Abstract
The β2-integrin lymphocyte function-associated antigen-1 (LFA-1) plays a crucial role within the immune system. It regulates the interaction between T cells and antigen-presenting cells and facilitates T-cell adhesion to the endothelium, a process that is important for lymphocyte extravasation and homing. Signals mediated via the T-cell receptor and the chemokine receptor CCR7 activate LFA-1 through processes known as inside-out signaling. The molecular mechanisms underlying inside-out signaling are not completely understood. Here, we have assessed the role of the ADAP/SKAP55 module for CCR7-mediated signaling. We show that loss of the module delays homing and reduces intranodal T-cell motility in vivo. This is probably because of a defect in CCR7-mediated adhesion that affects both affinity and avidity regulation of LFA-1. Further analysis of how the ADAP/SKAP55 module regulates CCR7-induced integrin activation revealed that 2 independent pools of the module are expressed in T cells. One pool interacts with a RAPL/Mst1 complex, whereas the other pool is linked to a RIAM/Mst1/Kindlin-3 complex. Importantly, both the RAPL/Mst1 and the RIAM/Mst1/Kindlin-3 complexes require ADAP/SKAP55 for binding to LFA-1 upon CCR7 stimulation. Hence, 2 independent ADAP/SKAP55 modules are essential components of the signaling machinery that regulates affinity and avidity of LFA-1 in response to CCR7.
Introduction
Within the immune system, the β2-integrin LFA-1 (lymphocyte function-associated antigen; αLβ2) mediates T-cell adhesion to the endothelium, homing of T cells to secondary lymphoid organs, and T-cell activation through the interaction of lymphocytes with antigen-presenting cells (APCs).1,2 On resting T cells, LFA-1 is expressed in an inactive form and adopts a low-affinity conformation for its ligands, the ICAM molecules (inter cell adhesion molecules).3,4 Triggering of the T-cell receptor (TCR) by Ag/MHC complexes or of the chemokine receptor CCR7 by CCL21 induces a conformational change of LFA-1 that increases its affinity for ICAM-1 (affinity regulation). Furthermore, these stimuli also facilitate clustering of LFA-1, a process termed avidity modulation.3,4 The molecular events leading to LFA-1 activation have collectively been termed “inside-out signaling.”
Research of the last decade has revealed that the small GTPase Rap1, the 2 Rap1 effector proteins RIAM (Rap1 interacting adapter molecule) and RAPL (regulator for cell adhesion and polarization enriched in lymphoid tissues), and the RAPL-interacting mammalian Ste20-like kinase (Mst1) are critically involved in TCR- and CCR7-mediated signaling events regulating T-cell adhesion, LFA-1 affinity and avidity modulation, and T-cell–APC interactions.3,4 Indeed, RAPL- and Mst1-deficient T cells show defects in adhesion, homing, and intranodal migration in vivo.5-7
In addition to RAPL, RIAM, and Mst1, the cytosolic adapter proteins ADAP (adhesion and degranulating promoting adapter protein), and SKAP55 (Src-kinase associated phosphoprotein of 55 kDa) are crucial for inside-out signaling.3,8 ADAP-deficient T cells display impaired interactions with APCs, altered TCR-mediated adhesion, and diminished LFA-1 activation.9-11 Similar defects are seen in SKAP55-deficient human and mouse T cells.12-15 Besides the similar functional properties, biochemical work of recent years has shown that (1) SKAP55 and ADAP constitutively interact with each other; (2) all SKAP55 molecules expressed in T cells are associated with ADAP16 ; and (3) expression of ADAP is required for stable expression of SKAP55 in T cells.9,12,17 Collectively, these data suggest that ADAP and SKAP55 form a functional signaling unit that regulates inside-out signaling in T cells. This unit has been termed the ADAP/SKAP55 module.12
In an effort to understand how the ADAP/SKAP55 module regulates TCR-mediated LFA-1 activation, we recently found RIAM to constitutively associate with SKAP55.18 Disruption of this interaction diminished TCR-mediated adhesion and conjugate formation with APCs. Further, we had shown that the ADAP/SKAP55 module is required for plasma membrane targeting of RIAM and its upstream effector Rap1.12,18 In addition, others have demonstrated that SKAP55 directly interacts with RAPL.19 Disruption of this interaction blocks the binding of RAPL/Rap1 to LFA-1 and also impairs TCR-mediated adhesion and T cell–APC conjugation.19 Hence, the associations of RAPL and RIAM with the ADAP/SKAP55 module are crucial for T-cell adhesion and T-cell interactions with APCs. However, it has not yet been investigated whether RAPL and RIAM bind to the same or to distinct pools of the ADAP/SKAP55 module.
In addition, until now only few studies have examined the role of the ADAP/SKAP55 module for chemokine-mediated adhesion and migration,20-23 and it is currently unclear how ADAP and SKAP55 regulate these processes. Here we show that the ADAP/SKAP55 module controls CCR7-mediated adhesion and activation of LFA-1, as well as T-cell migration and motility in vivo. Moreover, we identified 2 independent pools of the ADAP/SKAP55 module, one of which associates with RAPL, Mst1, and Rap1, whereas the other interacts with RIAM, Mst1, Kindlin-3, and Talin. We further show that the 2 complexes are independently recruited to the α- or β-chain of LFA-1 and, hence, identify 2 ADAP/SKAP55 modules as essential signaling components that coordinate CCR7-mediated activation of LFA-1 as well as T-cell adhesion and migration.
Methods
cDNA construct and antibodies
The cDNA of human RAPL (German Science Center for Genome Research) was cloned into the pGEX4T1 vector (GE Healthcare). Purified GST-RAPL fusion protein was used to generate a polyclonal rabbit serum and mAbs as previously described12,23 (the mAbs RAPL 104B4G12 and 67C12E4 were selected). The GST fusion protein for the cytoplasmic domain of CD18 has been described.24 The cDNA of human CD11a (provided by S. Fagerholm, University of Dundee, United Kingdom) was used as template to clone the cytoplasmic domain of CD11a into the pGEX6P-1 vector (GE Healthcare). The following antibodies for surface expression were used in this study: allophycocyanin- or FITC-conjugated anti-CD4, anti-CD8, anti-CD3 (BD Biosciences), anti-CD18–biotin, anti-CD11a–biotin, anti-CD62L–allophycocyanin, and anti-CCR7–allophycocyanin (eBioscience) were used for mouse T cells. CCR7 (eBioscience) and FITC-conjugated CD18 or CD11a (Immunotools) were used for human primary T cells. The anti-CD3 antibodies (145-2C11 and OKT3; eBioscience) and human/mouse CCL21 (Tebu) were used for activation of T cells. The anti–human ADAP sheep serum25 (provided by Gary A. Koretzky, University of Pennsylvania, Philadelphia, PA), anti-RIAM rabbit serum26 (provided by T. E. Stradal, University of Münster, Münster, Germany), anti-RAPL rabbit serum, anti-SKAP55 rabbit serum,27 anti-CD18, and CD11a goat sera (Santa Cruz Biotechnology) were used for immunoprecipitation studies. For immunoblot analysis, the following antibodies were used: anti-SKAP55 rat mAb,12 anti-ADAP mAb, anti-SKAP55 mAb, anti-Mst1 mAb, and anti-Rap1 mAb (BD Biosciences), anti-RIAM rat mAb,23 anti-Talin mAb, and anti–β-actin mAb (Sigma-Aldrich), anti-human/mouse kindlin-3 rabbit sera,28,29 anti–phosphop(p)-ERK1/2, anti-S473 Akt rabbit sera (Cell Signaling Technology), anti-GST mAb (Santa Cruz Biotechnology). HRP-, FITC-, or allophycocyanin-labeled secondary antibodies, and allophycocyanin-conjugated strepavidin were purchased from Dianova.
Mice, T-cell purification, electroporation of siRNAs
ADAP-deficient mice were provided by Gary A. Koretzky.11 SKAP-HOM-deficient mice have been described.30 All experiments involving mice were performed according to the guidelines of the State of Sachsen-Anhalt, Germany. Primary human T cells, splenic T cells, or CD4+ lymphocytes were purified using T-cell isolation kits and AutoMacs magnetic separation system (Miltenyi Biotec). Approval for these studies was obtained from the Ethics Committee of the Medical Faculty at the Otto-von-Guericke University, Magdeburg, Germany. Informed consent was obtained in accordance with the Declaration of Helsinki. Electroporation of siRNAs for ADAP (GAAGATTCCAAACCTACAT) and Renilla luciferase (CCAAGTAATGTAGGATCAA) into human primary T cells have been described.23
Flow cytometry, soluble ICAM-1–binding assay, and integrin clustering
To analyze the cell surface expression of LFA-1, CCR7, CD4, CD8, TCR, or CD62L, cells were stained with indicated Abs and analyzed using a FACSCalibur flow cytometer and CellQuestPro xxx Version software (BD Biosciences). Soluble Fc-ICAM-1 (R&D Systems) binding of human primary T cells after various stimuli was assessed as previously described.23 Intracellular staining for flow cytometry of ADAP has been described.23 To assess chemokine-induced clustering of integrins, human T cells were stimulated with CCL21 (1 μg/mL) for 30 minutes at 37°C and fixed in 1% ice-cold paraformaldehyde. After labeling with FITC-conjugated CD18 or CD11a, the cells were imaged with a LEICA TCS SP2 laser scanning confocal system (Leica Microsystems) using a plan apochromatic oil emerging 63× objective (NA 1.4). T cells were analyzed for the maximum diameter at the z-position. For each experiment, 30 randomly selected cells in triplicates were analyzed to calculate the percentage of cells with CD18 or CD11a clusters
Chemotaxis, adhesion assay, in vivo homing, and intranodal motility of T cells
Chemotaxis and adhesion assays have been described.23 For T-cell homing, purified T cells from wild-type (WT) or ADAP−/− mice were differentially labeled with 0.5μM DDAO-SE (Invitrogen) and 0.5μM 5,6-carboxyfluorescein diacetate (CFSE; Invitrogen). A total of 1 × 107 DDAO-SE–labeled WT were mixed with an equal number of CFSE-labeled ADAP−/− T cells and injected intravenously into the tail veins of age- and sex-matched WT recipient mice. After 2 or 18 hours, recipient mice were killed and peripheral lymph nodes (pLNs; inguinal, mesenteric, and auxiliary), and splenocytes were analyzed by flow cytometry. The percentage of DDAO-SE+- and CFSE+-labeled cells was determined, and the T-cell homing index was calculated as the ratio of DDAO-SE–labeled to CFSE-labeled cells. In addition, the percentage of CD4+ or CD8+ T cells was determined by flow cytometry within the DDAO-SE+– and CFSE+-gated cells. Reversal of the fluorescent dyes gave the same results. Intravital imaging of intranodal T-cell migration by 2-photon laser scanning microscopy has been performed as previously described.31 To directly compare their motility within the paracortical T-cell zone of recipient LNs, purified CD4+ WT and ADAP−/− T cells were differentially labeled with Oregon Green or TAMRA (both Invitrogen); dye labeling was switched in every second experiment. Because of the delayed homing of ADAP−/− T cells to LNs, equal numbers of differential labeled T cells were adoptively transferred into WT recipient mice 24 hours before intravital imaging was performed. In total, popliteal LNs of 7 WT recipient mice were imaged, and data analysis was performed as previously described31,32 (supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Immunoprecipitation, Western blot analysis, preparation of plasma membrane, Rap1 GTPase assay, and GST fusion protein pull-down assay
Cell lysis and immunoprecipitation were performed as previously described.12,18,23 Equivalent amounts of protein (determined by Bradford assay; Roth) were used in precipitation studies (500 μg of total protein from human primary T cell). Cell lysates (50 μg of total protein) or immune complexes were separated by SDS-PAGE and transferred to nitrocellulose. Western blots were conducted with the indicated antibodies and developed with the appropriate horseradish peroxidase-conjugated secondary antibodies and the Luminol detection system (Roth). Isolation of plasma membrane fractions has been previously described.12,18,23 GTPase activity of Rap1 was assessed as previously described.12,18,23 The CD18 and CD11a cytoplasmic domains were expressed as GST-fusion proteins and bound to glutathione-Sepharose beads (GE Healthcare). For GST-pull-down experiments, GST-fusion proteins bound to glutathione-Sepharose beads were incubated with lysates of resting and stimulated primary human T cells for 2 hours at 4°C, washed, and analyzed by Western blotting.
Statistical analysis
Statistical differences were analyzed using Student t test. P < .05 was considered statistically significant.
Results
Altered chemotaxis, homing, and intranodal migration of ADAP-deficient T cells
We have recently shown that the ADAP/SKAP55 module is required in human T cells for CXCL12-mediated chemotaxis.23 To extend our study to mouse T cells and to CCL21, the ligand for CCR7 that regulates T-cell trafficking in vivo,33 we assessed migration of wild-type (WT) and ADAP−/− mouse T cells using a conventional in vitro chemotaxis assay. As shown in Figure 1A, compared with WT T cells ADAP−/− T cells displayed a 75% reduction of in vitro migration in response to CCL21. Disruption of the chemokine gradients decreased migration of WT and ADAP−/− lymphocytes to near-background levels, indicating that the migration was not the result of chemokinesis (Figure 1A). Flow cytometric analysis revealed normal levels of CCR7 on the surface of WT and ADAP−/− T cells (supplemental Figure 1A). Furthermore, the ratios of migrating CD4+ and CD8+ T cells were similar between ADAP−/− and WT T cells (supplemental Figure 1B), excluding the possibility that the attenuated migratory response in the absence of ADAP/SKAP55 (note that ADAP−/− T cells also lack expression of SKAP55 as well of its homolog, SKAP-HOM12 ) was the result of a migration defect of a particular T-cell subset. Western blot analysis further showed comparable levels of phosphorylated ERK1/2 and Akt of WT and ADAP−/− T cells upon CCR7 stimulation at various time points, indicating that the reduced migratory behavior of ADAP−/− T cells was not the result of a general unresponsiveness of the CCR7 receptor (supplemental Figure 2A). Similar results as shown here for mouse T cells were obtained when we suppressed the expression of ADAP in primary human T lymphocytes (supplemental Figure 3).
The ADAP/SKAP55 module controls T-cell migration in vitro and in vivo. (A) Purified splenic WT and ADAP−/− T cells were placed into the upper part of transwell chambers. Cells were incubated in the absence or presence of CCL21 in the upper and/or lower chamber for 2 hours. Migrated T cells (lower chamber) were counted and calculated as percentage of input cell numbers (mean ± SD; n = 8 mice each). **P ≤ .001. (B) A mixture of equal numbers of CFSE-labeled WT and DDAO-SE–labeled ADAP−/− purified T cells was adoptively transferred into 4 genetically matched WT recipients. After 2 or 18 hours, the percentage of labeled lymphocytes from pLN (mesenteric [MLN], auxiliary [ALN], and inguinal [ILN]), as well as the spleen was determined by flow cytometry, and the homing index was calculated as the ratio of ADAP−/− to WT T-cell numbers. The graph summarizes the data of 2 independent experiments (mean ± SD; n = 8 recipient mice).
The ADAP/SKAP55 module controls T-cell migration in vitro and in vivo. (A) Purified splenic WT and ADAP−/− T cells were placed into the upper part of transwell chambers. Cells were incubated in the absence or presence of CCL21 in the upper and/or lower chamber for 2 hours. Migrated T cells (lower chamber) were counted and calculated as percentage of input cell numbers (mean ± SD; n = 8 mice each). **P ≤ .001. (B) A mixture of equal numbers of CFSE-labeled WT and DDAO-SE–labeled ADAP−/− purified T cells was adoptively transferred into 4 genetically matched WT recipients. After 2 or 18 hours, the percentage of labeled lymphocytes from pLN (mesenteric [MLN], auxiliary [ALN], and inguinal [ILN]), as well as the spleen was determined by flow cytometry, and the homing index was calculated as the ratio of ADAP−/− to WT T-cell numbers. The graph summarizes the data of 2 independent experiments (mean ± SD; n = 8 recipient mice).
Because CCR7 and its ligand CCL21 regulate lymphocyte trafficking to lymphoid organs,33 we next investigated whether the ADAP/SKAP55 module is also required for homing of T cells into pLNs and the spleen. To this end, we adoptively transferred a 1:1 mixture of DDAO-SE-labeled WT and CFSE-labeled ADAP−/− T cells into WT recipient mice and subsequently assessed the homing of the transferred cells by flow cytometry. As shown in Figure 1B, although WT and ADAP−/− T cells express comparable levels of the homing receptor CD62L on the cell surface (supplemental Figure 1A), homing of ADAP-deficient T cells to pLNs and the spleen was reduced compared with WT T cells. This was most evident in short-term homing assays where we observed a 50% to 75% decrease in the homing capacity of ADAP−/− T cells. However, even after 18 hours, the numbers of ADAP−/− cells that had homed to the spleen and lymph nodes was approximately only 80% of that of WT T cells. Flow cytometric analysis of the migrated cells showed similar ratios of CD4+ and CD8+ ADAP-proficient or -deficient T cells, again indicating that the delayed homing capacity of ADAP−/− T cells was not the result of an impaired function of a specific T-cell subset (supplemental Figure 4).
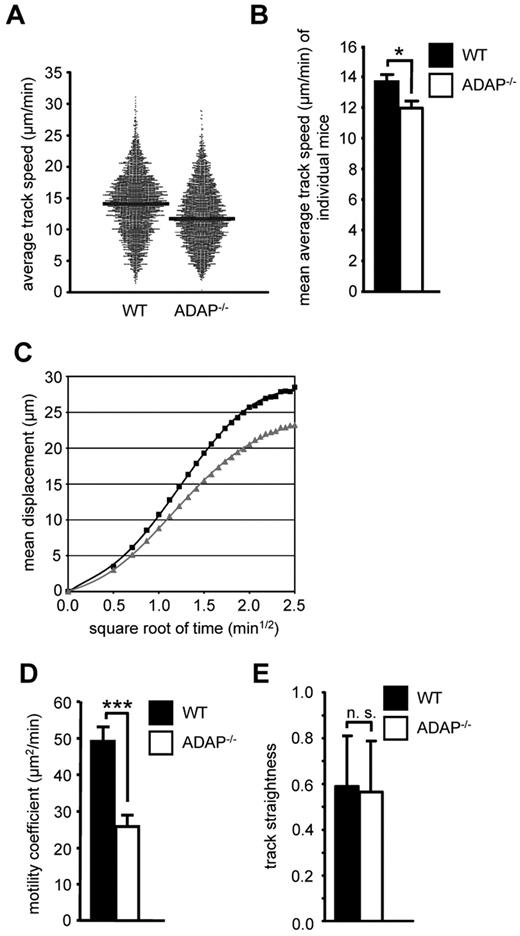
Because CCR7 also regulates migratory processes within lymph nodes,31 we next analyzed whether loss of ADAP would affect the intranodal migration of T cells. We adoptively transferred a mixture of Oregon Green–labeled WT and TAMRA-labeled ADAP−/− CD4+ T cells into recipient WT mice and used intravital 2-photon laser-scanning microscopy of the popliteal LN to visualize the movement of these T cells 24 hours after transfer. Under steady-state conditions, ADAP−/− T cells displayed a slightly reduced migration velocity within the paracortical T-cell zone compared with WT T cells (median average track speed, 11.7 vs 14.1 μm/min, Figure 2A, statistical comparison of individual experiments in Figure 2B). Similarly, ADAP−/− T cells also exhibited a reduced propensity to move away from their relative starting positions (mean displacement plot, Figure 2C), resulting in a 2-fold reduction of the motility coefficient (mean values, 25.8 vs 49.2 μm2/min, Figure 2D). In contrast, the overall directionality of ADAP−/− T-cell migration (measured as the track straightness) was not significantly altered (Figure 2E). Taken together, the ADAP/SKAP55 module regulates CCR7-mediated migration of mouse/human T cells in vitro as well as T-cell homing and basal intranodal motility in vivo.
The ADAP/SKAP55 module influences the intranodal motility of naive CD4+ T cells in vivo. (A) Equal cell numbers of Oregon Green–labeled WT and TAMRA-labeled ADAP−/− CD4+ T cells (and vice versa) were adoptively transferred into WT recipients. After 24 hours, the paracortical T-cell zone of the popLN was imaged by intravital microscopy, and T cells were tracked semiautomatically using Imaris. Each dot represents the average track speed of an individual cell; and black bars, median values. Graph summarizes all tracked cells from the popLN imaging of 7 WT recipient mice. (B) Statistical comparison of the mean average track speed values of WT and ADAP−/− CD4+ T cells, calculated separately for each imaging experiment (mean ± SEM; n = 6 for WT, n = 7 for ADAP−/−). *P ≤ .05. (C) Mean displacement plot of WT (black squares) and ADAP−/− (gray squares) CD4+ T cells. (D) Motility coefficient (mean ± SEM; n = 6 for WT, n = 7 for ADAP−/−). ***P ≤ .005. (E) Track straightness (mean ± SEM; n = 6 for WT, n = 7 for ADAP−/−). n.s. indicates not significant.
The ADAP/SKAP55 module influences the intranodal motility of naive CD4+ T cells in vivo. (A) Equal cell numbers of Oregon Green–labeled WT and TAMRA-labeled ADAP−/− CD4+ T cells (and vice versa) were adoptively transferred into WT recipients. After 24 hours, the paracortical T-cell zone of the popLN was imaged by intravital microscopy, and T cells were tracked semiautomatically using Imaris. Each dot represents the average track speed of an individual cell; and black bars, median values. Graph summarizes all tracked cells from the popLN imaging of 7 WT recipient mice. (B) Statistical comparison of the mean average track speed values of WT and ADAP−/− CD4+ T cells, calculated separately for each imaging experiment (mean ± SEM; n = 6 for WT, n = 7 for ADAP−/−). *P ≤ .05. (C) Mean displacement plot of WT (black squares) and ADAP−/− (gray squares) CD4+ T cells. (D) Motility coefficient (mean ± SEM; n = 6 for WT, n = 7 for ADAP−/−). ***P ≤ .005. (E) Track straightness (mean ± SEM; n = 6 for WT, n = 7 for ADAP−/−). n.s. indicates not significant.
The ADAP/SKAP55 module regulates T-cell adhesion and LFA-1 activation on chemokine stimulation
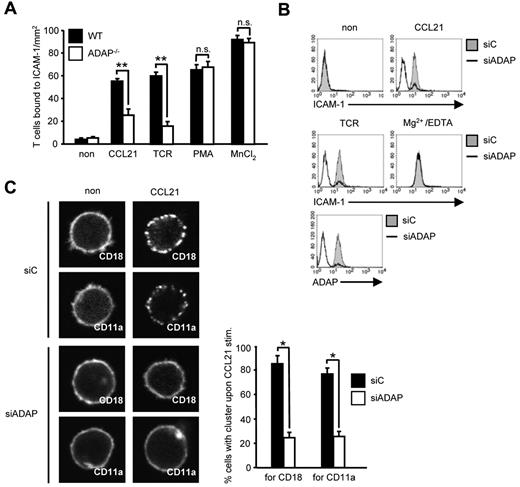
T-cell homing depends on CCR7-mediated activation of LFA-1 followed by arrest of T cells to the endothelium.1,2 Because the ADAP/SKAP55 module has previously been shown to be required for the activation of LFA-1 on TCR stimulation, we were next interested to determine whether the impaired homing of ADAP-deficient cells might be the result of altered T-cell adhesion in response to CCR7 stimulation. Figure 3A shows that this is indeed the case. Thus, on CCL21 treatment, WT T cells readily adhered to ICAM-1. In contrast, although ADAP-deficient T cells expressed similar amounts of CD11a and CD18 (the αL- and the β2-chain of LFA-1) on the surface (supplemental Figures 1A and 3B), they displayed a 60% reduced adhesion to ICAM-1. Treatment with PMA and Mn2+ bypassed this defect, indicating that the cells were not generally unresponsive to external stimuli (Figure 3A). Comparable data were obtained when the expression of ADAP was suppressed by siRNA in primary human T cells (supplemental Figure 5). Hence, ADAP is mandatory for CCR7-induced activation of LFA-1.
CCL21-induced T-cell adhesion to ICAM-1 and affinity/avidity regulation of LFA-1 depends on the ADAP/SKAP55 module. (A) Purified splenic WT and ADAP−/− T cells were left untreated or stimulated with CCL21, anti–CD3 mAb 145-2C11 (TCR), PMA, or MnCl2, respectively, and subsequently analyzed for their ability to bind plate-bound Fc-ICAM-1 (mean ± SD; n = 6 experiments). **P ≤ .001. n.s. indicates not significant. (B) Human primary T cells were transfected with either control siRNA (siC) or siRNAs against ADAP (siADAP). After 72 hours, cells were analyzed for their ability to bind soluble Fc-ICAM-1 in response to CCL21, anti-CD3 mAb OKT3 (TCR), or Mg2+/EDTA stimulation for 5 minutes. Suppression of ADAP expression was evaluated by flow cytometry. One representative experiment of 3 is shown. (C) Human T cells transfected as described in panel B were stimulated with CCL21, fixed, and stained with FITC-conjugated CD18 or CD11a antibodies. For each experiment, 30 randomly selected cells in triplicates were analyzed to calculate the percentage of cells with CD18 or CD11a clusters by confocal microscopy (mean ± SD; n = 4 experiments). *P ≤ .05.
CCL21-induced T-cell adhesion to ICAM-1 and affinity/avidity regulation of LFA-1 depends on the ADAP/SKAP55 module. (A) Purified splenic WT and ADAP−/− T cells were left untreated or stimulated with CCL21, anti–CD3 mAb 145-2C11 (TCR), PMA, or MnCl2, respectively, and subsequently analyzed for their ability to bind plate-bound Fc-ICAM-1 (mean ± SD; n = 6 experiments). **P ≤ .001. n.s. indicates not significant. (B) Human primary T cells were transfected with either control siRNA (siC) or siRNAs against ADAP (siADAP). After 72 hours, cells were analyzed for their ability to bind soluble Fc-ICAM-1 in response to CCL21, anti-CD3 mAb OKT3 (TCR), or Mg2+/EDTA stimulation for 5 minutes. Suppression of ADAP expression was evaluated by flow cytometry. One representative experiment of 3 is shown. (C) Human T cells transfected as described in panel B were stimulated with CCL21, fixed, and stained with FITC-conjugated CD18 or CD11a antibodies. For each experiment, 30 randomly selected cells in triplicates were analyzed to calculate the percentage of cells with CD18 or CD11a clusters by confocal microscopy (mean ± SD; n = 4 experiments). *P ≤ .05.
Chemokine-mediated inside-out signaling is known to alter both LFA-1 affinity (conformation) and avidity (clustering).4 To determine the role of the ADAP/SKAP55 module for CCR7-mediated affinity modulation of LFA-1, we assessed binding of soluble ICAM-1 to WT and ADAP−/− human T cells. Figure 3B shows that siRNA-mediated knockdown of ADAP impaired binding of soluble Fc-ICAM-1 after CCR7 (and TCR stimulation), whereas ICAM-1 readily bound to the transfectants when LFA-1 was pharmacologically activated by Mg2+/EDTA (Figure 3B). Next, we investigated avidity regulation of LFA-1 by analyzing the clustering of CD18 or CD11a in response to CCL21 stimulation. As shown in Figure 3C, ADAP−/− T cells do not display the typical clustering of LFA-1 upon CCR7 triggering. Thus, the ADAP/SKAP55 module is important for CCR7-mediated affinity and avidity regulation of LFA-1.
Two distinct pools of the ADAP/SKAP55 module regulate LFA-1 activity
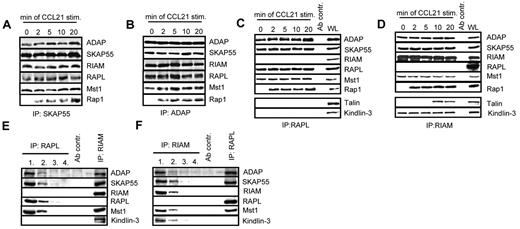
The Rap effector molecules RIAM and RAPL have recently been reported to interact with SKAP55.18,19 Moreover, 2 studies have demonstrated that this interaction is mandatory for TCR-mediated adhesion of ICAM-1.18,19 To assess whether the same holds true for CCL21 stimulation, anti-SKAP55 and anti-ADAP immunoprecipitates were obtained from resting or CCL21-stimulated human T cells and analyzed by anti-ADAP, -SKAP55, -RIAM, -RAPL, and -Rap1 Western blotting, respectively. Figure 4A and B show that, in resting and in CCL21-stimulated T cells RIAM and RAPL, constitutively interact with ADAP and SKAP55, whereas Rap1 associates with the complexes only on stimulation.
Two distinct pools of the ADAP/SKAP55 module were identified in T cells. (A) Human primary T cells were left untreated or stimulated with CCL21 for the indicated time points. Immunoprecipitations were performed using anti–SKAP55 or (B) anti–ADAP antibodies, and precipitates were analyzed by Western blotting using the indicated antibodies. One representative experiment of 2 is shown. (C-D) Purified T cells were stimulated as described in panel A, and lysates were subjected to anti-RAPL (C) or anti-RIAM (D) immunoprecipitation. Precipitates were analyzed by Western blotting using the indicated antibodies. One representative experiment of 3 is shown. (E-F) Primary human T cells were subjected to 4 sequential immunoprecipitations with anti–RAPL (E) or anti–RIAM (F) antibodies, respectively, followed by final immunoprecipitations using either anti-RIAM or anti-RAPL antibodies. Precipitates were analyzed byWestern blotting with the indicated antibodies. One representative experiment of 2 is shown.
Two distinct pools of the ADAP/SKAP55 module were identified in T cells. (A) Human primary T cells were left untreated or stimulated with CCL21 for the indicated time points. Immunoprecipitations were performed using anti–SKAP55 or (B) anti–ADAP antibodies, and precipitates were analyzed by Western blotting using the indicated antibodies. One representative experiment of 2 is shown. (C-D) Purified T cells were stimulated as described in panel A, and lysates were subjected to anti-RAPL (C) or anti-RIAM (D) immunoprecipitation. Precipitates were analyzed by Western blotting using the indicated antibodies. One representative experiment of 3 is shown. (E-F) Primary human T cells were subjected to 4 sequential immunoprecipitations with anti–RAPL (E) or anti–RIAM (F) antibodies, respectively, followed by final immunoprecipitations using either anti-RIAM or anti-RAPL antibodies. Precipitates were analyzed byWestern blotting with the indicated antibodies. One representative experiment of 2 is shown.
To investigate whether the binding of RAPL and RIAM occurs on the same or on distinct pools of the ADAP/SKAP55 module, anti-RAPL or anti-RIAM immunoprecipitates were prepared from resting or CCL21-stimulated T cells and subsequently analyzed byWestern blotting. As shown in Figure 4C and D, this approach clearly revealed that RAPL and RIAM bind to distinct pools of the ADAP/SKAP55 module. Indeed, although ADAP and SKAP55 were readily recovered in RAPL immunoprecipitates, RIAM was not detectable (Figure 4C). Conversely, Western blot analysis of RIAM immunoprecipitates showed a constitutive interaction between ADAP, SKAP55, and RIAM, whereas RAPL was undetectable (Figure 4D).
To further substantiate the existence of two distinct pools findings, we depleted RAPL or, alternatively, RIAM by sequential immunoprecipitations from T-cell lysates. The depleted lysates were then subjected to secondary anti-RIAM or anti-RAPL immunoprecipitations, respectively. Subsequently, all precipitates were analyzed by Western blotting for coprecipitation of ADAP, SKAP55, Mst1, RAPL, and RIAM. As demonstrated in Figure 4E, ADAP, SKAP55, and Mst1 coprecipitated with RIAM even after complete depletion of RAPL. Similarly, ADAP, SKAP55, Mst1, and RAPL coprecipitated with RAPL in RIAM-depleted lysates (Figure 4F). Thus, resting T cells express 2 distinct pools of the ADAP/SKAP55 module: one that selectively interacts with RAPL and Mst1 and one that exclusively interacts with RIAM and Mst1.
Chemokine- and TCR-mediated activation of LFA-1 has been reported to depend on the FERM domain-containing protein Talin, and it is thought that the association between RIAM and Talin regulates integrin activation.34 In line with these reports, we detected Talin in RIAM immunoprecipitates of CCL21-stimulated primary human T cells (Figure 4D). Importantly, however, the CCR7-mediated interaction between Talin and RIAM occurred with a delayed kinetics compared with the stimulus-dependent association of Rap1 with the complex. In addition to Talin, we found the FERM domain-containing protein Kindlin-3 to selectively and constitutively coprecipitate with RIAM, suggesting that Kindlin-3 is an additional component of the ADAP/SKAP55/RIAM/Mst1 module. In marked contrast to the RIAM immunoprecipitates, Western blot analysis of RAPL immunoprecipitates did not yield a constitutive or inducible interaction with either Talin or Kindlin-3 (Figure 4C).
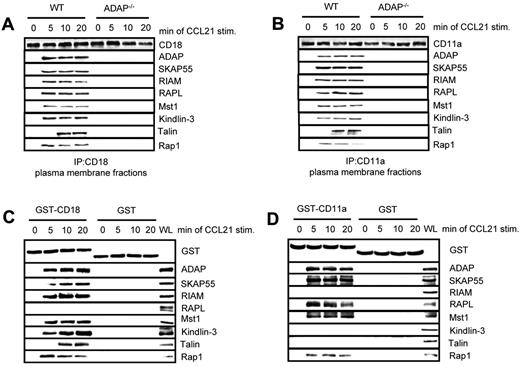
Previously, we have shown that the ADAP/SKAP55 module is required for plasma membrane recruitment of RIAM after T-cell activation.18 Furthermore, it has been shown that Kindlin-3 and Talin associate with the β-chain of LFA-1 (CD18),24,34 whereas RAPL has been reported to interact with the α-chain of LFA-1 (CD11a).35 Given our observation that RIAM/Mst1/Kindlin-3 complexes and RAPL/Mst1-complexes associate with distinct pools of ADAP/SKAP55, we speculated that the ADAP/SKAP55 modules might be required for recruitment of both signaling complexes to activated LFA-1. To assess this question, we isolated plasma membrane fractions from resting or CCL21-stimulated WT and ADAP−/− T cells and subjected them to anti–CD18 immunoprecipitation. Precipitates were subsequently analyzed by Western blotting for the presence of ADAP, SKAP55, RIAM, RAPL, Mst1, Kindlin-3, Talin, and Rap1. Figure 5A shows that all molecules only bound to CD18 in activated WT T cells but not in ADAP-deficient T cells. Similar data were obtained when we precipitated CD11a from membrane fractions of resting and CCL21-stimulated WT and ADAP-deficient T cells (Figure 5B).
Two distinct pools of the ADAP/SKAP55 module are recruited to LFA-1. (A) Purified splenic WT and ADAP−/− T cells were left untreated or stimulated with CCL21 for the indicated time points. Plasma membrane fractions were prepared, lysed, and subjected to anti-CD18 immunoprecipitation. Subsequently, precipitates were analyzed by Western blotting, using the indicated antibodies. One representative experiment of 2 is shown. (B) Plasma membrane fractions as described in panel A were subjected to CD11a immunoprecipitation and then analyzed by Western blotting using the indicated antibodies. One representative experiment of 2 is shown. (C-D) Purified human T cells were left untreated or stimulated with CCL21 for the indicated time points. Lysates were incubated with GST-fusion proteins bound to glutathione-Sepharose beads. Precipitates were analyzed by Western blotting using the indicated antibodies. One representative experiment of 2 is shown.
Two distinct pools of the ADAP/SKAP55 module are recruited to LFA-1. (A) Purified splenic WT and ADAP−/− T cells were left untreated or stimulated with CCL21 for the indicated time points. Plasma membrane fractions were prepared, lysed, and subjected to anti-CD18 immunoprecipitation. Subsequently, precipitates were analyzed by Western blotting, using the indicated antibodies. One representative experiment of 2 is shown. (B) Plasma membrane fractions as described in panel A were subjected to CD11a immunoprecipitation and then analyzed by Western blotting using the indicated antibodies. One representative experiment of 2 is shown. (C-D) Purified human T cells were left untreated or stimulated with CCL21 for the indicated time points. Lysates were incubated with GST-fusion proteins bound to glutathione-Sepharose beads. Precipitates were analyzed by Western blotting using the indicated antibodies. One representative experiment of 2 is shown.
To dissect whether the 2 ADAP/SKAP55 modules selectively associate with the CD18 and the CD11a chains of LFA-1, we performed a pull-down assay using the recombinant cytoplasmic domains of CD18 and CD11a, respectively. Figure 5C shows that, under these conditions, the ADAP/SKAP55/RIAM/Mst1/Kindlin-3/Talin complex only associates with CD18 upon CCR7 stimulation of T cells, whereas the ADAP/SKAP55/RAPL/Mst1 module selectively associates with the cytoplasmic domain of CD11a (Figure 5D). Collectively, these findings indicate that the ADAP/SKAP55 modules facilitate membrane recruitment and LFA-1 association of both the ADAP/SKAP55/RIAM/Mst1/Kindlin-3/Talin and the ADAP/SKAP55/RAPL/Mst1 complexes in T cells upon CCR7 stimulation. Moreover, they show that the ADAP/SKAP55/RIAM/Mst1/Kindlin-3/Talin complex is linked to the CD18 subunit, whereas the ADAP/SKAP55/RAPL/Mst1-complex is associated with the CD11a subunit of LFA-1. Besides this, the pull-down assays confirmed previously data showing that RAPL is selectively linked to the α-chain of LFA-1.35
Discussion
In this study, we show that, in addition to its central role in TCR-mediated adhesion and T-cell interaction with APCs,9-11 the cytosolic adaptor protein ADAP is also involved in controlling CCR7-mediated LFA-1 affinity/avidity regulation, adhesion, homing, as well as T-cell motility within in the lymph nodes.
Loss of ADAP strongly affected adhesion in vitro and short-term homing of T cells to the pLNs in vivo. In line with this finding are previous studies showing that loss of 2 interaction partners of the ADAP/SKAP55 module, the Rap1 effector molecule RAPL and the mammalian Ste20-like kinase Mst1 also impairs CCL21-mediated adhesion and homing.5-7 In the latter studies, RAPL and Mst1 have been shown to be required for the formation of stable arrest of T cells to the endothelium. Whether ADAP exerts a similar function during T-cell/endothelial interactions as RAPL and Mst1 is not known at present and requires further investigation.
One interesting observation of our study was that long-term homing of ADAP-deficient T cells was less impaired than short-term homing. Currently, we cannot provide a molecular basis for this finding. However, one possibility would be that ADAP preferentially affects LFA-1/ICAM-1 and, to a lesser extent, VLA-4/VCAM interactions that also play a role in adhesion of T cells to the endothelium and homing to the pLN.36 Alternatively, it might be possible that other ADAP- and/or integrin-independent signaling pathways are involved in T-cell homing. In this regard, one potential molecule could be the Rac nucleotide exchange factor DOCK2, which has been shown to regulate T-cell homing independently of adhesion of T lymphocytes.37 In this context, it is important to note that ADAP deficiency did not impair TCR- or chemokine-mediated Rac activation (S.K., unpublished data, November 2010). Finally, it was recently shown that expression of minute amounts of CCR7 on plasmacytoid dendritic cells is still sufficient to allow for homing.38 Because ADAP deficiency did not lead to a complete block in LFA-1 function, we thus hypothesized that the residual activity of CCR7-mediated LFA-1 activity mediates the delayed T-cell homing. Clearly, future studies are required to precisely determine how ADAP deficiency affects the recruitment of T cells to the pLN. Moreover, it will be equally important to compare the long-term homing capabilities of RAPL and Mst1-deficient T cells directly with those of ADAP-deficient T cells. Only then it will be possible to precisely judge the individual contributions of the 3 signaling molecules to adhesion and homing processes.
Besides T-cell homing, 2-photon intravital microscopy demonstrated that loss of ADAP attenuated the motility of T cells within pLNs. Again, this phenotype is similar to the one that has previously been described for RAPL- and Mst1-deficient T cells,6,7 which further underlines the functional interdependence of the 3 molecules. The reduced capability of ADAP−/− T cells to patrol the LN parenchyma byrandom walk might affect their efficiency to scan dendritic cells for presented antigen. Together with the delayed T-cell homing and the known defects in T-cell interaction with APCs that potentially compromise the establishment and/or maintenance of stable T cell–DC contacts, this could explain the attenuated T cell–dependent immune responses observed in ADAP−/− mice.10 Currently, it is not yet clear how the ADAP/SKAP55 module (as well as RAPL and Mst1) regulates the intranodal motility of T cells in vivo. One hypothesis could be that ADAP modulates the functions of CD18. In line with this hypothesis would be the recent finding that loss of CD18 induces a comparable reduction in T-cell velocity as loss of ADAP.39 Alternatively or concomitantly, loss of ADAP could affect actin-polymerization driven forces that regulate T-cell motility. In line with this idea are preliminary data obtained in our laboratory, which indicate that loss of ADAP affects the magnitude and the kinetics of CCL21-mediated F-actin polymerization as well as T-cell polarization (S.K., unpublished observations, August-September 2009 and September 2011).
Loss of the ADAP/SKAP55 module impaired CCL21-mediated T-cell affinity and avidity regulation of LFA-1. During our attempts to assess the molecular mechanisms underlying these findings, we identified 2 independent pools of the ADAP/SKAP55 module: one containing RIAM, Mst1, Talin, Kindlin-3, and Rap1 and the other containing RAPL, Mst1, and Rap1. Each of the 2 pools appears to independently interact with the α-chain (CD11a) and the β-chain (CD18) of LFA-1, respectively. Surprisingly, we found that Mst1, a known binding partner of RAPL,40 constitutively associates with the ADAP/SKAP55/RIAM complex. This finding was unexpected because none of the molecules within this complex contains a SARAH domain that has been reported to facilitate the interaction between Mst1 and RAPL (and hence explains the association of Mst1 with the ADAP/SKAP55/RAPL complex). This could indicate that the interaction between Mst1 and the ADAP/SKAP55/RIAM module is mediated via (a) so far unknown molecule(s) that we are currently trying to identify.
RIAM has been reported to inducibly associate with Talin. Moreover, this association is required for the activation of β3-integrins.41,42 Similarly, it has been reported that an interaction between constitutive active Rap1 (Rap1V12) and RIAM facilitates the binding of Talin to the integrin β3-chain.41,42 This suggests that a complex formed between RIAM and active Rap1 is required to mediate binding of Talin to the integrin β-chain. In line with this hypothesis, we found that Talin associates with the ADAP/SKAP55/RIAM complex only in response to CCR7 stimulation (Figure 4D). Importantly, the recruitment of Talin to the complex occurred with a delayed kinetics compared with the recruitment of Rap1 (Figures 4D and 5A-C). Because loss of the ADAP/SKAP55 module does not affect CCR7-mediated activation of Rap1 (supplemental Figure 2B), these findings collectively suggest a 2-step model of CCL21-mediated integrin activation. In the first step, Rap1 becomes activated by CCL21 and binds to the ADAP/SKAP55 module via RIAM. By this mechanism, the RIAM/Rap1-complex is brought to the cell membrane in close proximity to the CD18 subunit of LFA-1. In a second step, the membrane-associated ADAP/SKAP55/RIAM/Rap1 complex facilitates binding of Talin to the β-chain of LFA-1, thereby allowing the activation of LFA-1.
In addition to Talin, we found a constitutive association of the ADAP/SKAP55/RIAM module with the FERM domain-containing protein Kindlin-3 (Figure 4D). Currently, we do not know how Kindlin-3 interacts with the ADAP/SKAP55/RIAM module. Importantly, however, Kindlin-3 has previously been reported to be involved in the regulation of cellular adhesion and migration. Moreover, mutations in the kindlin-3 gene cause leukocyte adhesion deficiency-III.43,44 Intriguingly, Kindlin-3–deficient T cells display a loss of LFA-1 activation on chemokine- or TCR-mediated stimuli,45 although the expression of Talin is unaffected. This could suggest that Talin acts downstream of Kindlin-3 in T cells and that Kindlin-3 induces the association between Talin and LFA-1 in concert with the ADAP/SKAP55/RIAM/Rap1-signaling complex. Moreover, because loss of RIAM, Kindlin-3, or Talin impairs affinity regulation of LFA-1,7,24,46,47 it is probable that the pool of the ADAP/SKAP55 module that is linked to RIAM, Talin, and Kindlin-3 is required for CCR7-mediated inside-out signaling.
If the ADAP/SKAP55/RIAM module regulates inside-out signaling via the β-chain of LFA-1, what then could be the function of the ADAP/SKAP55/RAPL module that interacts with the LFA-1 α chain? Clearly, one possibility could be that the 2 ADAP/SKAP55 pools synergistically regulate inside-out signaling. However, in contrast to RIAM and Talin, loss of RAPL does not impair affinity modulation of LFA-1 after chemokine stimulation.7 This could indicate that RAPL is not involved in inside-out-mediated affinity regulation of LFA-1 on CCR7 stimulation but rather that it controls LFA-1–mediated outside-in signaling processes. Indeed, such a role for RAPL has been proposed by Ebisuno et al, which demonstrated that RAPL facilitates stable T-cell arrest at the endothelium via outside-in signaling.7 Similarly, ADAP has been reported to be involved in outside-in signaling via LFA-1.48 Hence, it is tempting to speculate that the 2 ADAP/SKAP55 modules differentially regulate inside-out and outside-in signaling processes on CCR7-mediated integrin activation.
Our biochemical experiments also showed that the ADAP/SKAP55 module is required for recruitment of both signaling complexes to LFA-1. In this context, it is important to note that LFA-1, Rap1, RAPL, and Mst1 are mostly present in endosomal vesicles.40 Moreover, upon activation of Rap1, LFA-1, Mst1, and RAPL translocate within the same compartment to the plasma membrane.40 The Rap1-dependent and LFA-1–mediated adhesion and vesicle transport have been shown to depend on the GTPase Rab11.49 Whether the ADAP/SKAP55 module also localizes within such a compartment and whether it plays a role in Rab11-dependent intracellular trafficking of LFA-1, Mst1, and RAPL (eg, by regulating the functional activity of Rab11) are not known at present. Similarly, it is not yet known whether RIAM and Kindlin-3 localize within in the same endosomal compartment as LFA-1, Rap1, RAPL, and Mst1. Clearly, further investigations are required to solve these issues.
Nevertheless, the data presented here suggest an important role of the ADAP/SKAP55 module for CCL21-mediated LFA-1 affinity/avidity regulation, T-cell adhesion, and migration in vitro and in vivo. They demonstrate the existence of 2 independent pools of ADAP/SKAP55 modules: one that is linked to RIAM, Mst1, Talin, Kindlin-3, and Rap1 and the other that associates with RAPL, Mst1, and Rap1. Each of the 2 pools independently interacts with the α- or β-chain of LFA-1 and together, the 2 ADAP/SKAP55 pools orchestrate inside-out and possibly also outside-in signaling via LFA-1 on CCR7-mediated activation of T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank G. A. Koretzky, T. E. Stradal, and S. Fagerholm for providing reagents, M. Gunzer and T. Beyer for critical reading of this manuscript, and A. Ramonat for excellent technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft (grant GRK1167 TP11, J.D., S.K., and B.S.; and grant SFB854 TP10/12, C.F., S.K., and B.S.).
Authorship
Contribution: S.K., B.S., X.W., J.D., A.R., T.W., R.F., and M.T. performed and/or designed research; S.K. and T.W. analyzed data; B.M., C.F., I.P., F.K., and M.M. generated and contributed reagents; and S.K. and B.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefanie Kliche, Institute of Molecular and Clinical Immunology, Otto-von-Guericke University, D-39120 Magdeburg, Germany; e-mail: stefanie.kliche@med.ovgu.de.

![Figure 1. The ADAP/SKAP55 module controls T-cell migration in vitro and in vivo. (A) Purified splenic WT and ADAP−/− T cells were placed into the upper part of transwell chambers. Cells were incubated in the absence or presence of CCL21 in the upper and/or lower chamber for 2 hours. Migrated T cells (lower chamber) were counted and calculated as percentage of input cell numbers (mean ± SD; n = 8 mice each). **P ≤ .001. (B) A mixture of equal numbers of CFSE-labeled WT and DDAO-SE–labeled ADAP−/− purified T cells was adoptively transferred into 4 genetically matched WT recipients. After 2 or 18 hours, the percentage of labeled lymphocytes from pLN (mesenteric [MLN], auxiliary [ALN], and inguinal [ILN]), as well as the spleen was determined by flow cytometry, and the homing index was calculated as the ratio of ADAP−/− to WT T-cell numbers. The graph summarizes the data of 2 independent experiments (mean ± SD; n = 8 recipient mice).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/3/10.1182_blood-2011-06-362269/5/m_zh89991184800001.jpeg?Expires=1765886638&Signature=JWDeaJYIjdsGnNsJ74f7Jg9DTi17OUyebc3AFOhilvqy2Jf8CrNwSzFR6~SctxdDD72I8Msowz3mHo47yjn0ve7k0lKjgd6FLMmj-IJhslp-csOdjFQNE5tlmflDdYMlBNORBJATC~rROcxCPedfCanC8R69~Lpj9FSKgdEGlJegxojY9qJkysYqlCtWx9XyUc3O2gLK5MGPfgz3d2jeN8Kr2im7vz~aXwbduANACvpr2d7vlvirwR2Ajhy~JM~XiObiv0EV9Ot8ruH7Z0s7tGrD5LXzcGvfKtpEBb7cK3LlFm3NORpl-~wuavvNlG4qOtbpXJetOylMzu0IVlzgFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal