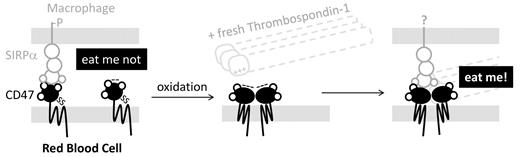
In this issue of Blood, Burger et al provide compelling in vitro evidence that phagocytic uptake of aged human erythrocytes is promoted by the marker of self CD47 in complex with the serum factor thrombospondin-1.1 CD47 is expressed on cells of all types and binds and signals to SIRPα on phagocytes.
The key in vivo evidence thus far for a marker-of-self role for CD47-SIRPα interactions comes from CD47-knockout mice: when red cells from these mice are injected into the circulation of control mice, the deficient cells are cleared within hours by macrophages in the spleen whereas normal red cells circulate for weeks.2 Surprisingly, the knockout mice are not anemic, but additional in vitro studies with both mouse and human red cells3 have largely confirmed that the “eat me not” signal can counter “eat me” signals on red cells—despite one report to the contrary.4 Loss of CD47's protective role had long been hypothesized to impact clearance of senescent cells,2 but data have been lacking. The studies of Burger et al suggest instead that aged cells add thrombospondin-1 to the CD47-SIRPα interaction (see figure), and this then prompts phagocytosis of red cells.
Oxidative aging of CD47 combines with thrombospondin-1 binding to switch an “eat me not” signal through SIRPa on macrophages to a strong “eat me” interaction.
Oxidative aging of CD47 combines with thrombospondin-1 binding to switch an “eat me not” signal through SIRPa on macrophages to a strong “eat me” interaction.
The biophysical nature of the binary CD47-SIRPα interaction must be suitably tuned to signal “self” through phosphorylation of SIRPα's inhibitory motif in the cytoplasmic tail. If the CD47-SIRPα interaction is too weak, then signaling will be insufficient. On the other hand, if the CD47-SIRPα interaction is too strong, then the macrophage cannot let go of the red cell, even when exposed to blood flow through the spleen that tends to disrupt erythrocyte-macrophage adhesion. In vitro studies of increasing CD47 density on microparticles have shown that signaling to SIRPα increases to saturation in correlation with CD47's real but limited ability to inhibit phagocytosis.3 The culture studies of Burger et al do not examine SIRPα signaling upon addition of thrombospondin-1, but their assays do suggest that the ternary interaction is stronger than the binary because aged cells, but not fresh cells, adhere strongly to SIRPα-expressing cells. This strong adhesion evidently favors “eat me.”
Living in an oxidative environment as we do, red cells accumulate oxidative damage that could include changes to CD47, according to Burger et al. Moreover, blood storage is thought to cause oxidative stress similar to cell aging in vivo, and stored blood is generally cleared faster than normal, perhaps contributing to clinical problems such as transfusion-related acute lung injury. Analysis of the membrane proteome of stored red cells has demonstrated dramatic alteration and cleavage of several proteins that include band 3 and band 4.2,5 which are notable as these proteins interact with CD47 in human red cells. In the same analysis, oxidative modifications of side-chains were also apparent in many other red cell membrane proteins. Oxidative stress, nonetheless, causes band 3 clustering with binding of IgG6 and phosphatidylserine exposure,7 signaling macrophages to clear these red cells. Although a chemically defined change in aged CD47 has not been identified, oxidation of antibodies may illustrate how interactions can be altered by oxidation that increases the anionic state and thus the tendency of antibodies to bind cationic surfaces.8 Burger et al indeed show that a small cationic peptide from thrombospondin-1, as well as the very large intact molecule, not only binds exclusively to aged red cells but is also largely blocked from binding by anti-CD47 antibodies. This indicates that CD47 is a likely target of the peptide, similar perhaps to the exposed phosphatidylserine, which is negative and binds divalent cation, namely calcium, that mediates binding of serum annexin V. Surprisingly, thrombospondin-1 needs to be fresh, which suggests that the relevant peptide is chemically altered with age or becomes cryptic, encouraging further study by methods such as mass spectrometry that can be adapted to assess conformational changes as well as oxidation state.9
CD47 is expressed on all cells, and so there are broader implications beyond erythrocytes. For example, blocking thrombospondin-1 from binding CD47 has been reported to make normal tissue nearly immune to radiation therapy of tumors and also to assist in tumor death in mice.10 While the mechanisms are not yet clear, one possible mechanism could involve protection from senescence-driven phagocytosis of tumor-infiltrating leukocytes. These cells ordinarily express CD47 and, by avoiding clearance, they can better attack the tumor. When considering translation of such ideas to humans, however, it should be kept in mind that detailed studies of red cells have shown some of CD47's interactions with multiple proteins differ both within and between species.11 The multifunctionality of CD47 is certainly a rich topic for deeper understanding in homeostasis and disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■