Case presentation
A 58-year-old man with a past medical history of underlying congenital aortic valve defect and hypertension was incidentally diagnosed with an isolated anemia (hemoglobin of 7.8 g/dL) when he presented to his cardiologist with symptoms of fatigue and exertional dyspnea. Evaluation for common causes of anemia, including assessment of serum creatinine, vitamin B12, folate, and iron levels, and evaluation of the gastrointestinal tract with a colonoscopy and esophagogastroduodenoscopy, were all normal. The patient was referred to a hematologist, and a bone marrow biopsy and aspirate demonstrated a normocellular marrow with trilineage hematopoeisis, increased number of megakaryocytes with dysmegakaryopoiesis, and 3% myeloblasts. Cytogenetic analysis revealed a normal karyotype with an isolated deletion 5q [del(5)(q22q35)] abnormality in 20 of 20 metaphase chromosomes, consistent with del (5q) myelodysplastic syndrome (MDS) according to the World Health Organization classification. He had an International Prognostic Scoring System (IPSS) score of 0 and was treated with recombinant humanized erythropoietin at a dose of 60 000 units weekly. He had a hematologic improvement, with a rise in hemoglobin of 3 g/dL, but lost his response 15 months later, at which time he started requiring packed red blood cell transfusions, progressing to 2 units every 2 to 3 weeks despite an increase in the dose of recombinant humanized erythropoietin by 25%. Three-and-a-half years after his MDS diagnosis, he was referred to the Cleveland Clinic.
At presentation to our institution, his peripheral blood counts showed a white blood cell count of 2300/mm3, absolute neutrophil count (ANC) of 1120/mm3, normal differential hemoglobin of 7.6 g/dL, mean corpuscular volume of 90.2 fL, and a platelet count of 428 000/mm3. A repeat bone marrow biopsy and aspirate revealed a markedly hypocellular marrow (10%), decreased granulopoiesis and erythropoiesis with dyserythropoietic cells, absence of ring sideroblasts, increased megakaryocytes with dysplastic morphology consisting of predominantly monolobate and occasional hyperlobate forms, and 2% myeloblasts. Cytogenetic analysis showed the previous del(5)(q22q35) abnormal karyotype. His IPSS score was 0.5. He was started on 10 mg of lenalidomide daily but had to be dose-reduced to 5 mg daily after the first cycle because of a drop in ANC (to 340/mm3) and platelets (to 64 000/mm3). Six weeks after starting treatment, his counts started improving; and by 12 weeks, his hemoglobin normalized to 13 to 15 g/dL and his platelet counts stabilized in the range of 120 000 to 150 000/mm3, with an ANC that fluctuated between 1200 and 1500/mm3. A follow-up bone marrow biopsy 6 months later confirmed complete morphologic, but continued, presence of del (5q) clone in 19 of 20 metaphases.
His response was sustained for 2.5 years, during which time the patient remained transfusion independent, in excellent physical health, and without any significant treatment-related adverse events. However, the patient then reported worsening fatigue interfering with his daily functioning, and his peripheral blood counts showed significant changes, with a drop in hemoglobin to 8 g/dL, decline in ANC to 660/mm3, and a drop in platelets to 56 000/mm3. A bone marrow examination showed dysplastic morphology involving all 3 lineages with 7% blasts, persistent del(5q) abnormality, and a new, separate −7 clone, indicating disease progression to refractory anemia with excess blasts and an IPSS score of 2.0. He was started on DNA hypomethylating therapy with azacitidine, and elected for an umbilical cord blood transplantation, which unfortunately was not successful. The patient died 7 years after his MDS diagnosis.
Discussion
Our patient with MDS and the del(5q) cytogenetic abnormality would be classified initially as having lower-risk disease, a categorization applied to approximately 75% of newly diagnosed patients (French-American-British categories of refractory anemia and refractory anemia with ring sideroblasts; World Health Organization categories of refractory anemia, refractory anemia with ring sideroblasts, refractory cytopenia with unilineage or multilineage dysplasia, MDS with del (5q), and MDS unclassified; and IPSS scores < 1.0). Those with the del(5q) lesion comprised 8% to 9% of all new diagnoses.1
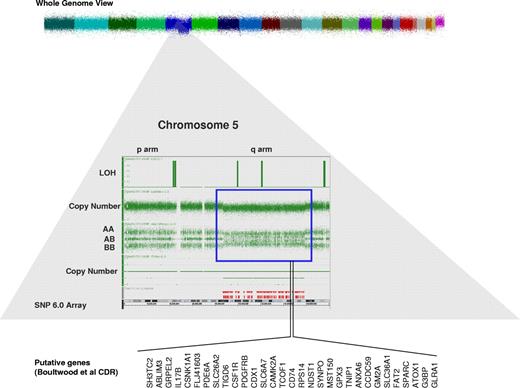
Within MDS, data are accumulating that the abnormalities traditionally observed with classic metaphase karyotyping, seen in approximately half of MDS patients and most commonly involving chromosomes 5, 7, 8, and 20, are only the tip of the iceberg of molecular changes in MDS.2 A recent collaboration among the King's College, Cleveland, Johns Hopkins, and Tampa groups examining 430 patients with MDS, MDS/myeloproliferative neoplasm, and acute myeloid leukemia (AML) arising from MDS used single nucleotide polymorphism array technology to identify chromosomal defects not seen with metaphase cytogenetics (Figure 1).3 The diagnostic yield of single nucleotide polymorphism arrays was 30% higher than with classic karyotyping, identifying abnormalities in 74% of patients, compared with 44% (P < .0001). These previously cryptic lesions also predicted for worse prognosis, including in patients with isolated del(5q) abnormalities. In addition, having a TP53 mutation impacted outcome among 37 del(5q) MDS patients treated with lenalidomide, as these patients had a trend toward increased rates of leukemia evolution and were less likely to have a complete cytogenetic response to the drug. At the time this patient was seen, these additional abnormalities were not being assessed.5
Single nucleotide polymorphism 6.0 arrays result in a patient with del 5q in the setting of the 5q− syndrome. There is loss of heterozygosity (LOH) and reduction in copy number in the q arm of chromosome 5. These changes represent a deletion in that region of chromosome 5 (blue box). In an attempt to identify the putative genetic defects that causes 5q− syndrome, several investigators defined different commonly deleted regions (CDRs) in chromosome 5. Shown here are the CDRs from Boultwood et al4 and the putative genes of interest.
Single nucleotide polymorphism 6.0 arrays result in a patient with del 5q in the setting of the 5q− syndrome. There is loss of heterozygosity (LOH) and reduction in copy number in the q arm of chromosome 5. These changes represent a deletion in that region of chromosome 5 (blue box). In an attempt to identify the putative genetic defects that causes 5q− syndrome, several investigators defined different commonly deleted regions (CDRs) in chromosome 5. Shown here are the CDRs from Boultwood et al4 and the putative genes of interest.
Patients in lower-risk subgroups have a predicted survival measured in years. Given the indolent disease course, lower-risk MDS patients often have the luxury of delaying treatment until disease-related symptoms, or need for frequent transfusions, outweigh the anticipated side effects of a drug. Hence, the use of erythropoiesis-stimulating agents in this cohort of patients, especially those with low erythropoietin levels as first-line treatment, is entirely appropriate considering the pharmacoeconomic profile of lenalidomide and the attendant adverse event profile compared with that of erythropoiesis-stimulating agents. In our patient's case, given his cytopenias and red blood cell transfusion dependency despite erythropoiesis-stimulating agent use, the time for waiting had passed.
It is speculated that lenalidomide functions by selectively suppressing the del (5q) clone through inhibition of haplodeficient cell cycle regulatory targets coded within the 5q31 commonly deleted region,6,7 complemented by effects on the bone marrow microenvironment. Typical of subjects enrolled into the MDS-003 registration study that resulted in the approval of lenalidomide for transfusion-dependent, lower-risk del(5q) MDS patients,8 our patient had MDS for 3.5 years (median duration of disease on study enrollment was 2.5 years) had previously been treated with an erythropoiesis-stimulating agent (73% of subjects), achieved red blood cell transfusion independence while on therapy (67% of subjects), and had a response duration of more than 2 years (median for study subjects, 2.5 years). Also similar to study subjects, our patient had therapy-related adverse events of neutropenia and thrombocytopenia, which necessitated lenalidomide dose reduction (seen in 84% of study subjects across lenalidomide dosing schedules)8 and which subsequently leveled off to a platelet count of 20% of baseline (typical of study subjects).9 The MDS-004 phase 3 study, in which del(5q) MDS patients were randomized 1:1:1 to lenalidomide 10 mg/day on days 1 to 21, lenalidomide 5 mg/day on days 1 to 28, or placebo on days 1 to 28 of 28-day cycles, largely confirmed the phase 2 findings, although with a transfusion independence response rate that was 11% to 25% lower.10 Once a patient responds to lenalidomide, the drug is continued until disease progression.
Could we have predicted that our patient was going to respond to lenalidomide? In some ways, yes. In multivariate analyses, with variables analyzed continuously, predictors of transfusion independence response included younger age, shorter MDS duration, lower baseline transfusion burden, and treatment-related thrombocytopenia; 3 of 4 categories would have been pertinent.11
But could we have predicted that he would lose his response and progress to higher-risk MDS? Our patient's IPSS score was reassessed on treatment failure and found to be 2.0, a substantial increase over his score of 0, at diagnosis. Acknowledging that the IPSS should only be used in an untreated MDS population to determine risk and prognosis at diagnosis, even if we used a dynamic prognostic scoring system, such as the one developed by the M. D. Anderson group, our patient would have earned a score predicting a median survival of 6 months. He sustained an increase in blast percentage and cytogenetic evolution: both harbingers of eventual leukemic evolution. In the MDS-004 phase 3 lenalidomide study, 52 patients (25.4%) progressed to AML with almost 3 years of follow-up, whereas in a separate analysis of 381 untreated del(5q) MDS patients, the rate of AML evolution was 17% at 5 years.12 Predictors of AML evolution among 286 del(5q) patients treated with lenalidomide from MDS-003 and MDS-004 included advanced disease, 2 or more additional cytogenetic abnormalities, and baseline high transfusion burden, none of which pertained to our patient when he started disease-modifying therapy. Taking a wider view, though, he had already carried his diagnosis for 6 years when he lost his response, about the time one would expect his disease to evolve, based on his IPSS score at diagnosis. It is also possible our patient had a phenotypically distinct population of del(5q) MDS stem cells with refractoriness to lenalidomide, as identified by Tehranchi et al.13 Once a patient fails lenalidomide (and this eventually happens to all treated patients, as the therapy is not curative), it is reasonable to try a hypomethylating agent, such as azacitidine; or with higher-risk MDS subtypes, proceed to transplantation. How these patients fare after lenalidomide failure has not been well characterized.
This case demonstrates the natural history, over 7 years, of a typical del(5q) MDS patient treated successfully with lenalidomide, particularly the predictors of response, the ability to treat through adverse events, and the inevitable disease progression.
Authorship
Contribution: S.M. and M.A.S. wrote the manuscript; and R.V.T. edited the manuscript and created the figure.
Conflict-of-interest disclosure: M.A.S. has served on an advisory board for Celgene Corp. The remaining authors declare no competing financial interests.
Correspondence: Mikkael A. Sekeres, Leukemia Program, Department of Hematologic Oncology and Blood Disorders, Cleveland Clinic Taussig Cancer Institute, Desk R35, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: sekerem@ccf.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal