Abstract
Natural killer (NK) cells can mediate the rejection of bone marrow allografts and exist as subsets based on expression of inhibitory/activating receptors that can bind MHC. In vitro data have shown that NK subsets bearing Ly49 receptors for self-MHC class I have intrinsically higher effector function, supporting the hypothesis that NK cells undergo a host MHC-dependent functional education. These subsets also play a role in bone marrow cell (BMC) allograft rejection. Thus far, little in vivo evidence for this preferential licensing across mouse strains with different MHC haplotypes has been shown. We assessed the intrinsic response potential of the different Ly49+ subsets in BMC rejection by using β2-microglobulin deficient (β2m−/−) mice as donors. Using congenic and allogeneic mice as recipients and depleting the different Ly49 subsets, we found that NK subsets bearing Ly49s, which bind “self-MHC” were found to be the dominant subset responsible for β2m−/− BMC rejection. This provides in vivo evidence for host MHC class I–dependent functional education. Interestingly, all H2d strain mice regardless of background were able to resist significantly greater amounts of β2m−/−, but not wild-type BMC than H2b mice, providing evidence that the rheostat hypothesis regarding Ly49 affinities for MHC and NK-cell function impacts BMC rejection capability.
Introduction
Natural killer (NK) cells mediate non-MHC–restricted killing of virally infected and neoplastic cells as well as playing a role in immune responses regulation.1,2 One of the original descriptions of NK function was the ability of nonsensitized lethally irradiated mice to reject bone marrow cell (BMC) allografts.3 Despite being described over 40 years ago, the mechanism(s) by which NK cells can detect and reject allogeneic BMC is still not completely clear.
NK cells are heterogeneous and exist as subsets based on the expression of various markers. One family of these molecules, Ly49 in mice and killer-cell immunoglobulin-like receptors (KIRs) in human, binds MHC class I and can exert potent inhibitory signals to NK cells.4-7 This has been postulated as a means to regulate NK function and prevent reactivity toward “self.”8,9 Interestingly, apart from the role of inhibitory Ly49+ NK subsets in BM graft rejection, there have been little reports demonstrating in vivo activity of the inhibitory Ly49 receptors with regard to tumor or viral clearance. Data from clinical BM transplantation (BMT) studies would suggest that KIRs may indeed mediate antitumor effects after allogeneic BMT in some leukemias.10,11
Recently, data were reported that inhibitory Ly49 molecules, and their interactions with MHC may play a role in the development of NK activity. It was shown that NK cells bearing Ly49 receptors to self-MHC preferentially produced interferon on stimulation through NK 1.1 in vitro.12 This suggested that NK cells bearing such inhibitory receptors were “licensed” and could selectively respond to physiologic stimuli to mediate effector functions because they had a mechanism to protect from self-reactivity. Thus far, the critical evidence for licensing has relied on in vitro activation of isolated NK subsets using interferon or lytic effects as the primary readouts.12 Clearly, although the role of MHC in NK cell recognition may be pivotal with regard to BMC allograft rejection, it is also complicated with regard to definitive evidence for licensing in vivo because of the powerful effects of MHC on triggering Ly49 molecules on NK cells.
To determine whether there was evidence for licensing in vivo, we sought to remove the complicating effects of MHC expression on the target population through the use of β2m−/− mice, which lack of MHC class I expression. These mice have markedly impaired NK activity and their BMC are universally rejected by irradiated mice, even ones from MHC-identical congenic strains, demonstrating the potent role of MHC in down-regulating NK activity.13 We therefore used donor β2m−/− BMC and assessed the ability of host NK subsets to reject them to ascertain whether differences in Ly49+ subsets exist to mediate BMC rejection in the absence of MHC class I binding. Studies have demonstrated that preferential β2m−/− BMC rejection by Ly49C and NKG2A using H2b recipients, suggesting that licensing can indeed be observed.14 We sought to extend these studies and to use mice with different MHC haplotypes to determine whether patterns of rejection by NK subsets existed, which were consistent with the licensing concept in which opposite effects of the different Ly49+ subsets may be predicted. We report here that differential effects do indeed exist between the Ly49+ subsets in BMC rejection consistent with licensing when B10 congenic mouse strains differing only in MHC were used. Importantly, the rejection capability of the different strains and haplotypes demonstrated a clear increased ability of H2d haplotype mice to resist β2m−/− BMC, even when rejection was not observed with wild-type BMC. These results indicate that operational differences exist in NK subsets, which may have implications in BMT, cancer, and in NK function in general.
Methods
Mice
Animal protocols were approved by the University of Nevada, Reno or the University of California Davis Animal Care and Use Committees. Female BALB/c (H2d) and C57BL/6 (B6, H2b) mice were obtained from the Animal Production Area, National Cancer Institute. Female MHC class I–deficient (β2m−/− B6: H-2b), B6D2F1 (H-2b×d), B6 (H2b), C57BL/10 (B10, H2b), and B10.D2 (H2d) mice were purchased from The Jackson Laboratory. All mice were kept under specific pathogen-free conditions until use at 10 to 14 weeks of age.
Flow cytometric analysis
Mice were injected intraperitoneally with 300 μg of rat-IgG, 300 μg anti-Ly49C/I (5E6), or 300 μg anti-Ly49G2 (4D11). After 48 hours, spleens were harvested and single-cell suspensions prepared. Flow cytometric analysis was performed to determine the percentage of NK cells (CD45+NK1.1+CD3−) and Ly49C/I+ and Ly49G2+ NK subsets. Cells were stained with PB–anti-CD45, PC7–anti-CD3, AF647–anti-NK1.1, PE– anti-Ly49C/I (5E6), PE–anti-Ly49I (YLI-90), FITC–anti-Ly49G2 (4D11 or Cwy-3), or PE–anti-NKG2A and analyzed on a LSR-Fortessa cytometer (BD Biosciences). Anti-CD45, anti-NK1.1, and anti-CD3 were obtained from BioLegend. Anti-Ly49C/I, anti-Ly49I, anti-Ly49G2 and anti-NKG2A were obtained from BD Biosciences.
BMT studies
Before bone marrow transplantation (BMT), recipient mice were treated with 300 μg anti-Ly49C/I, 300 μg anti-Ly49G2, 300 μg anti-Ly49A (YE1/32), 300 μg anti-NK1.1 (PK136), 20 μL anti–asialo-GM1 (anti-ASGM1, 1:20 dilution; Wako Chemicals), or 300 μg rat-IgG, 2 and 1 day before irradiation. In some experiments, NK-cell activation was performed by administration of 240 μg polyinosinic/polycytidylic (poly I:C), 2 days before BMT. Then recipients were lethally irradiated with a 137Cs source (B6, B10, and B10.D2 at 950 cGy, BALB/c at 800 cGy, and B6D2F1 at 1000 cGy). Donor BMCs were collected by flushing femurs and tibias under aseptic conditions, and single-cell suspensions were prepared in PBS. Depending on the host-donor BMT combination, 7.5 to 100 million BMCs were intravenously injected into the tail veins of irradiated recipients.
CFU-c assay
The engraftment of donor BMCs was assessed by an in vitro colony-forming unit (CFU-c) assay for hematopoietic cell growth. On day 7 after BMT, spleens were gently crushed and single-cell suspensions were prepared. Then, 106 spleen cells were plated in 0.3% sea-plaque agar in 35-mm dishes containing recombinant murine cytokines (10 ng/mL IL-3 and 10 ng/mL GM-CSF, obtained from PeproTech). Plates were incubated at 37°C for 7 days, and the colonies with more than 50 cells were counted. The data are presented as mean ± SEM total CFU-c per spleen, which was obtained by multiplying the number of colonies by the cellularity. One-way or 2-way ANOVA or Student t test was performed to determine whether the mean values were significantly different (P < .05) when appropriate. Each experiment was performed 2 to 4 times with 3 or 4 mice per group.
Histologic analysis
At day 7 after BMT, spleens from B6 recipient mice were collected and fixed in 10% neutral-buffered formalin. Samples were then embedded in paraffin, cut into 5-μm-thick sections, and stained with H&E. All tissues were stained at the Histology Consultation Services. All slides were coded and read in a blinded fashion. Images were captured with an Olympus BX4 microscope equipped with a Q-color 3 camera, using 4× numerical aperture objective lens. Magnification for each capture image is shown in Figure 3 legend. Images were processed for contrast and brightness using Adobe Photoshop CS3.
Results
Ly49+ NK-cell subsets in different mouse strains and effects of depletion
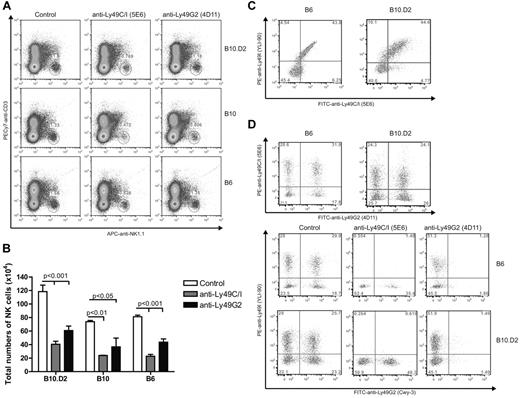
The original description of differential NK function consistent with licensing were seen with Ly49A (binds H2Dd) and Ly49C (binds H2Kb) NK subsets.12 Ly49G2+ NK cells compose 40% to 50% of the NK cells in B6, B10, and B10.D2 mice and have a similar MHC-binding capability as Ly49A. Therefore, although licensing effects in Ly49G2 subset have not been directly demonstrated for H2d strains, these cells would be expected to behave in a similar manner to Ly49A+ NK cells. We first determined the percentages of NK cells expressing Ly49G2 and Ly49C/I receptors in the different strains and the effects of in vivo depletion. Splenocytes from B6 and B10.D2 mice expressed comparable amounts and percentages of single- and double-positive Ly49G2 and Ly49C/I NK subsets (Figure 1D), in agreement with previous reports demonstrating that differences in only MHC did not affect the distribution of the subsets.15 Importantly, in vivo depletion of individual subsets using anti-Ly49G2 and anti-Ly49C/I was performed (Figure 1D). B10 mice did also show similar Ly49G2 and Ly49C/I distribution and effects of depletion (data not shown). In addition, the subset depletion was indicated by the reduction in total NK-cell numbers (CD45+CD3−NK1.1+) and did not alter the other single-positive subsets (Figure 1A-B,D). Therefore, in vivo depletion should allow for assessment of the function of the remaining subset.
Effect of anti-Ly49C/I and anti-Ly49G2 treatment on the frequency of NK Ly49 subsets in various strains of mice. Mice received 300 μg mAb 5E6 (anti-Ly49C/I), mAb 4D11 (anti-Ly49G2), or rat IgG for 2 consecutive days. Spleen cells were then assessed by 5-color flow cytometric analysis for Ly49C/I or Ly49I and Ly49G2 expression on NK cells as described in “Flow cytometric analysis.” (A) Representative dot plots for NK cell (CD45+NK1.1+CD3−) in spleen cells are presented. (B) Total number of CD45+NK1.1+CD3− after antibody depletion. (C) Frequency of Ly49C/I (5E6) and Ly49I (YLI-90) in control B6 and B10.D2 mice. (D) Distribution of Ly49G2 (Cwy-3) and Ly49I (YLI-90) NK subsets after antibody treatment in B6 and B10.D2 mice. Data are representative of 3 experiments (mean ± SEM). Two-way ANOVA was done to assess significance (P < .05).
Effect of anti-Ly49C/I and anti-Ly49G2 treatment on the frequency of NK Ly49 subsets in various strains of mice. Mice received 300 μg mAb 5E6 (anti-Ly49C/I), mAb 4D11 (anti-Ly49G2), or rat IgG for 2 consecutive days. Spleen cells were then assessed by 5-color flow cytometric analysis for Ly49C/I or Ly49I and Ly49G2 expression on NK cells as described in “Flow cytometric analysis.” (A) Representative dot plots for NK cell (CD45+NK1.1+CD3−) in spleen cells are presented. (B) Total number of CD45+NK1.1+CD3− after antibody depletion. (C) Frequency of Ly49C/I (5E6) and Ly49I (YLI-90) in control B6 and B10.D2 mice. (D) Distribution of Ly49G2 (Cwy-3) and Ly49I (YLI-90) NK subsets after antibody treatment in B6 and B10.D2 mice. Data are representative of 3 experiments (mean ± SEM). Two-way ANOVA was done to assess significance (P < .05).
Differential effects of Ly49 subset depletion on the ability of H2b, H2d, and F1 mice to reject β2m−/− BMC
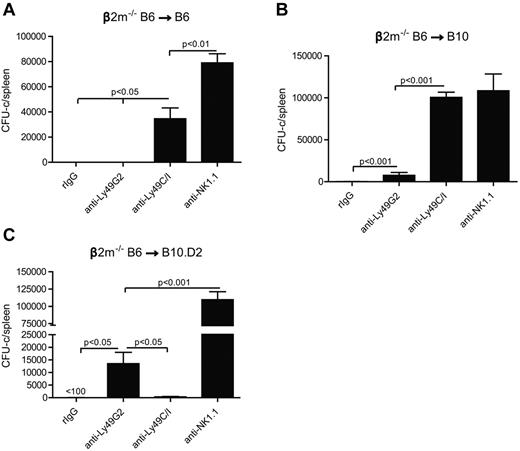
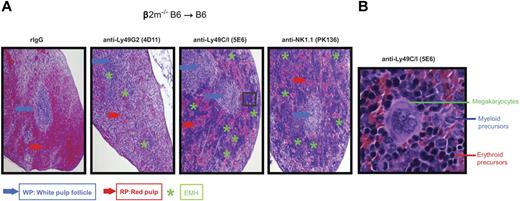
We first wanted to ascertain whether the in vitro licensing effects reported using NK cells from mice of different MHC haplotypes could be observed with regard to in vivo function by measuring NK-cell mediated allogeneic BMC rejection after lethal irradiation. We therefore examined the roles of the 2 predominant Ly49+ subsets (Ly49C/I and Ly49G2) on their ability to reject allogeneic BMC because the role of these subsets has been extensively characterized with regard to allogeneic BMC rejection. Because the expression of MHC on the donor BMC serves as a ligand for the Ly49 receptors and markedly inhibits subsequent functions of NK cells, we used β2m−/− BMCs, which lack MHC class I expression and are universally rejected by even syngeneic recipients. We performed BMT studies using the splenic CFU-c assay 7 days after BMT as a measure for hematopoietic recovery and an indicator for engraftment. We and others have previously demonstrated that the CFU-c effects observed are comparable with the shorter-term splenic 5-iodo-2′-deoxyuridine uptake assay and also correlate with long-term chimerism and recovery.16,17 However, because of the higher sensitivity of this assay, different amounts of BMC needed to be used for each host-recipient combination to determine engraftment/rejection. In the first study, lethally irradiated B6 recipients were infused with β2m−/− or control BMCs. The first observation regarded the extreme sensitivity of the B6 β2m−/− BMCs to rejection by lethally irradiated wild-type B6 recipients (Figure 2A), which was consistent with previous reports.13,18 As many as 15 × 106 BMC could be successfully resisted by the B6 recipients, whereas there was no rejection of wild-type syngeneic control BMCs (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), indicating the powerful inhibitory role of MHC in blocking NK-cell mediated rejection. We then assessed the ability of the different NK subsets to mediate BMC rejection by determining whether removal of a particular subset could result in the abrogation of rejection. Prior depletion of Ly49C/I+ cells resulted in significantly (P < .05) increased engraftment, whereas depletion of Ly49G2+ NK cells did not affect rejection (Figure 2A). This is also consistent with a previous study using this strain indicating a preferential ability of Ly49C+ NK cells in mediating BMC rejection in the absence of class I.19 The role of licensed NK cells in β2m−/− BMC rejection was also demonstrated by histologic examination (Figure 3) where the depletion of the Ly49C/I subset also led to an improvement in splenic engraftment with marked extramedullary hematopoiesis (EMH) in splenic red pulp. EMH, indicative of engraftment, was observed with erythroid islands, scattered myeloid precursors, and large megakaryocytes implying hematopoietic regeneration in the splenic red pulp (Figure 3B). Similar observations were found when anti-NK1.1 was used to deplete host NK cells (Figures 2A and 3A). In B10 mice, another H2b strain, removal of the Ly49C/I+ subset, also resulted in significantly increased engraftment compared with Ly49G2+ subset depletion (Figure 2B). In this experiment, depletion of Ly49C/I+ cells was nearly as effective as depleting all NK1.1+ cells in abrogating resistance, which could be explained by genetic background differences between B10 and B6 strains, which affects multiple immune parameters. Regardless, in H2b recipients, the removal of Ly49C/I+ cells resulted in increased engraftment of β2m−/− BMC.
Effects of Ly49G2+ or Ly49C/I+ NK-cell subset depletion on β2m−/− BMC rejection by B6, B10, and B10.D2 recipients. Recipient mice received mAbs for NK-cell subset depletion using anti-Ly49C/I (5E6), anti-Ly49G2 (4D11) mAbs or rIgG control 2 and one day before lethal irradiation. (A-B) A total of 15 million or (C) 50 million of β2m−/− BMCs were transferred intravenously in (A) B6 (H2b), (B) B10 (H2b), and (C) B10.D2 (H2d) recipients, respectively. Anti-NK1.1 (PK136) was used 2 and one day before BMT to remove all NK1.1-positive cells in some groups to demonstrate that BMC rejection was the result of host NK cells. In all experiments, 7 days after transplantation, hematopoietic progenitor content of the spleens (total CFU-c per spleen) was assessed (mean ± SEM). One-way ANOVA test was performed to determine whether the mean values were significantly different (P < .05). Each experiment was performed 2 or 3 times.
Effects of Ly49G2+ or Ly49C/I+ NK-cell subset depletion on β2m−/− BMC rejection by B6, B10, and B10.D2 recipients. Recipient mice received mAbs for NK-cell subset depletion using anti-Ly49C/I (5E6), anti-Ly49G2 (4D11) mAbs or rIgG control 2 and one day before lethal irradiation. (A-B) A total of 15 million or (C) 50 million of β2m−/− BMCs were transferred intravenously in (A) B6 (H2b), (B) B10 (H2b), and (C) B10.D2 (H2d) recipients, respectively. Anti-NK1.1 (PK136) was used 2 and one day before BMT to remove all NK1.1-positive cells in some groups to demonstrate that BMC rejection was the result of host NK cells. In all experiments, 7 days after transplantation, hematopoietic progenitor content of the spleens (total CFU-c per spleen) was assessed (mean ± SEM). One-way ANOVA test was performed to determine whether the mean values were significantly different (P < .05). Each experiment was performed 2 or 3 times.
Histologic evidence of BMC engraftment after Ly49G2 or Ly49C/I NK subset depletion of B6 recipients before β2m−/− BMC infusion. B6 recipient mice received mAbs for NK cell or NK-cell subset depletion using anti-NK1.1 (PK136), anti-Ly49C/I (5E6), anti-Ly49G2 (4D11) mAbs or rIgG control 2 and 1 day before lethal irradiation. A total of 15 million β2m−/− BMCs were transferred intravenously into B6 recipient mice. (A) H&E staining of formalin-fixed spleens (4×) for the different treatments after 7 days after transplantation is shown. (B) Enlarged image (40×) of a region that showed engraftment with marked EMH after anti–Ly49C/I (5E6) treatment. Blue and red arrows indicate the location of the white pulp follicle (WP) and red pulp (RP), respectively. Green asterisks indicate the EMH region. Images are representative of 2 independent experiments.
Histologic evidence of BMC engraftment after Ly49G2 or Ly49C/I NK subset depletion of B6 recipients before β2m−/− BMC infusion. B6 recipient mice received mAbs for NK cell or NK-cell subset depletion using anti-NK1.1 (PK136), anti-Ly49C/I (5E6), anti-Ly49G2 (4D11) mAbs or rIgG control 2 and 1 day before lethal irradiation. A total of 15 million β2m−/− BMCs were transferred intravenously into B6 recipient mice. (A) H&E staining of formalin-fixed spleens (4×) for the different treatments after 7 days after transplantation is shown. (B) Enlarged image (40×) of a region that showed engraftment with marked EMH after anti–Ly49C/I (5E6) treatment. Blue and red arrows indicate the location of the white pulp follicle (WP) and red pulp (RP), respectively. Green asterisks indicate the EMH region. Images are representative of 2 independent experiments.
We then sought to verify the licensing activity of NK subsets in BMC rejection by using H2d mice. We transferred β2m−/− BMC into lethally irradiated B10.D2 congeneic mice to compare BMC rejection capability with B10, which differs only at the MHC gene locus (H2d vs H2b). In marked contrast to H2b strains, the depletion of the Ly49G2+, but not the Ly49C/I+ subset, in B10.D2 resulted in increased engraftment (Figure 2C). This demonstrates marked intrinsic differences with regard to BMC rejection in the host NK subsets solely based on the MHC haplotype of the recipient. As Ly49G2 binds H2Dd, this subset would be considered licensed in H2d strain, whereas Ly49C preferentially binds H2Kb and therefore licensed in H2b recipients. Thus, this converse rejection pattern is consistent with the concept that the NK cells bearing Ly49 receptors specific for self-MHC are “licensed” as determined by their ability to reject BMCs. These are also the first results demonstrating licensing properties of the Ly49G2+ subset.
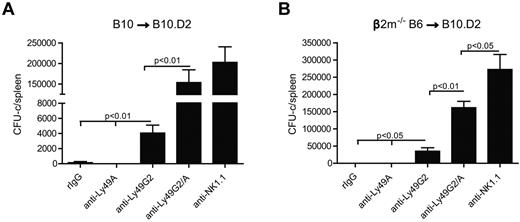
Similar to Ly49G2, Ly49A binds also H2Dd20,21 and therefore is considered licensed in H2d strains as previously suggested.12,22,23 To determine the impact of licensed NK cells from H2d mice in BM rejection, we performed double depletion of Ly49G2 and Ly49A subsets in vivo to investigate whether effects on engraftment could be observed. Interestingly, the administration of anti-Ly49A did not deplete this subset in H2d-strain mice but still could abrogate rejection of H2b allografts, presumably because of signaling by the antibody in vivo (data not shown). We have observed that anti-Ly49A (YE1/32) is capable of depleting Ly49A+ cells in H2b mice but not in H2d strain, possibly because of cis-binding of MHC (M.A. et al, manuscript in preparation). Regardless, and consistent with earlier reports that double depletion results in greater effects in the engraftment of allogeneic wild-type BMCs,24 additive effects were indeed observed using this combination in H2d recipients. We demonstrated that the use of anti-Ly49G2 and anti-Ly49A on B10.D2 recipients resulted in greater engraftment of allogeneic B10 and β2m−/− BMC compared with either subset alone (Figure 4). Thus, the MHC-binding capability of the Ly49 family members and the haplotype of the recipient correspond with rejection capability with regard to class I-deficient BMC rejection.
Combined effects of Ly49G2 and Ly49A NK-cell subset depletion on B10 or β2m−/− BMC rejection by B10.D2 recipients. Recipient mice received rIgG control, anti-NK1.1 (PK136), anti-Ly49G2 (4D11), and/or anti-Ly49A (YE1/32) for NK cell and NK-cell subset depletion 2 and one day before lethal irradiation. A total of 15 million of B10 (A) or 50 million of β2m−/− BMCs (B) were transfused to B10.D2 recipients. Seven days after transplantation, spleens were collected and CFU-c assay was performed (mean ± SEM). One-way ANOVA test was performed to determine whether the mean values were significantly different (P < .05). Each experiment was performed 2 or 3 times.
Combined effects of Ly49G2 and Ly49A NK-cell subset depletion on B10 or β2m−/− BMC rejection by B10.D2 recipients. Recipient mice received rIgG control, anti-NK1.1 (PK136), anti-Ly49G2 (4D11), and/or anti-Ly49A (YE1/32) for NK cell and NK-cell subset depletion 2 and one day before lethal irradiation. A total of 15 million of B10 (A) or 50 million of β2m−/− BMCs (B) were transfused to B10.D2 recipients. Seven days after transplantation, spleens were collected and CFU-c assay was performed (mean ± SEM). One-way ANOVA test was performed to determine whether the mean values were significantly different (P < .05). Each experiment was performed 2 or 3 times.
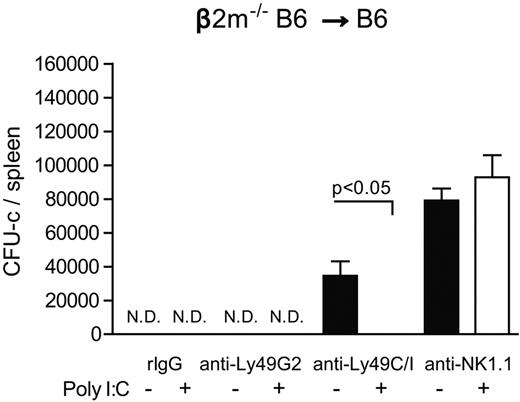
We then determined whether this relative inability of the unlicensed subset to reject β2m−/− BMCs could be overcome by prior activation of host NK cells. When B6 recipient mice were given poly I:C to augment NK function, BMC rejection was observed despite prior removal of the licensed Ly49C/I+ subset (Figure 5). These results indicate that, although differential abilities of Ly49+ NK subsets were observed in MHC-congenic recipients, prior activation of the unlicensed NK cell can override this and allow for continued rejection by the remaining unlicensed subset. This is also demonstrated by the ability of unlicensed Ly49G2+ NK cells to reject higher amounts of β2m−/− BMCs after poly I:C administration (Figure 5: 25 million vs Figure 2A: 15 million).
Effect of prior NK activation of recipient mice with β2m−/− BMC rejection. Two and 1 days before transplantation B6 recipient mice were treated with anti-NK1.1 (PK136), anti-Ly49G2 (4D11), anti-Ly49C/I (5E6), or rIgG control. In addition, some mice received poly I:C 2 days before BMT to boost BMC rejection. A total of 25 million β2m−/− BMCs were injected into lethal irradiated B6 recipients. The CFU-c formation from the spleens is shown (mean ± SEM). One-way ANOVA test was performed to determine whether the mean values were significantly different (P < .05). Each experiment was performed 2 or 3 times.
Effect of prior NK activation of recipient mice with β2m−/− BMC rejection. Two and 1 days before transplantation B6 recipient mice were treated with anti-NK1.1 (PK136), anti-Ly49G2 (4D11), anti-Ly49C/I (5E6), or rIgG control. In addition, some mice received poly I:C 2 days before BMT to boost BMC rejection. A total of 25 million β2m−/− BMCs were injected into lethal irradiated B6 recipients. The CFU-c formation from the spleens is shown (mean ± SEM). One-way ANOVA test was performed to determine whether the mean values were significantly different (P < .05). Each experiment was performed 2 or 3 times.
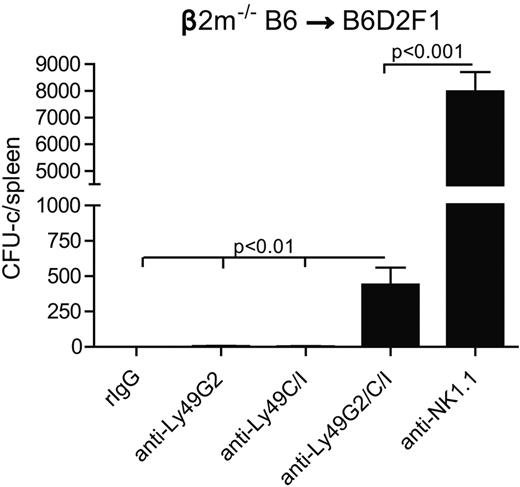
Next, we wanted to assess whether the licensing of Ly49+ NK subsets affected BM rejection ability in F1 hybrid mice (B6 × DBA2/J F1: B6D2F1). B6D2F1 (H2bxd) possess NK cells, which have been developed in the presence of both H2d and H2b MHC, suggesting that NK cells that bearing either Ly49G2 and Ly49C/I can potentially be licensed as a result. Therefore, both licensed NK subsets would be expected to play a role rejecting β2m−/− BMCs. We decided to assess the effects of only Ly49G2 depletion and not Ly49A subset in B6D2F1 recipients because Ly49G2 represents a greater NK population, and we have previously demonstrated that Ly49A plays a role in the rejection of parental H2b BMC by B6D2F1 recipients. Lethally irradiated B6D2F1 mice were transfused with β2m−/− BMCs after Ly49G2 and/or Ly49C/I depletion. As predicted, the depletion of either Ly49G2+ or Ly49C/I+ NK subsets still resulted in robust BM rejection; only when both subsets were simultaneously depleted was rejection impaired (Figure 6). In addition, B6D2F1 mice were able to resist a higher amount of β2m−/− BMC (30 million) compared with parental B6 (15 million; Figure 2A). These results suggest that Ly49G2 and Ly49C/I subsets are both licensed in the B6D2F1 mice with regard to BMC rejection. However, total NK depletion led to significantly higher engraftment compared with Ly49G2 and Ly49C/I double depletion, suggesting that other NK subsets, such as Ly49A, could also mediate BMC rejection. Thus, the MHC of the recipient can result in NK licensing with regard to their intrinsic ability to resist BMCs.
Effects of Ly49G2 or Ly49C/I NK-cell subset depletion on β2m−/− BMC rejection by B6D2F1 hybrid recipients. Two days after mAb treatment, 30 million of β2m−/− BMCs were injected into lethal irradiated B6D2F1 recipients. Seven days after transplantation, CFU-c assay was done from spleens (mean ± SEM). One-way ANOVA test was performed to determine whether the mean values were significantly different (P < .05). Data are representative of 2 experiments.
Effects of Ly49G2 or Ly49C/I NK-cell subset depletion on β2m−/− BMC rejection by B6D2F1 hybrid recipients. Two days after mAb treatment, 30 million of β2m−/− BMCs were injected into lethal irradiated B6D2F1 recipients. Seven days after transplantation, CFU-c assay was done from spleens (mean ± SEM). One-way ANOVA test was performed to determine whether the mean values were significantly different (P < .05). Data are representative of 2 experiments.
Differential ability of B10 and B10.D2 strain mice to reject allogeneic and β2m−/− BMCs
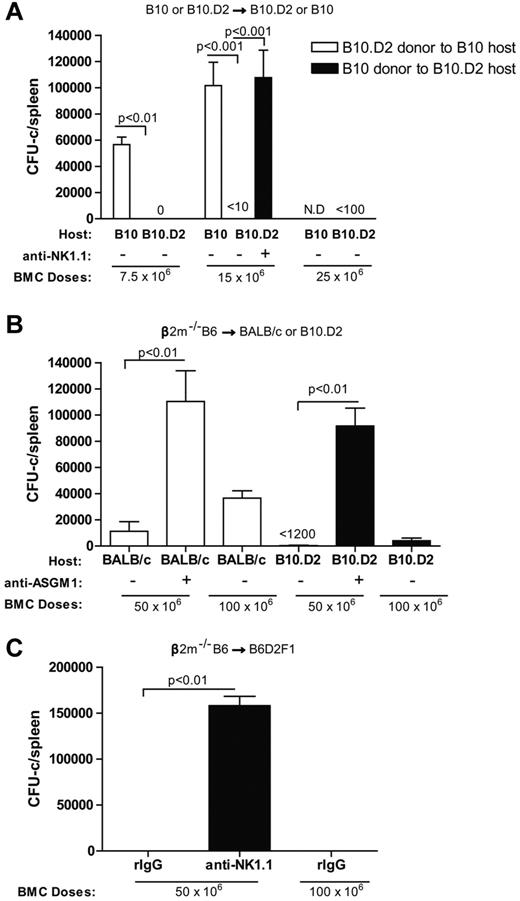
Interestingly, we found that higher numbers of β2m−/− BMCs (up to 5 × 107) had to be given to the B10.D2 recipients to observe the effects of subset depletion in contrast to their B10 counterparts where resistance could be overridden by giving as few as 1.5 × 107 BMCs despite the fact that MHC class I molecules were absent (Figure 2B-C). This is consistent with 3 possibilities: (1) a rheostat model in which mouse haplotypes with greater numbers of NK cells whose Ly49 receptors have a greater overall affinity for self-MHC molecules would allow them to become licensed and resist more BMCs19 ; (2) the Ly49A subset of NK cells plays a larger role in rejection; or (3) there are molecules within the H2b MHC but distinct from class I that can be detected by H2d NK cells in a positive manner. With regard to the first possibility, Ly49C has been reported to have affinity not only for H2Db but also Dd20 and therefore could possibly become licensed in H2d mice and contribute to rejection. Removal of this subset alone would not be sufficient to detect increased engraftment as the Ly49G2+ subset has a higher affinity for H2Dd and would dominate. It has been reported that H2d mice have more licensed NK cells because of the ability of more Ly49 (Ly49G2/A and, to a lesser extent, Ly49C) molecules to bind H2d compared with H2b mice.19 We first determined whether this heightened ability to resist β2m−/− BMCs could also be seen with class I–expressing donor BMCs. Both B10 and B10.D2 recipients were assessed for their ability to reject fully allogeneic H2-disparate B10.D2 and B10 BMC, respectively. The results show that B10.D2 mice were capable of resisting markedly more allogeneic BMCs than their B10 counterparts despite having identical background genes (Figure 7A). These results indicate differential abilities do indeed exist between the NK cell rejection ability of the 2 congenic strains, differing only within the MHC.
Differential ability of B10, B10.D2, BALB/c, and B6D2F1 recipients to reject allogeneic and β2m−/− BMC. (A) The engraftment of allogeneic BMCs after transfer of 7.5 or 15 million B10.D2 BMCs into B10 recipients or 7.5, 15, or 25 million B10 BMCs into B10.D2 recipients is shown. (B) The engraftment of 50 or 100 million MHC class I deficient BMCs (β2m−/−) in H2d strains (BALB/c and B10.D2) is shown. (C) The engraftment of 50 or 100 million MHC class I-deficient BMC (β2m−/−) after transfer into B6D2F1 (H2bxd) recipients is shown. In all experiments, some groups were treated with anti-NK1.1 (PK136) or anti-ASGM1 to promote engraftment by removing host NK cells 2 days before BMT. Seven days after transplantation, hematopoietic progenitor content of the spleens (total CFU-c per spleen) was assessed (mean ± SEM) to determine engraftment. N.D. indicates not done. One-way ANOVA was performed to determine whether the mean values were significantly different (P < .05). Each experiment was performed 2 or 3 times.
Differential ability of B10, B10.D2, BALB/c, and B6D2F1 recipients to reject allogeneic and β2m−/− BMC. (A) The engraftment of allogeneic BMCs after transfer of 7.5 or 15 million B10.D2 BMCs into B10 recipients or 7.5, 15, or 25 million B10 BMCs into B10.D2 recipients is shown. (B) The engraftment of 50 or 100 million MHC class I deficient BMCs (β2m−/−) in H2d strains (BALB/c and B10.D2) is shown. (C) The engraftment of 50 or 100 million MHC class I-deficient BMC (β2m−/−) after transfer into B6D2F1 (H2bxd) recipients is shown. In all experiments, some groups were treated with anti-NK1.1 (PK136) or anti-ASGM1 to promote engraftment by removing host NK cells 2 days before BMT. Seven days after transplantation, hematopoietic progenitor content of the spleens (total CFU-c per spleen) was assessed (mean ± SEM) to determine engraftment. N.D. indicates not done. One-way ANOVA was performed to determine whether the mean values were significantly different (P < .05). Each experiment was performed 2 or 3 times.
We then determined whether this heightened ability to resist β2m−/− BMCs could extend to other H2d strains. BALB/c (H2d) mice are classically considered to have lower NK activity and reject allogeneic BMCs poorly, especially compared with B6 or B10 background mice.16,17,25 The number of β2m−/− BMCs capable of being resisted by irradiated B10.D2 and BALB/c strain mice was compared (Figure 7B). As opposed to B10 mice, which could resist only 1.5 × 107 BMCs (Figure 2B), both BALB/c and B10.D2 recipients rejected as much as 5 × 107 BMCs (Figure 7B). Surprisingly, B10.D2 mice were also capable of completely rejecting as many as 1 × 108 BMCs. These data indicate that there is also an effect of background genes on NK cell-mediated rejection ability because the B10.D2 strain was capable of rejecting greater numbers of BMC than the H2-identical BALB/c recipients, but both were greater than H2b recipients. Furthermore, the data that B10.D2 recipients are still capable of rejecting greater amounts of BMCs from the same donor (β2m−/−) compared with B10 recipients exclude donor BMC capabilities as a possible factor in the differential engraftment.
Finally, to rule out that positive recognition of some determinants within MHC but not class I of the H2b haplotype was occurring by the H2d NK cells, we assessed the ability of B6D2F1(H2bxd) mice to mediate hybrid resistance to β2m−/− BMCs. B6D2F1 recipients should express any determinants encoded within the B6 MHC and thus be tolerated to self. Therefore, rejection capability should be decreased if positive recognition was occurring. However, if the rheostat hypothesis were correct, B6D2F1 mice should be able to resist as much as any H2d strain despite the observations that hybrid resistance to MHC class I-expressing BMCs is much weaker than allogeneic resistance.3,17,25-27 The results demonstrate that B6D2F1 recipients were indeed capable of mediating far greater resistance compared with the other H2b strains (Figure 7C). Similar to B10.D2, B6D2F1 recipients were capable of completely resisting 1 × 108 β2m−/− BMCs. These results are therefore consistent with the rheostat hypothesis regarding Ly49 binding abilities on NK activity.19 The data also support the idea that background genes can play a role in rejection as B6D2F1 recipients could resist similar amounts of BMC than B10.D2.
Discussion
NK subsets have long been shown to play a role in BMC rejection. The mechanisms by which NK cells can detect and reject allogeneic HSCs are still unclear.17 NK cells express different receptors that allow them not only to actively recognize tumors and other cell types (ie, NKG2D) but also inhibit their activity (mouse Ly49s, human KIRs, and CD94/NKG2A). There has been significant interest as to the role of the inhibitory Ly49 molecules, particularly with in vivo activities of NK cells, but little is known. Efforts to deplete NK subsets based on inhibitory Ly49 markers have not yielded discernable effects on tumor cell resistance (B.R.B., W.J.M., and M. Bennett, unpublished data, 2002), but blockade of these receptors has yielded increased antitumor effects, suggesting that they can indeed play a role.28 It is difficult to draw conclusions from depletion studies that target multiple subsets because a reduction in total NK-cell numbers occurs, which can also affect overall rejection as well as the fact that all subsets may contribute to some degree. It has been postulated that inhibitory receptors to self-MHC protect the host from autoreactive attack by NK cells, yet clearly there are NK subsets that do not bear self-MHC binding molecules. In vitro activation of the different NK subsets has shown that NK cells that had inhibitory Ly49+ receptors to self-MHC display higher activity when stimulated. It has been proposed that NK cells undergo an education or “licensing” in which NK cells expressing self-MHC binding Ly49 molecules either proceeded in development and/or were more effectively triggered by physiologic activating signals, resulting in heightened functional activity.6,9,12 Similar licensing effects have been reported with human NK cells based on KIR expression.29 The in vivo significance of licensing, however, is unclear as the only data showing differential effects of these subsets in different haplotypes of mice have been through in vitro readouts.12
The data indicate that recipient MHC can contribute to the intrinsic activity of NK subsets consistent with licensing and BMC rejection capability. We report here that functional support for “licensed” NK subsets can indeed be demonstrated in vivo as evidenced by the differential abilities of these Ly49 subsets to mediate BMC rejection in mice whose differences were only within MHC. Only by removing the powerful inhibitory effects of MHC class I on donor BMCs could this evidence of differential activity of the different NK cell Ly49+ subsets be observed in different recipients. The data clearly demonstrated that MHC expression on the donor cells is by far the largest factor in affecting rejection as orders of magnitude higher β2m−/− BMCs could be rejected compared with wild-type BMCs. However, the data also show that molecules encoded outside the MHC can also affect BMC rejection, as illustrated by our comparison between B10.D2 and BALB/c that share the same MHC alleles but have different NKC alleles.
We have previously reported that removal of the Ly49+ subset not responsible for mediating the rejection of normal BMC allograft will result in an enhanced ability of the remaining subset to resist the graft.16 It is interesting to note that, when β2m−/− BMCs were used, this effect did not occur and the removal of the opposite subset did not enhance but actually slightly reduced rejection. This suggests that both subsets can mediate resistance in the absence of class I on the donor BMC, but it is a matter of degree with the licensed subset predominating. This pattern of NK cell licensing could be observed with H2b and H2d strains on the B10 background. However, B10.D2 mice had the greatest ability to resist BMC and yet still demonstrated licensing effects. This would indicate that various baselines of NK cell activity exist, of which licensing is just one. Furthermore, in B6D2F1 mice, where a greater number of NK cells are licensed, single NK subset depletion did not affect rejection as other remaining licensed NK subsets can overrule the deficit of one subset.
We have also shown, even with heightened rejection capability, that the Ly49G2+ and Ly49A+ NK cells in B10.D2 mice are more effective with regard to BMC rejection than to other NK cells in any other strain. The dual role of these subsets in H2d mice significantly increased allogeneic BMC engraftment compared with targeting a single subset, which was close to the engraftment observed after total NK depletion. According to initial studies that analyzed Ly49 binding properties,20 Ly49A and Ly49G2 have little affinity for H2b, whereas the strongest affinity is for H2d. Therefore, because both licensed subsets could mediate strong rejection against B10 and β2m−/− BMCs, when both subsets are blocked, rejection is reduced. The ability of B10.D2 recipients to resist larger numbers of β2m−/− BMCs compared with H2b recipients also indicates that there are degrees of activation of the NK cells depending on the alleles of Ly49 and MHC in the recipient. The stronger rejection observed in H2d strains can also be explained by the rheostat model.19 This model postulates that the higher the number of inhibitory receptors that bind self-MHC present on a cell, the higher the self-recognition abilities and effector functions of the NK cell. Thus, the affinity of the inhibitory receptors for MHC class I determinants can have a marked impact on the overall rejection capability of the recipient. In addition, there were clearly background gene effects as B10.D2 and B6D2F1 recipients resisted up to 100 million BMCs, whereas the BALB/c recipients resisted less, indicating that the overall rejection ability of a recipient is contingent on the haplotype, background genes, and NK activation status.
These data then demonstrate that NK licensing, as determined by Ly49 typing, can have an impact on BMC outcomes in vivo and is observed in the absence of MHC recognition on the target cell. However, other non-MHC determinants can also play a role in overall BMC rejection. NKG2A is also an inhibitory receptor that binds to the nonclassic MHC class I Qa-1 (mouse) and HLA-E (human)30,31 and has also been shown to mediate NK education19,22,32 and BMC rejection14 in an independent and additive fashion with regards to KIRs or Ly49s. Qa-1 associates with β2 microglobulin and the peptide derived in TAP-dependent fashion from H2Db leader sequence Qdm.33-36 The interaction between Qa-1 and CD94/NKG2A blocks NK-mediated lysis37 ; therefore, NKG2A+ NK cells could reject β2m−/− BMCs because of the inadequate Qa-1-NKG2A interaction. The percentage of NKG2A+ cells within the Ly49C/I− or Ly49G2− population is approximately 41% and 45% in B6 mice and 36% and 44% in B10.D2, respectively (data not shown). Therefore, it is probable that NKG2A also plays a role in BMC rejection as depletion of Ly49G2 and/or Ly49C/I is not sufficient in eliminating the NKG2A+ population. The presence of NKG2A+ NK cells could also account for the decreased BMC rejection when anti-NK1.1 is used compared with single Ly49 subset depletion.
The data demonstrating that poly I:C treatment of B6 allowed the unlicensed cells to mediate rejection is interesting in that this strain now exhibited rejection patterns similar to the F1 hybrid recipient, suggesting that the licensing effect can be overridden. A similar effect was also observed when IL-2 was administered to the recipients before transplantation (data not shown), suggesting that any situation where the cytokine environment leads to NK activation could activate both licensed and unlicensed NK cells and together participate in immune responses. These data are supported by several groups that have proposed effector functions of the unlicensed NK subset after in vitro stimulation12 or murine cytomegalovirus infection.38 It is possible that, during NK reconstitution after allogeneic BMT, both activated licensed and unlicensed NK subsets collaborate in the resistance to opportunistic infections.35 Additional NK stimulation by cytokines (IL-15 or IL-2) could further improve the outcomes not only by accelerating immune reconstitution but also by further improving NK effector functions by bypassing licensing.
In allogeneic HSCT settings, NK alloreactivity has been associated with reduced tumor relapse risk and increased tumor survival for AML patients.10,39 These studies suggest that donor selection based on licensing patterns of the NK-cell populations could also have significant effects on outcome.11,40-42 This protective effect was associated with an early expansion and hyper-responsiveness of unlicensed NK cells that lasted 3 to 6 months after HSCT and then slowly acquired tolerance, representing a rare population in the donor levels.40 Similarly, in HLA-matched allogeneic HSCT where NK phenotype tends to recapitulate the donor type,40,43,44 an unrelated HLA-mismatched HSCT study demonstrated that NK education is driven by donor ligands supporting the hematopoietic origin for this process. This study suggested that expression of KIRs to a ligand presented in the donor but absent in the recipient remain responsive long after HSCT and could promote long-term antitumor responses.32 In contrast, in an HLA-matched sibling HSCT study, NK cells expressing KIR for non–self-HLA were less responsive, whereas NKG2A+ NK cells showed the highest response against tumor targets with no differences between the expression of self/non–self-KIRs.44 Other studies have identified a preferential expansion of immature NK cells characterized as CD56bright and NKG2A+ after HLA-matched HSCT.45,46 The discrepancies observed in these studies can be the result of the donor HLA, presence of T cells before HSCT, the immunosuppressive regimen followed, HSC origin, and myeloablation doses that could affect NK reconstitution and education.
Despite the elevated NK alloreactivity, the highly immunosuppressive and myeloablative conditioning regimen necessary to successfully allow HLA-mismatched allogeneic HSCT has been associated with significant regimen-related toxicities and mortality because of the delay in immune-cell recovery. As a result, there is a high risk of tumor relapse and opportunistic infections, such as CMV and EBV early HSCT.47 Therefore, the identification of licensed NK cells before HSCT and selective depletion using monoclonal antibodies against those specific KIRs could result in improved engraftment. These results indicate that host NK subsets can indeed exhibit properties consistent with licensing in allogeneic HSCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lisbeth Welniak, Cherish Agutos and Sean Bissing for assisting in the manuscript preparation, Can Sungur and Anna Peter for helpful review of the manuscript, and Weihong Ma and Monja Metcalf for their technical assistance.
This work was supported by the National Institutes of Health (grant R01-HL089905).
National Institutes of Health
Authorship
Contribution: K.S., M.A., E.A., I.B., D.R., and W.J.M. had experimental oversight, analyzed data, and assisted in the writing of the manuscript; M.C. conducted histologic analysis; and D.L.L. assisted with preparation of the manuscript and experimental design.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William J. Murphy, Departments of Dermatology and Internal Medicine, University of California–Davis School of Medicine, CTSC-IRC Suite 1630, 2921 Stockton Blvd, Sacramento, CA 95817; e-mail: wmjmurphy@ucdavis.edu.
References
Author notes
K.S. and M.A. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal