Abstract
Agrin, an extracellular matrix protein belonging to the heterogeneous family of heparan sulfate proteoglycans (HSPGs), is expressed by cells of the hematopoietic system but its role in leukocyte biology is not yet clear. Here we demonstrate that agrin has a crucial, nonredundant role in myeloid cell development and functions. We have identified lineage-specific alterations that affect maturation, survival and properties of agrin-deficient monocytic cells, and occur at stages later than stem cell precursors. Our data indicate that the cell-autonomous signals delivered by agrin are sensed by macrophages through the α-DC (DG) receptor and lead to the activation of signaling pathways resulting in rearrangements of the actin cytoskeleton during the phagocytic synapse formation and phosphorylation of extracellular signal-regulated kinases (Erk 1/2). Altogether, these data identify agrin as a novel player of innate immunity.
Introduction
Agrin is an extracellular matrix protein belonging to the heterogeneous family of heparan sulfate proteoglycans (HSPGs), which are key molecules involved in skeletal development, hematopoiesis, and inflammation,1,2 as well as in leukocyte adhesion and motility.3 Agrin has been extensively studied in the context of the neuromuscular junction (NMJ), where the protein exerts a key role as regulator of postsynaptic differentiation.4 However, although agrin is broadly expressed during development,5,6 little is known approximately its role at sites other than the NMJ. In the context of leukocytes, a previous study suggested that agrin may be a regulator of the T-cell immunologic synapse, where it may act as a costimulatory molecule by recruiting lipid rafts.7 More recently it was reported by our group that agrin deficiency on bone marrow (BM) stroma leads to a defect in CD34+CD135− LSK cell differentiation resulting in reduced cell numbers of all hematopoietic cell lineages.8 Our studies, as well as previous ones, indicated that the agrin receptor in hematopoietic cells is α-dystroglycan (α-DG), a broadly expressed cell-surface receptor with high affinity for extracellular matrix (ECM) proteins.9
Although both agrin and α-DG are expressed in mature hematopoietic cells,7,10 the in vivo functional significance of their expression is still unclear. The aim of this study was to analyze the involvement of agrin in leukocyte biology. By using mice that are null for agrin,11 we demonstrate that agrin is a critical player in the development and function of monocytes and macrophages.
Methods
Mice
Agrin-deficient mice have been described elsewhere.11 Musk-LAgrn−/+ mice (on C57BL/6 background) were bred at the animal facility of the Humanitas Clinical Institute. Mutant and control mice were genotyped by polymerase chain reaction (PCR) of tail DNA as already described.11 Congenic B6(CD45.1) mice, purchased from Jackson ImmunoResearch Laboratories, were maintained in the Charles River animal facility and used as recipients of BM transplantation experiments. Procedures involving animals and their care conformed to institutional guidelines in compliance with national (4D.L. N.116, G.U., suppl. 40, 18-2-1992) and international (EEC Council Directive 86/609, OJ L 358,1,12-12-1987; National Institutes of Health Guide for the Care and Use of Laboratory Animals) law and policies. All efforts were made to minimize the number of animals used and their suffering.
BM transfer assays
For BM transfer experiment 1 × 106 BM cells from P5 Musk-LAgrn−/− or control mice were transferred into 8- to 12-week-old B6(CD45.1) recipients, that were placed under antibiotic treatment 1 week before and 2 weeks after irradiation (950 cGy). Mice were killed and analyzed 9 weeks after transfer; a second BM transfer was performed as the first and analyzed 9 weeks later.
Histology
Tissues were fixed in 4% (weight/volume) formalin, embedded in paraffin, sectioned, and stained with H&E.
Immunohistochemistry and immunofluorescence
Sections (5-μm) of OCT-embedded femur from ctrl and Musk-L;Agrn−/− mice fixed in 4% (weight/volume) paraformaldehyde and decalcified, as previously reported,12 were incubated with anticathepsin K (1:400; gift from Prof D. Bromme, Department of Biochemistry and Molecular Biology, The University of British Columbia, Vancouver, BC)13 antibody and the appropriate secondary antibody. Staining was visualized by 3,3′-diaminobenzidine (DAB; Dako) and counterstained with hematoxylin. Images were acquired with Olympus BX51 microscope equipped with Colorview III digital camera (Olympus).
Resident peritoneal macrophages incubated with opsonized bioparticles were fixed with 10% formalin, permeabilized with ice-cold acetone as already reported,14 blocked and stained with Alexa 488–phalloidin (1:100; Invitrogen) or with anti–p-Syk (1:100, Cell Signaling Technologies) followed by Alexa 488 anti–rabbit (1:500; Invitrogen). Nuclei were counterstained with Hoechst 33342 (1 mg/mL) and mounted with ProLong (Molecular Probes).
Sorted monocytes and macrophages were fixed with 1% or 4% formaldehyde, blocked with bovine serum albumin (BSA) 1% and incubated with the primary antibodies antiagrin (1:2000; gift from Prof M. Ferns, Department of Physiology and Membrane Biology, University of California, Davis School of Medicine, Davis, CA), anti–α-DG (1:250; AbCam) and anti–Grb-2 (1:100; R&D systems). The appropriate Alexa-conjugated secondary antibodies were used (Molecular Probes). The nuclei were counterstained with Hoechst 33342 (Molecular Probes). To induce capping, macrophages were first incubated for 30 minutes at 4°C with the primary monoclonal antibodies (mAbs) to α-DG (1:250; AbCam), CD71 (1:100l; BioLegend), or MHCII (1:100; BD Pharmingen) and then at 37°C with the appropriate Alexa-conjugated secondary mAbs (Molecular Probes). Cells were fixed with 4% formaldehyde and labeled with anti–Grb-2 as previously described. Images were acquired by a confocal microscope Fluoview FV1000 (Olympus) with an oil immersion objective (40× or 60 × 1.4 NA Plan-Apochromat; Olympus) using laser excitation at 405, 488, 594, or 633 nm. Images were processed using Adobe Photoshop 9.0.2. Colocalization was measured on single confocal sections using the “colocalization” module of Imaris 5.0.1, 64-bit version (Bitplane AG). Colocalization coefficients used were (1) Pearson (describes the correlation of the intensity distributions between channels. Its values range between −1.0 and 1.0, where 0 indicates no significant correlation and 1.0 indicates complete positive correlation); and (2) Manders (values are in the range from 0 to 1.0. A value of zero means that there are no overlapping pixels).
FACS sorting and analysis
BM cell suspensions were obtained by flushing of multiple bones (femur, tibia, humerus, ulna) in sterile staining buffer (PBS containing 2% FCS). Peripheral blood (PB) was obtained by retro-orbital blood withdrawal. Cell surface, 4/6 color stainings were performed in staining buffer for 30 minutes, on ice, in the dark, with the appropriate combinations of saturating concentrations of the following conjugated monoclonal antibodies (mAbs) obtained from either BD Pharmingen, eBioscience, or BioLegend: CD45.2 (104), CD45.1 (A20), Mac1/CD11b (M1/70), CD3e (145-2C11), CD127/IL-7Rα (A7R34), CD115 (AFS98), CD19 (MB19-1), CD45 (R0-F11), Ly6G (1A8), Ly-6C (AL-21), Gr-1 (RB6-8C5), B220 (RA3-6B2), Sca-1 (D7), CD16/32 (2.4G2), and CD34 (RAM34). The lineage cocktail that included the Mac1, Gr1, B220, TER119, CD5, 7/4 biotin-conjugated mAbs and CD117/c-Kit (3C1) were purchased from MACS Mylteni. F4/80 (A3-1) was purchased from Serotech. Phospho-ERK expression was detected using anti-ERK1/2(T202/Y204) mAb (BD Pharmigen). All macrophage fluorescence-activated cell sorter (FACS) analyses have been performed with the exclusion of SSChi eosinophil population. Dead cells were excluded by low angle and orthogonal light scatter.
Absolute count of leukocytes was performed using TruCount tubes (BD Bioscience) according to the manufacturer's instructions. For annexin V staining, freshly isolated BM cells were first stained with the appropriate mAbs, then washed in binding buffer and incubated with annexin V (BD Pharmingen).
Stained cells were analyzed with a FACSCanto I (2 lasers) or FACSCanto II (3 lasers) flow cytometer (BD Bioscience). Cell sorting experiments were performed using a FACSAria (3 lasers; BD Bioscience). Diva software (BD Pharmingen) was used for data acquisition and analysis. Granulocyte-monocyte progenitor (GMP), common myeloid progenitors (CMP), and megakaryocyte-erythroid progenitor (MEP) progenitor analysis was performed according to published protocols.15
In vitro apoptosis assay
In vitro apoptosis assay was performed with total splenocytes from agrin-deficient (Musk-LAgrn−/−) or control (ctrl) mice incubated with anti–α-DG antibody IIH6C4 (Millipore) or isotype control. Annexin+/propidium iodide (PI)+staining was analyzed within the CD11b+/F4/80+ population after a 24 hours incubation.
In vitro and in vivo phagocytosis assay
In vitro Fc-R mediated phagocytosis was assessed with paraformaldehyde-inactivated Alexa Fluor 594 zymosan (Saccaromyces cerevisiae) bioparticles opsonized with purified rabbit polyclonal immunoglobulin (Ig)G antibody specific for S cerevisiae. Opsonized IgG-594 S cerevisiae were added to cells at a ratio of 15/particles/macrophage cell in the dark at 37°C for 1 or 45 minutes. To stop phagocytosis, chamber slides were transferred onto ice. Undigested serum-opsonized zymosan was removed by rinsing with chilled PBS. Slides were stained with Alexa 488–conjugated phalloidin (Molecular Probes) and the phagocytic index was scored as the total number of ingested serum-opsonized zymosan per macrophage.
For in vivo assays heat inactivated Aspergillus fumigatus conidia (kindly provided by L. Romani, Department of Experimental Medicine and Biochemical Sciences, University of Perugia, Perugia, Italy) were used.16 Conidia were labeled with fluorescin 5(6)-isothiocynate (FITC; Sigma-Aldrich; 5 mg/mL in DMSO). Mice were injected intraperitoneally with 8 × 107 conidia and sacrified 30 minutes later. PEC macrophages were analyzed by FACS for phagocytic index determination (% of ctrl). Cytospins were prepared and stained with Diff Quick (Dade).
For frustrated phagocytosis assays macrophages were overlayed onto human IgG-opsonized coverslips. After 60 minutes cells were washed, fixed with 4% paraformaldehyde/PBS, stained for Alexa 488–conjugated phalloidin (Molecular Probes), and processed for immunofluorescence.
In vitro monocyte and macrophage differentiation
For monocyte differentiation total BM cells or equal number of sorted CMPs, GMPs or monoblasts from agrin-deficient (Musk-L;Agrn−/−) or control (ctrl) mice were cultured for 5 or 7 days in IMDM supplemented with 10% FCS + glu + 15mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid) + PenStrep with 50 ng/mL recombinant macrophage colony-stimulating factor (M-CSF) or 20 ng/mL granulocyte CSF (G-CSF). Sorted Ly6Chigh BM cells (7 × 104 sorted cells per well in 48-well plates) were cultured for 3 days in IMDM supplemented with 10% FCS+glu+15mM Hepes+ PenStrep with 50 ng/mL of recombinant M-CSF. Three populations could be distinguished: Ly-6ChighF4/80−, Ly-6ChighF4/80+, and Ly-6ClowF4/80+, resembling monocytes, monocytes in the process of differentiating into macrophages, and mature macrophages, respectively.17
Statistical analysis
Statistical analyses were performed using the unpaired Student t test; (GraphPad Prism Version 4.0), or where indicated, by ANOVA followed by the nonparametric Student Newman-Keuls test for multiple comparisons. Differences were considered significant when P ≤ .05, very significant when P ≤ .01, and extremely significant when P ≤ .0001.
Results
Agrin is predominantly expressed by cells of the monocytic lineage
Previous publications have described agrin as broadly expressed across different hematopoietic cell types, such as T cells and B cells.7,10 However, flow cytometric analysis of PB leukocytes showed that agrin is predominantly expressed in monocytes, whereas it is significantly lower in lymphocytes and granulocytes (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Further analyses showed that expression of agrin and its receptor α-DG are developmentally regulated in monocytes, with mature developmental stages showing higher levels of agrin and α-DG expression compared with their BM myeloid progenitors (Figure 1).
Expression of agrin and its receptor in monocytic cell populations. Representative flow cytometric analyses and confocal images of agrin and α-DG expression in myeloid progenitors and monocytic cell populations (the gray solid curve represents the isotype control). Cells were stained for agrin or α-dystroglycan (α-DG; green) and nuclear DNA (blue). Scale bar, 5 μm.
Expression of agrin and its receptor in monocytic cell populations. Representative flow cytometric analyses and confocal images of agrin and α-DG expression in myeloid progenitors and monocytic cell populations (the gray solid curve represents the isotype control). Cells were stained for agrin or α-dystroglycan (α-DG; green) and nuclear DNA (blue). Scale bar, 5 μm.
Defective generation of monocytes in agrin deficient mice
We took advantage of agrin knockout mice to investigate the functions of agrin in monocytes and macrophages. To overcome the neonatal lethality of agrin knockout mice because of neuromuscular synapse dysfunctions,18 we used mice that are null for all agrin isoforms but carry a transgene that increases Musk expression selectively in skeletal muscle.11 Musk expression in skeletal muscle allows agrin knockout mice to live longer, but still most Musk-L;Agrn−/− mice die between postnatal day (P) 7 and P10 for unknown reasons11 and our experiments were therefore performed with P4 to P6 mice. Musk-L;Agrn+/+ or Musk-L;Agrn−/+ (referred hereafter as control mice) did not differ in any of the analyzed phenotypes, and hence, will not be further distinguished.
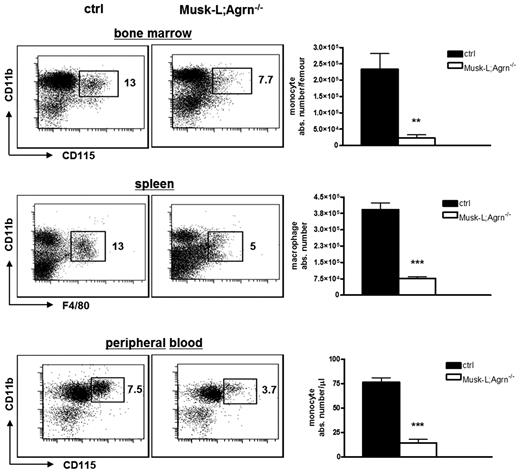
In a previous study,8 we reported that the absolute number of total leukocytes in all hematopoietic and peripheral compartments is reduced in MuSK-L;Agrn−/− mice compared with control mice. Interestingly, in addition to the general reduction of hematopoietic cell numbers, agrin-deficient mice showed a significantly reduced frequency of monocytic cells in BM, spleen, and PB (Figure 2), in comparison with control mice. This reduction in relative frequency was not observed in lymphocytes (T or B) or granulocytes (supplemental Figure 2 and not shown), indicating that the absence of agrin specifically affects monocytes and macrophages.
Reduced frequency and numbers of monocytic cells in Musk-L;Agrn−/− mice. Representative flow cytometric analyses and absolute numbers of monocytic cells in control and Musk-L;Agrn−/− mice. Values in dot plots represent the percentage among CD45+ cells (ctrl, n = 10; Musk-L;Agrn−/−, n = 10; **P ≤ .01, ***P ≤ .0001; error bars represent SEM).
Reduced frequency and numbers of monocytic cells in Musk-L;Agrn−/− mice. Representative flow cytometric analyses and absolute numbers of monocytic cells in control and Musk-L;Agrn−/− mice. Values in dot plots represent the percentage among CD45+ cells (ctrl, n = 10; Musk-L;Agrn−/−, n = 10; **P ≤ .01, ***P ≤ .0001; error bars represent SEM).
To determine whether agrin deficiency differentially impairs specific subsets of monocytes, we evaluated the percentage of peritoneal cavity macrophages and liver Kupffer cells, 2 macrophage subtypes that differ in their dependency on M-CSF.19 Flow cytometry analysis revealed decreased frequency and absolute numbers of both M-CSF dependent (peritoneal cavity macrophages) and independent (liver Kupffer cells) tissue myeloid cells in agrin knockout mice (supplemental Figure 3A). In accordance with the general impairment in the monocytic compartment, agrin-deficient mice had fewer osteoclasts than control littermates (supplemental Figure 3B), although this did not result in alterations of the bone cavity.8
The observed reduction of mature monocytic cells in agrin-deficient mice could be the consequence of developmental arrest(s) during the differentiation process and/or reduced viability of mature cells. In terms of differentiation, we observed a significant increase in the frequency of the oligopotent population of CMPs, as well as of the more differentiated GMPs in agrin-deficient mice (Figure 3A). Because these data suggested the presence of a developmental arrest at later stages of the differentiation process, specific developmental stages of monocytic cells were analyzed in in vitro experiments. Equal numbers of BM cells obtained from either agrin-deficient or control mice were cultured with M-CSF20 and the in vitro differentiation of monocytes was evaluated. CD11b expression was monitored to verify its decrease in promonocytes and monocytes compared with monoblasts (data not shown). We found that lack of agrin inhibited the transition of promonocytes (CD31int/Ly6C+) into monocytes (CD31−/Ly6Chigh; supplemental Figure 4A). This result was further confirmed and expanded in in vitro differentiation experiments in which equal numbers of sorted CMPs (Figure 3B), GMPs (Figure 3C), and monoblasts (Figure 3D) from either Musk-L;Agrn−/− or control mice were stimulated with M-CSF to give rise to monocytes. Moreover, to substantiate the claim that the observed defect is specific for the monocyte/macrophage lineage, sorted GMPs were also cultured in the presence of G-CSF (Figure 3E). Cell analysis after 7 days demonstrated that neutrophil differentiation was not affected by the absence of agrin.
Agrin deficiency impairs monocytic cell maturation. (A) Representative flow cytometric analysis of the myeloid hematopoietic progenitors in control and agrin-deficient mice and relative frequencies among total BM cells (ctrl, n = 20; Musk-L;Agrn−/−, n = 5). FACS analysis is referred to the Lin−/IL7R−/cKit+/Sca1− gate, in which CMPs are defined as CD34+/low/CD16/32int, whereas GMPs as CD34+/CD16/32+. Equal numbers of sorted CMP(B) or GMP BM progenitors (C) and (E) or CD31+/Lin− BM monoblasts (D) from P5 control and Musk-L;Agrn−/− mice were cultured for 7 (B-C,E) or 5 days (D) with 50 ng/mL recombinant M-CSF (B-C,D) or with 20 ng/mL recombinant G-CSF (E) and total cell numbers relative to control were calculated. Data are from 2 (B-C,E) or 3 (D) independent experiments. Gate strategy was performed as shown in representative plots; R1: c-Kit+/CD31high/Ly6C−/Ly6G−; R2: c-Kit+/CD31+/Ly6C+/Ly6G−; R3: c-Kitlow/CD31−/Ly6Chigh/Ly6G−;R4: c-Kitlow/CD31−/Ly6Cint/Ly6G+. (F) Equal numbers of sorted Ly6Chigh BM cells from P5 control and Musk-L;Agrn−/− mice were cultured for 3 days with 50 ng/mL recombinant M-CSF and total monocytic cell numbers relative to control were calculated. Shown are a representative flow cytometric analysis of Ly6ChighF4/80− (monocytes), Ly-6ChighF4/80+ (monocytes in the process of differentiating into macrophages), and Ly6CloF4/80+ (mature macrophages) and the statistical analysis of the frequency of the 3 populations, (ctrl, n = 14; Musk-L;Agrn−/−, n = 7). (G) Annexin V analysis on P5 BM monocytes and splenic macrophages (ctrl, n = 10; Musk-L;Agrn−/−, n = 7;). In all panels, error bars represent SEM and *P ≤ .05, **P ≤ .01, ***P ≤ .0001.
Agrin deficiency impairs monocytic cell maturation. (A) Representative flow cytometric analysis of the myeloid hematopoietic progenitors in control and agrin-deficient mice and relative frequencies among total BM cells (ctrl, n = 20; Musk-L;Agrn−/−, n = 5). FACS analysis is referred to the Lin−/IL7R−/cKit+/Sca1− gate, in which CMPs are defined as CD34+/low/CD16/32int, whereas GMPs as CD34+/CD16/32+. Equal numbers of sorted CMP(B) or GMP BM progenitors (C) and (E) or CD31+/Lin− BM monoblasts (D) from P5 control and Musk-L;Agrn−/− mice were cultured for 7 (B-C,E) or 5 days (D) with 50 ng/mL recombinant M-CSF (B-C,D) or with 20 ng/mL recombinant G-CSF (E) and total cell numbers relative to control were calculated. Data are from 2 (B-C,E) or 3 (D) independent experiments. Gate strategy was performed as shown in representative plots; R1: c-Kit+/CD31high/Ly6C−/Ly6G−; R2: c-Kit+/CD31+/Ly6C+/Ly6G−; R3: c-Kitlow/CD31−/Ly6Chigh/Ly6G−;R4: c-Kitlow/CD31−/Ly6Cint/Ly6G+. (F) Equal numbers of sorted Ly6Chigh BM cells from P5 control and Musk-L;Agrn−/− mice were cultured for 3 days with 50 ng/mL recombinant M-CSF and total monocytic cell numbers relative to control were calculated. Shown are a representative flow cytometric analysis of Ly6ChighF4/80− (monocytes), Ly-6ChighF4/80+ (monocytes in the process of differentiating into macrophages), and Ly6CloF4/80+ (mature macrophages) and the statistical analysis of the frequency of the 3 populations, (ctrl, n = 14; Musk-L;Agrn−/−, n = 7). (G) Annexin V analysis on P5 BM monocytes and splenic macrophages (ctrl, n = 10; Musk-L;Agrn−/−, n = 7;). In all panels, error bars represent SEM and *P ≤ .05, **P ≤ .01, ***P ≤ .0001.
In accordance with the in vitro data, the ex vivo analysis of BM monocytic subsets showed an increased frequency of monoblast population that paralleled with a decreased frequency of monocytes in agrin-deficient mice compared with controls (supplemental Figure 4B).
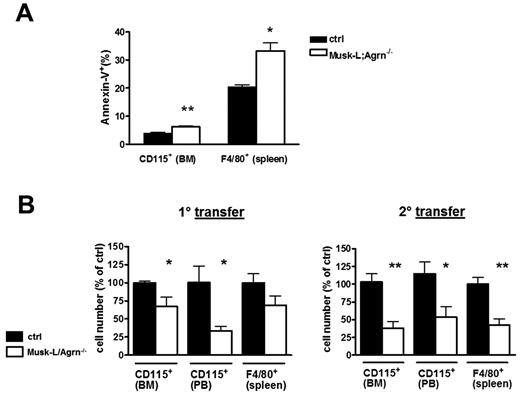
Next, we analyzed the in vitro differentiation of sorted Ly6Chigh BM cells into macrophages. Interestingly, we found that agrin is required not only for the generation of monocytes, but also for the further differentiation of monocytes into macrophages (Figure 3F). The total number of agrin-deficient monocytic cells recovered after 3 days of culture with M-CSF was 2-fold reduced compared with controls (data not shown). This fact prompted us to further analyze the contribution of agrin to the monocytic compartment and to speculate that, in addition to the developmental problems already discussed, reduced viability of monocytic cells could contribute to the decline of monocyte/macrophage populations observed in agrin-deficient mice. Indeed, annexin V staining demonstrated increased ex vivo apoptosis of myeloid cells in Musk-L;Agrn−/− mice compared with control (Figure 3G).
Cell-autonomous effects of agrin on differentiation and survival of monocytic cells
Agrin-deficient mice might have multiple defects that could indirectly cause the observed monocytic cells phenotype. Moreover, it was important to determine whether monocytic cells are dependent on agrin signals in a cell-autonomous manner or if they can sense agrin provided by the microenvironment. Thus, we performed BM transfer experiments in which lethally irradiated wild-type (WT) recipients (CD45.1) were reconstituted using total BM cells from either Musk-L;Agrn−/− or control mice (CD45.2) and subsequently a second serial 9 weeks transfer. The frequency of PB hematopoietic cells expressing CD45.2 was quantified by flow cytometry, demonstrating comparable reconstitution in all recipients in both transfers (supplemental Figure 5A). When the viability and frequency of myeloid cells was analyzed, even in an agrin-expressing environment, a higher percentage of apoptotic cells was detected among agrin-deficient myeloid cells (Figure 4A), suggesting the existence of a cell-autonomous agrin signal required for monocyte survival. These experiments also indicated that, although BM cells from agrin-deficient mice were able to engraft and reconstitute lethally irradiated recipients,8 agrin-deficient monocytic cells were less abundant than their control counterpart both in the BM and in periphery (Figure 4B). The specific defect in monocytic lineage development was maintained after a second serial transfer (9 weeks plus 9 weeks; Figure 4B). The absolute number and normalized count of PB T, B cells, and peripheral mononuclear cells (PMNs) showed no significant differences between mice reconstituted with ctrl or Musk-L;Agrn−/− BM cells (supplemental Figure 5A-B), supporting once again the specificity of the monocytic cell lineage defect. Altogether, these results confirm the data obtained with Musk-L;Agrn−/− mice and, in addition, indicate that agrin provides cell-autonomous signals for the development and survival of monocytic cells. Moreover, because the defective phenotype was maintained in reconstituted WT mice that were otherwise healthy, we can exclude the possibility that systemic effects and generalized poor health of Musk-L;Agrn−/− mice are responsible for the monocytic cell defects.
Cell-autonomous requirement for agrin in myeloid cell development. (A) Annexin V analysis on monocytes and macrophages of donor origin determined 9 weeks after BM transfer (ctrl, n = 4; Musk-L;Agrn−/−, n = 4). (B) Analyses of donor monocytic cells in BM, PB, and spleen 9 weeks after BM transfer (n = 4), and after a second serial (9 weeks plus 9 weeks) BM transfer (n = 6). In all panels, error bars represent SEM and *P ≤ .05, **P ≤ .01.
Cell-autonomous requirement for agrin in myeloid cell development. (A) Annexin V analysis on monocytes and macrophages of donor origin determined 9 weeks after BM transfer (ctrl, n = 4; Musk-L;Agrn−/−, n = 4). (B) Analyses of donor monocytic cells in BM, PB, and spleen 9 weeks after BM transfer (n = 4), and after a second serial (9 weeks plus 9 weeks) BM transfer (n = 6). In all panels, error bars represent SEM and *P ≤ .05, **P ≤ .01.
The cell-autonomous requirement for agrin was confirmed in in vitro experiments in which Ly6Chigh BM cells sorted from either control or Musk-L;Agrn−/− mice were cocultured with an equal number of either Ly6Chigh or Ly6C− cells (negative fraction) from control mice (CD45.1), in the presence of M-CSF. When differentiated CD45.2+/Ly6ClowF4/80+ macrophages were counted, we found that among WT BM cells, only monocytes partially improve the differentiation of agrin-deficient monocytes, whereas the other cell populations have no effects (supplemental Figure 6).
Defective phagocytosis by agrin-deficient macrophages
The data described in Figure 3 indicate that the loss of agrin results in defective maturation and reduced viability of monocytic cells. However, although at a reduced rate, monocytes and macrophages were generated in the absence of agrin and this allowed us to determine whether mature monocytic cells require agrin for their functional activities.
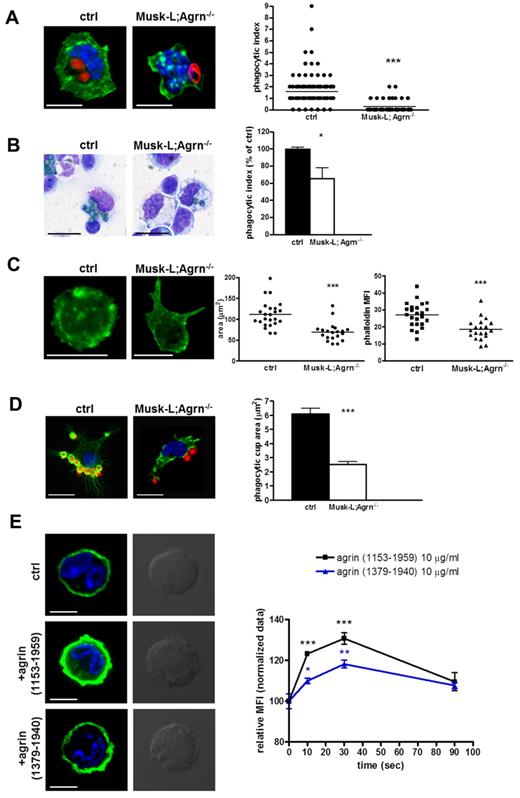
Macrophage phagocytosis is critical for defense against pathogens and we thus analyzed the ability of agrin-deficient macrophages to perform phagocytosis in vitro and in vivo. First, we compared spleen-sorted macrophages from control and Musk-L;Agrn−/− mice for their ability to engulf IgG-opsonized zymosan bioparticles (cerevisiae) in vitro. Although most of the control macrophages engulfed bioparticles, Musk-L;Agrn−/− macrophages showed defective phagocytosis, with more than one-half of the cells being unable to engulf any bioparticle (Figure 5A). Then, we performed an in vivo assay using A fumigatus conidia to explore the macrophage response to a pathogenic saprophytic fungus. CD45.1 mice were reconstituted with control or Musk-L;Agrn−/− (CD45.2) BM cells and, 8 weeks after the transfer, were injected intraperitoneally with 5 × 107 heat-inactivated, FITC-labeled conidia. Flow cytometric analysis performed on the CD45.1−/CD11b+/F4/80+ population revealed a 40% of reduction in the phagocytic index of Musk-L;Agrn−/− macrophages, compared with control ones (Figure 5B).
Impaired phagocytosis in agrin-deficient macrophages. (A) Confocal images and phagocytic index of CD11b+/F4/80+ macrophages sorted from control or Musk-L;Agrn−/− spleens and incubated with opsonized Alexa Fluor 594–conjugated zymosan A bioparticles (red) for 1 hour at 37°C (green: Alexa 488–Falloidin). The phagocytic index represents the number of red particles per macrophage. Scale bar, 10 μm. (B) Representative images and phagocytic index of an in vivo phagocytosis assay. Irradiated recipients (CD45.1) reconstituted with control or Musk-L;Agrn−/− BM cells 9 weeks after the transfer were injected intratraperitoneally with 5 × 107 heat inactivated FITC-labeled conidia and killed 30 minutes later. Peritoneal macrophage phagocytosis was analyzed by FACS on the CD45.1−/CD11b+/F4/80+ population. Alternatively, cytospins were prepared and stained with Diff Quick (images). Scale bar, 10 μm. (C) CD11b+/F4/80+ macrophages sorted from ctrl and Musk-L;Agrn−/− were plated onto hIgG-coated coverslips and fixed after 60 minutes. Representative images are shown; scale bar, 10 μm. Analyses of adherent membrane surface area (μm2) and phalloidin MFI are shown. (D) F-actin response (phalloidin staining, green) in peritoneal macrophages during formation of phagocytic synapses with opsonized zymosan A bioparticles (red) for 1 minute. Representative images and statistical analysis of the phagocytic cup area (μm2) are shown. Scale bar, 10 μm. (E) Resident peritoneal CD11b+/F4/80high macrophages sorted from control mice were treated with 10 μg/mL of soluble, recombinant agrin (1153-1959; R&D) or (1379-1940; kindly provided by Panos Kabourdis, William Harvey Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London), as indicated. Induction of actin polymerization (phalloidin staining) was analyzed by confocal microscopy (left) and flow cytometry (right). Scale bar, 5 μm. Error bars represent SEM and *P ≤ .05, **P ≤ .01, ***P < .0001.
Impaired phagocytosis in agrin-deficient macrophages. (A) Confocal images and phagocytic index of CD11b+/F4/80+ macrophages sorted from control or Musk-L;Agrn−/− spleens and incubated with opsonized Alexa Fluor 594–conjugated zymosan A bioparticles (red) for 1 hour at 37°C (green: Alexa 488–Falloidin). The phagocytic index represents the number of red particles per macrophage. Scale bar, 10 μm. (B) Representative images and phagocytic index of an in vivo phagocytosis assay. Irradiated recipients (CD45.1) reconstituted with control or Musk-L;Agrn−/− BM cells 9 weeks after the transfer were injected intratraperitoneally with 5 × 107 heat inactivated FITC-labeled conidia and killed 30 minutes later. Peritoneal macrophage phagocytosis was analyzed by FACS on the CD45.1−/CD11b+/F4/80+ population. Alternatively, cytospins were prepared and stained with Diff Quick (images). Scale bar, 10 μm. (C) CD11b+/F4/80+ macrophages sorted from ctrl and Musk-L;Agrn−/− were plated onto hIgG-coated coverslips and fixed after 60 minutes. Representative images are shown; scale bar, 10 μm. Analyses of adherent membrane surface area (μm2) and phalloidin MFI are shown. (D) F-actin response (phalloidin staining, green) in peritoneal macrophages during formation of phagocytic synapses with opsonized zymosan A bioparticles (red) for 1 minute. Representative images and statistical analysis of the phagocytic cup area (μm2) are shown. Scale bar, 10 μm. (E) Resident peritoneal CD11b+/F4/80high macrophages sorted from control mice were treated with 10 μg/mL of soluble, recombinant agrin (1153-1959; R&D) or (1379-1940; kindly provided by Panos Kabourdis, William Harvey Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London), as indicated. Induction of actin polymerization (phalloidin staining) was analyzed by confocal microscopy (left) and flow cytometry (right). Scale bar, 5 μm. Error bars represent SEM and *P ≤ .05, **P ≤ .01, ***P < .0001.
Altogether, these findings indicate that agrin-deficient macrophages have defective performance in terms of phagocytosis. Notably, flow cytometric analysis indicated that these defects could not be attributable to differences in the surface expression of the main Fcγ receptor or other key molecules, such as CD44, CD36, or CD11a (supplemental Figure 7).
Previous studies have shown that remodeling of cytoskeleton and plasma membrane are required for efficient phagocytosis.21 Therefore, we performed the frustrated phagocytosis assay which is analogous to the phagocytosis of an infinitely large substrate22 and allows to evaluate macrophage spreading onto opsonized substrata. The immunofluorescence analysis revealed that control macrophages displayed the dense, circumferential F-actin staining typical of spreading cells and that this phenotype was not present in Musk-L;Agrn−/− macrophages (Figure 5C). Actin polymerization, measured as phalloidin mean fluorescence intensity (MFI), and the adherent membrane surface area were indeed reduced in agrin-deficient macrophages compared with control (Figure 5C).
Interestingly, when agrin-deficient neutrophils were analyzed, we did not observe any defect in either phagocytosis or cytoskeletal remodeling (supplemental Figure 8), again indicating that agrin has a specific relevance for monocytes and macrophages.
Bidirectional information exchange between antigen presenting cells and the target T cells passes through sites of functional contact called the immunologic synapse.23 Recent studies have concentrated on a new model of synapse called the phagocytic synapse that is crucial for triggering phagocytosis in innate immune cells and is characterized by a localized expansion of the plasmalemmal membrane, coupled to highly active remodeling.14
We therefore examined the formation of the phagocytic synapse with IgG-opsonized zymosan bioparticles in control and agrin-deficient macrophages. Peritoneal resident macrophages from mice reconstituted with either Musk-L;Agrn−/− or control BM cells showed comparable activation and recruitment of Syk kinase to the synapse, independently of agrin expression (supplemental Figure 9). However in agreement with the data shown in Figure 5A, the number of synapses was markedly reduced in agrin-deficient macrophages, and the analysis of F-actin staining revealed a defective progress of the phagocytic cup, with pseudopode extension and phagosomal closure barely detectable (Figure 5D).
Altogether, these data indicate that the defective phagocytosis of agrin-deficient macrophages may be explained, at least in part, by cytoskeletal defects occurring in these cells. In addition, the experiments reported above suggest that the F-actin response occurring during phagocytosis may be regulated by agrin activity. In support of this hypothesis, soluble, recombinant agrin led to a potent F-actin response in both control (Figure 5E) and agrin-deficient (not shown) peritoneal resident macrophages.
Agrin delivers survival signals through the α-DG receptor
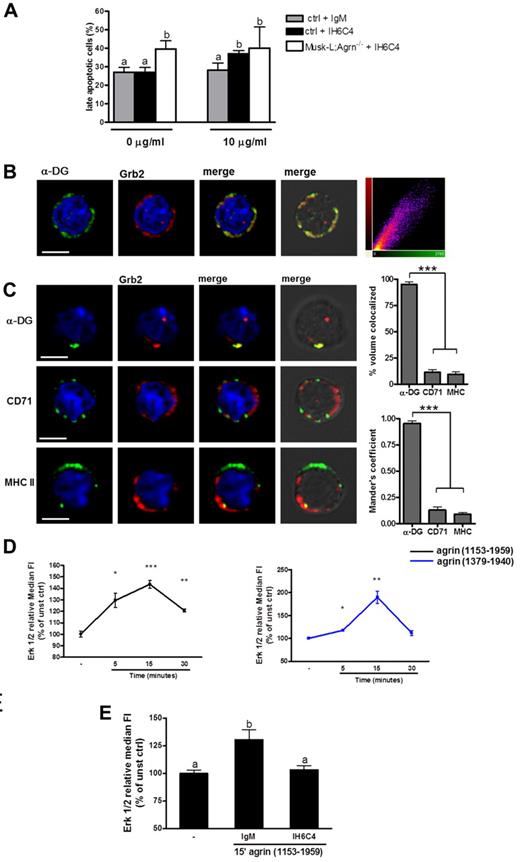
To explore the involvement of α-DG in agrin-mediated survival of myeloid cells, total splenocytes from control mice were incubated with an anti–α-DG antibody that inhibits the binding of agrin to the receptor.24,25 After 24 hours, the percentage of late apoptotic F4/80+ cells was evaluated by flow cytometry. This experiment showed that the antibody-mediated blocking of agrin–α-DG interactions enhanced apoptosis (Figure 6A) and that this effect required agrin expression, because the antibody failed to enhance apoptosis in splenocyte cultures obtained from agrin-deficient mice (Figure 6A).
Agrin delivers signals through the α-DG. (A) Control and Musk-L;Agrn−/− splenocytes were incubated with (10 μg/mL) or without (0 μg/mL) the anti-α-DG blocking antibody IIH6C4 or the same concentration of its isotype control (IgM). Annexin+/PI+ analysis was performed on CD11b+/F4/80+ macrophages after 24 hours (ctrl, n = 10; Musk-L;Agrn−/−, n = 6.) Bars with different letters are significantly different from each other (student-Newman-Keuls test, P < .05). (B-C) Representative confocal images (scale bar, 5 μm) of macrophages isolated from P5 control mice and quantitative colocalization analysis. (B) Cells were fixed and stained for α-DG (green), Grb2 (red), and DNA (blue). Corresponding pictures were merged (yellow). Each colocalized pixel was plotted on a scatter diagram to give correlation plots, with colocalizing pixels falling around the diagonal line. Data from at least 30 cells were used to calculate the Pearson coefficient (P) as mean ± SEM. (C) Cells were incubated with primary mAbs to α-DG, CD71, or MHCII and then secondary mAbs (green) to induce patching. Fixed cells were stained for Grb-2 (red) and DNA (blue). Corresponding pictures were merged (yellow). Percentage of colocalized volume and the Mander colocalization coefficient with Grb2 were determined as described in “Immunochemistry and immunofluorescence.” Data from at least 30 cells were used. (D) Total splenocytes were stimulated with commercial agrin (1153-1959; black line), or laboratory-produced agrin (1379-1940; blue line) and the phosphorylation of Erk 1/2 was evaluated in CD11b+ /F4/80+ cells. (E) Total splenocytes preincubated with the anti–α-DG blocking antibody IIH6C4 or its isotypic control (IgM) were stimulated with commercial agrin (1153-1959) for 15 minutes and the phosphorylation of Erk 1/2 was evaluated in CD11b+ /F4/80+ cells. Changes in Erk phosphorylation were expressed as fold increase of MFI of stimulated over unstimulated cells (time 0); n = 3. Bars with different letters are significantly different from each other (Student Newman-Keuls test, P < .05).
Agrin delivers signals through the α-DG. (A) Control and Musk-L;Agrn−/− splenocytes were incubated with (10 μg/mL) or without (0 μg/mL) the anti-α-DG blocking antibody IIH6C4 or the same concentration of its isotype control (IgM). Annexin+/PI+ analysis was performed on CD11b+/F4/80+ macrophages after 24 hours (ctrl, n = 10; Musk-L;Agrn−/−, n = 6.) Bars with different letters are significantly different from each other (student-Newman-Keuls test, P < .05). (B-C) Representative confocal images (scale bar, 5 μm) of macrophages isolated from P5 control mice and quantitative colocalization analysis. (B) Cells were fixed and stained for α-DG (green), Grb2 (red), and DNA (blue). Corresponding pictures were merged (yellow). Each colocalized pixel was plotted on a scatter diagram to give correlation plots, with colocalizing pixels falling around the diagonal line. Data from at least 30 cells were used to calculate the Pearson coefficient (P) as mean ± SEM. (C) Cells were incubated with primary mAbs to α-DG, CD71, or MHCII and then secondary mAbs (green) to induce patching. Fixed cells were stained for Grb-2 (red) and DNA (blue). Corresponding pictures were merged (yellow). Percentage of colocalized volume and the Mander colocalization coefficient with Grb2 were determined as described in “Immunochemistry and immunofluorescence.” Data from at least 30 cells were used. (D) Total splenocytes were stimulated with commercial agrin (1153-1959; black line), or laboratory-produced agrin (1379-1940; blue line) and the phosphorylation of Erk 1/2 was evaluated in CD11b+ /F4/80+ cells. (E) Total splenocytes preincubated with the anti–α-DG blocking antibody IIH6C4 or its isotypic control (IgM) were stimulated with commercial agrin (1153-1959) for 15 minutes and the phosphorylation of Erk 1/2 was evaluated in CD11b+ /F4/80+ cells. Changes in Erk phosphorylation were expressed as fold increase of MFI of stimulated over unstimulated cells (time 0); n = 3. Bars with different letters are significantly different from each other (Student Newman-Keuls test, P < .05).
The DC complex is known to bind the growth factor receptor binding protein 2 (Grb2),26-28 a widely expressed signaling protein associated with son of sevenless (SOS), an exchange factor of Ras GTPase.29,30 The Grb2-SOS-Ras signaling pathway is involved in development, proliferation and survival of hematopoietic cells and, in particular, it is required for monocyte/macrophage differentiation.31,32 Interaction between α-DG and Grb2 in primary myeloid cells from P5 control mice was confirmed by confocal microscopy. Quantitative colocalization analysis revealed constitutive association between the 2 molecules, as indicated by the Pearson correlation coefficient (P = .86 ± 0.01; Figure 6B.) This was confirmed by the fact that crosslinking of α-DG, but not of CD71 or MHC class II, resulted in redistribution of Grb2 in an intensely fluorescent, colocalization cap at the plasma membrane (Figure 6C). Similar results were obtained in agrin-deficient macrophages (not shown).
The activation of the agrin-induced Grb-2 signaling pathway was analyzed by measuring Erk 1/2 phosphorylation in myeloid cells. In agreement with results previously reported on myotubes,33 soluble agrin induced detectable Erk 1/2 phosphorylation in both control macrophages (Figure 6D) and agrin-deficient ones (supplemental Figure 10). However the Erk 1/2 phosphorylation 15 minutes peak was significantly reduced compared with control cells (ctrl relative MFI: 143.6 ± 3.5; Musk-L;Agrn−/− relative MFI: 120.7 ± 1.55, ***P ≤ .0001), suggesting that agrin-deficient macrophages express a functional agrin receptor but are hyporesponsive to α-DG stimulation. Spleen cells stimulated with phorbol-12-myristate-13-acetate (PMA) were used as positive control (not shown). In accordance with these data, preincubation of control macrophages with an α-DG blocking antibody resulted in a significant reduction in Erk 1/2 phosphorylation on addition of exogenous agrin (Figure 6E).
Discussion
Monocyte production, part of the highly regulated process called myelopoiesis, is based on the coordinated proliferation, survival and differentiation of hematopoietic stem cells and committed myeloid progenitors.34 Monocytes originate in the BM from a CMP that is shared with neutrophils, and once released into the PB, they circulate for several days before entering tissues and giving rise to mature macrophages.35 In this study, we discovered that agrin regulates monocyte and macrophage biology at multiple levels.
Agrin has been studied extensively at the NMJ, where it acts in trans as a key regulator of neuromuscular postsynaptic signal,4 but it has also been implicated in the formation of the immunologic synapse.7 More recently we have reported a novel, nonredundant and unexpected in trans role of agrin at the hematopoietic stem cell niche.8
In this study, we observed that agrin-deficient mice showed specific defects in the monocyte lineage, with reduced frequency and absolute number of mature monocyte-derived cells in hematopoietic and peripheral compartments. Moreover, monocytes and macrophages displayed ex vivo reduced viability, compared with WT littermates. CMP and GMP frequencies were slightly higher in agrin-deficient mice than in control, and, together with the in vitro differentiation data and the fact that granulocytes were not reduced in frequency, this indicates that agrin is required for monocytic developmental stages later than precursor proliferation/differentiation. Indeed, we could identify at least 2 arrests during in vitro stimulation of BM cells with M-CSF, one occurring during the maturation of monoblasts to monocytes and the other affecting differentiation of monocytes into macrophages. Thus, the HSPG glypican has been shown to mediate a role in lineage-specific differentiation of myeloid cells at the GMP stage,36 agrin seems to control downstream pathways specific for monocytic cells. Together, these data indicate that HSPGs are key players of myeloid development.
The BM transfers performed in this study not only exclude the contribution of systemic effects on monocyte homeostasis, but also provide in vivo evidence that monocytes maturation and survival require agrin signals delivered in a cell-autonomous manner. Lack of agrin, however, did not abolish macrophage production and, although at low frequencies, mature macrophages could be isolated from agrin-deficient mice and were further analyzed. We could not detect any difference in terms of expression of several maturation markers or receptors between agrin-deficient and control macrophages, suggesting that the macrophages that could survive and differentiate in the absence of agrin signals were similar to those obtained in control mice. However, agrin-deficient mature macrophages exhibited impaired in vitro and in vivo phagocytosis, which could not be explained by reduced or altered expression of phagocytic surface molecules. The impaired phagocytosis of agrin-deficient macrophages paralleled with the inability of these cells to spread onto an opsonized substratum, suggesting the presence of important cytoskeletal defects in the absence of agrin. The rapid and dynamic reorganization of the actin cytoskeleton to form the phagocytic synapse is indeed one of the most important features of phagocytosis.37 In addition to the spreading defects, when placed onto an opsonized substratum, agrin-deficient macrophages were also less responsive than control ones in terms of cytoskeletal rearrangements. In accordance with the known role of agrin as cytoskeleton organizer in muscle cells38 and T lymphocytes,7 we found that agrin is a very strong activator of actin polymerization in macrophages. However, the role of agrin in phagocytosis must be very specific because agrin-deficient macrophages were able to migrate in response to the chemokine CCL2 (data not shown). The investigation of the specific pathways activated by agrin during macrophage phagocytosis will be the subject of future studies.
The in vitro data obtained using an antibody that inhibits agrin interaction with α-DG allow us to propose that, as already established for T lymphocytes10 and hematopoietic stem cells,8 monocytic cells sense agrin through α-DG receptors.
Agrin-deficient monocytes express a functional α-DG receptor as shown by their capacity to respond to soluble agrin. However, their response in terms of pErk signaling is reduced, suggesting that agrin-deficient cells may be biochemically less responsive to α-DG. Furthermore, in physiologic conditions agrin is not soluble and the BM transfer results, together with the in vitro data, clearly indicate that agrin expressed by nonmonocytic cells cannot deliver signals necessary for monocyte/macrophage survival, differentiation, and phagocytosis. In agreement with this, the results from our cocolture assay (supplemental Figure 6) indicated that among WT BM cells, only monocytes partially improve the in vitro differentiation of agrin-deficient monocytes, whereas the other cell populations have no effects.
We cannot formally exclude the possibility that other receptors besides α-DG may also be involved in agrin signaling. However, our data indicate that α-DG and Grb2 colocalize in unstimulated macrophages and that the 2 molecules move together in coclusters induced by anti–αDG antibody, thus confirming DG-Grb2 interaction in primary monocytic cells. Grb2-SOS-Ras pathway results in activation of the MAPK pathway.39 In agreement, we found that stimulation of macrophages with recombinant, soluble agrin induces Erk phosphorylation. Interestingly, the Grb2-SOS-Ras signaling pathway is known to be involved in development, proliferation and survival of hematopoietic cells, and in particular, in monocyte/macrophage differentiation.32 Ras mutations occur in approximately 25% of human myeloid leukemia40 and lethally irradiated mice that received transplants of BM cells infected with mutated NRAS (N12) develop a myeloproliferative/acute myeloid leukemia (AML)–like disease.41 Thus, altogether these data allow us to propose that the developmental defects observed in the monocytic compartment of agrin-deficient mice are because of lack of agrin signals that are delivered through the DG complex and induce Erk phosphorylation.
In addition to a better comprehension of the biology of HSPGs, this study provides the first evidence for a direct, specific, and nonredundant role of agrin in monocyte and macrophage biology and thus identifies agrin as a key player of innate immunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Marta Lezama, Cecilia Garlanda, Anna Elisa Trovato, Simone Cenci, and Elisabetta Mariani for assistance; Chiara Buracchi for cell sorting; Michael Ferns, Panos Kabourdis, Dieter Bromme, and Luigina Romani for providing reagents; and Massimo Locati and Alberto Mantovani for helpful discussions and critical reading of the paper.
This work was funded by grants from the Italian Association for Cancer Research (AIRC), Ministero dell'Università e della Ricerca and Ministero della Salute and by EU-FP7 Sybilla n° 201106 to A.V., and by grants from the National Institutes of Health to M.L.D. and S.B. A.S. was funded by Inserm, France, and Cassa di Risparmio delle Provincie Lombarde Foundation, Italy.
National Institutes of Health
Authorship
Contribution: C.M. and A.A. designed and performed experiments and analyzed the data; C.S. performed confocal microscopy experiments; J.C. and C.P. performed in vivo experiments; F.M. performed in vivo phagocytosis assay; S.J.B. provided the animal model; S.J.B. and M.L.D. reviewed the paper; A.S. and A.V. supervised the study; C.M. and A.V. wrote the paper; and A.V. provided funds.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cristina Mazzon, Istituto Clinico Humanitas, Istituto Di Ricovero e Cura a Carattere Scientifico, via Manzoni 113, Rozzano, Milan, Italy; e-mail: cristina.mazzon@humanitasresearch.it.
References
Author notes
C.M. and A.A. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal