Abstract
Somatic mosaicism has been described in several primary immunodeficiency diseases and causes modified phenotypes in affected patients. X-linked anhidrotic ectodermal dysplasia with immunodeficiency (XL-EDA-ID) is caused by hypomorphic mutations in the NF-κB essential modulator (NEMO) gene and manifests clinically in various ways. We have previously reported a case of XL-EDA-ID with somatic mosaicism caused by a duplication mutation of the NEMO gene, but the frequency of somatic mosaicism of NEMO and its clinical impact on XL-EDA-ID is not fully understood. In this study, somatic mosaicism of NEMO was evaluated in XL-EDA-ID patients in Japan. Cells expressing wild-type NEMO, most of which were derived from the T-cell lineage, were detected in 9 of 10 XL-EDA-ID patients. These data indicate that the frequency of somatic mosaicism of NEMO is high in XL-ED-ID patients and that the presence of somatic mosaicism of NEMO could have an impact on the diagnosis and treatment of XL-ED-ID patients.
Introduction
X-linked anhidrotic ectodermal dysplasia with immunodeficiency (XL-EDA-ID) is a disease with clinical features including hypohidrosis, delayed eruption of teeth, coarse hair, and immunodeficiency associated with frequent bacterial infections.1-5 The gene responsible for XL-EDA-ID has been identified as NF-κB essential modulator (NEMO).6-8 NEMO is necessary for the function of IκB kinase, which phosphorylates and degrades IκB to activate NF-κB.9,10 Defects in NEMO cause various abnormalities in signal transduction pathways involving NF-κB, and affect factors such as the IL-1 family protein receptors, the TLRs, VEGFR-3, receptor activator of nuclear factor κB (RANK), the ectodysplasin-A receptor, CD40, and the TNF receptor I.7 Whereas a complete loss of NEMO function in humans is believed to cause embryonic lethality,11 NEMO mutations in XL-EDA-ID patients are hypomorphic,8 causing a partial loss of NEMO functions.
In XL-EDA-ID, NEMO defects lead to diverse immunologic features including susceptibility to pathogens, impaired Ab response to polysaccharides,2,4,12 hypogammaglobulinemia,13 hyper IgM syndrome,14 and impaired NK-cell activity,15 with a large degree of variability in phenotypes among the patients. For example, approximately one-tenth of XL-EDA-ID patients exhibit reduced mitogen-induced proliferation of T lymphocytes.12 Moreover, one-fourth suffer from inflammatory disorders such as inflammatory bowel disease and rheumatoid arthritis,12 although the inflammatory process usually relies on NF-κB activation.16 One explanation for this clinical variability is that the XL-EDA-ID phenotype is NEMO genotype-specific. Although the XL-EDA-ID database reported by Hanson et al succeeds to some extent in linking the specific clinical features to NEMO genotype,12 the penetrance of some clinical features is not high and the mechanism accounting for this variability is unknown.
Recently, we have reported a case of spontaneous reversion mosaicism of the NEMO gene in XL-EDA-ID, which showed an atypical phenotype involving decreased mitogen-induced T-cell proliferation along with decreased CD4 T cells (patient 1).17 There have been no subsequent reports on somatic mosaicism in XL-EDA-ID, and its prevalence and impact on the clinical features of the disease is unknown. In this study, we describe the younger brother of patient 1, who suffered from XL-EDA-ID with the same mutation and somatic reversion mosaicism of NEMO. Patient 2 showed intriguing laboratory findings in that mitogen-induced T-cell proliferation varied in accordance with the rate of detected reversion in the peripheral blood. These 2 cases led us to perform a nationwide study of XL-EDA-ID patients in Japan that revealed a high incidence of somatic mosaicism of NEMO.
Methods
Informed consent
Informed consent was obtained from the patients and their families following the Declaration of Helsinki according to the protocol of the Internal Review Board of Kyoto University, which approved this study.
Patients
Patient 1 was an XL-EDA-ID patient with a duplication mutation of the NEMO gene spanning intron 3 to exon 6. This patient has been reported previously17 and died from an Aspergillus infection at the age of 4. Patient 2, born at term, was the younger brother of patient 1. This patient was also diagnosed as XL-EDA-ID with the same duplication mutation as patient 1 by genetic study. He received trimethoprim-sulfamethoxazole prophylaxis and a monthly infusion of immunoglobulin from the age of 1 month. The patient maintained good health and had a body weight of 7899g at 6 months when he started to fail to thrive. Except for poor weight gain, patient 2 appeared active with a good appetite, negative C-reactive protein, normal white blood cell counts, and no apparent symptoms. At 19 months of age, Mycobacterium szulgai was detected by venous blood culture, and the patient was treated with multidrug regimens including ethambutol, rifabutin, and clarithromycin based on the treatment of systemic Mycobacterium avium complex infection. The patient responded well to the treatment and his weight increased from 7830g to 9165g within a month after the treatment was initiated. Patient 2 received an unrelated cord blood cell transplantation at 26 months of age, containing 8.5 × 107 nucleated cells/kg (4.4 × 105 CD34+ cells/kg), which was matched at 5 of 8 loci: mismatches occurred at 1 HLA-B and 1 HLA-C allele (according to serology), and at 1 HLA-A, 1 HLA-B, and 1 HLA-C allele (according to DNA typing). The preconditioning regimen consisted of fludarabine (30 mg/m2/d) on days −7 to −3, melphalan (70 mg/m2/d) on days −6 to −5, and rabbit anti-thymocyte globulin (2.5 mg/kg/d) on days −6 to −2. At first, Tacrolimus (0.024 mg/kg/d) was used to prevent GVHD, but this was switched to cyclosporin A (3 mg/kg/d) on day 9 because of drug-induced encephalopathy. Neutrophil (> 0.5 × 109/L) and platelet (> 50 × 109/L) engraftment were examined on days 13 and 40, respectively. Although CD19+ cells (2042/μL, 94% donor chimerism), CD56+ cells (242/μL, 97% donor chimerism), and monocytes (557/μL, 69% donor chimerism) were successfully generated, CD3+ cells were not detected in the peripheral blood by day 54. The patient suffered from septic shock and died on day 60. Patients 3 to 10 were XL-EDA-ID patients recruited nationwide in Japan. Clinical details of patients 3, 4, and 10 have been reported previously.18-20 These patients had clinical phenotypes characteristic of XL-EDA-ID such as ectodermal dysplasia, innate and/or acquired immunity defects, and susceptibility to pyogenic bacteria and Mycobacterium infection. Every patient had a mutation in the NEMO gene that caused reduced NF-κB activation in a NEMO reconstitution assay, as described in “Proliferation of NEMOnormal and NEMOlow T cells.” Patient profiles are listed in Table 1.
Clinical and genetic features of XL-EDA-ID patients
| Patient . | Mutation . | Ectodermal dysplasia . | Mitogen-induced proliferation . | Infections . | Complications . | Therapy . | Sex chromosome chimerism . |
|---|---|---|---|---|---|---|---|
| 1 | Duplication | + | Reduced | Sepsis (S.P. and P.A.) | Chronic diarrhea | IVIG | 100% XY |
| Disseminated M.A.C. | Failure to thrive | RFP, CAM, AMK, EB | |||||
| Skin abscess (S.A.) | Small intestinal stenosis | Rifabutin | |||||
| Invasive Aspergillus | Lymphedema | ||||||
| 2 | Duplication | + | Reduced | Sepsis (E coli) | Failure to thrive | IVIG, ST, EB, CAM | 99.8% XY 0.2% X |
| Disseminated M.S. | Rifabutin, SCT | ||||||
| 3 | D311E | − | Normal | Disseminated B.C.G. | IVIG, INH | 100% XY | |
| Sepsis (S.P.) | RFP, SCT | ||||||
| 4 | A169P | + | Normal | Meningitis (S.P.) | IBD | IVIG, ST, PSL | 99% XY |
| Interstitial pneumonia | CyA, MTX, Infliximab | ||||||
| Rheumatoid arthritis | |||||||
| 5 | L227P | + | Normal | Recurrent pneumonia | IBD | ST, mesalazine | Not done |
| Pyogenic coxitis | Infliximab | ||||||
| Recurrent otitis media | |||||||
| 6 | R182P | + | Not done | Recurrent otitis media | IBD | ST, mesalazine | 99.8% XY 0.2% X |
| UTI, Recurrent stomatitis | |||||||
| Subepidermal abscess | |||||||
| 7 | R175P | + | Normal | Recurrent sepsis (S.P.) | IVIG | 100% XY | |
| 8 | Q348X | + | Normal | Disseminated B.C.G. | IBD | IVIG, ST | 100% XY |
| 9 | R175P | + | Normal | Recurrent pneumonia | IBD | IVIG | 100% XY |
| Recurrent otitis media | 5-aminosalicylic acid | ||||||
| Kaposi varicelliform eruption | |||||||
| 10 | 1167 ins C | + | Normal | Sepsis and Enteritis (E.A) | Failure to thrive | IVIG, SCT | Not done |
| Sepsis (C.G.) | Pyloric stenosis, colon polyps | ||||||
| UTI (K.P.) |
| Patient . | Mutation . | Ectodermal dysplasia . | Mitogen-induced proliferation . | Infections . | Complications . | Therapy . | Sex chromosome chimerism . |
|---|---|---|---|---|---|---|---|
| 1 | Duplication | + | Reduced | Sepsis (S.P. and P.A.) | Chronic diarrhea | IVIG | 100% XY |
| Disseminated M.A.C. | Failure to thrive | RFP, CAM, AMK, EB | |||||
| Skin abscess (S.A.) | Small intestinal stenosis | Rifabutin | |||||
| Invasive Aspergillus | Lymphedema | ||||||
| 2 | Duplication | + | Reduced | Sepsis (E coli) | Failure to thrive | IVIG, ST, EB, CAM | 99.8% XY 0.2% X |
| Disseminated M.S. | Rifabutin, SCT | ||||||
| 3 | D311E | − | Normal | Disseminated B.C.G. | IVIG, INH | 100% XY | |
| Sepsis (S.P.) | RFP, SCT | ||||||
| 4 | A169P | + | Normal | Meningitis (S.P.) | IBD | IVIG, ST, PSL | 99% XY |
| Interstitial pneumonia | CyA, MTX, Infliximab | ||||||
| Rheumatoid arthritis | |||||||
| 5 | L227P | + | Normal | Recurrent pneumonia | IBD | ST, mesalazine | Not done |
| Pyogenic coxitis | Infliximab | ||||||
| Recurrent otitis media | |||||||
| 6 | R182P | + | Not done | Recurrent otitis media | IBD | ST, mesalazine | 99.8% XY 0.2% X |
| UTI, Recurrent stomatitis | |||||||
| Subepidermal abscess | |||||||
| 7 | R175P | + | Normal | Recurrent sepsis (S.P.) | IVIG | 100% XY | |
| 8 | Q348X | + | Normal | Disseminated B.C.G. | IBD | IVIG, ST | 100% XY |
| 9 | R175P | + | Normal | Recurrent pneumonia | IBD | IVIG | 100% XY |
| Recurrent otitis media | 5-aminosalicylic acid | ||||||
| Kaposi varicelliform eruption | |||||||
| 10 | 1167 ins C | + | Normal | Sepsis and Enteritis (E.A) | Failure to thrive | IVIG, SCT | Not done |
| Sepsis (C.G.) | Pyloric stenosis, colon polyps | ||||||
| UTI (K.P.) |
S.P. indicates Streptococcus pneumoniae; P.A., Pseudomonas aeruginosa; IVIG, intravascular immunoglobulin infusion; M.A.C., Mycobacterium avium complex; S.A., Staphylococcus aureus; E coli, Escherichia coli; ST, trimethoprim-sulfamethoxazole; M.S., Mycobacterium szulgai; AMK, amikacin; EB, ethambutol; CAM, clarithromycin; SCT, stem cell transplantation; B.C.G., Bacille de Calmette et Guerin; INH, isoniazid; RFP, rifampicin; IBD, inflammatory bowel disease; PSL, prednisolone; CyA, cyclosporine A; MTX, methotrexate; UTI, urinary tract infection; E.A., Enterobacter aerogenes; C.G., Candida glabrata; and K.P., Klebsiella pneumonia.
Flow cytometric analysis
NEMO intracellular staining was performed as previously described.17 The cells were stained for the following lineage markers before staining for NEMO: CD4, CD8, CD14, CD15, CD19, CD56, CD45RA (BD Biosciences/BD Pharmingen), and CCR7 (R&D Systems Inc). Intracellular staining of human IFN-γ, TNF-α, and NEMO was performed as previously described.18 The stained cells were collected using a FACSCalibur flow cytometer (BD Biosciences) and analyzed using the FlowJo software (TreeStar).
Reporter assay
Wild-type and mutant NEMO cDNAs were generated from a healthy volunteer and the recruited XL-EDA-ID patients by RT-PCR; the cDNAs were subcloned into the p3xFLAG-CMV14 vector (Sigma-Aldrich). NEMO null rat fibroblast cells (kindly provided by Dr S. Yamaoka, Department of Molecular Virology, Graduate School of Medicine, Tokyo Medical and Dental University, Tokyo, Japan) were plated at a density of 3 × 104 cells/well in a 24-well culture dish and were transfected with 40 ng of NF-κB reporter plasmid (pNF-κB-Luc; BD Biosciences/BD Clontech), 2 ng of NEMO mutant expression construct, 10 ng of internal control for the normalization of transfection efficiency (pRL-TK; Toyo Ink), and 148 ng of mock vector using FuGENE HD Transfection Reagent (TOYO-B-Net) according to the manufacturer's protocol. Twelve hours after transfection, the cells were stimulated with 15 ng/mL lipopolysaccharide (LPS; Sigma-Aldrich) for 4 hours and the NF-κB activity was measured using the PicaGene Dual SeaPansy assay kit (TOYO-B-Net). Experiments were performed in triplicate and firefly luciferase activity was normalized to Renilla luciferase activity.
Subcloning analysis of cDNA
Cell sorting of the various cell lineages was performed by FACSVantage (BD Biosciences). The purity of each lineage was > 95%. The cDNA from sorted cells was purified and reverse transcribed by Super Script III (Invitrogen) with random hexamers and amplified by the proofreading PCR enzyme KOD, as previously described.17,21 The PCR primers used were NEMO2 (5′-AGAGACGAAGGAGCACAAAGCTGCCTTGAG-3′) and NEMO3 (5′-ACTGCAGGGACAATGGTGGGTGCATCTGTC-3′). The PCR products were subcloned using a TA cloning kit (Invitrogen) and sequenced by ABI 3130xl Genetic analyzer (Applied Biosystems). To determine whether additional mutations occurred in revertant subclones that had wild-type sequence in the original mutation site, the entire coding region of the NEMO gene was sequenced and an additional mutation was considered present when the same mutation was detected in multiple subclones.
Allele-specific PCR
The mRNA purified from sorted T cells and monocytes was reverse-transcribed by SuperScript III (Invitrogen) with the gene-specific primer NEMO2 and amplified by the proofreading PCR enzyme KOD (Toyobo) using the primers NEMO3 and NEMO 4 (5′-TGTGGACACGCAGTGAAACGTGGTCTGGAG-3′). The PCR products were used as templates for allele-specific PCRs with Ex Taq polymerase (Takara Bio). Mutant and wild-type NEMO DNA was generated from each NEMO expression plasmid, mixed at graded ratios, and used as controls. PCR conditions and primer sequences are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Proliferation of NEMOnormal and NEMOlow T cells
To obtain PHA-induced T-cell blasts, PBMCs were stimulated with PHA (1:100; Invitrogen) and cultured in RPMI 1640 supplemented with 5% FCS and recombinant human IL-2 (50 IU/mL; kindly provided by Takeda Pharmaceutical Company) at 37°C for 7 days. Subcloning analysis of the cDNA obtained from the T-cell blasts was performed as described in “Subcloning analysis of cDNA.”
Results
Reversion mosaicism of NEMO occurred in siblings with similar immunologic phenotypes
We previously reported patient 1 with a duplication mutation of the NEMO gene spanning intron 3 to exon 6, who was diagnosed as XL-EDA-ID at 1 year of age after suffering from recurrent infections.17 At first, genetic diagnosis of the patient was difficult because the expression of aberrant NEMO mRNA was masked by the expression of normal NEMO mRNA by the revertant cells. Flow cytometric analysis of intracellular NEMO expression revealed cells with normal (NEMOnormal) and reduced (NEMOlow) levels of NEMO expression, indicating the presence of reversion mosaicism of the NEMO gene, and further analysis revealed that the NEMO mutation was disease-causing. PCR across the mutated region and sequencing of the PCR products revealed a duplication extending from intron 3 to exon 6, which was confirmed by Southern blot analysis. Additional copy number analysis of the NEMO gene of patient 1 and his mother excluded the possibility of a complex chromosomal aberration such as multiple duplication of the NEMO gene (supplemental Figure 1). Furthermore, polymorphism analysis using variable number tandem repeats on NEMOnormal and NEMOlow cells from patient 1 revealed that these cells were derived from the same origin (supplemental Table 2), indicating that the NEMO gene mosaicism was less likely because of amalgamation. The genomic analysis of the NEMOnormal cells revealed a complete reversion of the NEMO gene with no additional mutations. The clinical phenotype of patient 1 was combined immunodeficiency with a reduced number of T cells and mitogen-induced proliferation (Tables 2–3). We previously determined that reduced NEMO expression in the mutant T cells caused impairment of T-cell development and mitogen-induced proliferation.
Surface marker analysis of peripheral mononuclear cells of patients 1 and 2
| . | Patient 1 . | Patient 2 . | Healthy controls . | |
|---|---|---|---|---|
| Age at analysis | 2 y | 2 mo | 19 mo | |
| CD3 | 1503 | 2366 | 1014 | 2997 ± 1751 |
| CD4 | 292 | 1583 | 374 | 1683 ± 874 |
| CD8 | 1160 | 783 | 547 | 1114 ± 976 |
| TCRαβ | 1386 | 2295 | 439 | 2620 ± 1612 |
| TCRγδ | 109 | 74 | 574 | 343 ± 177 |
| CD4+CD45RA | 58 | 1336 | 105 | 1471 ± 890 |
| CD4+CD45RO | 263 | 307 | 266 | 497 ± 189 |
| CD8+CD45RA | 1178 | 783 | 297 | 1083 ± 1078 |
| CD8+CD45RO | 361 | 21 | 250 | 385 ± 442 |
| CD4+CD25 | 80 | 427 | 93 | 210 ± 99 |
| CD19 | 1200 | 941 | 1543 | 1252 ± 1145 |
| CD20 | 1189 | 931 | 1536 | 1125 ± 837 |
| CD19+Sm-IgG | 7 | 18 | 17 | 54 ± 21 |
| CD19+Sm-IgA | 15 | 4 | 14 | 18 ± 14 |
| CD19+Sm-IgM | 1171 | 910 | 1505 | 1057 ± 881 |
| CD19+Sm-IgD | 1171 | 906 | 1495 | 1052 ± 884 |
| CD16 | 912 | 176 | 24 | 287 ± 200 |
| CD56 | 908 | 176 | 24 | 306 ± 207 |
| . | Patient 1 . | Patient 2 . | Healthy controls . | |
|---|---|---|---|---|
| Age at analysis | 2 y | 2 mo | 19 mo | |
| CD3 | 1503 | 2366 | 1014 | 2997 ± 1751 |
| CD4 | 292 | 1583 | 374 | 1683 ± 874 |
| CD8 | 1160 | 783 | 547 | 1114 ± 976 |
| TCRαβ | 1386 | 2295 | 439 | 2620 ± 1612 |
| TCRγδ | 109 | 74 | 574 | 343 ± 177 |
| CD4+CD45RA | 58 | 1336 | 105 | 1471 ± 890 |
| CD4+CD45RO | 263 | 307 | 266 | 497 ± 189 |
| CD8+CD45RA | 1178 | 783 | 297 | 1083 ± 1078 |
| CD8+CD45RO | 361 | 21 | 250 | 385 ± 442 |
| CD4+CD25 | 80 | 427 | 93 | 210 ± 99 |
| CD19 | 1200 | 941 | 1543 | 1252 ± 1145 |
| CD20 | 1189 | 931 | 1536 | 1125 ± 837 |
| CD19+Sm-IgG | 7 | 18 | 17 | 54 ± 21 |
| CD19+Sm-IgA | 15 | 4 | 14 | 18 ± 14 |
| CD19+Sm-IgM | 1171 | 910 | 1505 | 1057 ± 881 |
| CD19+Sm-IgD | 1171 | 906 | 1495 | 1052 ± 884 |
| CD16 | 912 | 176 | 24 | 287 ± 200 |
| CD56 | 908 | 176 | 24 | 306 ± 207 |
Surface markers expressed by XL-EDA-ID patients' PBMCs are shown as absolute counts per microliter of peripheral blood. Healthy control values are based on children aged 1 to 6 years and are shown as the mean ± SD.
Sm indicates the surface membrane.
Immunologic analysis of patients 1 and 2
| . | Patient 1 . | Patient 2 (treated with IVIG) . | |
|---|---|---|---|
| Age at analysis, mo | 9 | 9 | 20 |
| Serum immunoglobulin levels, g/L (control) | |||
| IgG | 10.63 (4.51-10.46) | 8.44 (4.51-10.46) | 10.37 (7.15-9.07) |
| IgA | 1.36 (0.14-0.64) | 1.88 (0.14-0.64) | 3.93 (0.22-1.44) |
| IgM | 0.4 (0.33-1.00) | 0.17 (0.33-1.00) | 0.20 (0.34-1.28) |
| Age at analysis | 2 y | 2 mo | 23 mo |
| T-cell proliferation, SI (control) | 9.3 (206.9 ± 142.5) | 55.3 (64.8 ± 8.1) | 7.2 (89.4 ± 31.2) |
| . | Patient 1 . | Patient 2 (treated with IVIG) . | |
|---|---|---|---|
| Age at analysis, mo | 9 | 9 | 20 |
| Serum immunoglobulin levels, g/L (control) | |||
| IgG | 10.63 (4.51-10.46) | 8.44 (4.51-10.46) | 10.37 (7.15-9.07) |
| IgA | 1.36 (0.14-0.64) | 1.88 (0.14-0.64) | 3.93 (0.22-1.44) |
| IgM | 0.4 (0.33-1.00) | 0.17 (0.33-1.00) | 0.20 (0.34-1.28) |
| Age at analysis | 2 y | 2 mo | 23 mo |
| T-cell proliferation, SI (control) | 9.3 (206.9 ± 142.5) | 55.3 (64.8 ± 8.1) | 7.2 (89.4 ± 31.2) |
Control values of serum immunoglobulin levels are based on children aged either 7 to 9 months or 1 to 2 years and are shown as the mean ± SD. The T-cell proliferation assay was performed as described previously17 with at least three healthy adults as controls.
SI indicates stimulation index; and IVIG, 2.5 g of monthly IV immune globulin infusion.
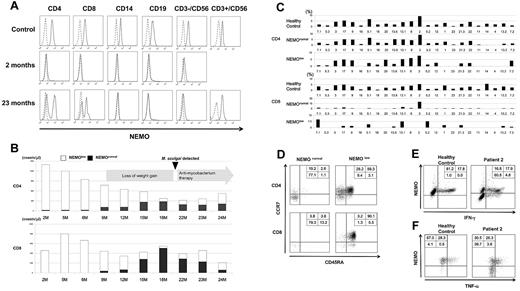
Patient 2, the younger brother of patient 1, was diagnosed as XL-EDA-ID with the same duplication mutation as his brother. Flow cytometric analysis of intracellular NEMO expression performed at diagnosis showed that most of his PBMCs had reduced NEMO expression (Figure 1A). At 2 months of age, when most of the T cells were NEMOlow, absolute counts of the patient's T cells and the mitogen-induced proliferation of the patient's PBMCs were comparable with those of the healthy controls (Figure 1A-B; Table 2). These findings indicated that the NEMO mutation had no effect on T-cell development and mitogen-induced proliferation during early infancy in patient 2.
Identification and characterization of NEMO revertant T cells in patient 2. (A) Intracellular expression of NEMO in various PBMC lineages from a healthy adult control and patient 2 were evaluated by flow cytometry. For the patient, results of the analyses performed at 2 months and 23 months are shown. Solid lines indicate staining with the anti-NEMO mAb, and dotted lines indicate the isotype control. (B) Time-course variations in the absolute count of NEMOnormal and NEMOlow T cells in patient 2. M indicates age in months. (C) TCR-Vβ repertoire analysis of the patient's CD4+ and CD8+ T cells. PBMCs from the patient and a healthy adult control were stained for the TCR-Vβ panel, CD4, CD8, and NEMO, and analyzed by flow cytometry. (D) Phenotype analysis of T cells in patient 2. PBMCs from the patient and a control were stained for the expression of NEMO, CCR7, CD45RA, and CD4 or CD8. Data shown were gated on NEMOnormal or NEMOlow CD4+ or CD8+ cells. (E-F) Cytokine production from NEMOnormal and NEMOlow T cells. PBMCs from the patient and a control were stimulated with PMA and ionomycin for 6 hours and stained for intracellular (E) IFN-γ or (F) TNF-α along with NEMO. Cells shown are gated on the CD3+ population.
Identification and characterization of NEMO revertant T cells in patient 2. (A) Intracellular expression of NEMO in various PBMC lineages from a healthy adult control and patient 2 were evaluated by flow cytometry. For the patient, results of the analyses performed at 2 months and 23 months are shown. Solid lines indicate staining with the anti-NEMO mAb, and dotted lines indicate the isotype control. (B) Time-course variations in the absolute count of NEMOnormal and NEMOlow T cells in patient 2. M indicates age in months. (C) TCR-Vβ repertoire analysis of the patient's CD4+ and CD8+ T cells. PBMCs from the patient and a healthy adult control were stained for the TCR-Vβ panel, CD4, CD8, and NEMO, and analyzed by flow cytometry. (D) Phenotype analysis of T cells in patient 2. PBMCs from the patient and a control were stained for the expression of NEMO, CCR7, CD45RA, and CD4 or CD8. Data shown were gated on NEMOnormal or NEMOlow CD4+ or CD8+ cells. (E-F) Cytokine production from NEMOnormal and NEMOlow T cells. PBMCs from the patient and a control were stimulated with PMA and ionomycin for 6 hours and stained for intracellular (E) IFN-γ or (F) TNF-α along with NEMO. Cells shown are gated on the CD3+ population.
NEMOnormal T cells gradually increased as patient 2 grew older, while the absolute count of NEMOlow T cells decreased (Figure 1A-B). Accordingly, normal full-length NEMO cDNA, which had been undetectable in cord blood, was detectable in the patient's peripheral blood at 12 months of age. However, while NEMOnormal T cells were increasing, mitogen-induced T-cell proliferation started to decrease (Table 3), and the patient started to show poor weight gain from 6 months of age. When patient 2 was 17 months old, a blood culture revealed an M szulgai bacteremia. At this time, the absolute count of NEMOnormal T cells peaked, and NEMOlow T cells were at a minimum. He began to gain weight after anti-Mycobacterium medication was initiated, although NEMOnormal T cells started to decrease and NEMOlow T cells began to increase (Figure 1B). When the patient was 23 months old, mitogen-induced T-cell proliferation was still low and a roughly equal number of NEMOlow and NEMOnormal T cells were detected (Table 3). Overall, as patient 2 grew older, NEMOnormal T cells increased as the total number of T cells and the mitogen-induced T-cell proliferation decreased, similar to what had occurred in patient 1 at a similar age.
Various analyses were performed to compare the immunologic phenotype of NEMOlow and NEMOnormal T cells in detail. Both NEMOnormal and NEMOlow CD4+ T cells carried a diverse Vβ repertoire, but CD8+ T cells had a skewed Vβ repertoire regardless of NEMO expression level (Figure 1C). Surface marker analysis revealed that most of the NEMOnormal T cells were CD45RA−/CCR7− and most of the NEMOlow T cells were CD45RA+/CCR7+ (Figure 1D). The NEMOnormal T cells produced similar amounts of IFN-γ and TNF-α as healthy control cells, while the production of these cytokines were reduced in NEMOlow T cells (Figure 1E-F). Taken together, these data implied that the immunologic phenotype of T cells from patient 2 converged with that of patient 1 as patient 2 grew older.
High incidence of somatic mosaicism of the NEMO gene in XL-EDA-ID patients
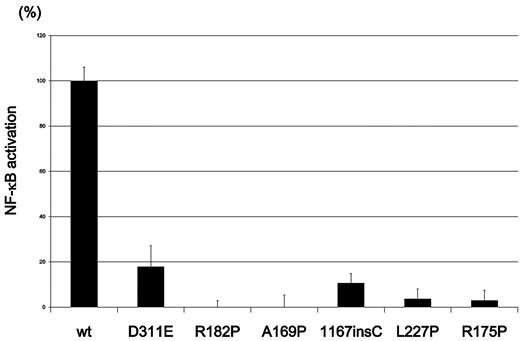
It is worth noting that somatic reversion mosaicism of the NEMO gene occurred in both of the 2 XL-EDA-ID siblings carrying a duplication mutation. To determine whether a high frequency of reversion is a specific event for this type of NEMO duplication mutation22-25 or if the reversion of the NEMO gene occurs commonly in XL-EDA-ID patients, we recruited an additional 8 XL-EDA-ID patients from throughout Japan (Table 1) and analyzed the presence of NEMO reversion. These patients had various combinations of clinical phenotypes characteristic of XL-EDA-ID such as ectodermal dysplasia, innate and acquired immunity defects, and susceptibility to pyogenic bacteria and Mycobacterium infections. Every patient had a mutation of the NEMO gene with reduced NF-κB activation potential, as evaluated in a NEMO reconstitution assay (Figure 2).
NF-κB transactivation by NEMO mutants from the XL-EDA-ID patients. NF-κB transactivation induced by NEMO mutants in the XL-EDA-ID patients. Mock vectors and wild-type (wt) NEMO were used as controls. The NF-κB activation index of NEMO variants were calculated as (NF-κB activation by each NEMO variant − NF-κB activation of the mock vector)/(NF-κB activation by wild-type NEMO − NF-κB activation of the mock vector). The data shown are the mean ± SD of triplicate wells and are representative of 3 independent experiments with similar results.
NF-κB transactivation by NEMO mutants from the XL-EDA-ID patients. NF-κB transactivation induced by NEMO mutants in the XL-EDA-ID patients. Mock vectors and wild-type (wt) NEMO were used as controls. The NF-κB activation index of NEMO variants were calculated as (NF-κB activation by each NEMO variant − NF-κB activation of the mock vector)/(NF-κB activation by wild-type NEMO − NF-κB activation of the mock vector). The data shown are the mean ± SD of triplicate wells and are representative of 3 independent experiments with similar results.
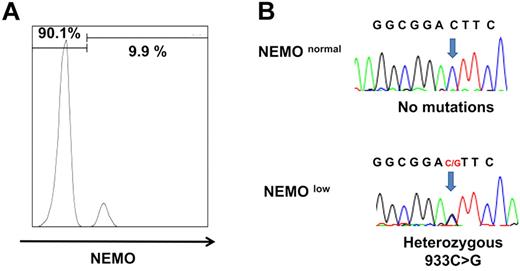
Among the 8 patients, only patient 3 had a large proportion of NEMOlow cells by flow cytometric analysis. The majority of patient 3's PBMCs were NEMOlow, whereas 10% of the patient's CD8+ cells were NEMOnormal (Figure 3A). This patient was identified as carrying the D311E mutation. Because missense mutations of the NEMO gene often do not result in the reduced expression of NEMO protein, subcloning and sequencing analysis was performed on the NEMO cDNA isolated from the remaining patients, and 6 of the 7 patients had normal NEMO subclones (Table 3). Expansion of maternal cells after fetomaternal transfusion was ruled out in these patients by FISH analysis with X and Y probes (Table 1).
NEMO revertant T cells in patient 3. (A) Intracellular expression of NEMO in CD8+ cells from patient 3. (B) Sequencing chromatograms of DNA from NEMOnormal or NEMOlow CD8+ cells of patient 3. Arrows indicate the mutated base position at c. 931.
NEMO revertant T cells in patient 3. (A) Intracellular expression of NEMO in CD8+ cells from patient 3. (B) Sequencing chromatograms of DNA from NEMOnormal or NEMOlow CD8+ cells of patient 3. Arrows indicate the mutated base position at c. 931.
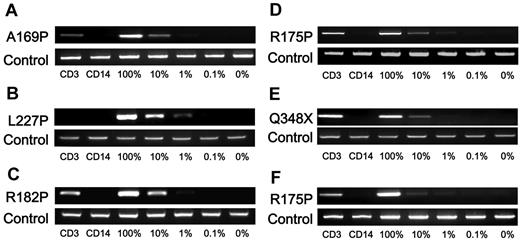
Additional genetic analysis of the entire coding region of the NEMO gene was performed on NEMOnormal cells from patient 3 and on reverted subclones from the other patients, except for patient 10 who had already received stem cell transplantation. The NEMO gene in these samples had reverted to wild-type with no additional mutations (Figure 3B and data not shown). To specifically determine in which cell lineages the reversion occurred, subcloning and sequencing analysis of cDNA in various cell lineages was performed. This analysis revealed that all the revertant cells were of the T-cell lineage and that no reversion occurred in monocytes and very little occurred in B cells (Table 4). Allele-specific PCR confirmed that reversion occurred in T cells but not in monocytes (Figure 4).
Analysis of NEMO gene mosaicism in various cell lineages for each patient
| Patient . | Mutation . | Age at analysis . | CD4, % (proportion) . | CD8, % (proportion) . | CD14, % (proportion) . | CD19, % (proportion) . |
|---|---|---|---|---|---|---|
| 1 | Duplication | 2 y | 90 | 100 | 0 | 4.0 |
| 2 | Duplication | 15 mo | 45 | 66 | 0 | 4.0 |
| 3 | D311E | 3 y | 2.4 | 9.9 | 0 | 1.2 |
| 4 | A169P | 12 y | 0 (0/19) | 24 (9/37) | 0 (0/19) | 0 (0/47) |
| 5 | L227P | 3 y | 0 (0/25) | 0 (0/35) | 0 (0/30) | 0 (0/25) |
| 6 | R182P | 4 y | 18 (5/28) | 17 (9/52) | 0 (0/27) | 0 (0/33) |
| 7 | R175P | 6 y | 0.4 (1/25) | 39 (11/28) | 0 (0/28) | 0 (0/25) |
| 8 | Q348X | 8 y | 38 (6/16) | 47 (9/19) | 0 (0/33) | 0 (0/25) |
| 9 | R175P | 15 y | 30 (9/30) | 36 (12/33) | 0 (0/23) | 0 (0/14) |
| 10 | 1167 ins C | 9 mo | PBMC 9.3 (4/43) | |||
| Patient . | Mutation . | Age at analysis . | CD4, % (proportion) . | CD8, % (proportion) . | CD14, % (proportion) . | CD19, % (proportion) . |
|---|---|---|---|---|---|---|
| 1 | Duplication | 2 y | 90 | 100 | 0 | 4.0 |
| 2 | Duplication | 15 mo | 45 | 66 | 0 | 4.0 |
| 3 | D311E | 3 y | 2.4 | 9.9 | 0 | 1.2 |
| 4 | A169P | 12 y | 0 (0/19) | 24 (9/37) | 0 (0/19) | 0 (0/47) |
| 5 | L227P | 3 y | 0 (0/25) | 0 (0/35) | 0 (0/30) | 0 (0/25) |
| 6 | R182P | 4 y | 18 (5/28) | 17 (9/52) | 0 (0/27) | 0 (0/33) |
| 7 | R175P | 6 y | 0.4 (1/25) | 39 (11/28) | 0 (0/28) | 0 (0/25) |
| 8 | Q348X | 8 y | 38 (6/16) | 47 (9/19) | 0 (0/33) | 0 (0/25) |
| 9 | R175P | 15 y | 30 (9/30) | 36 (12/33) | 0 (0/23) | 0 (0/14) |
| 10 | 1167 ins C | 9 mo | PBMC 9.3 (4/43) | |||
For patients 1 to 3, data represent the percentages of NEMOnormal cells in each lineage, as assessed by flow cytometry. For patients 4 to 10, the ratio indicates the number of wild-type NEMO clones in various cell lineages as compared with the total number of clones analyzed, based on subcloning and sequencing analysis.
NEMO reversion selectively occurs in T cells of XL-EDA-ID patients. Allele-specific PCR for NEMO on CD3+ or CD14+ cells from (A) patient 4, (B) patient 5, (C) patient 6, (D) patient 7, (E) patient 8, and (F) patient 9. Numbers below each figure indicate the percentages of wild-type NEMO cDNA mixed with each mutant. Primers used in each PCR are shown on the left.
NEMO reversion selectively occurs in T cells of XL-EDA-ID patients. Allele-specific PCR for NEMO on CD3+ or CD14+ cells from (A) patient 4, (B) patient 5, (C) patient 6, (D) patient 7, (E) patient 8, and (F) patient 9. Numbers below each figure indicate the percentages of wild-type NEMO cDNA mixed with each mutant. Primers used in each PCR are shown on the left.
Selective advantage of NEMOnormal cells in XL-EDA-ID carriers
The high frequency of somatic mosaicism in T cells of XL-EDA-ID patients indicated a strong selective advantage of wild-type NEMO T cells over T cells carrying mutant NEMO. To confirm this hypothesis, NEMO cDNA analysis was performed on various cell lineages from the mothers of the patients who are heterozygous for NEMO mutation and thus have mosaicism because of X-chromosome inactivation. This analysis assumes that the percentage of cDNA for wild-type NEMO reflects the percentage of cells expressing wild-type NEMO. A high proportion of wild-type NEMO cDNA was observed in T cells from the mothers of patients 1/2, 3, 8, and 10, although wild-type NEMO cDNA was not predominant in T cells from the mother of patient 4 (Table 5). Similarly, there was an apparent high proportion of wild-type NEMO cDNA in monocytes and B cells from the mothers of patients 1/2, 8, and 10 (Table 5). These findings suggested a general selective advantage of NEMOnormal cells over NEMOlow cells in vivo, especially in T cells.
Expression of mutant NEMO in various cell lineages for the mother of each XL-EDA-ID patient
| Sample . | Mutation . | Analysis . | Subtype . | Mutant type, % (proportion) . |
|---|---|---|---|---|
| Mother of patients 1 and 2 | Duplication | FACS | CD3 | 0 |
| CD14 | 0 | |||
| CD19 | 0 | |||
| Mother of patient 3 | D311E | FACS | CD3 | 13 |
| CD3− | 54 | |||
| Subcloning | CD3 | 22 (6/27) | ||
| CD3− | 55 (12/22) | |||
| Mother of patient 4 | A169P | Subcloning | CD3 | 52 (11/21) |
| CD14 | 58 (11/19) | |||
| CD19 | 42 (5/12) | |||
| Mother of patient 8 | Q348X | Subcloning | CD3 | 0 (0/26) |
| CD14 | 17 (3/18) | |||
| CD19 | 0 (0/18) | |||
| Mother of patient 10 | 1167insC | Subcloning | CD3 | 18 (7/39) |
| CD14 | 12 (5/43) | |||
| CD19 | 27 (12/44) |
| Sample . | Mutation . | Analysis . | Subtype . | Mutant type, % (proportion) . |
|---|---|---|---|---|
| Mother of patients 1 and 2 | Duplication | FACS | CD3 | 0 |
| CD14 | 0 | |||
| CD19 | 0 | |||
| Mother of patient 3 | D311E | FACS | CD3 | 13 |
| CD3− | 54 | |||
| Subcloning | CD3 | 22 (6/27) | ||
| CD3− | 55 (12/22) | |||
| Mother of patient 4 | A169P | Subcloning | CD3 | 52 (11/21) |
| CD14 | 58 (11/19) | |||
| CD19 | 42 (5/12) | |||
| Mother of patient 8 | Q348X | Subcloning | CD3 | 0 (0/26) |
| CD14 | 17 (3/18) | |||
| CD19 | 0 (0/18) | |||
| Mother of patient 10 | 1167insC | Subcloning | CD3 | 18 (7/39) |
| CD14 | 12 (5/43) | |||
| CD19 | 27 (12/44) |
Data are shown as either the percentages of NEMOnormal cells, as assessed by flow cytometry, or as the ratio of clones containing wild-type NEMO to the total number of clones, as analyzed by subcloning and sequencing analysis.
Proliferation capacity of NEMOnormal and NEMOlow T cells
T-cell proliferation stimulated by mitogens such as PHA is usually not reduced in XL-EDA-ID patients. However, the emergence of NEMOnormal cells coincided with a reduction in mitogen-induced proliferation in patient 2. To further determine the effect of NEMOnormal cells on mitogen-induced proliferation of peripheral T cells, the proportions of T cells carrying the wild-type and mutant were examined before and after PHA stimulation in XL-EDA-ID patients and their mothers (Table 6). In patients 2, 4, and 8, the percentage of the NEMOnormal cells decreased after PHA stimulation, while NEMOnormal cells prevailed in patient 9. In the mothers of patient 4 and 10, the percentage of NEMOnormal cells increased after PHA stimulation, while the percentage of the NEMOnormal cells decreased in the mother of patient 3. These results indicated that the NEMO mutation does not directly affect the mitogen-induced proliferation capacity of T cells and factors other than the NEMO genotype determine the proliferation capacity of NEMOnormal and NEMOlow T cells.
Expression of mutant NEMO in CD3-positive cells and PHA blasts
| Sample . | Mutations . | Analysis . | Subtype . | Mutant type, % (proportion) . |
|---|---|---|---|---|
| Mother of patient 3 | D311E | FACS | CD3 | 13 |
| PHA blast | 47 | |||
| Subcloning | CD3 | 22 (6/27) | ||
| PHA blast | 48 (11/23) | |||
| Mother of patient 4 | A169P | Subcloning | CD3 | 52 (11/21) |
| PHA blast | 18 (9/49) | |||
| Mother of patient 8 | Q348X | Subcloning | CD3 | 0 (0/26) |
| PHA blast | 0 (0/21) | |||
| Mother of patient 10 | 1167insC | Subcloning | CD3 | 18 (7/39) |
| PHA blast | 9 (4/43) | |||
| Patient 2 | Duplication | FACS | CD3 | 73 |
| PHA blast | 93 | |||
| Patient 4 | A169P | Subcloning | CD3 | 79 (19/24) |
| PHA blast | 100 (37/37) | |||
| Patient 8 | Q348X | Subcloning | CD3 | 56 (18/32) |
| PHA blast | 100 (16/16) | |||
| Patient 9 | R175P | Subcloning | CD3 | 87 (34/39) |
| PHA blast | 0 (0/28) |
| Sample . | Mutations . | Analysis . | Subtype . | Mutant type, % (proportion) . |
|---|---|---|---|---|
| Mother of patient 3 | D311E | FACS | CD3 | 13 |
| PHA blast | 47 | |||
| Subcloning | CD3 | 22 (6/27) | ||
| PHA blast | 48 (11/23) | |||
| Mother of patient 4 | A169P | Subcloning | CD3 | 52 (11/21) |
| PHA blast | 18 (9/49) | |||
| Mother of patient 8 | Q348X | Subcloning | CD3 | 0 (0/26) |
| PHA blast | 0 (0/21) | |||
| Mother of patient 10 | 1167insC | Subcloning | CD3 | 18 (7/39) |
| PHA blast | 9 (4/43) | |||
| Patient 2 | Duplication | FACS | CD3 | 73 |
| PHA blast | 93 | |||
| Patient 4 | A169P | Subcloning | CD3 | 79 (19/24) |
| PHA blast | 100 (37/37) | |||
| Patient 8 | Q348X | Subcloning | CD3 | 56 (18/32) |
| PHA blast | 100 (16/16) | |||
| Patient 9 | R175P | Subcloning | CD3 | 87 (34/39) |
| PHA blast | 0 (0/28) |
PHA blasts were obtained by incubating PBMCs with PHA and soluble IL-2 for 7 days. Data are shown as either the percentages of NEMOnormal cells, as assessed by flow cytometry, or as the ratio of clones containing wild-type NEMO to the total number of clones, as analyzed by subcloning and sequencing analysis.
Discussion
Somatic reversion mosaicism has been described in several disorders affecting the hematopoietic system, the liver, and the skin.23,26 Reports of somatic reversion cases have been particularly abundant in patients with immunodeficiency diseases, including Wiskott-Aldrich syndrome (WAS)27 and SCID, which occur because of mutations in the interleukin receptor common γ chain,28 CD3ζ,29 RAG-130 , and ADA genes.31 Patients with somatic reversion mosaicism may present with significantly milder clinical phenotypes compared with nonrevertant patients with the same germline mutation, although this is not always the case.26 One common feature in most cases where the somatic reversion mosaicism has been observed is a strong in vivo selective advantage of the revertant cells that express the wild-type gene product. One of the most intensively investigated diseases associated with somatic reversion mosaicism is WAS.32-34 A report showed that up to 11% of WAS patients have presented with somatic reversion mosaicism.33
In our investigation, 9 of 10 XL-EDA-ID patients presented with somatic mosaicism. Two of the 9 were cases of reversion from a duplication mutation, while the others exhibited true back-reversion from a substitution or insertion mutation. This finding calls for caution when diagnosing XL-EDA-ID patients. Because the existence of a NEMO pseudogene makes it difficult to perform genetic analysis using genomic DNA, diagnosis of the disease is often confirmed by sequencing analysis of NEMO cDNA, and the presence of somatic mosaicism can cause misdiagnosis of XL-EDA-ID patients either when NEMOnormal cells make up the majority of the patients' PBMCs or when the cDNA of the mutated NEMO gene cannot be amplified by PCR.17 In fact, mutated NEMO cDNA could not be amplified from the PBMCs of patient 2 even when NEMOnormal cells were absent (during early infancy), and only wild-type NEMO cDNA was amplified after the appearance of NEMOnormal cells (data not shown), probably because of the instability of the mutated NEMO mRNA. Flow cytometric analysis of intracellular NEMO protein is of help in identifying the NEMOlow cells in some patients, but the technique is not applicable when the NEMO mutation does not cause reduced expression of NEMO protein. Thus, some cases of XL-EDA-ID patients with reversion may be difficult to diagnose.
The high frequency of somatic mosaicism observed in XL-EDA-ID patients indicates a strong in vivo selective advantage for NEMOnormal cells, which express the wild-type gene product. Patient 2 presented with a high mutant T-cell count at birth that gradually decreased over time (Figure 1B). This finding indicates that wild-type NEMO expression is critical for the survival of certain cell lineages, including T cells, after birth. On the other hand, no NEMOnormal monocytes and very few NEMOnormal B cells were detected in the recruited XL-EDA-ID patients (Table 4). This specific feature is similar to other somatic reversion mosaicism seen in primary immunodeficiency patients26 and indicates that the expression of NEMO is less critical for the survival of monocytes or B cells compared with that of T cells. There is also an apparent concordance between the degree of the disruption of NEMO gene and the proportion of reverted NEMOnormal cells compared with NEMOlow cells. The high proportion of reverted T cells seen in patients 1 and 2 as well as in patient 8 was associated with a highly disruptive mutation of the NEMO gene (ie, a duplication mutation in patients 1 and 2, and a truncation mutation in patient 8). In addition, the highly selective X-chromosome inactivation observed in the mothers of XL-EDA-ID patients indicated a strong selective advantage for NEMOnormal cells over NEMOlow cells. It is also noteworthy that reverted T cells were not detected in patient 5, who carried an L227P mutation that was not localized to either of the functional domains in the NEMO protein. Other reported cases with the same mutation presented with polysaccharide-specific humoral immunodeficiency and autoinflammatory diseases, but were spared complications such as cellular immunodeficiency and susceptibility to Mycobacterium (similar to patient 5).4,8 This may reflect the fact that the L227P mutation in NEMO has less influence on T-cell growth than NEMO mutations that occur in functional domains, and suggests that reversion of the mutation has little impact on T-cell survival. Although the number of cases in our study is limited, it appears that the more disruptive NEMO mutations favor the survival of NEMOnormal cells after reversion and X-chromosome inactivation.
Regarding the gradual decline in the number of NEMO-deficient T cells, one candidate trigger could be infection. Because the dominance of the memory phenotype and the skewed TCR repertoire among CD8+ T cells in NEMOnormal cells were observed in both patients 1 and 2 (Figure 1C and Mizukami et al18 ), continuous infection of pyogenic bacteria in patient 1 and M szulgai in patient 2 could be a reason for the emergence of NEMOnormal cells and the elimination of NEMOlow cells. The decrease in NEMOnormal cells and restoration of NEMOlow cells after anti-mycobacterial therapy in patient 2 support this hypothesis. In the case of patient 1, the predominance of NEMOnormal T cells with an effector/memory phenotype at diagnosis (Table 4 and Mizukami et al18 ) is likely to be the result of chronic infection, and it is possible that NEMOlow cells were predominant during his early infancy. Because some reports have indicated that TNF-α–induced programmed cell death of several cell types, including a human T-cell line, was enhanced by hypomorphic NEMO mutations,12,35 and considering our finding that the levels of TNF-α expressed in revertant T cells were similar to levels in healthy control T cells in vitro (Figure 1F), TNF-α produced from these cells in response to infection could be involved in mutant T-cell elimination.
Unexpectedly, T-cell proliferation in patient 2 was equivalent to that of normal controls at the age of 2 months and was reduced after NEMOnormal T cells increased (Figure 1B; Table 3). This finding indicates that the NEMOlow T cells did not have intrinsically impaired mitogen-induced proliferation. One reasonable explanation for the reduced proliferation observed after the increase in NEMOnormal T cells is a reduction in the absolute number of T cells (naive T cells in particular), probably because of the infection. Therefore, to identify other mechanisms underlying reduced T-cell proliferation, the impact of NEMO mutation on PHA-induced T-cell proliferation was indirectly examined in vitro by comparing the response of NEMOnormal and NEMOlow cells derived from XL-EDA-ID patients and their mothers. After PHA stimulation and proliferation, the proportion of NEMOlow T cells increased in patients 2, 4, and 8, while the opposite result was observed in patient 9 and in the mother of patient 4 (Table 6). Although the precise mechanism is unclear, a reduction in the proportion of NEMOnormal cells after PHA stimulation would reflect the lower proliferative capacity of NEMOnormal cells compared with that of NEMOlow cells, which may be another explanation for the reduced T-cell proliferation observed in patient 2 at 23 months of age when NEMOnormal T cells were dominant. In the reports on reversion mosaicism of IL2RG gene mutations,28,36 the restoration of T-cell function and clinical symptoms varied among patients. Therefore, other factors besides the genotype of the mutations, such as the developmental stage where reversion occurred and the frequency of reversion, affect the clinical impact of somatic mosaicism of NEMO gene mutations.
In this study, the effect of somatic mosaicism of the NEMO gene on clinical phenotype could not be fully evaluated. However, cytokines produced by revertant T cells could influence the development of clinical symptoms of XL-EDA-ID, such as inflammatory bowel disease. In a mouse model, intestinal epithelial cell-specific inhibition of NF-κB through the conditional ablation of NEMO resulted in the development of chronic bowel inflammation sensitized intestinal epithelial cells to TNF-α–induced apoptosis.37 In this model, the first phase of intestinal inflammation was initiated by epithelial cell death and was followed by a second phase of TNF-α–induced intestinal inflammation, the latter being dependent on T cells. Another report showed that HSCT in XL-EDA-ID patients exacerbated the patients' inflammatory bowel disease.38 Indeed, in patient 4, the percentage of reverted T cells was reduced after repeated administrations of anti-TNFα blocking Ab, and the symptoms of inflammatory bowel disease improved.18 Considering this evidence, somatic mosaicism in T cells might be an important factor leading to inflammatory disease in XL-EDA-ID patients with defective NF-κB activation. However, our study did not show a tight association between inflammatory bowel disease and somatic mosaicism, and further investigation is needed to determine whether the NEMOnormal T cells play a role in inflammatory processes in XL-EDA-ID.
In conclusion, this study has identified a high frequency of somatic mosaicism in XL-EDA-ID patients, particularly in T cells, and has revealed important insights into human T-cell immunobiology in XL-EDA-ID. Although we could not demonstrate the clinical impact of somatic mosaicism in XL-EDA-ID patients, our findings suggest that care is required when making molecular diagnoses of XL-EDA-ID, and might shed light on the mechanisms underlying the variability in the clinical manifestation of XL-ED-ID and facilitate the search for appropriate treatments.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to all the XL-EDA-ID patients and their families for their participation. They also thank Shoji Yamaoka (Department of Molecular Virology, Graduate School of Medicine, Tokyo Medical and Dental University, Tokyo, Japan) for kindly providing NEMO-null rat fibroblast cells, and Takeda Pharmaceutical Company Limited for kindly providing the recombinant human IL-2.
This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science, and Technology, and by grants from the Japanese Ministry of Health, Labor and Welfare.
Authorship
Contribution: Tomoki Kawai wrote the manuscript and performed research; R.N., T.Y., T.N., and T.H. edited the manuscript and supervised this work; K.I., Y.M., N.T., H.S., M.S., and Y.T. cultured cells; and T. Mizukami, H.N., Y.K., A.Y., T. Murata, S.S., E.I., H.A., Toshinao Kawai, C.I., S.O., and M.K. treated patients and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ryuta Nishikomori, MD, PhD, Department of Pediatrics, Kyoto University Graduate School of Medicine, 54 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: rnishiko@kuhp.kyoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal