In 2008, we reported favorable 5-year outcomes of nonmyeloablative allogeneic stem cell transplantation after fludarabine, cyclophosphamide, rituximab (FCR) conditioning for relapsed and chemosensitive follicular lymphoma. However, innovative strategies were still needed to treat patients with chemorefractory disease. We therefore subsequently performed a trial in which 90Y-ibritumomab tiuxetan (0.4 mCi/kg) was added to the fludarabine, cyclophosphamide conditioning regimen (90YFC). Here, we report updated results of the FCR trial and outcomes after 90YFC. For the FCR group (N = 47), since the last update, one patient developed recurrent disease. With a median follow-up of 107 months (range, 72-142 months), the 11-year overall survival and progression-free survival rates were 78%, and 72%, respectively. For the 90YFC group (N = 26), more patients had chemorefractory disease than did those in the FCR group (38% and 0%, P < .001). With a median follow-up of 33 months (range,17-94 months), the 3-year progression-free survival rates for patients with chemorefractory and chemosensitive disease were 80% and 87%, respectively (P = .7). The low frequency of relapse observed after a long follow-up interval of 9 years in the FCR group suggests that these patients are cured of their disease. The addition of 90Y to the conditioning regimen appears to be effective in patients with chemorefractory disease. This trial was registered at www.clinicaltrials.gov as NCT00048737.
Introduction
Conventional chemoimmunotherapy and radioimmunotherapy for advanced, relapsed follicular lymphoma (FL) has improved patient outcome but is not curative.1,2 Allogeneic stem cell transplantation (SCT) offers the advantages of lymphoma-free grafts and the immunologic graft-versus-lymphoma (GVL) effect, which have been found to lead to long-term remission.3,4 To exploit the GVL effect without the toxicity associated with myeloablative SCT, we evaluated the use of nonmyeloablative SCT (NST) in patients with advanced FL.
In 2008, we published the results of a prospective phase 2 trial to determine the efficacy of NST and fludarabine, cyclophosphamide, and rituximab (FCR) in patients with relapsed FL.5 We reported progression-free (PFS) and overall survival (OS) rates of 83% and 85%, respectively. It has been suggested that such favorable outcomes are the result of selective inclusion criteria; all patients had relapsed and chemosensitive disease, and most patients had matched related donors.
Various strategies are being investigated to improve the outcome of patients with chemorefractory FL after NST. Radioimmunotherapy with an anti-CD20 antibody conjugated with90yttrium-ibritumomab tiuxetan (90Y) has been associated with a superior response rate compared with rituximab in patients with relapsed or chemorefractory FL.6 In view of its β emission, 90Y delivers radiation not only to the tumor cells that bind the antibody but also, to neighboring tumor cells that are inaccessible to the antibody or have insufficient antigen expression as a result of a crossfire effect. Thus, we hypothesized that the addition of 90Y to the NST conditioning regimen would enhance initial disease control and that remission could be later sustained via the GVL effect of the graft.
Here, we report updated results of the FCR study, with a median follow-up duration of 9 years. We also evaluated the effectiveness of NST with 90Y-containing conditioning in relapsed FL patients, including those with chemorefractory disease.
Methods
Study design
FCR group.
The FCR trial included 47 patients with relapsed FL. All patients had undergone NST between March 1999 and April 2005 after a conditioning regimen of FCR. The eligibility criteria included age 19 to 70 years; chemosensitive, relapsed disease; and a partial response or better to salvage chemotherapy. Patients with symptomatic cardiac or pulmonary disease, active infections, or pregnancy were excluded. In addition, patients were required to have a 6 of 6 HLA-compatible sibling donor or HLA-A, -B, -C, and -DRB1 identical unrelated donor if no sibling donors were available, according to our department Standard Practice Guidelines.
90YFC group.
This trial (www.clinicaltrials.gov; NCT00048737) included 26 consecutive FL patients who had undergone NST at our institution between April 2004 and July 2010. Patients in the 90YFC group had the same eligibility criteria as did those in the FCR group, except that a single allele disparity for HLA-A, -B, or -C and patients with refractory disease were allowed in this trial. There were no count restrictions.
Written informed consent was obtained from all patients for both studies in accordance with the Declaration of Helsinki. The 2 studies were reviewed and approved by The University of Texas MD Anderson Cancer Center Institutional Review Board.
Clinical evaluation
In both groups, patients were evaluated 1, 3, 6, and 12 months after NST; every 6 months up to 5 years; and yearly thereafter. Responses were scored using standard criteria for patients with lymphoma.7,8 In addition, functional imaging functional imaging with 18F-fluoro-deoxyglucose positron emission tomography (PET) scans was repeated after NST in patients with avid scans at study entry. PET scans were visually reviewed at our facility by the same nuclear medicine specialist (H.A.M.) and scored according to the scale described by Barrington et al.9
Preparative regimen
FCR.
All FCR patients received 30 mg/m2 of fludarabine and 750 mg/m2 of cyclophosphamide intravenously for 3 days (−5 to −3 before NST).5 They were then given 375 mg/m2 of rituximab on day −13 and 1000 mg/m2 on days −6, +1, and +8. NST was performed on day 0.
90YFC.
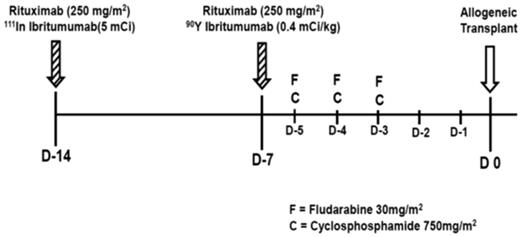
On day −14 before NST, patients were treated with 250 mg/m2 of rituximab, followed by a tracer of 5 mCi of In-111-ibritumomab tiuxetan (111In), as previously described.10 Five planar whole-body γ-camera images were obtained within the first hour and at 4, 24, 72, and 144 hours after injection for both dosimetric analysis and to confirm the expected biodistribution of the radiotracer. Dosimetry was performed on day −15 to ensure that normal organ (liver, lung, kidney) doses would not exceed 20 Gy, and that less than 1 cGy/hr of 90Y-ibritumomab tiuxetan was being absorbed by the red marrow at the time of stem cell infusion. On day −7, patients were infused with 250 mg/m2 of rituximab, followed by 0.4 mCi/kg (maximum total dose, 32 mCi) of 90Y. On days −5 to −3, patients received FC at the dose and schedule described in Figure 1.
Treatment schema of 90Y-ibritumomab tiuxetan, fludarabine (30 mg/m2 per day), and cyclophosphamide (750 mg/m2 per day; 90YFC).
Treatment schema of 90Y-ibritumomab tiuxetan, fludarabine (30 mg/m2 per day), and cyclophosphamide (750 mg/m2 per day; 90YFC).
SCT and GVHD
Donor chimerism and engraftment were assessed using PCR-based methods, as previously described in detail.11 Donor lymphocyte infusions (DLIs) were administered, in combination with rituximab, to patients with persistent or progressive disease after NST, if GVHD was not present.5 Tacrolimus doses were rapidly tapered. Rituximab was then given at a dose of 375 mg/m2 intravenously followed by 3 weekly doses of 1000 mg/m2. A DLI of 1 × 107 CD3-positive T cells/kg was given after the first 2 doses of rituximab if no GVHD occurred. An escalated DLI dose was given at 6-week intervals if there was persistent active disease and no GVHD. DLIs were not routinely administered in patients with stable mixed chimerism if they remained in remission. Patients experiencing a rapid decrease of donor cells received DLIs with the goal of achieving complete donor chimerism.
GVHD prophylaxis consisted of tacrolimus and methotrexate.5 Patients who underwent transplantation from a matched unrelated or mismatched donor received 1 mg/kg of rabbit antithymocyte globulin intravenously on days −2 and −1 before NST. Acute and chronic GVHD were graded according to consensus criteria that have been reported.12,13
Statistical methods
Actuarial estimates of OS and PFS rates were calculated using the Kaplan-Meier method.14 OS was estimated from the date of transplantation to the date of death or last follow-up. The incidence of disease progression and GVHD were estimated using the cumulative incidence method,15 considering death in remission and death without GVHD as competing risks. P values ≤ .05 were considered statistically significant.
Results
Patient characteristics
Patients in both the FCR and the 90YFC groups had similar age and baseline characteristics (Table 1), including sex distribution, time from diagnosis, serum lactate dehydrogenase and β2-microglobulin levels, and number of prior chemotherapies. However, more 90YFC patients had chemorefractory disease (nonresponsive or progression while on chemo-immunotherapy) at transplantation (P < .001), were PET+ (P = .01), and had a matched unrelated or mismatched donor (P < .001; Table 1).
Patients' demographic and clinical characteristics
| Variable . | FCR . | 90YFC . | P . |
|---|---|---|---|
| No. of patients | 47 | 26 | |
| Median age, y (range) | 53 (33-68) | 55 (29-66) | .3 |
| Sex, no. (%) | |||
| Male | 25 (53) | 16 (61) | .3 |
| Female | 22 (47) | 10 (38) | |
| Lactate dehydrogenase > normal, no. (%) | 12 (25) | 5 (20) | .4 |
| Median β2-microglobulin, Mg/L (range) | 2 (1.2-6.5) | 2 (0.6-3.3) | .2 |
| PET+, no./total (%) | 7 (15) | 11/25 (44) | .01 |
| 111Ibrituximab-positive scan, no./total (%) | 0 | 10/25 (40) | |
| No. of prior chemotherapies, median (range) | 3 (2-7) | 3 (2-7) | .7 |
| Prior autologous transplantation, no. (%) | 9 (19) | 0 | |
| Grade, no. (%) | .6 | ||
| 1 or 2 | 37 (79) | 21 (81) | |
| 3 | 10 (21) | 3 (12) | |
| Nonspecified | 0 | 2 (7) | |
| Median time from diagnosis to transplantation, y (range) | 3 (0.7-24) | 2 (1.1-13.1) | .2 |
| Disease status at transplantation, no. (%) | < .001 | ||
| CR | 18 (38) | 8 (31) | |
| Partial remission | 29 (52) | 8 (31) | |
| Chemorefractory | 0 | 10 (38) | |
| Stem cell source, no. (%) | < .001 | ||
| Matched related | 45 (96) | 15 (58) | |
| Matched unrelated | 2 (4) | 8 (31) | |
| Mismatched related | 0 | 1 (4) | |
| Mismatched unrelated | 0 | 2 (8) |
| Variable . | FCR . | 90YFC . | P . |
|---|---|---|---|
| No. of patients | 47 | 26 | |
| Median age, y (range) | 53 (33-68) | 55 (29-66) | .3 |
| Sex, no. (%) | |||
| Male | 25 (53) | 16 (61) | .3 |
| Female | 22 (47) | 10 (38) | |
| Lactate dehydrogenase > normal, no. (%) | 12 (25) | 5 (20) | .4 |
| Median β2-microglobulin, Mg/L (range) | 2 (1.2-6.5) | 2 (0.6-3.3) | .2 |
| PET+, no./total (%) | 7 (15) | 11/25 (44) | .01 |
| 111Ibrituximab-positive scan, no./total (%) | 0 | 10/25 (40) | |
| No. of prior chemotherapies, median (range) | 3 (2-7) | 3 (2-7) | .7 |
| Prior autologous transplantation, no. (%) | 9 (19) | 0 | |
| Grade, no. (%) | .6 | ||
| 1 or 2 | 37 (79) | 21 (81) | |
| 3 | 10 (21) | 3 (12) | |
| Nonspecified | 0 | 2 (7) | |
| Median time from diagnosis to transplantation, y (range) | 3 (0.7-24) | 2 (1.1-13.1) | .2 |
| Disease status at transplantation, no. (%) | < .001 | ||
| CR | 18 (38) | 8 (31) | |
| Partial remission | 29 (52) | 8 (31) | |
| Chemorefractory | 0 | 10 (38) | |
| Stem cell source, no. (%) | < .001 | ||
| Matched related | 45 (96) | 15 (58) | |
| Matched unrelated | 2 (4) | 8 (31) | |
| Mismatched related | 0 | 1 (4) | |
| Mismatched unrelated | 0 | 2 (8) |
90YFC patients with chemorefractory disease (n = 10) at study entry had been treated with a median of 4 lines of therapy (range, 2-7 lines of therapy; Table 2). Disease bulk of more than 7 cm (range, 7-29.4 cm) was present in 5 patients (50%). Two patients (20%) had lung involvement, 1 with recurrent pleural effusion. One other patient (10%) had spleen and bone involvement. None of these patients had histologic evidence of FL transformation.
Chemorefractory patients at study entry: prior treatments and current status
| Patient no. . | Prior chemotherapy . | Current status . | ||
|---|---|---|---|---|
| Type . | Response . | DR/prior treatments . | ||
| 1 | R-CVP, R-CHOP → MR | PR | 6 mo | |
| FDR | PR | 2 mo | ||
| RIE | PD | NA | ||
| XRT | PD | NA | CCR 80+ mo | |
| 2 | R-CHOP → MR | CR | 11 mo | CR1 42 mo |
| R-CVP | PD | NA | CCR2 23+ mo | |
| 3 | R-CHOP | SD | NA | |
| RICE | SD | NA | ||
| R-ESHAP | PD | NA | ||
| R-Hyper-CVAD | PD | NA | CCR 60+ mo | |
| 4 | XRT | CR | 48 mo | |
| R-CHOP | CR | 9 mo | ||
| R-FND | PR | 17 mo | ||
| RICE | NR | NA | ||
| R-Hyper-CVAD | NR | NA | CCR 48+ mo | |
| 5 | R-CHOP | CR | 8 mo | |
| R-CHOP → MR | CR | 36 mo | ||
| R-ESHAP | NR | NA | ||
| R-Hyper-CVAD | NR | NA | ||
| R-GEMOX | NR | NA | CCR 26+ mo | |
| 6 | R-CHOP | CR | 12 mo | |
| R-ESHAP | NR | NR | CCR 36+ mo | |
| 7 | R-CHOP | NR | NA | |
| RICE | NR | NA | ||
| VBR | NR | NA | CCR 26+ mo | |
| 8 | R-CHOP | PR | 3 mo | |
| R-ESHAP | PR | 4 mo | Death 5 mo (GVHD) | |
| PD with cytopenia | ||||
| 9 | R-FND → IFN | CR | 24 mo | |
| FCR | NR | NA | ||
| VBR | NR | NA | ||
| Anti-CD19 immunotoxin | NR | NA | ||
| XRT | PD | NA | ||
| R-ESHAP | PD | NA | PD, death | |
| 5 mo | ||||
| 10 | FR | CR | Unknown | |
| R-FND | PD | NA | ||
| R-CHOP → MR | PR | 9 mo | ||
| RICE | NR | NA | ||
| VBR | NR | NA | ||
| R-GEMOX | NR | NA | ||
| R-Hyper-CVAD | NR | NA | CCR 22+ mo | |
| Patient no. . | Prior chemotherapy . | Current status . | ||
|---|---|---|---|---|
| Type . | Response . | DR/prior treatments . | ||
| 1 | R-CVP, R-CHOP → MR | PR | 6 mo | |
| FDR | PR | 2 mo | ||
| RIE | PD | NA | ||
| XRT | PD | NA | CCR 80+ mo | |
| 2 | R-CHOP → MR | CR | 11 mo | CR1 42 mo |
| R-CVP | PD | NA | CCR2 23+ mo | |
| 3 | R-CHOP | SD | NA | |
| RICE | SD | NA | ||
| R-ESHAP | PD | NA | ||
| R-Hyper-CVAD | PD | NA | CCR 60+ mo | |
| 4 | XRT | CR | 48 mo | |
| R-CHOP | CR | 9 mo | ||
| R-FND | PR | 17 mo | ||
| RICE | NR | NA | ||
| R-Hyper-CVAD | NR | NA | CCR 48+ mo | |
| 5 | R-CHOP | CR | 8 mo | |
| R-CHOP → MR | CR | 36 mo | ||
| R-ESHAP | NR | NA | ||
| R-Hyper-CVAD | NR | NA | ||
| R-GEMOX | NR | NA | CCR 26+ mo | |
| 6 | R-CHOP | CR | 12 mo | |
| R-ESHAP | NR | NR | CCR 36+ mo | |
| 7 | R-CHOP | NR | NA | |
| RICE | NR | NA | ||
| VBR | NR | NA | CCR 26+ mo | |
| 8 | R-CHOP | PR | 3 mo | |
| R-ESHAP | PR | 4 mo | Death 5 mo (GVHD) | |
| PD with cytopenia | ||||
| 9 | R-FND → IFN | CR | 24 mo | |
| FCR | NR | NA | ||
| VBR | NR | NA | ||
| Anti-CD19 immunotoxin | NR | NA | ||
| XRT | PD | NA | ||
| R-ESHAP | PD | NA | PD, death | |
| 5 mo | ||||
| 10 | FR | CR | Unknown | |
| R-FND | PD | NA | ||
| R-CHOP → MR | PR | 9 mo | ||
| RICE | NR | NA | ||
| VBR | NR | NA | ||
| R-GEMOX | NR | NA | ||
| R-Hyper-CVAD | NR | NA | CCR 22+ mo | |
DR indicates duration of response; R, rituximab; CVP, cyclophosphamide, vincristine, and prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; MR, maintenance rituximab; PR, partial remission; FDR, fludarabine, dexamethasone, rituximab; RIE, rituximab, ifosfamide, etoposide; PD, progressive disease; NA, not applicable; XRT, involved-field radiation therapy; CCR, continuous complete remission; SD, stable disease; ICE, ifosfamide, carboplatin, and etoposide; ESHAP, etoposide, cisplatin, cytarabine, and methylprednisolone; Hyper-CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone; FND, fludarabine, mitoxantrone, and dexamethasone; F, fludarabine; GEMOX, gemcitabine and oxaliplatin; and VB, bortezomib and bendamustine.
Patients in the 90YFC group who received a matched sibling donor had similar baseline characteristics to those who received a matched unrelated transplant. In particular, median age was 58 years (range, 29-66 years) and 52 (range, 44-62 years), respectively (P = .3), and chemorefractory disease was present in 40% and 38% of patients (P = .6) at transplantation.
Transplantation and engraftment
All patients received unmanipulated grafts from peripheral blood (45 patients in the FCR group and 25 in the 90YFC group) or bone marrow (2 patients and 1 patient, respectively).
The median number of CD34+ cells infused in the FCR and 90YFC groups was 4.5 and 4.7 × 106/kg, respectively. Neutrophil counts recovered to more than 0.5 × 109/L a median of 11 days after NST (range, 8-17 days) in both groups. Platelet counts recovered to more than 20 × 109/L after a median of 10 days (range, 6-17 days) in the FCR group and 12 days (range, 10-80 days) in the 90YFC group (P = .08).
Donor chimerism
Forty-six patients (98%) in the FCR group and all patients in the 90YFC group experienced donor cell engraftment. By day 30 after NST, the median values of donor T cells in the 2 groups were 89% and 87%, respectively (P = .6), whereas the median values of donor myeloid cells were 83% and 97%, respectively (P = .01). One patient in the FCR group and no patients in the 90YFC group developed primary graft failure. Secondary graft failure occurred in 2 FCR patients and 1 90YFC patient. The latter underwent a mismatched unrelated donor transplant.
DLI and final response
All patients achieved complete remission (CR) in the FCR group after NST. The patient with a primary graft failure experienced CR after a second NST. Three relapses occurred, 2 of which were previously reported. The third relapse was observed in a patient 6 years after the initial transplant.
A total of 4 patients received DLI in the FCR group. Two were related to a rapid decrease in donor chimerism without lymphoma relapse: they achieved complete donor chimerism. Two of the relapsed patients described in the preceding paragraph underwent DLI plus rituximab for management of their disease progression and experienced CR. Response remains continuous in 1 patient at 24+ months; the other patient recently experienced recurrent disease, 7 years after his initial response. He was recently reinduced into CR with DLI plus rituximab.
Twenty-five patients (96%) achieved CR in the 90YFC group, including 9 of 10 who had refractory disease before NST. Two patients experienced relapsed disease. One patient (no. 2; Table 2) experienced progression 42 months after NST. She underwent a second NST (rather than DLI, per personal preference) and was in remission at last follow-up, 20 months later. A second heavily pretreated chemorefractory patient (no. 9; Table 2) did not experience CR after NST; he progressed and died 3 months later with diffuse large B-cell lymphoma. Only 1 patient received a DLI. This patient was in CR but experienced a secondary graft failure. He remained in CR at last follow-up.
Survival
FCR group.
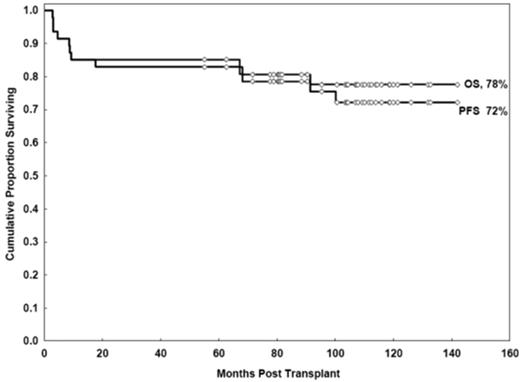
With a median follow-up duration of 107 months (range, 72-142 months), the estimated OS and PFS rates at 11 years were 78% (95% confidence interval [CI], 62%-87%) and 72% (95% CI, 56%-83%), respectively (Figure 2).
90YFC group.
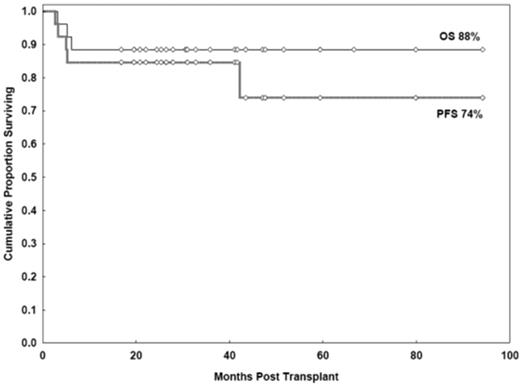
With a median follow-up duration of 33 months (range, 17-94 months), the estimated OS and PFS rates at 3 years were 88% (95% CI, 68%-96%) and 85% (95% CI, 64%-94%), respectively (Figure 3). Results were similar to those in the FCR group, in whom the 3-year OS and PFS estimates were 85% (95% CI, 71%-93%, P = .7) and 83% (95% CI, 69%-91%, P = .9), respectively.
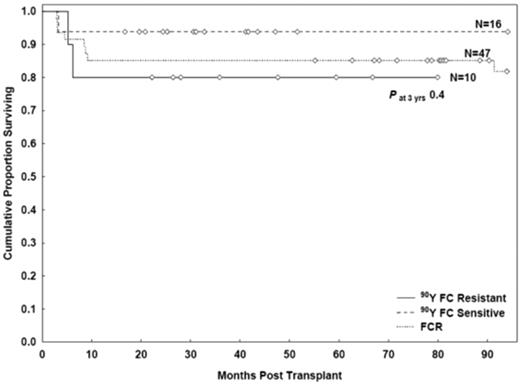
A similar outcome was observed in the 90YFC group patients with chemosensitive and chemorefractory disease at study entry. The 3-year OS rates were 94% (95% CI, 63%-99%) and 80% (95% CI, 41%-95%), respectively (P = .3); the 3-year PFS rates were 87% (95% CI, 59%-97%) and 80% (95% CI, 41%-94%), respectively (P = .7; Figure 4). Patients with chemorefractory disease had a longer median follow-up than chemosensitive patients (42 vs 31 months, respectively). The 6-year OS estimates for chemorefractory patients was 80% (95% CI, 41%-95%).
PFS rates after FCR and 90YFC conditioning in patients with chemosensitive and chemorefractory disease.
PFS rates after FCR and 90YFC conditioning in patients with chemosensitive and chemorefractory disease.
PET status and outcomes
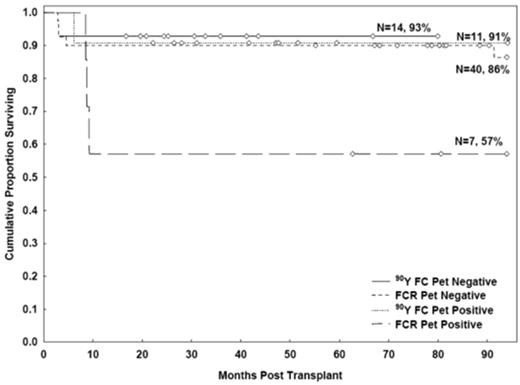
In the 90YFC group, 25 patients underwent PET at study entry; 11 (44%) were FDG-avid (Table 1). The 3-year OS rates were 93% (95% CI, 55%-99%) and 91% (95% CI, 51%-99%) for PET− and PET+ patients, respectively (P = .9). The 3-year PFS rates were 86% (95% CI, 54%-96%) and 91% (95% CI, 51%-99%; P = .7; Figure 5).
In the FCR group, 7 of 47 patients (15%) had positive functional images by PET scan. The 3-year OS rates were 90% (95% CI, 75%-96%) and 57% (95% CI, 17%-84%) for the PET− and PET+ patients, respectively (P = .06). The 3-year PFS rates were 87% (95% CI, 72%-95%) and 57% (95% CI, 17%-84%; P = .08).
GVHD and toxicity
The incidences of grade 2 or 3 acute GVHD in the FCR and 90YFC groups was 13% and 23%, respectively (P = .2). In the FCR group, grade 3 acute GVHD was observed in only 1 patient, and no patients had grade 4 acute GVHD. Two patients in the 90YFC group developed grade 3 acute GVHD; none had grade 4.
The 3-year cumulative incidence of chronic GVHD in the FCR and 90YFC groups was 58% and 39%, respectively (P = .2), and the incidence of its extensive form was 40% and 24% (P = .3).
We previously reported 7 deaths in the FCR group. Six were secondary to infections, and 1 of unknown causes. Four of the 6 fatal infections occurred in patients with a history of acute (n = 1) or chronic GVHD (n = 3). One patient had herpes Zoster infection 4 months before initiating his transplantation procedure; this recurred in a disseminated form 5 months after his transplantation. One additional patient died of viral pneumonia. Since our report in 2008, 3 additional deaths have occurred in patients in CR: 1 secondary to pancreatic cancer (a parent died of the same cause), 1 related to infection in a patient with a history of Bronchilitis obliterans, and 1 a sudden death of unknown origin. The disease in the last case had been restaged 6 months earlier and was found to still be in CR. None of the patients died with recurrent FL.
Three patients died in the 90YFC group (Table 2): 1 patient died of disease progression (day 188; patient no. 9), 1 of acute GVHD (day 98; patient no. 8), and 1 patient who had undergone a mismatched transplant and died of chronic GVHD (day 159). Thus, the treatment-related mortality rates at 100 days and 1 year were 4% and 8%, respectively, in the 90YFC group and 2% and 13%, respectively, in the FCR group.
Discussion
Our median 9-year follow-up data revealed that NST is effective against relapsed FL. Only one additional relapse has occurred since our original publication in 2008. The addition of 90Y to the conditioning regimen overcame the strong negative prognostic factor of disease chemorefractoriness at transplantation, where the 6-year OS has significantly improved to 80%. This occurred despite the presence of bulky disease of more than 7 cm in 50% of refractory patients, and no additional toxicities or GVHD risk were observed, even in patients who received transplants from unrelated donors. These results fulfill our ultimate objective of curing patients with relapsed FL with no undue transplantation-related mortality.
The main cause of failure after NST with FCR has been related to infections in patients with a history of GVHD. The use of high-dose rituximab has not been associated with an increased risk of fatal infections in patients with lymphoma who received an autologous SCT at our institution.16 In a multivariate analysis, we have recently found that decreased peripheral blood immunoglobulin (Ig) levels during the early periods after NST in patients with chronic lymphocytic leukemia have been associated with an increased risk of infection and inferior survival.17 Studying the role of IgG monitoring and replacement in these patients is under investigation.
Favorable outcomes after FC or FCR have been reported by other researchers. The Cancer and Leukemia Group B 109901 trial reported outcomes in 23 patients with relapsed chemosensitive FL.18 The nonmyeloablative conditioning regimen consisted of 20 mg/m2 of fludarabine for 5 days and 1000 mg/m2 of cyclophosphamide daily for 3 days. With a median follow-up of 31.2 months, the 2-year OS and PFS rates were 71% and 76%, respectively. More recently, Tomblyn et al reported on 8 FL patients who were randomly assigned to undergo NST with FCR conditioning.19 With a median follow-up of 3 years, the OS and PFS rates were 100% and 84%, respectively. The treatment of patients with chemorefractory disease remains challenging. The GELTAMO study group,20 for example, recently reported the outcomes of 37 FL patients who underwent sibling donor transplants with melphalan and fludarabine conditioning. At a median 52 months of follow-up, the 4-year OS and nonrelapse mortality rates for patients with progressive disease were 29% and 71%, respectively.
90Y for FL is the latest use of anti-CD20 monoclonal antibody immunotherapy. The conditioning regimen we used with 90Y was more effective than we expected in heavily pretreated and, in some cases, chemorefractory patients. Nevertheless, the use of 90Y alone does not explain the clinical response responses observed, especially in refractory patients. In a randomized phase 3 trial comparing the effectiveness of single-dose 90Y and rituximab in relapsed or chemorefractory FL, the overall response rate was 86% in the 90Y group versus 55% in the rituximab group (P < .001).6 However, despite this compelling finding, relapses were still observed in patients who had undergone 2 prior treatments. Indeed, the median times to progression in FL patients were 12.6 months in the 90Y group and 10.2 months in the rituximab group (P = .062).
We recently reported the results of autologous SCT in patients with relapsed, chemosensitive FL who had undergone 2 lines of prior therapy and for whom no donor was available for NST.21 These patients also received 90Y (0.4 mCi/kg), with carmustine, etoposide, cytarabine, and melphalan myeloablative conditioning. Their PFS rate at 5 years was 45%, with no plateau of the curve. These findings, together with the short times to progression after therapy with single-agent 90Y, suggest that the responses seen in chemorefractory FL patients who received allogeneic transplants after 90YFC were related to a GVL effect of NST facilitated by the improved initial disease control with 90Y. Patients who had relapsed chemosensitive FL had similar outcomes after NST with FCR and 90YFC.
The use of 90Y as a nonmyeloablative conditioning for lymphoma was recently evaluated by Bethge et al in 40 patients with lymphoid malignancies, including 17 FL patients.22 90Y (0.4 mCi/kg) was combined with fludarabine (days −4 to −2) and 200 cGy of total body irradiation. The 2-year nonrelapse mortality rate was 45% for the whole group. The incidence of grade 2 to 4 acute GVHD was 45%, resulting in a 2-year event-free survival rate of 57% for FL patients. Gopal et al used a similar regimen.23 Their 2-year nonrelapse mortality rate of 15.9% was significantly lower than that of Bethge et al.22 The cumulative incidence of acute GVHD was not provided, but 10% of patients developed grade 3 acute GVHD. However, that report included only 6 FL patients. Interpreting these disparate results remains difficult; they may be related to selection bias (single center in the study of Gopal et al23 vs multicenter in the study of Bethge et al22 ), patient heterogeneity, or other inherent unknown causes.
In conclusion, the long follow-up period of 9 years in this study provides evidence that NST after FCR conditioning can induce a complete response that lasts more than a decade in most patients with relapsed FL; this finding suggests that these patients are cured of their disease. The addition of 90Y-ibritumumab tiuxetan to the preparative regimen appears to be particularly effective in those patients with relapsed, chemorefractory disease and could be extended to patients with unrelated donors with no additional toxicities or GVHD risk. It seems prudent that community oncologists refer patients with relapsed FL to NST clinical trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by Biogen Idec, Bayer Healthcare, and Spectrum Pharmaceuticals.
Authorship
Contribution: I.F.K., R.E.C., and H.A.M. designed the study; I.F.K., R.M.S., W.D.E., B.I.S., R.V., R.E.C., and H.A.M. collected, analyzed, and interpreted the data; and all authors cared for patients and the wrote the paper.
Conflict-of-interest disclosure: I.F.K. served as an advisor for Spectrum Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Issa F. Khouri, Department of Stem Cell Transplantation and Cellular Therapy, Unit 423, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: ikhouri@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal