Antithymocyte globulin (ATG) + cyclosporine is effective in restoring hematopoiesis in severe aplastic anemia (SAA). We hypothesized that the humanized anti-CD52 mAb alemtuzumab might be active in SAA because of its lymphocytotoxic properties. We investigated alemtuzumab monotherapy from 2003-2010 in treatment-naive, relapsed, and refractory SAA in 3 separate research protocols at the National Institutes of Health. Primary outcome was hematologic response at 6 months. For refractory disease, patients were randomized between rabbit ATG + cyclosporine (n = 27) and alemtuzumab (n = 27); the response rate for alemtuzumab was 37% (95% confidence interval [CI], 18%-57%) and for rabbit ATG 33% (95% CI, 14%-52%; P = .78). The 3-year survival was 83% (95% CI, 68%-99%) for alemtuzumab and 60% (95% CI, 43%-85%) for rabbit ATG (P = .16). For relapsed disease (n = 25), alemtuzumab was administered in a single-arm study; the response rate was 56% (95% CI, 35%-77%) and the 3-year survival was 86% (95% CI, 72%-100%). In treatment-naive patients (n = 16), alemtuzumab was compared with horse and rabbit ATG in a 3-arm randomized study; the response rate was 19% (95% CI 0%-40%), and the alemtuzumab arm was discontinued early. We conclude that alemtuzumab is effective in SAA, but best results are obtained in the relapsed and refractory settings. The present trials were registered at www.clinicaltrials.gov as NCT00195624, NCT00260689, and NCT00065260.
Introduction
Severe acquired aplastic anemia (SAA) is a hematologic disease characterized by pancytopenia and a hypoproliferative BM. Although the ultimate etiology of aplastic anemia is not known, clinical experience and laboratory data implicate a proximate mechanism of immune-mediated destruction of hematopoietic progenitor and stem cells.1 Therapies directed at suppressing the immune system are an alternative to hematopoietic stem cell transplantation (HSCT) in SAA.2,3 Horse antithymocyte globulin (ATG) + cyclosporine, the most well-studied regimen, produces a hematologic response in 60%-70% of patients when used as first therapy. However, relapse occurs in approximately 30% of responding patients, and clonal evolution occurs in approximately 10%-15%.1 Therefore, unresponsiveness to initial immunosuppression, relapse, and clonal evolution have limited the success of horse ATG + cyclosporine in SAA.4
Although ATG + cyclosporine can be administered to the majority of patients, the associated toxicities are not minor. ATG administration causes: (1) infusion-related toxicity, manifested as fevers, rigors, urticarial cutaneous eruption, and in some cases, hypotension and hypoxemia; (2) serum sickness 1-2 weeks after administration of ATG, characterized by fever, a cutaneous eruption, arthralgia, myalgia, and nonspecific gastrointestinal and neurologic symptoms; and (3) transient blood count depression, which may lead to a temporary increase in transfusion requirements. Hypertension and azotemia are serious toxicities of cyclosporine, and hirsutism, gingival hyperplasia, hypomagnesemia, and neurologic symptoms are also common.
Horse ATG is considered moderately lymphocytotoxic through the action of polyclonal Abs that produce transient lymphodepletion (usually 1-2 weeks duration) and longer elimination of activated T cells, which are assumed to contribute to the induction of tolerance.5,–7 Rabbit ATG is more efficient at depleting peripheral blood lymphocytes in vivo and is more cytotoxic on a weight basis in vitro.8,9 In randomized studies, rabbit ATG has been reported to be more effective than horse ATG in preventing and reversing acute renal allograft rejection,10,11 and in SAA, rabbit ATG + cyclosporine has been shown to be effective in the relapse and refractory settings.12,13 However, the efficacy of rabbit ATG + cyclosporine as first line was disappointing, with a lower hematologic response rate compared to that of horse ATG + cyclosporine.14
Lymphocytotoxic therapies that are better tolerated and do not require concomitant cyclosporine use are an attractive alternative to ATG + cyclosporine. As a successful example, daclizumab, a genetically engineered human IgG1 specific to the α subunit of the IL-2 receptor, has resulted in responses of approximately 40% in patients with moderate aplastic anemia.15 In SAA, we hypothesized that alemtuzumab (Campath-1H), a humanized IgG1 mAb directed against the CD52 protein, might have activity. Alemtuzumab produces more durable lymphopenia (compared with horse ATG), which has made it an attractive agent in a wide range of autoimmune diseases, lymphoid malignancies, and in transplantation.9,16,,,,–21 Furthermore, in a diverse population of 21 patients with severe autoimmune cytopenias resistant to standard therapies, alemtuzumab was used as salvage therapy with some success: responses were observed in 15 patients.22 Based on these experiences, we conducted prospective studies using alemtuzumab in various settings. We report herein the largest prospective experience of alemtuzumab in SAA patients in the treatment-naive, relapsed, and refractory settings.
Methods
Patients
All consecutive patients who fulfilled protocol entry criteria were enrolled into 3 different treatment protocols from November 2003 to August 2010 at the Warren Grant Magnuson Clinical Center and the Mark O. Hatfield Clinical Research Center at the National Institutes of Health. All adult patients or parents (or legal guardians) of children < 18 years of age signed informed consent following the Declaration of Helsinki, according to protocols approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute (NHLBI). In the randomized studies, assignment to treatment was in blocks, with the assignment probability fixed over the course of the trial. Construction of the randomization schedule was done using a table of random numbers and was conducted by the Pharmacy Department at the Clinical Center of the National Institutes of Health. The trials were registered at www.clinicaltrials.gov as NCT00195624, NCT00260689, and NCT00065260.
All patients older than 2 years with SAA were eligible for these studies. For protocol entry purposes, SAA was defined as BM cellularity < 30% and severe pancytopenia with at least 2 of the following peripheral blood count criteria: absolute neutrophil count < 0.5 × 109/L, absolute reticulocyte count < 60 × 109/L, or platelet count < 20 × 109/L.23 Exclusion criteria were: creatinine > 2.5 mg/dL, underlying carcinoma, a diagnosis of Fanconi anemia, prior history of immunosuppressive therapy with alemtuzumab, human immunodeficiency virus seropositivity, evidence of a clonal disorder on BM cytogenetics, pregnancy, inability to understand the investigational nature of the study, or significant comorbidities such that imminent death was likely.
BM biopsy and aspiration for morphology and cytogenetics were performed before enrollment, 6 and 12 months after immunosuppressive therapy, and then yearly thereafter. Children and young adults (< 40 years of age) had chromosomes assayed after in vitro exposure of peripheral blood lymphocytes to diepoxybutane and mitomycin C to exclude Fanconi anemia. All patients were tested for paroxysmal nocturnal hemoglobinuria with a flow cytometric assay24 ; the presence of a clone was defined as the absence of glycosylphosphatidylinositol-anchored surface proteins in > 1% of neutrophils or RBCs.
Study design
Refractory SAA.
For patients who were nonresponders (or had a nonrobust response4 ) to initial horse ATG + cyclosporine, a second course of immunosuppression was administered after randomization between rabbit ATG + cyclosporine (the reference arm) or alemtuzumab (investigational arm; refractory study). Patients who did not respond to rabbit ATG were offered the option to “cross over” to the alemtuzumab arm in the same protocol and, conversely, those unresponsive to alemtuzumab could receive rabbit ATG + cyclosporine (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Relapsed SAA.
In patients who relapsed after horse or rabbit ATG–based immunosuppression, alemtuzumab was administered in a nonrandomized, single-arm study (relapse study; supplemental Figure 1B). If relapse reoccurred after successful treatment with alemtuzumab, cyclosporine could be instituted in the same protocol.
Treatment-naive SAA.
Alemtuzumab also was administered to treatment-naive patients as part of a 3-arm randomized study that was designed to compare horse ATG + cyclosporine, rabbit ATG + cyclosporine, and alemtuzumab as initial therapy (treatment-naive study; supplemental Figure 1C).
Immunosuppressive regimens
Rabbit ATG (thymoglobulin; Genzyme) was administered at a dose of 3.5 mg/kg/d for 5 consecutive days and cyclosporine at 10 mg/kg/d by mouth, as described previously.12 Dosing was adjusted to maintain cyclosporine plasma levels between 200 and 400 ng/mL. After a 1 mg test dose, alemtuzumab (Campath; Genzyme) was administered at 10 mg/dose/d for 10 days as an IV infusion over 2 hours.22 Children < 50 kg received alemtuzumab 0.2 mg/kg/d for 10 days (not to exceed 10 mg/d). In contrast to horse or rabbit ATG, cyclosporine was not administered with alemtuzumab. The alemtuzumab regimen was identical in all protocols.
As prophylaxis for Pneumocystis carinii pneumonia, all patients received aerosolized pentamidine monthly for at least 6 months. Daily valacyclovir at a dose of 500 mg daily for at least 8 weeks was used as Herpes simplex virus prophylaxis. In the alemtuzumab-treated patients, Pneumocystis carinii and antiviral prophylaxis were continued until CD4+ T cells were > 0.2 × 109/L, and ciprofloxacin 500 mg twice daily was given until the absolute neutrophil count was > 0.2 × 109/L. G-CSF and prophylactic antifungal therapy were not routinely administered with any of the immunosuppression regimens. Molecular monitoring for EBV and CMV was performed at baseline, weekly for the first month after each treatment course, every 2 weeks in the second month, and monthly thereafter for another 6 months.9 EBV and CMV quantitative real-time PCR was performed as described previously.25,26 A positive PCR was defined as > 250 EBV copies/106 mononuclear cell genome equivalents or > 250 CMV copies/mL blood. Because of reports of cardiotoxicity in alemtuzumab-treated patients,27 a 2D echocardiogram, 24-hour Holter monitoring, and troponin levels were performed before and after alemtuzumab infusion.
Study end points
Hematologic response was defined as no longer meeting the criteria for SAA,23 and a robust response was defined as platelet or absolute reticulocyte count of > 50 × 109/L at 3 months.4 Patients who relapsed by definition required reinstitution or augmentation of the dose of cyclosporine or administration of another course of immunosuppression.4 The majority of relapsed patients again met criteria for SAA, but in some, pancytopenia was moderate when further immunosuppression was instituted for declining blood counts. In these cases, hematologic response was defined by prespecified improvements in blood counts (supplemental Figure 2).
The primary end point for all studies was hematologic response at 6 months. Secondary end points included the hematologic response at 3 months and yearly, robustness of hematologic response, relapse, clonal evolution to myelodysplasia or acute leukemia, and overall survival.
Statistical methods
For the refractory study, sample size was based on the primary end point, hematologic response at 6 months. At 5% significance level and 80% power in a 2-sided test, 50 patients per treatment arm were required to detect a 30% difference for the response rate (our hypothesis). To allow for possible attrition such as early withdrawals, a total accrual of 120 patients was intended originally with a planned interim analysis when 25 patients per arm were accrued. For the relapsed study, sample size was calculated using a 2-stage Minimax design28 based on the hypothesis that response at 6 months (the primary end point) the alemtuzumab level would be > 70%. Sample size was determined by testing the null hypothesis (response rate < 50%) versus the alternative (response rate > 70%) at 5% significance level and 80% power in a 2-sided test. For the 2-stage design, 23 patients were accrued in the first stage and if 13 or more responded, a subsequent 13 patients would be entered into a second stage. For the treatment-naive study, the sample size was computed based on the assumption that the response rate at 6 months (primary end point) for rabbit ATG + cyclosporine and alemtuzumab would be 25% higher than the historical response rate for horse ATG + cyclosporine in this setting at our institution (60%). Based on normal approximations for testing proportions and Bonferroni adjustment for 2-way multiple comparisons, the study design required 60 subjects per arm to have a power of 80% (2-sided) at 5% significance. However, following recommendations from the NHLBI Data Safety and Monitoring Board, the alemtuzumab arm of the study was terminated early because of a low response rate at 6 months, and thus comparison between alemtuzumab and other arms of the treatment-naive study was not performed.
Patient characteristics were described using summary statistics including means, proportions, standard errors, and 95% confidence intervals (CIs). The significance for comparing these patient characteristics between the 2 treatment groups was calculated based on the t tests. Survival analyses based on the Kaplan-Meier method and the Cox proportional hazard models were used to draw inferences about the distributions of the overall survival time between the 2 treatments. Cumulative event distributions were analyzed using the Kaplan-Meier method and the Cox proportional hazard models for time to relapse among patients who responded to the first immunosuppression treatments and time to evolution for all patients. For the analyses of time-to-relapse and time-to-evolution distributions, patients who had died or who underwent SCT before these events were counted as censored. Log-rank P values were based on the Cox proportional hazard models, and were used to compare the survival and cumulative event curves between the 2 treatments. Stopping rules for toxicity and futility were described in all protocols. Numerical results were computed using the S-PLUS 8.0 software package (TIBCO). A 2-sided P value was used throughout and considered statistically significant if < .05. Analysis was based on intention to treat.
Results
A total of 90 patients 3-75 years of age received alemtuzumab in 3 different research protocols to investigate the role of alemtuzumab in SAA. The distribution of patients who received alemtuzumab were as follows: 27 were in the refractory study (which randomized between alemtuzumab and rabbit ATG + cyclosporine), 25 were in the relapsed study (single arm), 16 in the treatment-naive study, 12 patients received alemtuzumab (as salvage) after unresponsiveness to initial rabbit ATG + cyclosporine, and 10 patients received alemtuzumab after failing both horse and rabbit ATG (supplemental Figure 1). Patient characteristics for all studies are shown in Table 1, and a summary of the serious adverse events (requiring hospitalization) is shown in Table 2. There were 7 cases of thyroid abnormalities in alemtuzumab-treated patients: 5 developed hypothyroidism and 2 hyperthyroidism. All patients with thyroid toxicities were managed as outpatients and became euthyroid with conventional therapies. In all hypothyroid patients, alemtuzumab was administered for refractory disease. Of the hyperthyroid patients, one had relapsed SAA and the other had received alemtuzumab after unresponsiveness to initial rabbit ATG + cyclosporine.
Patient characteristics
| Characteristic . | Refractory study . | P . | Relapsed study . | Treatment-naive study . | |
|---|---|---|---|---|---|
| Rabbit ATG (n = 27) . | Alemtuzumab (n = 27) . | Alemtuzumab (n = 25) . | Alemtuzumab (n = 16) . | ||
| Age, y | 40.1 ± 3.8 | 37.2 ± 4.5 | .63 | 39.9 ± 4.5 | 39.2 ± 6.1 |
| Age < 18 y, % | 11.1 ± 6.2 | 25.9 ± 8.6 | .17 | 12 ± 6.6 | 25.0 ± 11.2 |
| Male sex, % | 59.3 ± 9.6 | 51.9 ± 9.8 | .59 | 44 ± 10.1 | 75.0 ± 11.2 |
| Etiology, % | |||||
| Idiopathic | 100 | 92.6 ± 5.1 | .16 | 100 | 93.8 ± 6.3 |
| Posthepatitis | 0 | 7.4 ± 5.1 | .16 | 0 | 6.2 ± 6.3 |
| Blood counts, × 109/L | |||||
| ARC | 21.267 ± 4.064 | 27.048 ± 4.815 | .36 | 35.852 ± 6.452 | 27.394 ± 5.980 |
| ALC | 1.078 ± 0.053 | 0.956 ± 0.086 | .24 | 1.329 ± 0.126 | 1.676 ± 0.170 |
| ANC | 0.616 ± 0.137 | 0.717 ± 0.149 | .62 | 0.595 ± 0.079 | 0.372 ± 0.065 |
| ANC < 0.2, % | 25.9 ± 8.6 | 11.1 ± 6.2 | .168 | 8.0 ± 5.5 | 31.3 ± 12.0 |
| Platelets | 10.654 ± 1.482 | 14.037 ± 1.722 | .146 | 14.880 ± 1.820 | 9.188 ± 2.118 |
| PNH clone < 1%, % | 56.0 ± 9.7 | 77.7 ± 8.2 | .10 | 64.0 ± 9.8 | 50.0 ± 12.9 |
| PNH clone ≥ 1%, % | 44.0 ± 9.7 | 22.3 ± 8.2 | .10 | 36.0 ± 9.8 | 50.0 ± 12.9 |
| Characteristic . | Refractory study . | P . | Relapsed study . | Treatment-naive study . | |
|---|---|---|---|---|---|
| Rabbit ATG (n = 27) . | Alemtuzumab (n = 27) . | Alemtuzumab (n = 25) . | Alemtuzumab (n = 16) . | ||
| Age, y | 40.1 ± 3.8 | 37.2 ± 4.5 | .63 | 39.9 ± 4.5 | 39.2 ± 6.1 |
| Age < 18 y, % | 11.1 ± 6.2 | 25.9 ± 8.6 | .17 | 12 ± 6.6 | 25.0 ± 11.2 |
| Male sex, % | 59.3 ± 9.6 | 51.9 ± 9.8 | .59 | 44 ± 10.1 | 75.0 ± 11.2 |
| Etiology, % | |||||
| Idiopathic | 100 | 92.6 ± 5.1 | .16 | 100 | 93.8 ± 6.3 |
| Posthepatitis | 0 | 7.4 ± 5.1 | .16 | 0 | 6.2 ± 6.3 |
| Blood counts, × 109/L | |||||
| ARC | 21.267 ± 4.064 | 27.048 ± 4.815 | .36 | 35.852 ± 6.452 | 27.394 ± 5.980 |
| ALC | 1.078 ± 0.053 | 0.956 ± 0.086 | .24 | 1.329 ± 0.126 | 1.676 ± 0.170 |
| ANC | 0.616 ± 0.137 | 0.717 ± 0.149 | .62 | 0.595 ± 0.079 | 0.372 ± 0.065 |
| ANC < 0.2, % | 25.9 ± 8.6 | 11.1 ± 6.2 | .168 | 8.0 ± 5.5 | 31.3 ± 12.0 |
| Platelets | 10.654 ± 1.482 | 14.037 ± 1.722 | .146 | 14.880 ± 1.820 | 9.188 ± 2.118 |
| PNH clone < 1%, % | 56.0 ± 9.7 | 77.7 ± 8.2 | .10 | 64.0 ± 9.8 | 50.0 ± 12.9 |
| PNH clone ≥ 1%, % | 44.0 ± 9.7 | 22.3 ± 8.2 | .10 | 36.0 ± 9.8 | 50.0 ± 12.9 |
P refers to the comparison between rabbit ATG and alemtuzumab in the refractory study.
ARC indicates absolute reticulocyte count; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; and PNH, paroxysmal nocturnal hemoglobinuria.
Summary of serious adverse events
| . | Refractory study . | Relapsed study . | Treatment-naive study . | |
|---|---|---|---|---|
| Rabbit ATG (n = 27) . | Alemtuzumab (n = 27) . | Alemtuzumab (n = 25) . | Alemtuzumab (n = 16) . | |
| Serum sickness, n | 2 | 0 | 0 | 0 |
| Hemorrhage, n | ||||
| Gastrointestinal | 2 | 0 | 2 | 0 |
| Gingival | 2 | 0 | 0 | 0 |
| Epistaxis | 1 | 0 | 0 | 0 |
| Infection, n | ||||
| Neutropenic fever, negative cultures | 6 | 8 | 11 | 10 |
| Neutropenic fever, positive cultures | 4 | 5 | 4 | 3 |
| Pneumonia | 0 | 2 | 1 | 3 |
| Urinary tract | 0 | 1 | 1 | 1 |
| Clostridium difficile | 0 | 1 | 0 | 2 |
| Candidemia | 0 | 0 | 1 | 0 |
| Septic shock | 0 | 1 | 0 | 0 |
| Perirectal abscess | 0 | 0 | 2 | 0 |
| Cervical abscess | 0 | 0 | 1 | 0 |
| Skin abscess | 0 | 0 | 1 | 0 |
| Cholecystitis | 0 | 1 | 0 | 0 |
| Tonsillitis/pharyngitis | 0 | 1 | 0 | 0 |
| PML | 0 | 0 | 1 | 0 |
| Cellulitis | 0 | 0 | 1 | 0 |
| Viral exanthema | 0 | 1 | 0 | 0 |
| Upper respiratory infection | 2 | 2 | 2 | 1 |
| Parotitis | 0 | 1 | 0 | 0 |
| Other | 3 | 1 | 7 | 3 |
| . | Refractory study . | Relapsed study . | Treatment-naive study . | |
|---|---|---|---|---|
| Rabbit ATG (n = 27) . | Alemtuzumab (n = 27) . | Alemtuzumab (n = 25) . | Alemtuzumab (n = 16) . | |
| Serum sickness, n | 2 | 0 | 0 | 0 |
| Hemorrhage, n | ||||
| Gastrointestinal | 2 | 0 | 2 | 0 |
| Gingival | 2 | 0 | 0 | 0 |
| Epistaxis | 1 | 0 | 0 | 0 |
| Infection, n | ||||
| Neutropenic fever, negative cultures | 6 | 8 | 11 | 10 |
| Neutropenic fever, positive cultures | 4 | 5 | 4 | 3 |
| Pneumonia | 0 | 2 | 1 | 3 |
| Urinary tract | 0 | 1 | 1 | 1 |
| Clostridium difficile | 0 | 1 | 0 | 2 |
| Candidemia | 0 | 0 | 1 | 0 |
| Septic shock | 0 | 1 | 0 | 0 |
| Perirectal abscess | 0 | 0 | 2 | 0 |
| Cervical abscess | 0 | 0 | 1 | 0 |
| Skin abscess | 0 | 0 | 1 | 0 |
| Cholecystitis | 0 | 1 | 0 | 0 |
| Tonsillitis/pharyngitis | 0 | 1 | 0 | 0 |
| PML | 0 | 0 | 1 | 0 |
| Cellulitis | 0 | 0 | 1 | 0 |
| Viral exanthema | 0 | 1 | 0 | 0 |
| Upper respiratory infection | 2 | 2 | 2 | 1 |
| Parotitis | 0 | 1 | 0 | 0 |
| Other | 3 | 1 | 7 | 3 |
Serious adverse events depicted are those that resulted in prolonged hospitalization, hospital admission, or death. Events shown are those that occurred after the initial cycle of immunosuppression for each study. Repeated hospitalizations in the same subject were counted as separate events.
PML indicates progressive multifocal leukoencephalopathy.
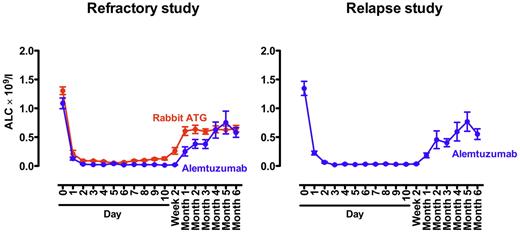
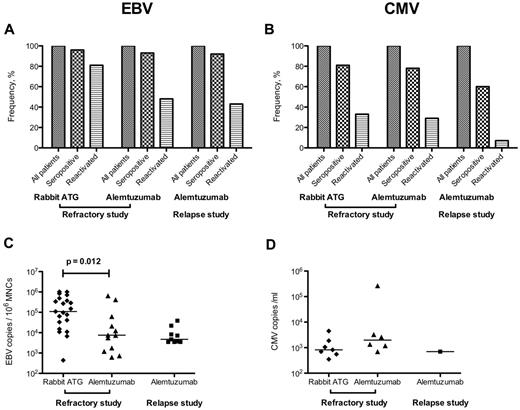
Alemtuzumab generally was well tolerated. Labeled infusion-related reactions were common and managed symptomatically. All patients completed the planned 10-day infusion of alemtuzumab. Increases in liver function tests with alemtuzumab infusion were common and in the randomized refractory study were comparable to those observed with rabbit ATG + cyclosporine (supplemental Figure 3). Liver function tests normalized over time in both groups. Lymphodepletion was universal and prolonged (Figure 1). In the refractory randomized study, the degree of lymphopenia was similar between both arms (rabbit ATG and alemtuzumab; Figure 1), with the lymphocyte count not yet reaching pretreatment levels by 6 months. Subclinical EBV and CMV reactivations were common, as described previously.9 There were no cases of EBV or CMV disease and prophylactic or preemptive therapy was not used in any case. An updated depiction of EBV and CMV reactivations for both the refractory and relapsed study is shown in Figure 2. There was no evidence of cardiotoxicity from alemtuzumab in any protocol. Troponin levels did not increase during or after the 10-day infusion, and the ejection fraction remained essentially unchanged pretreatment, after the 10-day infusion, and at 3 months after alemtuzumab treatment (supplemental Figure 4).
Absolute lymphocyte count (ALC) after rabbit ATG and alemtuzumab. In the refractory study (left panel), the degree and duration of lymphopenia was similar between the rabbit ATG (red) and alemtuzumab arms (blue). The baseline ALC had not been reached for either group by 6 months. In the relapsed study (right panel), the pattern of lymphopenia was similar to that observed in the alemtuzumab arm in the refractory study. The mean ± SEM is shown.
Absolute lymphocyte count (ALC) after rabbit ATG and alemtuzumab. In the refractory study (left panel), the degree and duration of lymphopenia was similar between the rabbit ATG (red) and alemtuzumab arms (blue). The baseline ALC had not been reached for either group by 6 months. In the relapsed study (right panel), the pattern of lymphopenia was similar to that observed in the alemtuzumab arm in the refractory study. The mean ± SEM is shown.
EBV and CMV reactivations after immunosuppression in the refractory and relapse studies. Nearly all patients were seropositive for EBV (A), whereas CMV seropositivity ranged from 60%-80% (B). Of the seropositive patients, EBV reactivation in the rabbit ATG arm was observed in approximately 80%, with median peak copy numbers of approximately 100 000 copies per 106 mononuclear cell (MNC) genome equivalents (C). In the alemtuzumab arm, EBV reactivations were less frequent and the median peak copy numbers were much lower compared with rabbit ATG (C). There was no difference in the likelihood or degree of reactivation after alemtuzumab in the refractory and relapsed studies. CMV reactivations were less common for both rabbit ATG and alemtuzumab, with median peak copy numbers of approximately 1000/mL. Only one patient in the relapsed study had reactivated CMV after alemtuzumab. All reactivations were self-limited and subclinical with prophylactic or preemptive therapies not used in any case. A positive PCR was defined as > 250 EBV copies/106 MNC genome equivalents or > 250 CMV copies/mL of blood.
EBV and CMV reactivations after immunosuppression in the refractory and relapse studies. Nearly all patients were seropositive for EBV (A), whereas CMV seropositivity ranged from 60%-80% (B). Of the seropositive patients, EBV reactivation in the rabbit ATG arm was observed in approximately 80%, with median peak copy numbers of approximately 100 000 copies per 106 mononuclear cell (MNC) genome equivalents (C). In the alemtuzumab arm, EBV reactivations were less frequent and the median peak copy numbers were much lower compared with rabbit ATG (C). There was no difference in the likelihood or degree of reactivation after alemtuzumab in the refractory and relapsed studies. CMV reactivations were less common for both rabbit ATG and alemtuzumab, with median peak copy numbers of approximately 1000/mL. Only one patient in the relapsed study had reactivated CMV after alemtuzumab. All reactivations were self-limited and subclinical with prophylactic or preemptive therapies not used in any case. A positive PCR was defined as > 250 EBV copies/106 MNC genome equivalents or > 250 CMV copies/mL of blood.
Alemtuzumab in patients unresponsive to initial horse ATG therapy (refractory study)
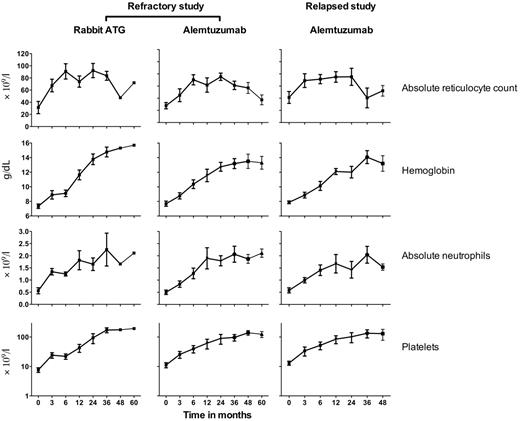
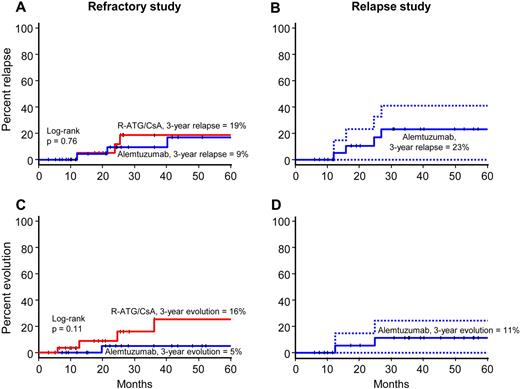
The activity of alemtuzumab in patients unresponsive to initial horse ATG + cyclosporine was evaluated in a study conducted between 2003 and 2010, in which patients were randomized between rabbit ATG + cyclosporine and alemtuzumab. Rabbit ATG was considered the reference arm. After a planned interim analysis conducted when 25 patients were evaluable for 6-month response, the study was closed by the Data Safety and Monitoring Board because there was no statistical difference in the primary outcomes between the 2 groups and the probability of detecting a significant difference with further accrual was remote. In total, 54 patients were enrolled; 27 were randomized to rabbit ATG + cyclosporine and 27 to alemtuzumab (supplemental Figure 5). All patients in the rabbit ATG arm were evaluable at 3 and 6 months, and all but 1 patient was evaluable at 3 and 6 months in the alemtuzumab arm (because of a single early death from infectious complications). The overall response rates for alemtuzumab was 37% (95% CI, 18%-57%) and for rabbit ATG 33% (95% CI, 14%-52%; Table 3; log-rank P = .78). One patient in the alemtuzumab arm responded between 6 and 12 months. The increment in blood counts among responders in both arms was comparable (Figure 3). Three patients in each group later relapsed. The cumulative incidence of relapse at 3 years for rabbit ATG was 19% (95% CI, 0%-36%) and for alemtuzumab 9% (95% CI, 0%-21%; Figure 4; P = .76). Dispositions among nonresponders in both arms are shown as supplemental Figure 6.
Response rate for alemtuzumab in refractory, relapsed, and treatment-naive SAA
| Response . | Refractory study (n = 54) . | P . | Relapse study (n = 25) . | Treatment-naive study (n = 16) . | |
|---|---|---|---|---|---|
| Rabbit ATG (95% CI) . | Alemtuzumab (95% CI) . | Alemtuzumab (95% CI) . | Alemtuzumab (95% CI) . | ||
| 3-mo | 19% (3-34) | 19% (3-34) | 1.00 | 48% (27-69) | 19% (0-40) |
| 6-mo | 33% (14-52) | 37% (18-57) | .78 | 56% (35-77) | 19% (0-40) |
| Response . | Refractory study (n = 54) . | P . | Relapse study (n = 25) . | Treatment-naive study (n = 16) . | |
|---|---|---|---|---|---|
| Rabbit ATG (95% CI) . | Alemtuzumab (95% CI) . | Alemtuzumab (95% CI) . | Alemtuzumab (95% CI) . | ||
| 3-mo | 19% (3-34) | 19% (3-34) | 1.00 | 48% (27-69) | 19% (0-40) |
| 6-mo | 33% (14-52) | 37% (18-57) | .78 | 56% (35-77) | 19% (0-40) |
In the refractory study, patients initially unresponsive to horse ATG + cyclosporine were randomized to rabbit ATG + cyclosporine (n = 27) and alemtuzumab (n = 27). In patients with relapsed disease, alemtuzumab was administered in a single-arm (nonrandomized) study. P is for the comparison between rabbit ATG and alemtuzumab in the refractory study.
Increase in blood counts after immunosuppression among responders in the refractory and relapsed studies. In the refractory study, the increment in blood counts was similar in the rabbit ATG and alemtuzumab arms. In the relapsed study, the increment in blood counts after alemtuzumab was similar to the same regimen in the refractory study. Only 1 responder in the rabbit ATG arm is shown for 4- and 5-year follow-up. Blood counts after relapse are not shown because alternative therapies were sought. The mean ± SEM is shown.
Increase in blood counts after immunosuppression among responders in the refractory and relapsed studies. In the refractory study, the increment in blood counts was similar in the rabbit ATG and alemtuzumab arms. In the relapsed study, the increment in blood counts after alemtuzumab was similar to the same regimen in the refractory study. Only 1 responder in the rabbit ATG arm is shown for 4- and 5-year follow-up. Blood counts after relapse are not shown because alternative therapies were sought. The mean ± SEM is shown.
Cumulative incidence or relapse and clonal evolution. In the refractory study (left panels), the rate of relapse was comparable between arms (A), whereas the rate of clonal evolution (C) was higher with rabbit ATG (red) compared with alemtuzumab (blue), but this difference was not statistically significant. In the relapsed study (right panels), the rate of a second relapse after alemtuzumab was 23% (B) and the clonal evolution rate 11% (D). For the relapse study panels, dotted lines represent 95% CI.
Cumulative incidence or relapse and clonal evolution. In the refractory study (left panels), the rate of relapse was comparable between arms (A), whereas the rate of clonal evolution (C) was higher with rabbit ATG (red) compared with alemtuzumab (blue), but this difference was not statistically significant. In the relapsed study (right panels), the rate of a second relapse after alemtuzumab was 23% (B) and the clonal evolution rate 11% (D). For the relapse study panels, dotted lines represent 95% CI.
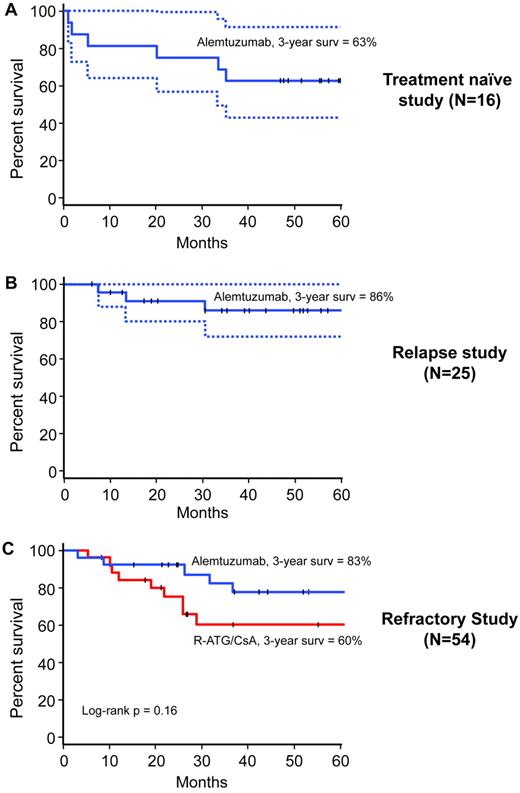
Clonal evolution was observed in 5 patients, 4 in the rabbit ATG arm (2 with monosomy 7, 1 with t(6;14), and 1 with trisomy 6), and 1 in the alemtuzumab arm (with monosomy 7). The 3-year cumulative incidence of clonal evolution for rabbit ATG was 16% (95% CI, 0%-32%) and for alemtuzumab 5% (95% CI, 0%-14%; Figure 4; P = .11). The 3-year survival of patients treated with rabbit ATG was 60% (95% CI, 43%-85%) and for those who received alemtuzumab 83% (95% CI, 68%-99%; log-rank P = .16; Figure 5). Median survival had not been reached in either group at a median follow-up of 36 months (range, 3-87) for all patients and 63 months (range, 8-87) in surviving patients (Figure 5). In the rabbit ATG arm, causes of death were: clonal evolution (n = 1), septicemia (n = 1), fungal infection (n = 2), heart failure (n = 1), HSCT related (n = 1), CNS hemorrhage (n = 1), traffic accident (n = 1), and unknown (n = 1); and in the alemtuzumab arm, HSCT-related (n = 1), septicemia (n = 1), pneumonia (n = 1), and unknown (n = 2). Therefore, alemtuzumab has activity in SAA and the hematologic response rate observed in those refractory to initial horse ATG + cyclosporine is comparable to that of rabbit ATG + cyclosporine.
Overall survival. In the treatment-naive study (A), 3-year survival was 63%. In the relapsed study (B), 3-year survival was 86%. In the refractory study (C), 3-year survival in the alemtuzumab arm (blue) was approximately 20% higher than rabbit ATG (red), but this difference was not statistically significant. The dotted lines in the treatment-naïve and relapse study panels represent 95% CI. The median survival was not reached in any of the treatment arms in any of the studies.
Overall survival. In the treatment-naive study (A), 3-year survival was 63%. In the relapsed study (B), 3-year survival was 86%. In the refractory study (C), 3-year survival in the alemtuzumab arm (blue) was approximately 20% higher than rabbit ATG (red), but this difference was not statistically significant. The dotted lines in the treatment-naïve and relapse study panels represent 95% CI. The median survival was not reached in any of the treatment arms in any of the studies.
Alemtuzumab in patients who relapsed after successful treatment with initial horse ATG regimens
The activity of alemtuzumab in relapsed SAA was evaluated in a single-arm, nonrandomized study that began in 2005 in patients who had relapsed after successful treatment with initial ATG + cyclosporine. A total of 25 patients 8-75 years of age were enrolled; 22 had relapsed after an initial horse ATG regimen and 3 had relapsed twice, once after the initial horse ATG regimen and then again after the rabbit ATG regimen (administered for the first relapse). The overall response rate to alemtuzumab was 56% (95% CI, 35%-77%; Table 3). The increment in blood counts among responders is shown in Figure 3. Of the 11 nonresponders, 5 were retreated with rabbit ATG + cyclosporine, 2 underwent HSCT, and 4 are on supportive care. Four of the 14 responders later relapsed; further immunosuppression was pursued in 3, of whom 2 responded. The cumulative incidence of relapse at 3 years was 23% (95% CI, 0-41; Figure 4). There were 2 clonal evolution events in the relapsed cohort, 1 to complex cytogenetics and 1 to deletion 13q (Figure 4). The cumulative incidence of clonal evolution at 3 years was 11% (95% CI, 0%-25%; Figure 4). The median follow-up was 39 months (range, 6-63) for all patients and for those surviving, 42 months (range, 6-63). The overall survival at 3 years was 86% (95% CI, 72%-100%; Figure 5). Therefore, alemtuzumab is also active in relapsed SAA with a response rate comparable to previously reported experience with rabbit ATG + cyclosporine in this setting.12
Alemtuzumab in treatment-naive patients
Based on these encouraging results in refractory and relapsed SAA, alemtuzumab was investigated in treatment-naive patients in a 3-arm study that began in 2006. This study randomized patients among horse ATG + cyclosporine, rabbit ATG + cyclosporine, and alemtuzumab monotherapy. However, after 16 patients had been randomized to alemtuzumab, this part of the study was discontinued at the recommendation of the Data Safety and Monitoring Board. Hematologic response had been observed in only 3 patients and there were 3 early deaths. Eight patients who were unresponsive to initial alemtuzumab then received rabbit ATG + cyclosporine and 5 have responded. At 3 years, the survival was 63% (95% CI, 43%-91%; Figure 5). The causes of death were: clonal evolution (n = 1), fungal pneumonia (n = 1), septicemia (n = 2), necrotizing fasciitis after HSCT (n = 1), and metastatic lung cancer (n = 1). These data suggest that alemtuzumab may not be as effective in treatment-naive patients compared with standard treatment with horse ATG + cyclosporine.
Alemtuzumab in other salvage settings
Alemtuzumab was also investigated in other settings in the context of these clinical trials (supplemental Figure 1). In the treatment-naive study, 13 patients unresponsive to initial rabbit ATG + cyclosporine received salvage alemtuzumab, of whom 1 responded. In total, 10 patients refractory to both horse and rabbit ATG immunosuppression received alemtuzumab as a salvage third course, and hematologic response was observed in 2 (both had shown small but incremental improvement with each prior ATG courses).
Discussion
The introduction of horse ATG + cyclosporine for SAA approximately 20 years ago has led to hematologic recovery in 60%-70% of patients and to long-term survival among responders of > 80%.1 The majority of responses occur within 3 months, with occasional improvement observed between 3 and 6 months (approximately 5%) and even fewer between 6 and 12 months.4 Our efforts (and those of others) have been to develop regimens that address the problems with standard horse ATG + cyclosporine: toxicity, unresponsiveness, relapse, and clonal evolution. In refractory cases, the response rate to a second course of rabbit ATG + cyclosporine has been reported to be 77%,13 but in our experience, was approximately 30% in the same setting.12 In relapsed SAA, reported response rates with retreatment have been more consistent, approximately 50%-60%.12,29,30 Unfortunately, the use of more potent immunosuppressive therapy has been controversial because of toxicity31,32 and the addition of a third drug to horse ATG + cyclosporine has not led to better hematologic recovery or to a decrease in the rate of relapse or clonal evolutions.33,34
In the present study, we report that alemtuzumab is an effective treatment in SAA, with hematologic recovery in 37% in those patients who were unresponsive to initial horse ATG + cyclosporine and 55% in patients in relapse. The response rate in refractory patients was comparable to that observed in the reference arm (rabbit ATG + cyclosporine), and the response rate in relapsed disease is comparable to published results for a repeat course of rabbit ATG + cyclosporine in this setting.12,29,30 Alemtuzumab, as administered in our protocols, has the significant advantage of not requiring cyclosporine, which is associated with significant morbidities, especially with chronic use and in older patients.
Overall, alemtuzumab was well tolerated, with serious adverse events primarily related to complications from cytopenias. There were 7 cases of thyroid abnormalities in alemtuzumab-treated patients, which have been described with this agent in other settings.35,36 All were successfully managed on an outpatient basis. There were no cases of clinically relevant EBV or CMV reactivations. However, 1 patient in the relapse study developed progressive multifocal leukoencephalopathy 1 year after completing alemtuzumab. This patient had a partial hematologic response and achieved transfusion independence soon after completing the alemtuzumab therapy. The neurologic diagnosis 1 year later was based on clinical findings, imaging, and detection of JC virus in cerebrospinal fluid (by PCR). No brain biopsy was performed. The patient received an experimental oral formulation of cidofovir with significant improvement; she is currently followed as an outpatient and has no need for transfusions. This serious neurologic toxicity in this patient appears to be a very rare event, because alemtuzumab has not been particularly associated with this infectious complication. In a recent survey of drug associations with progressive multifocal leukoencephalopathy, drug-induced cases were analyzed in 2 spontaneous adverse drug report (ADR) databases, the US FDA Adverse Event Reporting System (AERS) and the WHO Adverse Drug Reaction Database (VigiBase).37 The US FDA-AERS tracks postmarketing safety surveillance for all FDA-approved drugs, and the WHO-VigiBase tracks reported cases suspected of ADRs from 82 monitoring centers worldwide. In this study, the number of cases associated with alemtuzumab was 3 in FDA-AERS and 8 in WHO-VigiBase. However, the strength of the association cannot be determined by this reporting, because the setting in which the encephalopathy occurred and the associated drugs administered sequentially or concomitantly with alemtuzumab are not provided. In the same study, there were 78 cases of progressive multifocal leukoencephalopathy associated with rituximab and 20 with natalizumab, which is consistent with prior reports associating these mAbs to this infection.38,39
Our conclusions regarding the activity of alemtuzumab in SAA is supported by recent small and preliminary reports from investigators in South Korea, Europe, and Mexico. In a pilot dose-escalating study, Kim et al administered alemtuzumab IV (60 mg in 10 and 90 mg in 4 patients) + cyclosporine to 14 patients with SAA, of whom 11 were ATG naive, and hematologic response was observed in 3 of the treatment-naive patients, all at the lower dose of alemtuzumab.40 Risitano et al administered subcutaneous alemtuzumab (103 mg total) + low dose cyclosporine in 6 treatment-naive and 11 refractory patients with SAA; hematologic response was seen in 4 and 3 cases, respectively.41 More recently, in a Mexican pilot study, 14 treatment-naive patients received 50 mg of alemtuzumab with low-dose cyclosporine and hematologic response was observed in 8.42 The Korean experience in treatment-naive patients (3 of 11 responses) resembles our data: 3 of 16 responses in untreated SAA patients. The modest activity of alemtuzumab in treatment-naive patients in our study was surprising and possible explanations are currently being investigated. However, lower doses of alemtuzumab might be more effective in treatment-naive patients, because the Mexican study used half the alemtuzumab dose used in our study and the response rate neared 60%. There are no obvious explanations in patient demographics or pretreatment blood counts that could account for the lower response rate in treatment-naive cases in our study. The reported experience of alemtuzumab in relapsed and refractory SAA is more limited to a few cases, but our data in 52 patients who received alemtuzumab for relapse or refractory disease suggest that this agent might be more effective in these settings. Furthermore, the role for low-dose cyclosporine, which is frequently used in the other pilot studies, requires definition because a cyclosporine-free regimen, as was used in our study, has the advantage of improving tolerability of the immunosuppression. However, it is possible that cyclosporine might have a role in preventing relapse.
In the present study, alemtuzumab in patients who were refractory to both horse and rabbit ATG was not as effective, which is consistent with the reported experience of lack of benefit of a third course of immunosuppression in refractory cases.43 In this setting, our experience with alemtuzumab in 10 patients yielded response in 2, both of whom had increments in the blood counts with previous courses of ATG that were not sufficient for a response. In the 3-arm randomized study, 13 patients unresponsive to an initial course of rabbit ATG + cyclosporine received salvage therapy with alemtuzumab, and response was observed in only one. This suggests that alemtuzumab may not be as effective in those who fail an initial course of rabbit ATG + cyclosporine compared with those who are refractory to initial horse ATG + cyclosporine.
Our study has limitations and strengths. The National Institutes of Health Clinical Center is a federal research hospital and functions as a tertiary referral institution. Our patients may differ in important demographic features such as socioeconomic status and ethnicity and in clinical features from other patient cohorts.44 The Hematology Branch specializes in the diagnosis and treatment of BM failure syndromes, resulting in consistent and advanced care (such as the ready availability of histocompatible blood products, particularly granulocytes), which may influence outcomes.45 Aggregation of sufficient patients has made serial interventional studies feasible,1 but the low incidence of aplastic anemia46 limits the numbers of patients in specific disease categories, and protocols generally require years to accrue sufficient patients prospectively defined to answer important clinical questions.
As described, a few reports on the use of alemtuzumab in aplastic anemia have been published.40,–42 These were small pilot case series initiated after our protocols began and involved heterogeneous patient groups and treatment regimens that were not prospectively registered at www.clinicaltrials.gov. In contrast, the current reporting include trials that were prospectively designed and registered at the outset, with a much larger number of patients in well-defined disease settings in SAA (treatment-naive, relapse, and refractory), which included randomization in patients with treatment-naive (against 2 polyclonal ATGs) and refractory disease (against rabbit ATG). As a result, our studies allow for important inferences on the response rates of alemtuzumab in SAA. Trial registration minimizes the problem of selective or incomplete reporting and helps to avoid publication bias in the reporting of mainly positive results (the “winner's curse”).47 Therefore, our reporting is inclusive of consecutive patients who enrolled in research protocols, all study parameters were defined at the outset, and all patients were accounted for in the analysis and final reporting. Because of the more robust nature of our study design, the greater number of patients accrued in different disease settings, and longer follow-up, these results should allow the rational incorporation of this agent into clinical practice.
In summary, alemtuzumab is an active agent in SAA as monotherapy, with the best results observed in the refractory and relapsed settings. Either rabbit ATG or alemtuzumab can rescue approximately one-third of patients unresponsive to initial horse ATG + cyclosporine (in direct comparison). The response rate of alemtuzumab in relapsed SAA is comparable to the reported response rate of 50%-60% in this setting. The salvage rate with alemtuzumab in those unresponsive to initial rabbit ATG + cyclosporine appears low, and alemtuzumab cannot be recommended as first-line therapy for SAA outside of a clinical research protocol. Alemtuzumab is an appealing alternative to ATG + cyclosporine in the setting of relapsed or refractory disease, especially in those who experienced significant toxicity with prior ATG courses, in older patients, and in those who require chronic use of cyclosporine to maintain adequate blood counts.4,48 Our data also provide a basis for future research. Alemtuzumab should be as effective when administered subcutaneously as when administered intravenously, and the former route would permit outpatient use. The avoidance of cyclosporine in our regimen may make it especially appealing in the treatment of patients with comorbidities (especially renal disease) and in the elderly.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, Bethesda, MD.
National Institutes of Health
Authorship
Contribution: Phillip Scheinberg was the principal investigator for all studies and participated in the primary conception of the study, writing the protocols, execution of the study, data collection, analysis, and interpretation, and drafting of the manuscript; O.N. and B.W. participated in data collection and execution of the study and cared for the patients; Priscila Scheinberg tracked the toxicity data and attended to the protocol's regulatory requirements; C.O.W. participated in the primary conception and design of the study, performed all of the statistical analysis, and critically revised the manuscript; and N.S.Y. participated in the primary conception of the study, writing the protocols, interim discussions, data analysis and interpretation, and critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Phillip Scheinberg, Hematology Branch, NHLBI, 10 Center Dr, Bldg 10 CRC, Rm 3-5140, MSC 1202, Bethesda, MD 20892-1202; e-mail: scheinbp@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal