Abstract
Tissue inhibitor of metalloproteinase-3 (TIMP-3) is one of a family of proteins inhibiting matrix metalloproteinases, which has also been identified as a mediator for checking inflammation. Meanwhile, it is well known that inflammation causes the activation of the immune response. However, it is not clear whether TIMP-3 plays a role in the immune system. In the present study, we demonstrated a novel function of TIMP-3 in Th1/Th2 polarization through its influence on the antigen-presenting cells. First, TIMP-3 was found strikingly up-regulated by IL-4 during the differentiation of human dendritic cells via the p38MAPK pathway. Second, the expression of costimulatory molecule-CD86 was repressed by TIMP-3. Besides, the induction of IL-12 in matured dendritic cells was significantly inhibited in a PI3K-dependent manner. Furthermore, dendritic cells matured in the presence of TIMP-3 could stimulate allogeneic naive T helper (Th) cells to display a prominent Th2 polarization. Importantly, in an autoimmune disorder–primary immune thrombocytopenia, TIMP-3 showed a statistically positive correlation with IL-4 and platelet count, but a negative correlation with IFN-γ in patient blood samples. Collectively, these in vitro and in vivo data clearly suggested a novel role of TIMP-3 in Th1/Th2 balance in humans.
Introduction
Dendritic cells (DCs) are thought to be indispensable to initiate primary immune responses and play a pivotal role in linking the innate immunity and the adaptive immunity.1,2 Their functions directly determine the outcome of T helper (Th) cell–mediated immune responses.3,4 Normally, DCs reside in almost every tissue where they keep an immature state (imDCs) and serve as sentinels for the “dangerous” antigens. On stimulation with antigens or other signals, they undergo maturation and the matured DCs (mDCs) then migrate to the draining lymph nodes, where they present antigens to naive T cells.5,6 In addition to their antigen presenting capability executed by the MHC class II molecules and the costimulatory molecules (CD40, CD80, and CD86), DCs also manipulate the nature and extent of the T cell–mediated immune responses through producing a variety of cytokines in response to the TLR ligands.7 IL-12, which is deemed as a critical signal in T-cell polarization, together with IFN-γ, instructs naive T cell to shift toward a Th1 response, whereas IL-4 and a low level of IL-12 cause a Th2 response. In addition, the generation of regulatory T (Treg) cells requires IL-10.3,5,8,9 Therefore, the tissue environmental factors profoundly influence the adaptive immune responses through their modulation on DCs.
Tissue inhibitors of metalloproteinases (TIMPs), specific inhibitors of matrix metalloproteinases (MMPs), consist of a family of 4 proteins that have been characterized so far. Independent of their inhibitory ability on MMPs, they also perform other biologic activities, such as promotion of cell proliferation, proapoptotic and antiapoptotic, and synaptic plasticity activities.10,11 TIMP-3, a unique member of this family, is mainly sequestered to tissue extracellular matrix and is the only TIMP capable of inhibiting membrane bound MMPs, transmembrane MMPs, and sheddases, such as TNF-α converting enzyme.12,13 TIMP-3 has been shown to act as a tumor suppressor in different cancer types, and loss of TIMP3 is closely associated with acquisition of tumorigenesis.14-17 TIMP-3 can inhibit invasion and induce cell apoptosis in some specific cancer cell strains.18-22 These observations indicate that TIMP-3 expressed within the tumor microenvironment exerts suppressive effects on tumorigenesis and metastatic colonization, mainly because of its antiangiogenic activity. Indeed, the immune system and the inflammatory factors have been reported crucial in regulation of angiogenesis.23,24 Moreover, TIMP-3 has been demonstrated to be a physiologic regulator of inflammation.25 Meanwhile, it is well known that inflammation causes the activation of the immune response. However, the function of TIMP-3 in the immune system, especially in the adaptive immunity, has not been studied explicitly until now.
Primary immune thrombocytopenia (ITP) is an immune-mediated hemorrhagic disease. Dysfunctional cellular immunity is considered as an important pathophysiologic mechanism in ITP, and an increased Th1/Th2 ratio has been reported to correlate with disease activity.26 In the present study, we investigated the role of TIMP-3 in maturation and function of human monocyte–derived DCs and the distribution of TIMP-3 in ITP patient plasmas as well as its correlation with the Th polarization status. It is found that blood monocytes lack TIMP-3 expression unless they are induced for differentiation by stimuli: IL-4 greatly up-regulated the TIMP-3 expression in a p38 MAPK-dependent manner. Furthermore, we show that DCs matured by lipopolysaccharide (LPS) in the presence of exogenous recombinant human TIMP-3 led to loss of the expression of CD86 and less production of IL-12, resulting in a Th2 polarization for naive allogeneic T cells. Further analysis of the underlying mechanisms indicated that the PI3K signaling pathway is involved in the inhibition of IL-12 production by TIMP-3. More importantly, in the Th1-polarized autoimmune disease ITP, there is clearly a negative correlation between TIMP-3 and IFN-γ as well as a positive correlation between TIMP-3 and IL-4 in patient plasmas. In addition, there was also a significant positive correlation between plasma TIMP-3 and platelet count. Therefore, further understanding of the impact of TIMP-3 on human DC functions will ultimately benefit the development of new TIMP-3–based immunomodulatory and antitumor strategies.
Methods
Generation and culture of monocyte-derived DCs
PBMCs were isolated from leukocyte-enriched buffy coats of healthy volunteers by centrifugation with Ficoll-Paque Plus (Sigma-Aldrich). Monocytes were enriched from PBMCs by positive selection using anti-CD14–conjugated magnetic microbeads (Miltenyi Biotec). The purity of CD14+ monocytes was more than 98%, as determined by flow cytometry (BD Biosciences). Monocytes were cultured at 1 × 106/mL in complete RPMI medium containing 1000 U/mL GM-CSF and 500 U/mL IL-4 (R&D Systems) for 5 days under 37°C, 5% humidified CO2 to generate imDCs. To induce maturation, 50 ng/mL TNF-α (R&D Systems) or 1 μg/mL LPS (Sigma-Aldrich) was added in imDCs for another 2 days. To investigate the influence of TIMP-3 on DC maturation, 50 ng/mL rhTIMP-3 (R&D Systems) was added during the maturation. In some instances, at day 5, the cultures were supplemented with indicated doses of PI3K inhibitor wortmannin (Cell Signaling Technology) for 1 hour before inducing maturation. Cell morphology was identified by light microscopy (Olympus IX81), and cell viability was determined by trypan blue stain.
For experiments using the signaling kinase inhibitors p38 MAPK inhibitor SB203580 (Cell Signaling Technology), the JAK3 inhibitor ZM 39 923 hydrochloride (Tocris Bioscience), or the PI3K inhibitor wortmannin, monocytes were preincubated with the aforementioned inhibitors for 60 minutes. Then the cells were washed extensively and incubated with GM-CSF and IL-4 for 3 days, with TIMP-3 examined by quantitative RT-PCR. p38 MAPK phosphorylation was analyzed with lysates from monocytes cultured with or without IL-4 (500 U/mL) or GM-CSF (1000 U/mL) for the indicated times (0, 5, 15, 30, and 60 minutes) by Western blot using phospho-specific antibodies (Cell Signaling Technology). p38 MAPK activity was detected with a commercial kinase assay kit (Cell Signaling Technology) according to the manufacturer's protocol. Akt phosphorylation was examined with lysates from DCs matured with LPS or LPS plus TIMP-3 for 30 minutes by Western blot using phospho-specific antibodies (Cell Signaling Technology).
RNA interference
Transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Day 5 imDCs were harvested, washed with PBS, and then resuspended in serum-free RPMI 1640 medium with a mixture of Lipofectamine 2000 and TIMP-3 siRNAs or negative control siRNAs (Santa Cruz Biotechnology) previously incubated at room temperature for 20 minutes. A total of 10% FBS, 1000 U/mL GM-CSF, 500 U/mL IL-4, and 1 μg/mL LPS were added after 4 hours. Forty-eight hours later, the transfected cells and supernatants were collected, and the phenotypes of the cells were detected using flow cytometry. Cytokine production was measured by the Bio-Plex Protein Array system. The transfection efficiency was monitored by Western blot.
Phenotype analysis
The expression of surface molecules on DCs was determined by FITC-, PE, PE-Cy5-labeled monoclonal antibodies: CD14, CD40, CD80, CD86, CD83, and HLA-DR (BD Pharmingen). Isotype controls were run in parallel. The samples were acquired on a FACSCalibur flow cytometer, and the mean fluorescence intensities were processed by CellQuest Version 3.3 software (BD Biosciences).
Determination of T-cell polarization by DCs
Naive human CD4+ T cells for the allogeneic mixed lymphocyte reaction were obtained from PBMCs using anti-CD4 and anti-CD45RA MACS beads (Miltenyi Biotec). The purity was more than 97% assessed by flow cytometry. Allogeneic naive CD4+ T cells (106 cells/well) were cocultured with DCs matured under different conditions (2 × 105 cells/well) in RPMI 1640 medium. After allogeneic DC–T-cell coculture for 6 days at 37°C, the supernatants were harvested and assessed for cytokine production by the Bio-Plex Protein Array system (Bio-Rad). The harvested T cells were restimulated with 10 ng/mL phorbol myristate acetate and 1 μg/mL ionomycin in the presence of 10 μg/mL brefeldin A (all Sigma-Aldrich) for 5 to 6 hours. Cells were then fixed (2% paraformaldehyde), permeabilized (0.5% saponin), and analyzed by flow cytometry using PE-conjugated anti–IL-4 and IFN-γ antibodies (eBioscience).
Cytokine measurement
Cytokine production of IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12 p70, TNF-α, and IFN-γ was determined by the Bio-Plex Protein Array system as described previously.27 Plasma TIMP-3, IFN-γ, and IL-4 from ITP patients and healthy controls were measured by standard sandwich ELISA using commercially available kits (Mai Bio).
Quantitative RT-PCR
Quantitative RT-PCR was performed with total RNA extracted from cells as described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Western blot analysis
Western blot was performed with cell lysates as detailed in supplemental Methods.
Immunofluorescence staining
Immunofluorescence staining and analysis of fixed cells were performed with standard protocols (supplemental Methods).
Human subjects
Twenty newly diagnosed primary ITP patients (3 males and 17 females with mean age 39 ± 20.8 years) at the Department of Hematology, Qilu Hospital, Shandong University, China, from January 2010 to December 2010 were enrolled in this study. Platelet counts were ranged between 2 and 20 × 109/L, with a median count of 10.5 × 109/L. Patients with diabetes, hypertension, cardiovascular diseases, pregnancy, active infection, or connective tissue diseases were excluded. None of them had received glucocorticoids or other immunosuppressive treatments previously. Normal control group consisted of 20 voluntary healthy persons (6 males and 14 females with mean age 35 ± 12.5 years). Conduct of human subjects for this study was approved by the Medical Ethical Committee of Qilu Hospital, Shandong University. Informed consent was obtained from each participating subject before sampling in accordance with the Declaration of Helsinki.
Statistical analysis
Data were presented as the mean ± SD. Student t test and ANOVA were used for statistical comparisons. Nonparametric Spearman correlation was used to quantify the relationships between TIMP-3 and IFN-γ or IL-4, as well as TIMP-3 between platelet counts. All statistical analyses were conducted using GraphPad Prism Version 5.01 for Windows (GraphPad Software). Statistical significance was considered as P less than .05.
Results
TIMP-3 expression was induced during DC differentiation
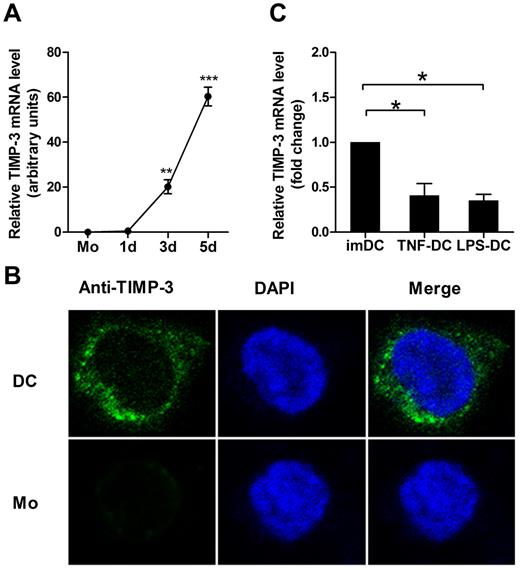
To clarify the role of TIMP-3 in regulation of the phenotype and function of DCs, we first determined the expression pattern of TIMP-3. The imDCs were generated from purified human CD14+ monocytes by GM-CSF and IL-4. During this differentiation, the TIMP-3 mRNA expression was found strikingly up-regulated (Figure 1A). To further detect this proteinase and its localization inside the cells, the immunofluorescence staining was performed. As shown in Figure 1B, the confocal laser scanning microscopy images clearly revealed that TIMP-3 was localized in both cytoplasm and nucleus in imDCs, but not or scarcely observed in monocytes. To determine whether there were some soluble forms of TIMP-3, which might be secreted into the culture supernatants, the cell products were then assayed by ELISA. However, TIMP-3 was detected neither in the supernatant of imDCs nor in the supernatant of monocytes (data not shown). Therefore, TIMP-3 was not expressed in monocytes until they underwent the differentiation toward DCs.
GM-CSF plus IL-4 induce TIMP-3 expression during differentiation of monocytes toward DCs. Freshly isolated monocytes (1 × 106 cells) were cultured in the presence of 1000 U/mL GM-CSF and 500 U/mL IL-4 for 5 days to generate imDCs, with 50 ng/mL TNF-α or 1 μg/mL LPS added for another 2 days to induce the DC maturation. (A) The TIMP-3 expression was assayed by quantitative RT-PCR in monocytes (Mo) and the cells after the induction for the indicated days. (B) Monocytes (Mo) and imDCs (DC) were stained with 4,6-diamidino-2-phenylindole (blue) and FITC-conjugated mAb (green) against TIMP-3 and then examined by confocal laser scanning microscopy system (Carl Zeiss LSM 710) with a Plan-Apochromat 63×/1.4 oil objective lens at room temperature. Images were processed using ZEN 2009 software (Carl Zeiss) and Adobe Photoshop CS5 (Adobe Systems). (C) Quantitative RT-PCR analysis of TIMP-3 mRNA in TNF-α–matured DCs and LPS-matured DCs, with imDCs as a control. Data are the mean ± SD of 3 independent experiments. *P < .05.
GM-CSF plus IL-4 induce TIMP-3 expression during differentiation of monocytes toward DCs. Freshly isolated monocytes (1 × 106 cells) were cultured in the presence of 1000 U/mL GM-CSF and 500 U/mL IL-4 for 5 days to generate imDCs, with 50 ng/mL TNF-α or 1 μg/mL LPS added for another 2 days to induce the DC maturation. (A) The TIMP-3 expression was assayed by quantitative RT-PCR in monocytes (Mo) and the cells after the induction for the indicated days. (B) Monocytes (Mo) and imDCs (DC) were stained with 4,6-diamidino-2-phenylindole (blue) and FITC-conjugated mAb (green) against TIMP-3 and then examined by confocal laser scanning microscopy system (Carl Zeiss LSM 710) with a Plan-Apochromat 63×/1.4 oil objective lens at room temperature. Images were processed using ZEN 2009 software (Carl Zeiss) and Adobe Photoshop CS5 (Adobe Systems). (C) Quantitative RT-PCR analysis of TIMP-3 mRNA in TNF-α–matured DCs and LPS-matured DCs, with imDCs as a control. Data are the mean ± SD of 3 independent experiments. *P < .05.
Next, we measured whether the DC maturation affected the TIMP-3 expression. LPS or TNF-α was applied for DC maturation in our experiments. Previously, it was shown that TIMP-3 mRNA was insignificantly up-regulated in TNF-α–matured DCs compared with imDCs.28 In contrast, we found that TIMP-3 mRNA was markedly down-regulated in mDCs, regardless of their stimulation by either LPS or TNF-α (Figure 1C). These results together suggested that there was a regulatory mechanism for TIMP-3 expression during DC differentiation.
IL-4 induced TIMP-3 expression during DC differentiation via p38 MAPK pathway
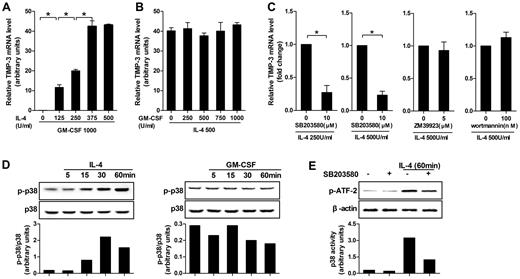
Five different doses of IL-4 or GM-CSF in combination with the standard dose of its counterpart were applied at the beginning of induction to identify their respective contributions in TIMP-3 induction. Quantitative RT-PCR results clearly showed that, although GM-CSF alone was not taking effect, the TIMP-3 expression was dose-dependent on IL-4 induction (Figure 2A). On the contrary, in the presence of the fixed dose of IL-4 (500 U/mL), different doses of GM-CSF (0-1000 U/mL) did not alter the expression of TIMP-3 (Figure 2B). Therefore, it was IL-4, but not GM-CSF, that determined the TIMP-3 expression during DC differentiation.
IL-4–induced TIMP-3 expression during DC differentiation was mediated by p38 MAPK pathway. (A) Monocytes were differentiated by standard dose of GM-CSF (1000 U/mL) in combination with gradient doses of IL-4 (0, 125, 250, 375, and 500 U/mL) for 5 days. (B) Alternatively, monocytes were stimulated by standard dose of IL-4 (500 U/mL) in combination with gradient doses of GM-CSF (0, 250, 500, 750, and 1000 U/mL) for 5 days. (C) Monocytes were preincubated with p38MAPK inhibitor SB203580 (10μM), JAK3 inhibitor ZM 39923 hydrochloride (5μM), or PI3K inhibitor wortmannin (100nM) for 1 hour. Then the cells were washed extensively and incubated with GM-CSF (1000 U/mL) and the indicated concentrations of IL-4 for 3 days. RNA extracts from the aforementioned cells differentiated under different conditions were analyzed for TIMP-3 mRNA by quantitative RT-PCR. (D) Monocytes were cultured with or without IL-4 or GM-CSF for the indicated times. Bottom insets: The quantitative analysis of p-p38/total p38 protein ratio, as measured by densitometry scanning of Western blots. Values are expressed in arbitrary units. (E) Monocytes were incubated with or without SB203580 (10μM) for 60 minutes and then stimulated with IL-4 (500 U/mL) for 60 minutes; kinase activity of p38 MAPK was measured with ATF-2 as a substrate. Data are the mean ± SD of 3 independent experiments. *P < .05.
IL-4–induced TIMP-3 expression during DC differentiation was mediated by p38 MAPK pathway. (A) Monocytes were differentiated by standard dose of GM-CSF (1000 U/mL) in combination with gradient doses of IL-4 (0, 125, 250, 375, and 500 U/mL) for 5 days. (B) Alternatively, monocytes were stimulated by standard dose of IL-4 (500 U/mL) in combination with gradient doses of GM-CSF (0, 250, 500, 750, and 1000 U/mL) for 5 days. (C) Monocytes were preincubated with p38MAPK inhibitor SB203580 (10μM), JAK3 inhibitor ZM 39923 hydrochloride (5μM), or PI3K inhibitor wortmannin (100nM) for 1 hour. Then the cells were washed extensively and incubated with GM-CSF (1000 U/mL) and the indicated concentrations of IL-4 for 3 days. RNA extracts from the aforementioned cells differentiated under different conditions were analyzed for TIMP-3 mRNA by quantitative RT-PCR. (D) Monocytes were cultured with or without IL-4 or GM-CSF for the indicated times. Bottom insets: The quantitative analysis of p-p38/total p38 protein ratio, as measured by densitometry scanning of Western blots. Values are expressed in arbitrary units. (E) Monocytes were incubated with or without SB203580 (10μM) for 60 minutes and then stimulated with IL-4 (500 U/mL) for 60 minutes; kinase activity of p38 MAPK was measured with ATF-2 as a substrate. Data are the mean ± SD of 3 independent experiments. *P < .05.
To further investigate the mechanism controlling the IL-4–induced TIMP-3 expression, we examined the possibilities of p38 MAPK, JAK3, and PI3K pathways using their selective inhibitors and found that TIMP-3 mRNA in monocytes was significantly blocked by the p38 MAPK inhibitor SB203580, regardless of the doses of IL-4 used (250 U/mL or 500 U/mL). However, JAK3 inhibitor ZM 39923 hydrochloride and PI3K inhibitor wortmannin did not show inhibitory effects (Figure 2C). To exclude the possibility that the inhibitors may have cytotoxic effects on the cells, trypan blue exclusion test was used to measure the cell viability, but the results did not indicate that the cytotoxic effect was involved (data not shown).
In line with the data in the preceding paragraph, it was not GM-CSF but IL-4 that enhanced the levels of phospho-p38 MAPK (Figure 2D). Then, we investigated the enzymatic activity of the immunoprecipitated p38 MAPK using GST–ATF-2 as substrate. A significant phosphorylation of ATF-2 was observed within 60 minutes of IL-4 treatment, whereas the IL-4–induced p38 MAPK kinase activity was inhibited by pretreatment with SB203580 in monocytes. However, SB203580 had minimal effects on cells without IL-4 treatment (Figure 2E). Together, our data demonstrated that p38 MAPK pathway played a critical role in IL-4–induced TIMP-3 expression.
Expression of the costimulatory molecule CD86 was altered by TIMP-3
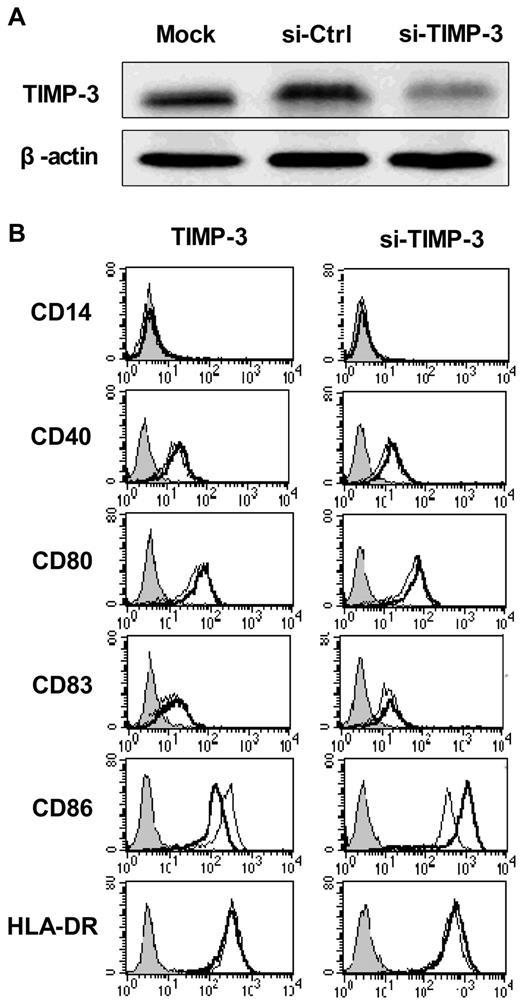
DCs display antigens with their surface molecules, including the MHC class II and the costimulatory molecules CD40, CD80, CD86, and CD83, all of which were greatly up-regulated during the DC maturation. To determine whether TIMP-3 could influence the maturation of DCs, we applied both exogenous rhTIMP-3 addition and the TIMP-3 gene silencing technique in DCs. As shown in Figure 3A, the TIMP-3 was efficiency removed with specific siRNA.
TIMP-3 modulated the DC phenotype. (A) The efficiency of TIMP-3 gene knockdown was confirmed by Western blot analysis. (B) rhTIMP-3 or TIMP-3 siRNA was applied during LPS-induced DC maturation. The expression of surface molecules was examined by flow cytometry. Histograms illustrate staining with isotype control (solid profiles) and with the indicated specific mAbs on untreated DCs (fine gray line) and treated DCs (bold black line). Results are representative of 3 separate donors.
TIMP-3 modulated the DC phenotype. (A) The efficiency of TIMP-3 gene knockdown was confirmed by Western blot analysis. (B) rhTIMP-3 or TIMP-3 siRNA was applied during LPS-induced DC maturation. The expression of surface molecules was examined by flow cytometry. Histograms illustrate staining with isotype control (solid profiles) and with the indicated specific mAbs on untreated DCs (fine gray line) and treated DCs (bold black line). Results are representative of 3 separate donors.
First, imDCs were exposed to rhTIMP-3 alone for 2 days, and we found, without LPS stimulation, rhTIMP-3 was unable to change the DC phenotype (data not shown). Furthermore, when exogenous rhTIMP-3 was supplemented to the LPS-matured DCs, there was a significant down-regulation of CD86, whereas other surface molecules (CD40, CD83, CD80, HLA-DR) remained unaltered (Figure 3B left). Consistent with this result, gene silencing of TIMP-3 in DCs led to an obviously increasing level of CD86 (Figure 3B right). Because CD86, other than CD80, is the initial costimulatory ligand expressed abundantly and early in DCs during antigen presentation,29 we think TIMP-3 could effectively modulate the following events after antigen presentation, such as T-cell differentiation, as we will discuss later.
TIMP-3 modulated the cytokine production in mature DCs
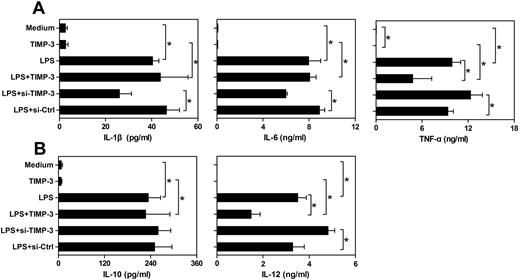
In addition to their antigen-presenting ability, another fundamental nature of DCs is to produce some specific cytokines for shaping the T-cell-mediated immune responses. Cytokines, such as IL-12 and IL-10, are considered to direct helper T-cell differentiation toward Th1 or Th2 cells, respectively.5 Meanwhile, proinflammatory cytokines IL-1β, IL-6, and TNF-α play a pivotal role in both innate and adaptive immune responses.30,31 Therefore, we sought to assess the effects of exogenous rhTIMP-3 and TIMP-3 silencing on the cytokines production pattern of LPS-matured DCs. We observed that imDCs spontaneously released relatively low levels of IL-1β, IL-6, IL-10, IL-12, and TNF-α (Figure 4). Exposure of imDCs to rhTIMP-3 alone did not change cytokine production significantly (Figure 4). As expected, LPS stimulation induced a significant up-regulation of all the cytokines. Interestingly, when rhTIMP-3 was added simultaneously with LPS, there was a dramatic decrease in the production of TNF-α and IL-12, whereas the levels of IL-10, IL-1β, and IL-6 were not markedly modulated by rhTIMP-3 (Figure 4). Moreover, we also detected a similarly inhibitory effect of TIMP-3 on IL-12 production in TNF-α–matured DCs (data not shown). Consistently, knockdown of endogenous TIMP-3 boosted LPS-induced TNF-α and IL-12 production in DCs, and loss of TIMP-3 had no apparent influence on IL-10, IL-1β, and IL-6 production (Figure 4). These observations indicated that TIMP-3 was involved in the regulation of inflammatory cytokines TNF-α and IL-12 during DC maturation, which might further endow DCs a different capacity in directing immune responses.
TIMP-3 down-regulated the production of IL-12 and TNF-α in DCs. imDCs were stimulated by LPS (1 μg/mL), TIMP-3 (50 ng/mL), or their combinations for maturation. Otherwise, LPS (1 μg/mL) was supplemented to the TIMP-3-knockdowned imDCs (si-TIMP-3) or their corresponding control (si-Ctrl). Two days later, the cell-free supernatants of different cultures were collected and analyzed for IL-1β, IL-6, IL-10, IL-12 p70, and TNF-α production by the Bio-Plex Protein Array system. (A) Proinflammatory cytokines IL-1β, IL-6, and TNF-α and (B) regulatory cytokines IL-10 and IL-12 p70 production. Data are the mean ± SD from 3 independent experiments. *P < .05.
TIMP-3 down-regulated the production of IL-12 and TNF-α in DCs. imDCs were stimulated by LPS (1 μg/mL), TIMP-3 (50 ng/mL), or their combinations for maturation. Otherwise, LPS (1 μg/mL) was supplemented to the TIMP-3-knockdowned imDCs (si-TIMP-3) or their corresponding control (si-Ctrl). Two days later, the cell-free supernatants of different cultures were collected and analyzed for IL-1β, IL-6, IL-10, IL-12 p70, and TNF-α production by the Bio-Plex Protein Array system. (A) Proinflammatory cytokines IL-1β, IL-6, and TNF-α and (B) regulatory cytokines IL-10 and IL-12 p70 production. Data are the mean ± SD from 3 independent experiments. *P < .05.
A crucial role of PI3K signaling pathway in TIMP-3–mediated IL-12 inhibition
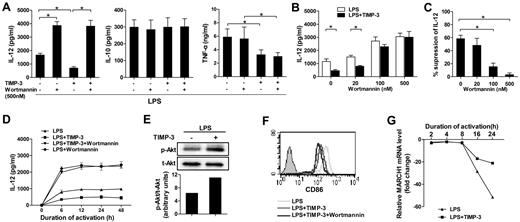
Previous reports have showed that PI3K negatively regulates IL-12 production in DCs.32,33 To determine the signaling mechanism(s) involved, we examined the effect of wortmannin, a pharmacologic global PI3K inhibitor, in TIMP-3-mediated down-regulation of IL-12. Pretreatment of imDCs with 500nM wortmannin could significantly augment LPS-induced IL-12 production (Figure 5A). Clearly, the inhibitory effect of rhTIMP-3 on IL-12 production in LPS-induced DCs was completely reversed by wortmannin (Figure 5A). By contrast, the production of IL-10 and TNF-α was not strikingly altered in wortmannin-pretreated DCs. In addition, the effect of wortmannin was dose-dependent: the efficient reversing function was observed at the concentrations higher than 100nM (Figure 5B-C). Because the kinetics of DC activation and IL-12 production are critical to determine the type of immune responses,34 we next investigated the effect of PI3K activity on the kinetics of TIMP-3–mediated IL-12 inhibition. As shown in Figure 5D, IL-12 production by DCs reached a plateau at 6 hours after LPS stimulation and maintained a high level up to 48 hours. After treatment with rhTIMP-3, IL-12 induction was always much lower than in those treated with LPS alone (Figure 5D). At each indicated time point, wortmannin could remarkably increase the IL-12 production in LPS-stimulated DCs. Meanwhile, in the presence of the PI3K inhibitor, TIMP-3 did not shown a significant change on IL-12 level (Figure 5D). In addition, Western blotting revealed that the level of phosphorylated Akt was significantly up-regulated within 30 minutes in imDCs without altering total amounts of Akt under TIMP-3 stress (Figure 5E). All these results suggested that PI3K signaling pathway was critically involved in the inhibition of IL-12 production by TIMP-3 in DCs.
PI3K signal activation was crucial for TIMP-3–mediated suppression of IL-12 production but not for TIMP-3–mediated repression of CD86 induction. Various concentrations of wortmannin or DMSO were used to treat imDC for 1 hour before their further stimulation with LPS or with LPS plus TIMP-3. (A) Two days later, production of IL-12, IL-10, and TNF-α in the cell-free supernatants was assayed by ELISA. (B-C) Reversal of TIMP-3–mediated IL-12 suppression by PI3K inhibitor wortmannin in DCs. Panel C was calculated based on the data collected in panel B. (D) The time-course tracking of TIMP–3 induced IL-12 down-regulation and its reversal by PI3K inhibitor wortmannin. The culture supernatants were collected at different time points for IL-12 assay by ELISA. (E) Whole-cell lysates from imDCs with LPS (1 μg/mL) with or without TIMP-3 (50 ng/mL) treatment for 30 minutes were subjected to Western blot analysis of phosphorylated Akt. (F) The same experiment was performed as in panel A, and the expression of CD86 was analyzed by flow cytometry. (G) Expression of MARCH1 mRNA in DCs matured with LPS (1 μg/mL) with or without TIMP-3 (50 ng/mL) for indicated times was analyzed by quantitative RT-PCR. Data are the mean ± SD from 3 independent experiments. *P < .05.
PI3K signal activation was crucial for TIMP-3–mediated suppression of IL-12 production but not for TIMP-3–mediated repression of CD86 induction. Various concentrations of wortmannin or DMSO were used to treat imDC for 1 hour before their further stimulation with LPS or with LPS plus TIMP-3. (A) Two days later, production of IL-12, IL-10, and TNF-α in the cell-free supernatants was assayed by ELISA. (B-C) Reversal of TIMP-3–mediated IL-12 suppression by PI3K inhibitor wortmannin in DCs. Panel C was calculated based on the data collected in panel B. (D) The time-course tracking of TIMP–3 induced IL-12 down-regulation and its reversal by PI3K inhibitor wortmannin. The culture supernatants were collected at different time points for IL-12 assay by ELISA. (E) Whole-cell lysates from imDCs with LPS (1 μg/mL) with or without TIMP-3 (50 ng/mL) treatment for 30 minutes were subjected to Western blot analysis of phosphorylated Akt. (F) The same experiment was performed as in panel A, and the expression of CD86 was analyzed by flow cytometry. (G) Expression of MARCH1 mRNA in DCs matured with LPS (1 μg/mL) with or without TIMP-3 (50 ng/mL) for indicated times was analyzed by quantitative RT-PCR. Data are the mean ± SD from 3 independent experiments. *P < .05.
Unfortunately, we found that CD86 was further down-regulated rather than reversed by wortmannin under TIMP-3 stress (Figure 5F), which is consistent with a previous finding that wortmannin could efficiently block CD86 expression on adenovirus-matured DCs.35 It has been reported that MARCH1-mediated ubiquitination plays an important role in human DCs to regulate their CD86 expression.36 We then comprehensively examined the dynamic expression pattern of MARCH1 during DC maturation. As shown in Figure 5G, the MARCH1 transcription in DCs was significantly down-regulated on LPS-induced maturation (16 and 24 hours). However, it tended to restrain the reduction of MARCH1 as the addition of TIMP-3, suggesting that CD86 ubiquitination may be a potential mechanism underlying TIMP-3-mediated CD86 suppression.
TIMP-3 altered the function of DCs in polarizing T-cell differentiation
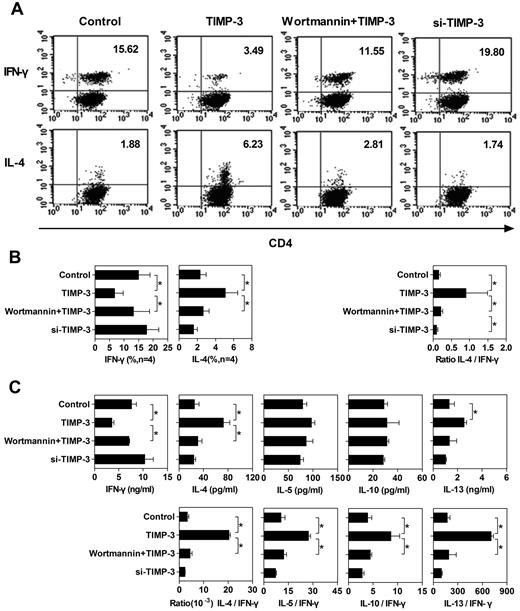
On interaction with the antigenic peptides presented by DCs, naive T cells proliferate and differentiate. It is well known that the cytokine milieu plays a crucial role in T-cell differentiation toward either Th1 or Th2 types.3 Effector Th1 cells acquire the capacity to secrete IFN-γ and mediate the cell-mediated immunity. Conversely, differentiated Th2 cells hold the capacity to produce IL-4, IL-5, IL-10, and IL-13 and regulate antibody-mediated humoral immune responses.37 The inhibitory effect of TIMP-3 on IL-12 production in DCs prompted us to evaluate the nature of primary allogeneic T-cell responses induced by DCs matured in TIMP-3 stress. To achieve this, allogeneic naive T cells were stimulated with differently conditioned DCs, as shown in Figure 6A. Six days later, cytokine production pattern in T cells was measured by intracellular cytokine staining and Bio-Plex Protein Array system. The intracellular staining assay revealed that allogeneic naive T cells primed by control DCs matured by LPS alone had a higher frequency of IFN-γ–producing cells than IL-4–producing cells, a bias toward Th1 cell differentiation (Figure 6A-B). In contrast, DCs matured by LPS in the presence of rhTIMP-3 programmed naive T cells toward a Th2 profile with a markedly reduced number of IFN-γ–producing cells and a strikingly increased number of IL-4–producing cells (Figure 6A-B). Preincubation of imDCs with wortmannin largely reversed the effect of TIMP-3: the Th1 profile was restored (Figure 6A-B), suggesting that the PI3K pathway was functionally involved in TIMP-3–induced DC modulation. In addition, siRNA silencing of TIMP-3 led DCs to generate a slightly increased number of IFN-γ–producing T cells (Figure 6A-B).
TIMP-3–treated DCs altered the Th1/Th2 polarization. To stimulate the naive T cells, 4 types of mDCs were applied: (1) imDCs stimulated with LPS (Control); (2) imDCs stimulated with LPS plus TIMP-3; (3) imDCs preincubated with 500nM wortmamin for 1 hour with the following maturation by LPS plus TIMP-3 (wortmamin + TIMP-3); and (4) TIMP-3–knockdown imDCs stimulated by LPS (si-TIMP-3). These mDCs were mixed with allogeneic naive T cells for 6 days to induce T-cell polarization at a DC:T ratio of 1:5. (A) Dot plots of IFN-γ and IL-4 production by CD4+ T cells from one representative donor. For intracellular analysis of cytokine production, CD4+ T cells were restimulated with 10 ng/mL phorbol myristate acetate and 1 μg/mL ionomycin in the presence of 10 μg/mL brefeldin A for 5 to 6 hours. Cells were then fixed, permeabilized, and stained for IFN-γ and IL-4 and analyzed by flow cytometry. (B) Average of percentage of CD4+ T cells positive for IFN-γ and IL-4 staining from 4 individual donors are presented in the bar charts. (C) Supernatants of the mixed lymphocyte reaction coculture were harvested, and the production of IFN-γ, IL-4, IL-5, IL-10, and IL-13 was detected by the Bio-Plex Protein Array system. Data are the mean ± SD from 3 independent experiments. *P < .05.
TIMP-3–treated DCs altered the Th1/Th2 polarization. To stimulate the naive T cells, 4 types of mDCs were applied: (1) imDCs stimulated with LPS (Control); (2) imDCs stimulated with LPS plus TIMP-3; (3) imDCs preincubated with 500nM wortmamin for 1 hour with the following maturation by LPS plus TIMP-3 (wortmamin + TIMP-3); and (4) TIMP-3–knockdown imDCs stimulated by LPS (si-TIMP-3). These mDCs were mixed with allogeneic naive T cells for 6 days to induce T-cell polarization at a DC:T ratio of 1:5. (A) Dot plots of IFN-γ and IL-4 production by CD4+ T cells from one representative donor. For intracellular analysis of cytokine production, CD4+ T cells were restimulated with 10 ng/mL phorbol myristate acetate and 1 μg/mL ionomycin in the presence of 10 μg/mL brefeldin A for 5 to 6 hours. Cells were then fixed, permeabilized, and stained for IFN-γ and IL-4 and analyzed by flow cytometry. (B) Average of percentage of CD4+ T cells positive for IFN-γ and IL-4 staining from 4 individual donors are presented in the bar charts. (C) Supernatants of the mixed lymphocyte reaction coculture were harvested, and the production of IFN-γ, IL-4, IL-5, IL-10, and IL-13 was detected by the Bio-Plex Protein Array system. Data are the mean ± SD from 3 independent experiments. *P < .05.
To confirm the single-cell level results of the cytokine production, we also conducted Bio-Plex Protein Array to measure the cytokine production by the whole-cell population. Consistent with the intracellular cytokine staining, TIMP-3 rendered DCs to stimulate naive T cells to produce less IFN-γ, but more IL-4, which could be reversed by the PI3K inhibitor wortmannin (Figure 6C). Although the TIMP-3 caused pattern of other Th2 cytokine (IL-5, IL-10, and IL-13) production was not as clear as IL-4, when we calculated the ratio of Th2 cytokine to IFN-γ, there were significantly higher values for all the Th2 cytokines (Figure 6C). Obviously, TIMP-3 indeed altered the capability of DCs to stimulate naive T-cell differentiation: a Th2 polarization was induced by TIMP-3 in a PI3K-dependent manner.
TIMP-3 displayed a statistically positive correlation with IL-4 but a negative correlation with IFN-γ in ITP patient plasma
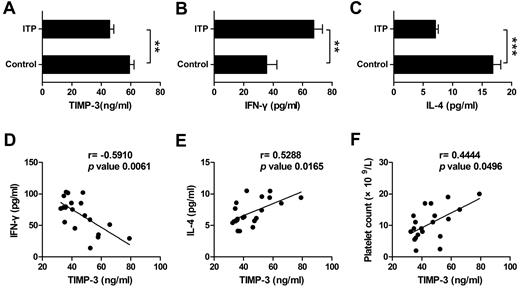
ITP has been manifested as a type 1 polarized immune response.26 To determine the potential role of TIMP-3 in vivo, especially its function in the diseases, we next monitored the expression of IFN-γ, IL-4, and TIMP-3 in ITP patient plasma, with the healthy persons as controls. On one hand, we found that the plasma level of TIMP-3 was significantly lower in ITP patients compared with that in healthy donors (Figure 7A). On the other hand, consistent with a recent report,38 the mean value of IFN-γ was significantly higher, but IL-4 was markedly lower in plasma from ITP patients compared with the healthy controls (Figure 7B-C). When we calculated the correlation of TIMP-3 with the Th1/Th2 cytokines, the results clearly showed a negative correlation between TIMP-3 and IFN-γ (r = −0.5910, P = .0061), as well as a positive correlation between TIMP-3 and IL-4 (r = 0.5288, P = .0165) in ITP patient plasma (Figure 7D-E). In addition, there was also a positive correlation between plasma TIMP-3 and platelet count (r = 0.4444, P = .0496) in ITP patients (Figure 7F). These data further supported the physiologic function of TIMP-3 in Th1/Th2 polarization, and targeting in TIMP-3 might be developed as a novel approach in treating autoimmune diseases.
TIMP-3 positively correlated with IL-4 and platelet count but negatively correlated with IFN-γ in ITP patient. Plasma levels of TIMP-3 (A), IFN-γ (B), and IL-4 (C) in newly diagnosed untreated ITP patients and the healthy controls were assayed by ELISA. *P < .05; **P < .01; ***P < .001. (D-F) The correlation between plasma levels of TIMP-3 and IFN-γ or TIMP-3 and IL-4 or TIMP-3 and platelet count in ITP patients, was calculated.
TIMP-3 positively correlated with IL-4 and platelet count but negatively correlated with IFN-γ in ITP patient. Plasma levels of TIMP-3 (A), IFN-γ (B), and IL-4 (C) in newly diagnosed untreated ITP patients and the healthy controls were assayed by ELISA. *P < .05; **P < .01; ***P < .001. (D-F) The correlation between plasma levels of TIMP-3 and IFN-γ or TIMP-3 and IL-4 or TIMP-3 and platelet count in ITP patients, was calculated.
Discussion
TIMP-3 has been reported to mediate a wide variety of biologic activities.14-22,39 However, it remains unclear how it influences the immune system, especially the adaptive immunity. In the present study, we investigated the expression and regulation of TIMP-3 during DC differentiation and elucidated the direct effects of TIMP-3 on DCs in terms of surface molecule expression, cytokine production, and Th1/Th2 shifting.
Previously, we had demonstrated the constitutive expression of TIMP-3 in both normoxic and hypoxic DCs.27 To determine the role of TIMP-3 in maturation and function of DCs, we first attempted to examine the kinetics of TIMP-3 expression during DC differentiation. And we found that TIMP-3 was not detectable in freshly isolated monocytes but gradually induced during their differentiation toward DCs, indicating that the differentiation stimulators were possibly involved. We then indeed identified that IL-4 was responsible for the induction of TIMP-3. However, the underlying molecular mechanisms were still unknown. The p38 MAPK activation has been demonstrated in the production of several cytokines induced by IL-4, and one study showed that IL-4 could up-regulate the expression of TIMP-2 in dermal fibroblasts through the activation of p38 MAPK.40 In our study, we observed that a specific inhibitor of p38 MAPK, SB203580, abolished the IL-4–induced TIMP-3 expression, and it was IL-4 rather than GM-CSF that enhanced the levels of phospho-p38 MAPK in monocytes, indicating that p38 MAPK at least played a partial role in this process.
TIMP-3 has been reported to minimally up-regulated during DC maturation by TNF-α.28 However, under our experimental conditions, TIMP-3 mRNA was markedly decreased in both LPS-matured DCs and TNF-α–matured DCs. Notably, we found that the soluble form of TIMP-3 was detected neither in the supernatant of imDCs nor in the supernatant of mDCs. Given that TIMP-3 has several unique features that distinguish it from the other TIMPs, we suggest that the newly synthesized and secreted TIMP-3 by DCs might bind to the proteoglycans of the cell membranes,41 or rapidly be endocytosed by the cell.42 Indeed, we analyzed the supernatants of imDCs, which were incubated 8 hours with 100 μg/mL heparin or extracted 5 minutes with 0.01% Triton X-100 or 1% SDS by ELISA, and found that these treatments caused an accumulation of TIMP-3 in the cell culture (supplemental Figure 1).
It is accepted that the type, intensity, and duration of a specific immune response depend on at least 3 signals provided by DCs. The antigen-specific signal 1 is generated during the interaction between MHC molecules-peptide complexes on DCs and T-cell receptor on naive T cells; the costimulatory signal 2 is transmitted through the interaction of costimulatory molecules (CD40, CD80, and CD86) on DCs with ligands on naive T cells; and the signal 3 was provided by various cytokines produced by DCs and other cell types.3,43 Our findings manifested that TIMP-3 at least impacted on 2 of the aforementioned signals in DCs: TIMP-3 altered the expression of costimulatory molecule CD86 and the production of cytokine IL-12. Our results showed that the expression of costimulator molecules and MHC molecules on DCs (data not shown) as well as the production of IL-10 and IL-12 were not significantly altered when TIMP-3 was delivered in the absence of LPS, suggesting that exposure to TIMP-3 alone does not induce activation of DCs. When DCs were stimulated simultaneously with TIMP-3 plus LPS, the presence of TIMP-3 resulted in a dramatic down-regulation of CD86. In addition, TIMP-3 treatment led to a suppression of IL-12 and TNF-α production in DCs, whereas IL-1β, IL-6, and IL-10 were not affected.
Autocrine IL-10 is involved in a regulatory mechanism in suppressing IL-12 production in DCs.44 However, there was no significant change in IL-10 expression detected in our study. Therefore, it is not likely that IL-10 was involved in the suppression of IL-12. Afterward, we provided evidence that TIMP-3–induced suppression of IL-12 during LPS/TLR4-mediated DC maturation was dependent on PI3K activation. It has been reported that a full response to LPS is mediated by the interaction with TLR4-MD2 (myeloid differentiation 2) complex, LPS-binding protein, and CD14 coreceptor.45,46 Although we found no evidence that TLR4 has a TIMP-3–binding site, we speculate that the target of TIMP-3 is TLR4 receptor complex or its related molecules. Whether TIMP-3 triggers the physical proximity and structural change or modulates functional interplay between TLR4-MD2 complex and CD14 to regulate LPS/TLR4/PI3K signaling pathway remains to be pursued in the future study. In contrast, PI3K activation appears not to be involved in TIMP-3–induced CD86 inhibition because PI3K inhibition resulted in a further loss of CD86 rather than a recovery of it. Interestingly, our study indicates that TIMP-3 restrain LPS-induced MARCH1 down-regulation, which is able to mediate ubiquitination of CD86 in DCs. However, whether the TIMP-3–mediated regulation of MARCH1 is directly responsible for down-regulation of CD86 in DCs remains a subject for further study. Thus, these data suggest that TIMP-3 is capable of modulating the activation and maturation of DCs.
ITP is a Th1 predominant disease with lower plasma TIMP-3 level. Previous studies have shown that there are no significant differences with respect to the absolute numbers of myeloid DCs and plasmacytoid DCs between ITP and healthy controls.47,48 We investigated the phenotype of DCs generated in vitro with purified CD14+ monocytes from ITP patients and found that the expression of CD86 was strikingly up-regulated in DCs from ITP patients, whereas HLA-DR and CD80 did not significantly alter between DCs from ITP patients and healthy subjects (supplemental Figure 2A). This observation is in agreement with that of Catani et al,48 which also demonstrated that CD86 was increased in DCs from ITP patients. This would be of particular interest because we observed that CD86 expression was significantly suppressed by TIMP-3 in LPS-primed DCs. Meanwhile, TIMP-3 levels were much lower in plasma from ITP patients than in healthy persons. Therefore, we consider that TIMP-3 may play an important role in alteration of DCs from ITP patients.
Activated DCs can differentiate naive T cells into at least 2 distinct Th types: Th1 cells produce large amounts of IFN-γ but little IL-4, IL-5, and IL-10, whereas Th2 cells secret little IFN-γ but large amounts of IL-4, IL-5, IL-10, and IL-13.5 Meanwhile, IL-12 is a critical cytokine for the differentiation of naive T cells into the Th1 cells, a T-cell subtype required for elimination of intracellular pathogens and transformed tumor cells.5,49 Therefore, we decided to investigate whether TIMP-3–mediated suppression of IL-12 production in DCs results in dysfunctional T-cell priming. As expected, intracellular staining results showed that TIMP-3 paved a way for the generation of Th2 immune response, with a higher number of IL-4–producing cells but lower number of IFN-γ–producing cells. It was further confirmed by the Bio-Plex Protein Array: TIMP-3–treated DCs generated T cells with higher ratios of IL-4/IFN-γ, IL-5/IFN-γ, IL-10/IFN-γ, and IL-13/IFN-γ than T cells primed by control DCs. The TIMP-3–skewed Th2 polarization could be efficiently reversed by PI3K inhibitor wortmannin, suggesting that the PI3K pathway plays a key role in the function of TIMP-3.
We then examined the Th profile in ITP patients in vitro and found that there was significant higher Th1/Th2 ratio in peripheral blood from ITP patients than that from healthy subjects (supplemental Figure 2B-C). This would be consistent with the findings of Wang et al.50 It is presently unclear what factors are mainly responsible for these abnormalities; however, all results mentioned herein support the possibility that the relatively low TIMP-3 levels in patients' plasma could partly account for these observations.
In conclusion, we found that TIMP-3 effectively suppressed the expression of CD86 and production of IL-12 in DCs, leading to a Th2 polarization from naive T cells, and the PI3K signaling activation was crucial for this process. Furthermore, in a Th1 polarized disease (ITP), TIMP-3 displayed a positive correlation with IL-4 and a negative correlation with IFN-γ, further supporting its role in suppressing the Th1 cell differentiation. Because TIMP-3 has been previously regarded as a major angiogenesis inhibitor, our findings will also provide novel information for current cancer therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30671902, 81072406, 30872321, and 30871088), the Natural Science Foundation of Shandong Province (Y2008C02), and the National Science Fund for Distinguished Young Scholars (81125002).
Authorship
Contribution: Q.S., H.N., Y.L., A.F., Q.X., and B.S. performed experiments, analyzed results, and made the figures; X.Z. and G.R. designed experiments and wrote the manuscript; J.S., Y.Y., W.G., M.Y., and K.D. collected data and provided crucial experimental advice; M.H. and J.P. contributed to the clinical diagnosis of patients; and X.Q. and J.L. conceived and supervised the research and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xun Qu, Institute of Basic Medical Sciences and Key Laboratory of Cardiovascular Proteomics of Shandong Province, Qilu Hospital, Shandong University, 107#, Wenhua Xi Road, Jinan 250012, Shandong, PR China; e-mail: quxun@sdu.edu.cn.
References
Author notes
Q.S., H.N., and J.L. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal