Abstract
MicroRNAs (miRs) are small RNAs that regulate gene expression at the posttranscriptional level. miR-27 is expressed in endothelial cells, but the specific functions of miR-27b and its family member miR-27a are largely unknown. Here we demonstrate that overexpression of miR-27a and miR-27b significantly increased endothelial cell sprouting. Inhibition of both miR-27a and miR-27b impaired endothelial cell sprout formation and induced endothelial cell repulsion in vitro. In vivo, inhibition of miR-27a/b decreased the number of perfused vessels in Matrigel plugs and impaired embryonic vessel formation in zebrafish. Mechanistically, miR-27 regulated the expression of the angiogenesis inhibitor semaphorin 6A (SEMA6A) in vitro and in vivo and targeted the 3′-untranslated region of SEMA6A. Silencing of SEMA6A partially reversed the inhibition of endothelial cell sprouting and abrogated the repulsion of endothelial cells mediated by miR-27a/b inhibition, indicating that SEMA6A is a functionally relevant miR-27 downstream target regulating endothelial cell repulsion. In summary, we show that miR-27a/b promotes angiogenesis by targeting the angiogenesis inhibitor SEMA6A, which controls repulsion of neighboring endothelial cells.
Introduction
MicroRNAs (miRNAs) are highly conserved, single-stranded noncoding short RNA molecules (18-24 nucleotides) that regulate gene expression at the posttranscriptional level. miRNAs silence gene expression by inhibiting the translation of proteins from mRNAs or by promoting the degradation of mRNAs. After transcription of the primary miRNA transcripts from the genome, their maturation is mediated by the 2 RNase III endonucleases Dicer and Drosha. Then, mature miRNAs are incorporated into the RNA-induced silencing complex,1 which mediates the binding of the miRNA to the 3′-untranslated region (3′-UTR) of the target mRNA leading either to translational repression or degradation of the target mRNA.2 Because miRNAs control specific expression patterns of target genes, miRNAs represent attractive candidates to interfere with neovascularization.
Increasing evidence indicates that miRNAs are important regulators of vascular development and angiogenesis.3,4 In this context, first studies addressed the function of the miRNA-processing enzymes Dicer and/or Drosha to explore the general role of miRNAs for angiogenesis. Depletion of Dicer in zebrafish or mice revealed an aberrant vessel growth, and silencing of Dicer in endothelial cells reduced in vitro angiogenesis.5-7 To date, several miRs that regulate endothelial cell function and angiogenesis have been identified,8 including the pro-angiogenic miRs miR-130a,9 miR-210,5,10,11 and miR-378.12 In addition, miR-126 was shown to regulate vascular integrity and angiogenesis during development and in ischemia-induced angiogenesis.13-15 In contrast, miR-221 and miR-222,7,16 miR-15 and miR-16,17,18 and members of the miR-17-92 cluster19,20 inhibit angiogenesis.
In our previous study, we found that the members of the miR-23∼27∼24 cluster, miR-27a and miR-27b, were highly expressed in endothelial cells.6 In addition, miR-27b was down-regulated after Dicer and Drosha silencing, and inhibition of miR-27b significantly reduced endothelial cell sprouting in vitro,6 indicating that miR-27b exerts pro-angiogenic effects. Recently, Zhou et al demonstrated that the miR-23∼27∼24 cluster regulates angiogenesis.21 In muscle stem cells, miR-27b down-regulates Pax3 expression during myogenic differentiation.22 Moreover, miR-27 down-regulates Runx1 expression during granulocyte differentiation23 and the nuclear receptor peroxisome proliferator-activated receptor-γ (PPAR-γ) in adipocytes.24 The myocyte enhancer factor 2C (MEF2C) is another important target of miR-27b during heart development.25 However, the specific functions and targets of miR-27 in endothelial cells are largely unexplored. As the family members miR-27a and miR-27b differ in only one nucleotide and share the same seed sequence, we investigated the specific role of both family members for the angiogenic activity of endothelial cells and determined the effects on neovascularization. Here we identified the angiogenesis inhibitor semaphorin 6A as a direct target of miR-27a/b in endothelial cells and provide novel evidence for the regulation of endothelial cell repulsion and vessel formation in mice and zebrafish by miR-27.
Methods
Cell culture
Pooled HUVECs were purchased from Lonza and cultured in endothelial basal medium (Lonza) supplemented with hydrocortisone, bovine brain extract, epidermal growth factor, gentamycin sulfate, amphotericin-B, and 10% FCS (Invitrogen) until the third passage. After detachment with trypsin, cells were grown in 6-cm culture dishes for at least 24 hours. Human embryonic kidney (HEK) 293FT cells (Invitrogen) were cultured in DMEM containing GlutaMAX and Pyruvat (Invitrogen) supplemented with 10% heat inactivated FCS (Invitrogen) and penicillin/streptomycin (Roche Diagnostics).
Transfection
HUVECs were transfected at 60% confluence using Lipofectamine RNAiMax (Invitrogen) according to the manufacturer's protocol. For overexpression of the individual miRNAs, specific precursor molecules for miR-27a and miR-27b or control (pre-miR-control, Ambion) were used. Inhibition of the different miRNAs in vitro was achieved by transfection of miRIDIAN Hairpin Inhibitors (Dharmacon RNAi Technologies) or miRCURY LNA microRNA Inhibitors (Exiqon). For siRNA-mediated gene knockdown, HUVECs were transfected with 67nM SEMA6A siRNA (5′-CAGCUAUGAUGGAGUCGAA[dT][dT]-3′, Sigma-Aldrich) or a control siRNA directed against firefly luciferase (5′-CGUACGCGGAAUACUUCGA[dT][dT]-3′).26
Western blot analysis
For Western blot analysis, HUVECs were lysed in RIPA lysis buffer (Sigma-Aldrich) for 15 minutes on ice. After centrifugation for 15 minutes at 20 000g (4°C), the protein content of the samples was determined according to the Bradford method. Equal amounts of protein were loaded onto SDS-polyacrylamide gels and blotted onto nitrocellulose or polyvinylidene difluoride membranes. Western blots were performed by using antibodies directed against SEMA6A (goat polyclonal anti–human SEMA6A; 1:500, R&D Systems), SEMA3B (rabbit polyclonal anti-SEMA3B, 1:5000, Thermo Scientific), tubulin (mouse monoclonal anti-tubulin, 1:1500, NeoMarkers), or ERK1 (rabbit anti-ERK1, 1:1000, Dianova). Secondary antibodies were purchased from Jackson ImmunoResearch Laboratories.
Quantitative real-time RT-PCR
Total RNA was isolated using the miRNeasy Mini Kit (QIAGEN) according to the manufacturer's protocol. Afterward, 1 μg of RNA from each sample was reverse transcribed into cDNA and subjected to quantitative SYBR Green PCR (StepOnePlus & Fast SYBR Green Mastermix; Applied Biosystems). Primer sequences are available on request.
MicroRNA expression analysis
Total RNA was isolated using miRNeasy Mini Kit (QIAGEN) according to the manufacturer's protocol. For miRNA detection, we used TaqMan MicroRNA Assays (Applied Biosystems). RNU48 served as control for HUVEC RNA, whereas snoRNA202 was used for murine samples. Quantitative real-time PCRs were done on a StepOnePlus device (Applied Biosystems). Gene expression data were normalized to U6, RNU48, or snoRNA202. The relative expression was determined using the formula 2−ΔCt.
Spheroid-based angiogenesis assay
Endothelial cell spheroids of defined cell number were generated as described previously.27 In vitro angiogenesis was quantified by measuring the cumulative length of the sprouts that had grown out of each spheroid using a digital imaging software (AxioVision Version 4.6; Carl Zeiss Imaging Solutions), analyzing 10 spheroids per group and experiment.
MTT viability assay
Assessment of cell viability was performed using the MTT assay. Forty hours after transfection, 0.5 mg/mL MTT was added to each well, and cells were incubated for 4 hours at 37°C. Cells were washed with PBS and lysed 30 minutes at room temperature with lysis buffer (40nM HCl in isopropanol). Absorbance was photometrically measured at 550 nm.
Luciferase cloning and transfection
Synthetic oligonucleotides bearing 4× the miR-27 binding sequence of the SEMA6A mRNA 3′-UTR or a mutated version of the sequence (see Figure 4D; mutations are shown in blue) containing HindIII and SpeI restriction sites were cloned into firefly luciferase reporter plasmid pMIR-Report (Ambion) according to the manufacturer's protocol. For measuring luciferase activity HEK293FT cells were grown in 24-well plates until 60% confluence. A total of 0.002 ng luciferase plasmid was cotransfected with 0.2 ng pGL4 Renilla plasmid (Promega) as control for the transfection efficiency and pre-miR-27a, pre-miR-27b, or pre-miR-control using Lipofectamine 2000 (Invitrogen). The activity of luciferase and Renilla was assessed after 48 hours with the Dual-Luciferase Reporter 1000 Assay System (Promega).
Antagomir experiments
Single-stranded RNAs were synthesized by VBC Biotech, Vienna as previously described,28 with the following sequences: Antagomir-27 (AM-27: 5′-GCAGAACUUAGCCACUGUGAA-3′) and Antagomir-Co (AM-Co: 5′-AAGGCAAGCUGACCCUGAAGUU-3′). C57BL/6 mice were used for all antagomir in vivo experiments. To analyze the specificity and efficacy of Antagomir-27, tissue was snap-frozen and stored at −80°C for RNA analysis. RNA isolation, cDNA synthesis, and real-time PCR were performed as described in “MicroRNA expression analysis.”
In vivo Matrigel experiments
All animal experiments were approved by the Regional Board of the state of Hessen, Germany. Eight-week-old mice were injected subcutaneously with 2 Matrigel basement matrix (BD Biosciences) plugs at day 0. Antagomir-Co or antagomir-27 was injected intravenously at day 0 (after Matrigel implantation) and at day 2 and day 4. All antagomirs were administered at doses of 12 mg/kg body weight in 0.2 mL per injection. Matrigel plugs were harvested at day 7. To analyze perfused capillaries, 200 μL FITC-conjugated lectin (1 mg/mL) was injected intravenously 30 minutes before harvest. Lectin-positive structures were counted manually in 5 microscopic fields (5×/0.25 objective) using a computer-assisted fluorescence microscope (Axiovert 100 M equipped with AxioCam camera; Carl Zeiss). Images were taken with an Axio Observer Z1 (Axio Vision Rel 4.8; Carl Zeiss) using the LD Plan-NeoFluar 20×/0.4 Corr Ph2 objective.
Zebrafish lines, antibodies, and reagents
Embryos of AB wild-type and the tg(fli1:EGFP) line29 were raised and staged as described.30 Embryos were kept in E3 solution at 28.5°C with or without 0.003% 1-phenyl-2-thiourea (Sigma-Aldrich) to suppress pigmentation and staged according to somite number or hours postfertilization (hpf).31 We used the following antibodies for this study: rabbit anti–green fluorescent protein (GFP, A-11122; Invitrogen) and HRP-conjugated antibodies (Dako North America).
Morpholino injection.
Morpholinos were diluted in 0.1M KCl. A total of 12 ng of microRNA morpholinos was injected through the chorion of 1-cell or 2-cell stage. An equal amount of standard control morpholino was used as control. The following antisense morpholinos (Gene Tools) were used: dre-mir-27a, 5′-GAGCGGAACTTAGCCACTGTGAACA-3′; dre-mir-27b, 5′- GATGCAGAACTTAGCCACTGTGAAC-3′; and standard Control-Mo (Co-Mo), 5′-CCTCTTACCTCAGTTACAATTTATA-3′.
RT-PCR.
Total RNA was isolated from zebrafish embryos using the miRNeasy Mini-Kit (QIAGEN) for microRNAs following the manufacturer's protocol and TaqMan MicroRNA Assays (Applied Biosystems) as described in “MicroRNA expression analysis.”
Analysis and quantification.
For each morpholino injection, the first 17 intersegmental vessels (ISVs) from the anterior part of 112 to 152 embryos (48 hpf) were analyzed for impaired ISV. For quantification of the dorsal longitudinal anastomotic vessel (DLAV), DLAV segments between 17 ISVs from the anterior part of 112 to 152 embryos (48 hpf) were counted. The parachordal vessel (PAV) was quantified in 112 to 152 embryos (48 hpf) regarding its partial or complete absence. Intracranial bleedings were quantified in 26 to 90 embryos at 48 hpf and 72 hpf.
Whole mount antibody staining
For whole mount antibody stainings tg(fli1:EGFP), embryos were processed as previously described.32
Repulsion assay
Untreated HUVECs were seeded in fibronectin-coated 24-well plates (4 × 104 cells/well). The following day, 1.5 × 103 HUVECs, which were previously transduced with GFP lentivirus and subsequently transfected with SEMA6A siRNA, miR-27a/b inhibitor (10nM), and the respective controls as indicated using Lipofectamine RNAiMax, were seeded on top of untreated endothelial cells. After 3 hours of attachment, cells were analyzed by video time lapse microscopy (Axio Observer.Z1, Carl Zeiss; Axiovision Version 4.7 software; Carl Zeiss Imaging Solutions) over 12.5 to 19 hours. The cell-free area was analyzed with the AxioVision Release Version 4.6.3 SP1 software (Carl Zeiss).
Repulsion assay
Untreated HUVECs were seeded in fibronetin-coated 24-well plates (4 × 104 cells/well). The following day, 1.5 × 103 HUVECs that were previously transduced with GFP lentivirus and subsequently transfected with SEMA6A siRNA, miR-27a/b-inhibitor (10nM) and the respective controls as indicated using Lipofectamine RNAiMax, were seeded on top of untreated endothelial cells. After 3 hours of attachment, cells were analyzed by video time lapse microscopy (Axio Observer.Z1, Carl Zeiss; Axiovision 4.7 software, Carl Zeiss Imaging Solutions GmbH) using a Plan-Apochromat objective lens (10×/0.45, Ph1; Carl Zeiss) and a AxioCam camera (MRm, Carl Zeiss) over 12.5 hours. The cell free area was analyzed with the AxioVision Release 4.6.3 SP1 software (Carl Zeiss).
Statistical analysis
Data are expressed as mean ± SEM. Two treatment groups were compared by Mann-Whitney U test, unpaired or paired Student t test as indicated (SPSS). Multiple group comparisons were done by ANOVA (posthoc analysis). Fisher exact test was used to compare the intracranial bleeding (see Figure 4D). Results were considered statistically significant when P was < .05.
Results
miR-27a/b regulates angiogenesis in vitro and in mice in vivo
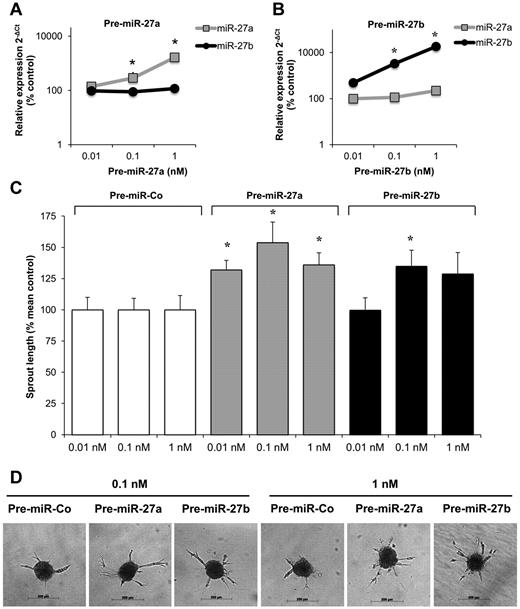
To study the effect of miR-27 on angiogenesis in vitro, precursor molecules were transfected into endothelial cells to overexpress the miR of interest. Transfection of HUVECs with precursors of miR-27a and miR-27b significantly increased the expression of the mature miR-27a and miR-27b, respectively (Figure 1A-B). Overexpression of miR-27a and miR-27b significantly increased endothelial cell sprouting in a 3-dimensional spheroid model (Figure 1C-D) and modestly augmented vascular endothelial growth factor (VEGF)–induced migration (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Although the overexpression of miR-27a was less efficient, the overall sprout length was higher at low concentrations (0.01nM) in miR-27a compared with miR-27b–overexpressing cells (Figure 1C), whereas miR-27b also increased the number of sprouts per spheroid (supplemental Figure 1B). In addition, miR-27a and miR-27b increased proliferation of endothelial cells (supplemental Figure 1C).
Effect of miR-27a/b overexpression on endothelial cell sprouting. (A-B) HUVECs were transfected with miR-27a precursor (pre-miR-27a, A) and miR-27b precursor (pre-miR-27b, B) as indicated. Expression of mature miR-27a/b was detected by quantitative RT-PCR compared with control-precursor (pre-miR-Co) transfected control cells after 24 hours. Data were normalized to RNU48; n = 3 to 7. *P < .05 versus pre-Co. (C-D) Effect of pre-miR-27a/b on endothelial cell sprout formation in the spheroid assay (n = 10 spheroids/experiment; n = 5-13 experiments). *P < .05 versus pre-miR-Co. Representative images of spheroids are shown in panel D.
Effect of miR-27a/b overexpression on endothelial cell sprouting. (A-B) HUVECs were transfected with miR-27a precursor (pre-miR-27a, A) and miR-27b precursor (pre-miR-27b, B) as indicated. Expression of mature miR-27a/b was detected by quantitative RT-PCR compared with control-precursor (pre-miR-Co) transfected control cells after 24 hours. Data were normalized to RNU48; n = 3 to 7. *P < .05 versus pre-Co. (C-D) Effect of pre-miR-27a/b on endothelial cell sprout formation in the spheroid assay (n = 10 spheroids/experiment; n = 5-13 experiments). *P < .05 versus pre-miR-Co. Representative images of spheroids are shown in panel D.
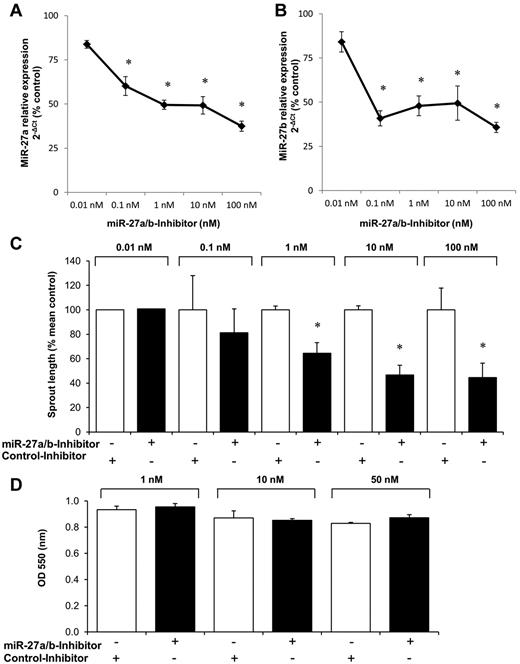
To determine whether inhibition of the endogenously expressed miR-27 reduces angiogenic sprouting, we inhibited miR-27b using a hairpin inhibitor. Inhibition of miR-27b significantly decreased the expression of miR-27b (Figure 2A-B). However, the inhibitor against miR-27b also reduced the expression of the related miR-27a (Figure 2A-B), indicating that the large overlap of the sequences (miR-27a and miR-27b differ only in 1 nucleotide) precludes specific inhibition of one of the miRNAs. Therefore, this miR inhibitor is referred to as miR-27a/b inhibitor from here on. Inhibition of miR-27a/b significantly blocked angiogenic sprouting (Figure 2C) but did not affect viability of endothelial cells (Figure 2D).
Effect of miR-27a/b inhibition on endothelial cell sprouting. HUVECs were transfected with miR inhibitors as indicated. (A-B) Expression of mature miR-27a (A) and miR-27b (B) was detected by quantitative RT-PCR compared with control-precursor (pre-miR-Co) transfected control cells after 24 hours. Data were normalized to RNU48. *P < .05 (paired t test). n = 3 to 8. (C) Effect of miR-27a/b inhibition on endothelial cell sprout formation in the spheroid assay (n = 10 spheroids/experiment, n = 1 for 0.01nM, and n = 3-8 experiments for 0.1-100nM). *P < .05 versus respective control inhibitors. (D) Effect of miR-27a/b inhibition on endothelial cell viability (MTT assay; n = 3).
Effect of miR-27a/b inhibition on endothelial cell sprouting. HUVECs were transfected with miR inhibitors as indicated. (A-B) Expression of mature miR-27a (A) and miR-27b (B) was detected by quantitative RT-PCR compared with control-precursor (pre-miR-Co) transfected control cells after 24 hours. Data were normalized to RNU48. *P < .05 (paired t test). n = 3 to 8. (C) Effect of miR-27a/b inhibition on endothelial cell sprout formation in the spheroid assay (n = 10 spheroids/experiment, n = 1 for 0.01nM, and n = 3-8 experiments for 0.1-100nM). *P < .05 versus respective control inhibitors. (D) Effect of miR-27a/b inhibition on endothelial cell viability (MTT assay; n = 3).
To assess the in vivo relevance of these findings, miR-27a/b was inhibited by antagomirs,28 and neovascularization was determined in implanted Matrigel plugs. Therefore, we injected antagomirs directed against miR-27b or control antagomir (12 mg/kg body weight each) at days 0, 2, and 4 after subcutaneous injection of Matrigel plugs (Figure 3A). Consistent with the in vitro experiments, the antagomir blocked both miR-27a and miR-27b (Figure 3B). Antagomir-27a/b decreased the number of perfused vessels that invaded the implanted Matrigel plug in vivo (Figure 3C-D). In summary, these data indicate that overexpression of miR-27a/b enhances angiogenesis in vitro, whereas inhibition of miR-27a/b reduces angiogenesis in vitro and in vivo.
Inhibition of miR-27a/b impairs angiogenesis in mice. (A-B) Effect of systemic infusion of 3 intravenous injections (each 12 mg/kg body weight) at day 0, day 2, and day 4 of antagomirs targeting miR-27a and miR-27b (AM-27a/b) or a control antagomir (AM-Co; n = 4-8 mice per group, 2 plugs/mouse) on miR expression in hearts, aorta, muscles, or Matrigel plugs harvested 7 days after the first injection. (C-D) Effect of antagomir infusion on the number of lectin-perfused vessels in Matrigel plugs in vivo after 7 days (n = 8 mice per group, 2 plugs/mouse: n = 15 plugs [AM-Co] and n = 16 plugs [AM-27a/b]). *P < .05 versus AM-Co. Representative images are shown in panel C.
Inhibition of miR-27a/b impairs angiogenesis in mice. (A-B) Effect of systemic infusion of 3 intravenous injections (each 12 mg/kg body weight) at day 0, day 2, and day 4 of antagomirs targeting miR-27a and miR-27b (AM-27a/b) or a control antagomir (AM-Co; n = 4-8 mice per group, 2 plugs/mouse) on miR expression in hearts, aorta, muscles, or Matrigel plugs harvested 7 days after the first injection. (C-D) Effect of antagomir infusion on the number of lectin-perfused vessels in Matrigel plugs in vivo after 7 days (n = 8 mice per group, 2 plugs/mouse: n = 15 plugs [AM-Co] and n = 16 plugs [AM-27a/b]). *P < .05 versus AM-Co. Representative images are shown in panel C.
miR-27a/b regulate embryonic vessel formation in zebrafish
To confirm our findings, we determined the effect of morpholinos against miR-27a and miR-27b on the formation of the ISV, DLAV, and the PAV in the tail region of zebrafish embryos at 48 hpf. The morpholinos against miR-27a and miR-27b decreased the expression of both miR-27a and miR-27b (data not shown), and both morpholinos significantly increased the number of defects per animal for the ISV (Figure 4A,C), DLAV (Figure 4A,C), and the PAV (Figure 4B-C). Furthermore, repression of miR-27a/b induced intracranial bleeding (Figure 4D-E).
miR-27a/b regulate embryonic vessel formation in zebrafish. (A-C) miR-27a and miR-27b morpholinos were injected into zebrafish at 1-cell or 2-cell stage. The 48-hpf vessel phenotype was quantified for ISV and DLAV (A), and PAV (B). n = 112 to 152. (A) Data are number of defects per animal. (B) Quantification of PAV phenotype: 0 indicates absent; 1, partially absent; and 2, present. (C) Representative images of panels A and B. Vasculature of tg(fli1:EGFP) zebrafish embryos were stained by antibody against GFP (48 hpf). Black arrows indicate ISV or DLAV defects. *PAV defects. (D-E) miR-27a and miR-27b morpholinos were injected into zebrafish, and intracranial bleeding was monitored at 48 hpf and at 72 hpf. (D) Quantification of intracranial bleedings (n = 26-90). (E) Representative images of intracranial bleedings (72 hpf). Black arrows indicate hemorrhages.
miR-27a/b regulate embryonic vessel formation in zebrafish. (A-C) miR-27a and miR-27b morpholinos were injected into zebrafish at 1-cell or 2-cell stage. The 48-hpf vessel phenotype was quantified for ISV and DLAV (A), and PAV (B). n = 112 to 152. (A) Data are number of defects per animal. (B) Quantification of PAV phenotype: 0 indicates absent; 1, partially absent; and 2, present. (C) Representative images of panels A and B. Vasculature of tg(fli1:EGFP) zebrafish embryos were stained by antibody against GFP (48 hpf). Black arrows indicate ISV or DLAV defects. *PAV defects. (D-E) miR-27a and miR-27b morpholinos were injected into zebrafish, and intracranial bleeding was monitored at 48 hpf and at 72 hpf. (D) Quantification of intracranial bleedings (n = 26-90). (E) Representative images of intracranial bleedings (72 hpf). Black arrows indicate hemorrhages.
miR-27 targets SEMA6A in endothelial cells
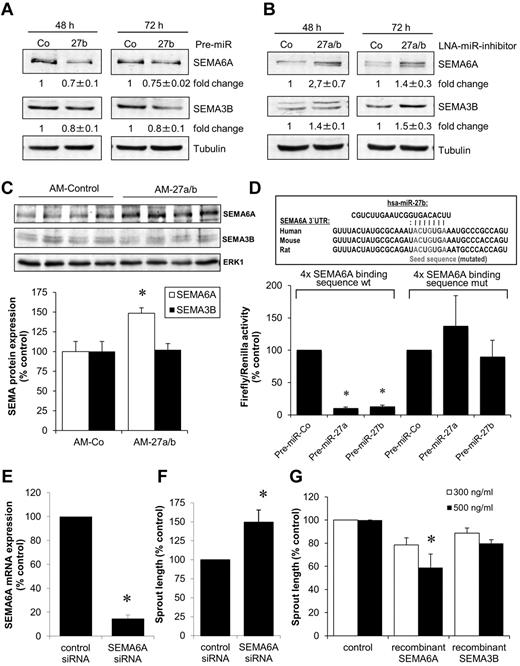
To determine the targets of miR-27, we first explored the regulation of known miR-27 targets in antagomir-27a/b–treated mice. Whereas sprouty2 and VEGF-C protein expression was significantly increased after antagomir-27a/b treatment, PPAR-γ was only modestly increased and MEF2C was not regulated (supplemental Figure 2). In addition, in silico prediction (PicTar and TargetScan Version 5.1) revealed that members of the semaphorin (SEMA) family, in particular SEMA6A and SEMA3B, are predicted targets of miR-27. Because SEMA6A and SEMA3B are described as inhibitors of angiogenesis,33,34 we further explored their function downstream of miR-27. As shown by Western blot analysis, overexpression of miR-27b decreased the protein expression of SEMA6A and SEMA3B (Figure 5A), whereas inhibition of miR-27a/b increased SEMA6A and SEMA3B protein expression (Figure 5B). In contrast to the regulation of SEMA6A protein expression, miR-27 did not significantly regulate the amount of SEMA6A mRNA (supplemental Figure 3A-B), a finding that is consistent with the ability of miRs to block protein translation without affecting mRNA levels. In vivo application of antagomir-27a/b significantly increased the expression of SEMA6A, but not SEMA3B, in mouse hearts (Figure 5C). These data indicate that SEMA6A is a potential target of miR-27 in endothelial cells. To confirm a direct regulation of SEMA6A by miR-27, we performed luciferase assays in which the miR-27a/b target sequence in the SEMA6A 3′-UTR was cloned in the luciferase 3′-UTR. Overexpression of miR-27a and miR-27b significantly reduced luciferase activity but exhibited no effect on a mutated construct (Figure 5D), demonstrating that miR-27a and miR-27b directly target the SEMA6A 3′-UTR.
miR-27 regulates the expression of semaphorin 6A and 3B. (A) Expression of SEMA6A and SEMA3B in HUVECs after transfection with miR-27 precursor (pre-miR-27b, 1nM) after 48 and 72 hours. Representative Western blots are shown. Tubulin serves as loading control. Blots were scanned, and protein expression was quantified by densitometric analysis. The ratio of SEMA6A/tubulin or SEMA3B/tubulin is shown as fold change ± SEM; n = 3. (B) Expression of SEMA6A and SEMA3B in HUVECs after transfection with LNA-miR-27 inhibitor (0.1nM) after 48 and 72 hours. Representative Western blots are shown. Tubulin serves as loading control. The ratio of SEMA6A/tubulin or SEMA3B/tubulin is shown as fold change ± SEM; n = 3. (C) Expression of SEMA6A and SEMA3B in hearts of mice treated with antagomir-27 or antagomir-control (see Figure 3 for details). Representative Western blots are shown. ERK1 serves as loading control. The ratio of SEMA6A/ERK1 or SEMA3B/ERK1 is shown as percentage of control; n = 4. P < .05. (D) Luciferase normalized to Renilla activity was measured in homogenates of HEK293FT cells transfected with luciferase constructs containing the wild-type (wt) or mutated (mut) seed sequences of miR-27 together with pre-miR-27a, pre-miR-27b, or control pre-miR. Measurements were done 48 hours after transfection; n = 6 or 7. (E-F) HUVECs were transfected with siRNA (67nM) targeting SEMA6A. A siRNA directed against firefly luciferase was used as a control. (E) Expression of SEMA6A was measured by quantitative RT-PCR after 24 hours; n = 3. *P < .05. (F) Effect of SEMA6A silencing on endothelial cell sprouting; n = 4. P < .05 (paired t test). (G) HUVECs were incubated with 300 or 500 ng/mL human recombinant SEMA6A or SEMA3B protein, and endothelial cell sprout formation was measured; n = 3 or 4. *P < .05 versus control.
miR-27 regulates the expression of semaphorin 6A and 3B. (A) Expression of SEMA6A and SEMA3B in HUVECs after transfection with miR-27 precursor (pre-miR-27b, 1nM) after 48 and 72 hours. Representative Western blots are shown. Tubulin serves as loading control. Blots were scanned, and protein expression was quantified by densitometric analysis. The ratio of SEMA6A/tubulin or SEMA3B/tubulin is shown as fold change ± SEM; n = 3. (B) Expression of SEMA6A and SEMA3B in HUVECs after transfection with LNA-miR-27 inhibitor (0.1nM) after 48 and 72 hours. Representative Western blots are shown. Tubulin serves as loading control. The ratio of SEMA6A/tubulin or SEMA3B/tubulin is shown as fold change ± SEM; n = 3. (C) Expression of SEMA6A and SEMA3B in hearts of mice treated with antagomir-27 or antagomir-control (see Figure 3 for details). Representative Western blots are shown. ERK1 serves as loading control. The ratio of SEMA6A/ERK1 or SEMA3B/ERK1 is shown as percentage of control; n = 4. P < .05. (D) Luciferase normalized to Renilla activity was measured in homogenates of HEK293FT cells transfected with luciferase constructs containing the wild-type (wt) or mutated (mut) seed sequences of miR-27 together with pre-miR-27a, pre-miR-27b, or control pre-miR. Measurements were done 48 hours after transfection; n = 6 or 7. (E-F) HUVECs were transfected with siRNA (67nM) targeting SEMA6A. A siRNA directed against firefly luciferase was used as a control. (E) Expression of SEMA6A was measured by quantitative RT-PCR after 24 hours; n = 3. *P < .05. (F) Effect of SEMA6A silencing on endothelial cell sprouting; n = 4. P < .05 (paired t test). (G) HUVECs were incubated with 300 or 500 ng/mL human recombinant SEMA6A or SEMA3B protein, and endothelial cell sprout formation was measured; n = 3 or 4. *P < .05 versus control.
SEMA6A inhibits endothelial cell sprouting
Because semaphorins were shown to mediate cell repulsion, we determined their function in endothelial cells. Silencing of SEMA6A with siRNA increased endothelial cell sprouting (Figure 5E-F), whereas incubation of endothelial cells with recombinant SEMA6A protein significantly decreased endothelial cell sprouting (Figure 5G), indicating that SEMA6A exerts anti–angiogenic activity. Recombinant SEMA3B also slightly, but not significantly, reduced endothelial cell sprouting (Figure 5G).
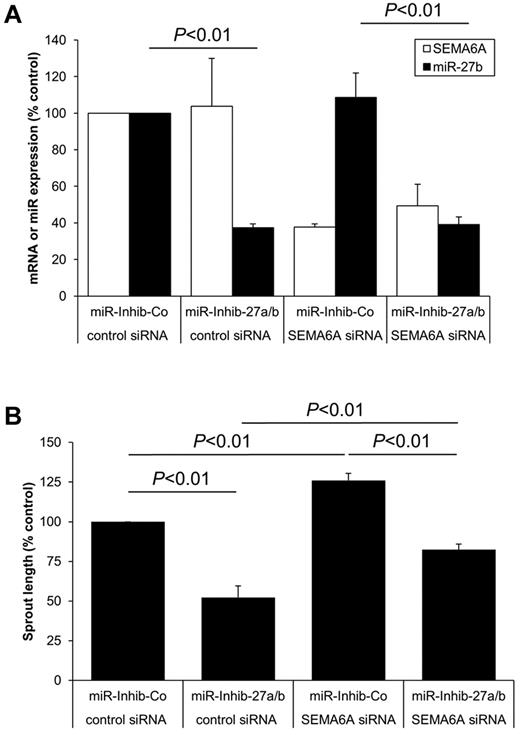
To test whether SEMA6A mediates the anti–angiogenic effect induced by inhibition of miR-27a/b, we simultaneously transfected endothelial cells with siRNA against SEMA6A and the miR-27a/b inhibitor (Figure 6A). Indeed, silencing of SEMA6A significantly reversed the anti–angiogenic effect mediated by the miR-27a/b inhibitor (Figure 6B), indicating that SEMA6A is one of the functionally relevant miR-27 downstream targets in endothelial cells.
SEMA6A contributes to the function of miR-27 in endothelial cells. HUVECs were transfected with SEMA6A siRNA (67nM), miR-27a/b inhibitor (10nM), and the respective controls as indicated. (A) Expression of SEMA6A or miR-27b was measured by quantitative RT-PCR after 24 hours; n = 4. (B) Spheroids were generated and sprouting was quantified; n = 4.
SEMA6A contributes to the function of miR-27 in endothelial cells. HUVECs were transfected with SEMA6A siRNA (67nM), miR-27a/b inhibitor (10nM), and the respective controls as indicated. (A) Expression of SEMA6A or miR-27b was measured by quantitative RT-PCR after 24 hours; n = 4. (B) Spheroids were generated and sprouting was quantified; n = 4.
Semaphorins either act as membrane anchored proteins or as secreted molecules. Therefore, we analyzed whether conditioned medium of miR-27a/b inhibitor-transfected endothelial cells affects endothelial cell sprouting. However, the conditioned medium did not reduce endothelial cell sprouting (supplemental Figure 3C), suggesting that the increased levels of SEMA6A after miR-27a/b inhibition control angiogenesis predominantly in its membrane-associated form.
miR-27a/b regulate endothelial cell repulsion
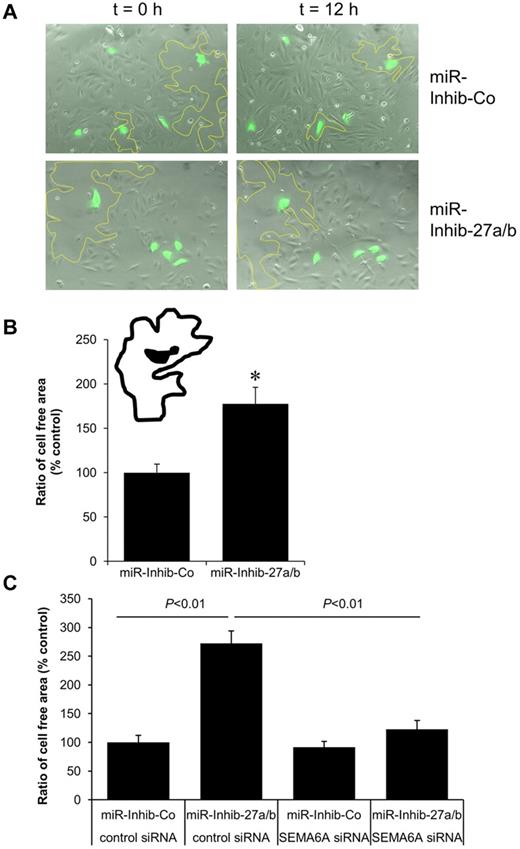
Semaphorins are known to regulate direct cell-to-cell communication and can induce repulsive signals. Therefore, we studied the role of miR-27a/b in endothelial cell repulsion using a previously described repulsion assay.35 For this assay, endothelial cells were transduced with lentivirus for stable GFP expression, subsequently transfected with the miR-27a/b inhibitor, mixed with untransfected endothelial cells, and analyzed by video time lapse microscopy. Tracking of single GFP-positive cells revealed that the cell-free areas surrounding miR inhibitor-27a/b-transfected GFP-positive endothelial cells were substantially larger than those surrounding miR inhibitor-control–transfected GFP-positive endothelial cells (Figure 7A-B; supplemental Videos 1-2). To test whether SEMA6A causally contributes to the repulsive effect of miR-27 inhibition, we transfected endothelial cells with siRNA against SEMA6A and the miR-27a/b inhibitor simultaneously. Silencing of SEMA6A significantly decreased repulsion mediated by the miR-27a/b inhibitor (Figure 7C), indicating that the miR-27a/b inhibitor-mediated increase of SEMA6A repels endothelial cells.
Inhibition of miR-27a/b increases the repulsive activity of endothelial cells. HUVECs were transduced with GFP lentivirus before transfection with the miR-27a/b inhibitor (10nM) or miR inhibitor-control (10nM). The following day, 1.5 × 103 transfected endothelial cells (green) were seeded on top of untreated endothelial cells (70% confluency). Cells were analyzed by video time lapse microscopy over 12.5 hours. (A) Representative images at time points 0 and 12 hours are shown. The cell-free areas surrounding single miR inhibitor/GFP-transfected endothelial cells are marked by a yellow line. (B) The cell-free areas surrounding single miR inhibitor/GFP-transfected endothelial cells were analyzed, and the ratio of the cell-free area at t = 12.5 hours and t = 0 hours is given as percentage of control. *P < .001. n = 32 cells and n = 3 experiments. (C) HUVECs were transfected with SEMA6A siRNA, miR-27a/b inhibitor, and the respective controls as indicated. Cells were tracked over 12.5 hours, and the ratio of the cell-free area is given as percentage of control. P < .01. n = 30 to 45 cells and n = 3 experiments.
Inhibition of miR-27a/b increases the repulsive activity of endothelial cells. HUVECs were transduced with GFP lentivirus before transfection with the miR-27a/b inhibitor (10nM) or miR inhibitor-control (10nM). The following day, 1.5 × 103 transfected endothelial cells (green) were seeded on top of untreated endothelial cells (70% confluency). Cells were analyzed by video time lapse microscopy over 12.5 hours. (A) Representative images at time points 0 and 12 hours are shown. The cell-free areas surrounding single miR inhibitor/GFP-transfected endothelial cells are marked by a yellow line. (B) The cell-free areas surrounding single miR inhibitor/GFP-transfected endothelial cells were analyzed, and the ratio of the cell-free area at t = 12.5 hours and t = 0 hours is given as percentage of control. *P < .001. n = 32 cells and n = 3 experiments. (C) HUVECs were transfected with SEMA6A siRNA, miR-27a/b inhibitor, and the respective controls as indicated. Cells were tracked over 12.5 hours, and the ratio of the cell-free area is given as percentage of control. P < .01. n = 30 to 45 cells and n = 3 experiments.
Discussion
The present study demonstrates that miR-27a and miR-27b are positive regulators of angiogenesis. Inhibition of miR-27a and miR-27b reduced endothelial cell sprouting in vitro and vascularization of implanted Matrigel plugs as well as vessel formation in zebrafish in vivo. These data are in accordance with the study by Zhou et al,21 which was published during preparation of this manuscript, demonstrating that the miR-23∼27∼24 cluster regulates angiogenesis. Besides demonstrating a pro-angiogenic activity of both miR-27 family members, we further showed that their inhibition regulates the repulsion of endothelial cells. Both attraction as well as repulsion are essential elements in the establishment of a functional vascular network; however, the control of these processes in endothelial cell is poorly understood. To our knowledge, this is the first study demonstrating the control of endothelial cell repulsion by a miR.
miR-27 targets the semaphorins SEMA6A and SEMA3B in vitro, whereas in our in vivo study SEMA6A, but not SEMA3B, was regulated. The semaphorin family consists of more than 20 genes encoding either for secreted or membrane-anchored ligands characterized by the presence of an amino-terminal sema domain that is essential for downstream signaling.36 Semaphorins were originally described as ligands mediating axon guidance during the development of the central nervous system. Meanwhile, it has been demonstrated that semaphorins and their receptors, the neuropilins and plexins, are also expressed in many other cell types, such as cancer cells and endothelial cells. Moreover, the various semaphorins (eg, SEMA3A, SEMA3E, SEMA3F, and SEMA4D) have been shown to either promote or inhibit tumor angiogenesis and progression.37-41 Little is known about the function of SEMA6A in angiogenesis and in endothelial cell biology. A recent report demonstrated that the SEMA6A-1 transcript is expressed in cancer cells and to a lesser extent in endothelial cells, and administration of recombinant SEMA6A-1 soluble extracellular domain blocked both growth factor- and tumor-induced angiogenesis.33 Our data confirmed that SEMA6A is indeed a repressor of sprouting angiogenesis. Interestingly, the addition of soluble recombinant SEMA6A repressed sprouting, but the supernatant of miR-27a/b inhibitor-treated endothelial cells did not. These data suggest that the inhibition of sprouting after miR-27a/b inhibition is predominantly caused by the up-regulation of the membrane bound form of SEMA6A. The receptors of the membrane-anchored SEMA6A Plexin A2 and A442 are expressed in endothelial cells43,44 (supplemental Figure 4), and the transmembrane form of SEMA6A was shown to repel embryonic neurons.45 Therefore, it is tempting to speculate that the transmembrane form of SEMA6A is increased on miR-27a/b inhibition and that this induces the repulsion of neighboring endothelial cells that express the SEMA6A receptors. Indeed, similar principles have been described for axon guidance molecules that control wiring neural and vascular networks, such as Plexin D1 and others.46,47 The function of SEMA3B is less clear. Although miR-27 represses SEMA3B in vitro, no change in protein expression was detected in antagomir-27a/b–treated mice, and SEMA3B only modestly affected endothelial cell sprouting. Therefore, it is likely that the effects of miR-27 in endothelial cells are predominantly mediated via SEMA6A repression. Indeed, inhibition of SEMA6A up-regulation partially reversed the effect of miR-27 on sprouting angiogenesis and fully blocked the effects of miR-27 on endothelial repulsion. Because the rescue of sprouting is not complete, additional targets may be involved in the complex regulation of vessel formation induced by miR-27.
Indeed, a recent study, which was published during revision of our manuscript, demonstrates that miR-23 and miR-27 not only target the protein SEMA6A, thus confirming our data, but additionally target the anti–angiogenic protein Sprouty2.21 In accordance, we found that Sprouty2 is up-regulated in mice treated with antagomir-27, indicating that SEMA6A and Sprouty2 are both targets of miR-27 in endothelial cells. Previous studies have additionally shown that miR-27b targets “suppression of tumorigenicity 14” (ST14), a transmembrane serine protease involved in extracellular matrix degradation that regulates cell proliferation, migration, and invasion in breast cancer cells.48 However, silencing of ST14 reduced sprouting of endothelial cells (supplemental Figure 5A-B), suggesting that it is a pro-angiogenic factor and rather not responsible for the biologic effects of miR-27 in endothelial cells. The nuclear receptor PPAR-γ is another reported target of miR-27 that controls adipocyte differentiation.24 Indeed, endothelial cells express PPAR-γ (albeit at low level) and silencing of PPAR-γ augmented endothelial cell sprouting (supplemental Figure 5C-D). In addition, the finding that shear stress decreases the expression of PPAR-γ in endothelial cells49 coincides with our data showing that miR-27a/b is increased in response to shear stress in endothelial cells (supplemental Figure 7). However, antagomir-27a/b only modestly and not significantly increased the expression of PPAR-γ in vivo. Thus, additional studies are required to determine whether this slight derepression of PPAR-γ by miR-27 inhibition contributes to the pro-angiogenic effects of the miR. Other potential targets contributing to the pro-angiogenic effect of miR-27 might be the lymphangiogenic growth factor VEGF-C, which is an in silico predicted target of miR-27, or the transcription factor MEF2C, which is targeted by miR-27 in cardiomyocytes25 and regulates vascular integrity and angiogenesis.50 Whereas VEGF-C was significantly up-regulated in antagomir-27a/b–treated mice, the expression of MEF2C was not regulated.
Taken together, our data indicate that repression of SEMA6A contributes to the pro-angiogenic effect of miR-27a/b in endothelial cells. To determine whether the regulation of other targets, such as Sprouty2 or VEGF-C, or a fine-tuned regulation of pro- and anti–angiogenic factors mediates the effects of miR-27a/b in endothelial cells in vivo needs further investigation.
The data of the present study demonstrate that pharmacologic modulation of miR-27 controls angiogenesis and endothelial cell repulsion in part via repressing SEMA6A. However, the physiologic regulation of miR-27 is unknown. In preliminary studies, we tested several stimuli that are known to control endothelial cell functions. Whereas the induction of hypoxia by exposure of the cells to an oxygen-reduced environment or the hypoxia-mimetic agent deferoxamine mesylate and VEGF did not significantly affect miR-27 levels (supplemental Figure 6), mechanical activation of endothelial cells by laminar shear stress increased miR-27a/b expression (supplemental Figure 7). Because endothelial cells are exposed to shear stress, particularly in the arterial wall where endothelial cells are expected to provide a tight barrier, one may speculate that the increase of miR-27 expression by shear stress may repress repulsive signals and thus help to maintain a continuous cell layer.
In conclusion, the findings of the present study provide novel insights into the complex posttranscriptional regulation of angiogenesis and endothelial cell communication. The manipulation of miR-27 may offer opportunities for the therapeutic modulation of angiogenesis in tumors or ischemic diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nicole Konecny, Ariane Fischer, Tino Röxe, and M. Muhly-Reinholz for expert technical assistance.
This work was supported by the European Research Council (Advanced grant “Angiomir”), the Deutsche Forschungsgemeinschaft (Excellence Cluster Cardiopulmonary System Exc 147-1, S.D.), the SFB 834 project B2 (C.U.), LOEWE program Lipid signaling (fellowship, C.D.), the SFB/TR23 project Z5 (J.K.), and Exc 147-1 and SFB 834 project A4 (I.F.).
Authorship
Contribution: D.K., T.F., A.K., R.A.B., A.B., C.D., J.-N.B., and E.H. performed and analyzed experiments; K.B. and J.K. performed the zebrafish study; C.U., S.D., I.F., A.M.Z., and J.K. designed experiments; and C.U., D.K., and S.D. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefanie Dimmeler, Institute for Cardiovascular Regeneration, Centre of Molecular Medicine, Goethe University, Theodor Stern-Kai 7, 60590 Frankfurt, Germany; e-mail: dimmeler@em.uni-frankfurt.de.
References
Author notes
C.U. and D.K. contributed equally to this study.

![Figure 3. Inhibition of miR-27a/b impairs angiogenesis in mice. (A-B) Effect of systemic infusion of 3 intravenous injections (each 12 mg/kg body weight) at day 0, day 2, and day 4 of antagomirs targeting miR-27a and miR-27b (AM-27a/b) or a control antagomir (AM-Co; n = 4-8 mice per group, 2 plugs/mouse) on miR expression in hearts, aorta, muscles, or Matrigel plugs harvested 7 days after the first injection. (C-D) Effect of antagomir infusion on the number of lectin-perfused vessels in Matrigel plugs in vivo after 7 days (n = 8 mice per group, 2 plugs/mouse: n = 15 plugs [AM-Co] and n = 16 plugs [AM-27a/b]). *P < .05 versus AM-Co. Representative images are shown in panel C.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/6/10.1182_blood-2011-08-373886/5/m_zh89991285880003.jpeg?Expires=1767702297&Signature=IJiYVgjpxHxiEdL9WfP8cuTZaMxxWr420NXbOLQkc20V5BSwSNHk7BDReifBQUSqxyMpTpM8GS~IUZFbF7-r6oVGMjzKDDrr3el6DthSqn123FYc2xy2iJkLBnTj9fr4UYbHferAiCB3vZ9Hl5564zhhr1-COSWOnCWs9RGmLb-yhFgt~YtaZQGvhd1YsOohOut1SxoyViVQUQowBk2OqeazoSfxCK1SCB0ECf3PoDNEpzup7OG~zHllwqx~EPZFwUmapTKk9eLszVnCC~C2pxrvGYGGpE-vjU3JrgiCTl1lKs4yMTk4DE2ZxFljWM5EjsaP7xSLVP60-UlDuIzAiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal