin ≥ 7.6 g/dL and ≤ 8.5 g/dL | 1.19 | 0.81 | 1.76 | .371 |
| Hemoglobin ≥ 8.6 g/dL (reference) | 1.00 | NA | NA | NA |
| SBP < 104 mmHg (reference) | 1.00 | NA | NA | NA |
| SBP ≥ 104 and ≤ 112 mmHg | 1.55 | 1.06 | 2.27 | .025 |
| SBP ≥ 113 mmHg | 1.73 | 1.18 | 2.54 | .005 |
| Sex (female, reference) | 1.42 | 1.04 | 1.93 | .026 |
| Constant | 0.26 | NA | NA | .001 |
| Covariate . | Odds ratio . | 95.0% CI for odds ratio . | P . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| Hemoglobin < 7.6 g/dL | 2.12 | 1.45 | 3.10 | < .001 |
| Hemoglobin ≥ 7.6 g/dL and ≤ 8.5 g/dL | 1.19 | 0.81 | 1.76 | .371 |
| Hemoglobin ≥ 8.6 g/dL (reference) | 1.00 | NA | NA | NA |
| SBP < 104 mmHg (reference) | 1.00 | NA | NA | NA |
| SBP ≥ 104 and ≤ 112 mmHg | 1.55 | 1.06 | 2.27 | .025 |
| SBP ≥ 113 mmHg | 1.73 | 1.18 | 2.54 | .005 |
| Sex (female, reference) | 1.42 | 1.04 | 1.93 | .026 |
| Constant | 0.26 | NA | NA | .001 |
The reference category for hemoglobin was the third tertile (hemoglobin ≥ 8.6 g/dL). Participants with hemoglobin in the second tertile had a higher risk of SCI compared with the third tertile. The reference category for SBP was the first tertile (SBP < 104 mmHg). Participants in the second and third tertiles had a higher risk of SCI compared with those in the first tertile.
NA indicates not applicable.
Joint effect of hemoglobin total (tertiles) and SBP (tertiles) on odds of SCI in 814 participants with HbSS or HbSβ° thalassemia between 5 and 15 years of age.
Joint effect of hemoglobin total (tertiles) and SBP (tertiles) on odds of SCI in 814 participants with HbSS or HbSβ° thalassemia between 5 and 15 years of age.
Given the association of SBP measurement with SCI, we tested the hypothesis that patients with documented hypertension would also have a higher rate of SCI. Approximately 1% (5 of 783) of the participants had diagnoses of hypertension and were being treated with antihypertensive medication at the time of their initial evaluation, although in one case the drug had been started for another indication (Table 4). During the screening phase for the trial, 5 of these 6 participants (83%) had SCI; this percentage was higher than expected compared with the remaining study participants (30% [237 of 778], P = .012).
Clinical characteristics of 6 participants (with either HbSS or HbSβ° thalassemia) between 5 and 15 years of age who had a clinical diagnosis of hypertension in the SIT Trial or started hypertensive medications
| Age, y . | Sex . | Medication . | BP . | Indication . | MRI results . |
|---|---|---|---|---|---|
| 6 | Female | Enalapril | 123/74 | Enalapril for hypertension and congenital single kidney | 1 faint lesion in the right frontal lobe white matter |
| Started 2 y before the diagnosis of SCI | 1 lesion in the posterior corpus callosum | ||||
| 9 | Male | Enalapril | 110/57 | Enalapril for mitral valve prolapse | 5-mm lesion in the left centrum semiovale |
| Started 8 y before the diagnosis of SCI | |||||
| 14 | Male | Lisinopril | 126/62 | Lisinopril for hypertension | High parietal white matter lesion on the right |
| Started 1.5 y before the diagnosis of SCI | |||||
| 11 | Female | Atenolol | 114/63 | Atenolol for hypertension | Diffuse, bihemispheric ischemic injury |
| Started 2 mo before the diagnosis of SCI | |||||
| 11 | Male | Enalapril | 104/58 | Enalapril for hypertension | 2 lesions in the left frontal lobe |
| Started 6 y before the diagnosis of SCI | Bilateral posterior perventricular white matter abnormality | ||||
| 5 | Male | Enalapril | 135/67 | Enalapril for hypertension | Does not have an infarct-like lesion |
| Started 2 y before the diagnosis of SCI |
| Age, y . | Sex . | Medication . | BP . | Indication . | MRI results . |
|---|---|---|---|---|---|
| 6 | Female | Enalapril | 123/74 | Enalapril for hypertension and congenital single kidney | 1 faint lesion in the right frontal lobe white matter |
| Started 2 y before the diagnosis of SCI | 1 lesion in the posterior corpus callosum | ||||
| 9 | Male | Enalapril | 110/57 | Enalapril for mitral valve prolapse | 5-mm lesion in the left centrum semiovale |
| Started 8 y before the diagnosis of SCI | |||||
| 14 | Male | Lisinopril | 126/62 | Lisinopril for hypertension | High parietal white matter lesion on the right |
| Started 1.5 y before the diagnosis of SCI | |||||
| 11 | Female | Atenolol | 114/63 | Atenolol for hypertension | Diffuse, bihemispheric ischemic injury |
| Started 2 mo before the diagnosis of SCI | |||||
| 11 | Male | Enalapril | 104/58 | Enalapril for hypertension | 2 lesions in the left frontal lobe |
| Started 6 y before the diagnosis of SCI | Bilateral posterior perventricular white matter abnormality | ||||
| 5 | Male | Enalapril | 135/67 | Enalapril for hypertension | Does not have an infarct-like lesion |
| Started 2 y before the diagnosis of SCI |
Discussion
Risk factors for SCI in SCA have been incompletely studied; however, they may provide insight into the pathogenesis and potential preventive strategies, not only in SCA, but also in the general population. We describe, for the first time, in a large multicenter setting that lower baseline hemoglobin concentration and relative high baseline SBP measurement, both risk factors for SCI in the general population, are associated with an increased risk of SCI in SCA in multivariable analysis. We also demonstrate that neither the incidence rates of pain nor acute chest syndrome (ACS) events, measures of SCA morbidity, are associated with SCI. Further, baseline WBC, HbF percentage, and oxygen saturation levels are not associated with an increased risk of SCI in multivariable analysis. There was biologic plausibility that relative high SBP was associated with end-organ disease in SCA, including the brain, based on the work of Rodgers et al14 and others.13,17 These findings, coupled with strong evidence that elevated SBP measurement was associated with SCI in older adults without SCA,18,19 provide support for the biologic basis and generalizability of our results.
Our results are different from the largest study to date evaluating risk factors for SCI among participants with SCA.12 In the CSSCD study, several variables, including seizure history, pain event rate, lower hemoglobin, high WBC, elevated pocked red blood cell counts, and SEN β-S globin gene haplotype (a measure of reduced splenic function), were associated in univariate analysis; however, only seizure history, low pain rate, higher WBC, and the presence of a SEN β-S globin gene haplotype were associated with risk of SCI in the final multivariable analysis.12 Several possible explanations exist to explain the differences between the current and previous studies. First, the current study has greater than 6 times more participants with SCI. Second, the two studies did not have the same eligibility criteria. The CSSCD was an infant cohort without selection based on previous comorbid condition; in contrast, the SIT Trial participants that were screened for SCI were enrolled between 5 and 15 years of age, and participants were excluded if they had an active nonfebrile seizure disorder or a stroke, or severe disease that led to treatment with hydroxyurea or blood transfusion therapy; consequently, our results can only be generalized to children with SCA who meet these criteria.
Our finding that low hemoglobin concentrations are associated with SCI in SCA is consistent with the findings in other populations without SCA in which SCI occurs, but which also have relative low hemoglobin concentrations, such as in patients receiving dialysis20 and patients with β-thalassemia intermedia.21,22 Although the etiology of SCI is unknown, the consistent finding that anemia is a risk factor strongly implicates cerebral hemodynamic insufficiency as a central component. All patients with chronic anemia have a compensatory mechanism in the brain (auto-regulation) that occurs with vasodilation of the cerebral vasculature and manifests initially as increased cerebral blood flow.23,24 Despite this compensatory mechanism, acute drops in hemoglobin are temporally associated with overt stroke25,26 and SCI27 in SCA. Given the strong association between anemia and SCI, the most likely explanation for the majority of SCI events in persons with SCA is a continuous state of delicate balance in supply and demand of oxygen to the brain; thus, any decrease in supply or increase in demand may result in ischemia or infarction in this vulnerable group of patients. Alternatively, the low hemoglobin may be a proxy for a higher rate of relative hemolysis, with subsequent endothelial dysfunction.28,29 We were unable to assess endothelial dysfunction, and our indirect measures of hemolysis (total bilirubin and reticulocyte count) were not associated with SCI in this cohort. Other potential mechanisms that are probably important and associated with hemodynamic insufficiency include vascular stenosis, dural venous sinus thrombosis, and posterior reversible encephalopathy syndrome, often referred to as PRES.30
Additional support for the precarious balance between oxygen delivery and demand that may result in SCI is the observation that infants with SCA may begin to develop their anemia as young as 10 weeks of age31 and that, at 3 months of age, the mean hemoglobin of infants with HbSS is approximately 1.5 g/dL below expected for children without SCA of the same age.31 The lower hemoglobin levels may explain in part why SCIs have been detected in an infant as young as 7 months32 and in several infants younger than 15 months.33 The majority of SCIs occur in children younger than 6 years,4 as the hemoglobin levels decline continuously until levels reach steady state.31 In addition to our findings that low hemoglobin levels are associated with SCI, two single-center studies of children with SCA have demonstrated similar findings. Using a multivariable analysis from a retrospective cohort, Bernaudin et al demonstrated that low hemoglobin was associated with an increased rate of SCI.5 In univariate analysis, lower hemoglobin levels were associated with SCI in the Kwiatkowski et al study of young children with SCA.4 Ultimately, studies of interventions to improve baseline hemoglobin concentration, such as hydroxyurea, will be necessary to evaluate this strategy for the primary prevention of SCI.
The biologic basis for the association between relative high SBP measurements and SCI is not known. The etiology of the lower blood pressure in SCA is not clear, but may be explained in part by compensatory systemic vasodilatation to increase oxygen delivery that occurs in adults and children with chronic anemia.14 We postulate that, in SCA, as with low hemoglobin, elevated SBP results in a compromise in this compensatory auto-regulation mechanism. Consistent with this concept is that the highest odds of SCI occurred in the group of children with both the highest SBP and the lowest hemoglobin measurements compared with all other groups (Figure 2).
Multiple studies in persons with SCA document an association between relatively elevated SBP and organ damage, including overt stroke,13,14 elevated tricuspid jet velocity measurement (a proxy for pulmonary hypertension),17 and renal insufficiency.17 The consistent relationship between organ injuries and relatively elevated SBP measurements in SCA suggests an underlying common mechanism that is unlikely to be elucidated in a clinical study and may require animal models or basic physiology studies for better understanding.
Our study has several limitations. First, SBP measurements were not standardized; however, a lack of a standard blood pressure approach across multiple sites would tend to increase the variance of these measurements and bias our results toward the null hypothesis. Although the vast majority of children with SCA were eligible to participate in the trial, the exclusion criteria eliminated some children with severe disease, including children with overt stroke or seizures, those known to have abnormal transcranial Doppler scan velocities, and those treated with hydroxyurea or chronic blood transfusion therapy. Thus, we may have limited our ability to detect other clinically significant associations because of restriction of the study population that was eligible for the SIT Trial. We considered the potential weakness that the SBP measurement was not standardized against height-, sex-, and ethnic-matched controls; however, we elected not to use this analytical strategy because children and adults with SCA have systematically lower blood pressures compared with the general population. Therefore, general population would represent a poor reference group. We did not adjust for age because the anticipated upper limits of normal for children between 5 and 15 years of age has a difference of less than 10 mmHg, and age was not statistically significant when evaluated in the multivariable model. We did not include analysis of magnetic resonance angiography (MRA) in this manuscript, as it was not mandatory at baseline and only a subgroup of patients received them.
In conclusion, we have provided evidence that both baseline hemoglobin concentration and relative high SBP are risk factors for SCI. Based on these findings, we cannot make definitive recommendations for preventing SCI; however, these findings provide the basis for further research focused on increasing the baseline hemoglobin concentration or attenuating factors that lead to relative high SBP measurements, as these are potentially modifiable risk factors for SCI.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grant U01-NS42804; M.R.D.).
The contents of this article represent the personal opinion of the authors and should not be construed as the opinion or position of the National Institutes of Health or its affiliates.
National Institutes of Health
Authorship
Contribution: M.R.D. designed and performed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; J.F.C. designed research and performed research, collected, analyzed, and interpreted data, and wrote the manuscript; M.J.R. performed statistical analysis; R.N.I. wrote the manuscript; S.A.S., C.P.M., T.H.H., R.V.I., B.I., P.T.T., M.K.-A., C.T.Q., F.B., G.A., G.M.W., JA.P., B.F., J.K.K., A.A.K., M.M.R., A.A.T., M.E.H., R.C.R.-L., F.J.K., H.S., C.E.G., S.L.S., K.A.K., J.J.S., and J.M.F. collected data and wrote the manuscript; M.J.N. wrote the manuscript, and performed analysis of stroke and silent cerebral infarct; and M.O.G. and J.P.M. analyzed and interpreted the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael R. DeBaun, Departments of Pediatrics and Medicine, Clinical Research, Pediatrics, and Vanderbilt-Meharry Center for Excellence in Sickle Cell Disease, Vanderbilt University School of Medicine, Monroe Carell Jr Children's Hospital at Vanderbilt, 2200 Children's Way, Nashville, TN 37232-9000; e-mail: m.debaun@vanderbilt.edu.
Appendix
SIT Trial Committees: Data and Safety Monitoring Board: David Schoenfeld (Chair), Massachusetts General Hospital; Thomas Adamkiewicz, Morehouse School of Medicine; Stephen Ashwal, Loma Linda University; H. Stacy Nicholson, Oregon Health & Science University; Guillaume Sebire, Université De Sherbrooke; Janice Cordell, National Institutes of Health. Advisory Committee: William Powers (Chair), University of North Carolina; Robert J. Adams, Medical University of South Carolina; Jeffrey Schatz, University of South Carolina; Yuko Palesch, Medical University of South Carolina. Neurology Committee: Michael J. Noetzel (Chair), Washington University School of Medicine; Michael Dowling, University of Texas Southwestern Medical Center; Deborah Hirtz, National Institutes of Health, National Institute of Neurological Disorders and Stroke; Rebecca N. Ichord, Children's Hospital of Philadelphia; E. Steve Roach, Nationwide Children's Hospital. Psychology Committee: Desiree White (Chair), Washington University Department of Psychology; T. David Elkin, University of Mississippi Medical Center; Mary M. George, Baylor College of Medicine; H. Gerry Taylor, Rainbow Babies and Children's Hospital. Neuroradiology Committee: Robert C. McKinstry III (Chair), Washington University School of Medicine; William S. Ball Jr, University of Cincinnati; Michael A. Kraut, Johns Hopkins Hospital; Marilyn Siegel, Washington University School of Medicine.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal