In this issue of Blood, Pegram and colleagues describe the ability to improve adoptive T-cell therapies by engineering T cells to secrete the potent proinflammatory cytokine IL-12.1 These exciting findings significantly extend previous observations made in a murine melanoma model targeting naturally occurring tumor antigens.2,3
Despite its discovery more than 20 years ago and the flurry of research related to its biologic function, IL-12 remains a controversial cytokine as a therapeutic anticancer agent.4 The benefits of administering IL-12 systemically were demonstrated in multiple preclinical studies through the early 1990s, but these promising findings never translated into successful human clinical trials.5 One of the major issues that limited the clinical development of IL-12 were the reported dose-limiting toxicities, which included fevers, elevated hepatic enzymes, hemodynamic instability, and in some cases death.4
Notwithstanding these setbacks, the importance of IL-12 for inducing an immune response against cancer is undeniable and the quest for using this potent cytokine for the immunotherapy of cancer continues.5,6 The findings by Pegram et al show a translatable method for harnessing the beneficial effects of IL-12 that may also minimize the hazardous outcomes associated with its use (see figure).
CD19 is an attractive tumor antigen target because it is robustly expressed by some leukemias and lymphomas. It is expressed by normal B lymphocytes, but not by other vital tissues. Although depleting CD19 results in the ablation of B cells, patients can be treated with infusions of immunoglobulins. CD19 has been recently targeted by a number of groups, including Brentjens.7,8 This approach is encouraging and it is conceivable that gene-engineered T cells targeting CD19 might eventually replace allogeneic bone marrow transplantation as a curative regimen for a subset of patients with B-cell malignancies. The use of a single-chain functional IL-12 molecule represents a welcome addition to this therapy, because it may eliminate the need for lymphodepletion and high-dose IL-2 used in current regimens.
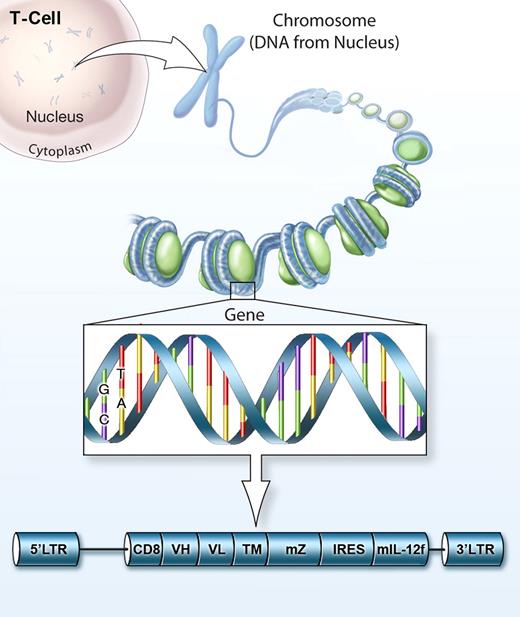
Gene-engineered T cells co-expressing CD19 CAR and IL-12 for adoptive cell therapy against cancer.
Gene-engineered T cells co-expressing CD19 CAR and IL-12 for adoptive cell therapy against cancer.
In their study, Pegram and colleagues show the critical need for autocrine production of IL-12 and IFN-γ to enhance the effects of adoptively transferred T cells. They also suggest that CD4+ T cells expressing IL-12 enhance the function of co-transferred CD8+ T cells and may be important for overcoming negative regulatory factors induced by tumors.1
Additional mechanisms ascribed to IL-12 include the ability to functionally reprogram myeloid-derived cells. Growing tumor masses can create an immunosuppressive microenvironment orchestrated by cellular infiltrates such as myeloid-derived suppressor cells, alternatively activated macrophages, and functionally impaired dendritic cells. Surprisingly, the majority of cells expressing the functional IL-12 receptor-β2 subunit within the tumor microenvironment are of myeloid origin.2 Sensitization of these cells by IL-12 likely triggers an acute inflammatory environment that enables improvements in antigen presentation and T-cell co-stimulation within tumors.2
Interestingly, Pegram et al noted that they did not observe toxicities in this study, although targeting human CD19 in this complex mouse might not fully capture all the toxicities. Others have observed toxicities from IL-12 production by T cells3 and so the issue might be seen in future clinical trials. It is difficult to predict the replicative potential of transferred T cells because every daughter cell of the gene-engineered T cell will also be producing supra-physiologic amounts of IL-12. Current safety measures that are being explored include the use of an inducible NFAT promoter, incorporation of suicide genes, or the use of molecular on/off promoter switches controlled by the systemic administration of a third-party drug.9,10 Carefully conducted clinical trials will be needed to assess whether the benefits of gene engineering T cells with IL-12 outweigh the risks.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal