Abstract
The bone morphogenetic protein (BMP) signaling pathway regulates survival, proliferation, and differentiation of several cell types in multiple tissues, including the thymus. Previous reports have shown that BMP signaling negatively regulates T-cell development. Here, we study the subpopulation of early human intrathymic progenitors expressing the type IA BMP receptor (BMPRIA) and provide evidence that CD34+CD1a−BMPRIA+ precursor cells mostly express surface cell markers and transcription factors typically associated with NK cell lineage. These CD34+ cells mostly differentiate into functional CD56+ natural killer (NK) cells when they are cocultured with thymic stromal cells in chimeric human-mouse fetal thymic organ cultures and also in the presence of SCF and IL-15. Moreover, autocrine BMP signaling can promote the differentiation of thymic NK cells by regulating the expression of key transcription factors required for NK cell lineage (eg, Id3 and Nfil3) as well as one of the components of IL-15 receptor, CD122. Subsequently, the resulting population of IL-15–responsive NK cell precursors can be expanded by IL-15, whose action is mediated by BMP signaling during the last steps of thymic NK cell differentiation. Our results strongly suggest that BMPRIA expression identifies human thymic NK cell precursors and that BMP signaling is relevant for NK cell differentiation in the human thymus.
Introduction
Natural killer (NK) cells are CD3−CD56+ large granular lymphocytes that function within the innate immune system to provide protection against infection and cancer, and they also can produce cytokines and chemokines to influence the adaptive immune response.1 NK cells develop from CD34+ hematopoietic precursor cells, and it is generally accepted that bone marrow is the main site of NK cell development in adult humans.1,2 However, several reports have shown that hematopoietic precursors and developing NK cells also can be found in human adult intestine,3 uterus,4,5 liver,6 and secondary lymphoid tissues, such as lymph nodes and tonsils.7 The thymus also is considered a site of NK cell development because mature NK cells and multipotent as well as bipotent T/NK and NK/dendritic cell (DC) precursors can be isolated from human postnatal thymus, but a complete pathway of NK cell differentiation at this site has not been defined in humans.8-11 By contrast, it has been recently demonstrated in mice the existence of a thymic pathway of NK cell development characterized by expression of CD127 and GATA-3.12 Thymic NK cell development also has been reported to rely on the expression of members of the inhibitor of differentiation/DNA binding (Id) protein family, such as Id2 and Id3, the down-regulation of Notch signaling and the balance between Nfil3/E4bp4 and Bcl11b transcription factors.13-16
The bone morphogenetic protein (BMP) family includes secreted signaling proteins that bind to a heteromeric receptor complex commonly constituted by the following type I and type II serine-threonine kinase receptors: type IA BMP receptor (BMPRIA)/ALK-3, type IB BMP receptor (BMPRIB)/ALK-6, type I Activin receptor (ActRIA)/ALK-2, and type II BMP receptor (BMPRII).17-19 On BMP binding, type II receptors phosphorylate type I receptors that then activate the BMP receptor-regulated Smads (BR-Smads) by phosphorylation. Subsequently, BR-Smads bind to the common partner Smad4, and the complex translocates into the nucleus where it regulates the transcription of BMP target genes, including Id proteins and Runx transcription factors.18,20,21
It is now known that BMPs are involved in the development of virtually all organs and the maintenance and renewal of several adult tissues, such as the hematopoietic and lymphoid tissues.22-26 In the thymus, both cortical and medullary epithelial cells express BMP receptors and produce BMP2 and BMP4 ligands.27-29 Thymocyte precursors also produce BMP4 and express the type I and type II BMP receptors as well as the BR-Smads required to initiate the intracellular signaling.27,28,30 The stimulation of BMP signaling pathway by treatment with BMP4 of fetal thymic organ cultures (FTOCs) enhances cell survival; inhibits thymocyte expansion; and, importantly, prevents differentiation along the T-cell lineage.27-31 On the contrary, the neutralization of the action of endogenously produced BMPs by treatment with different BMP inhibitors enhances thymocyte proliferation and promotes the differentiation of CD4+CD8+ thymocytes, suggesting that BMP signaling acts as a negative regulator of T-cell development.26,28,31
Here, we show that the subpopulation of early human intrathymic CD34+CD1a− progenitor cells expressing BMPRIA contains a large proportion of NK cell lineage–committed precursor cells. In addition, we provide evidence that autocrine BMP signaling is relevant for the differentiation of human thymic NK cells.
Methods
Cell isolation from human tissues
Human thymus samples from patients aged 1 month to 5 years undergoing corrective cardiac surgery were obtained and used according to the guidelines of the Medical Ethics Commission of Madrid-Montepríncipe and 12 de Octubre hospitals. Informed consent was provided according to the Declaration of Helsinki. Thymuses were dissected free of surrounding connective tissue and then gently disrupted with a potter homogenizer until completely disaggregated. To isolate thymic CD34+ precursors, thymocyte suspensions were enriched in immature thymocytes by using the sheep red blood cell rosetting technique followed by mAb-coupled magnetic bead treatment (Dynabeads; Invitrogen) to deplete T cells, B cells, NK cells, myeloid cells, and DCs, as described previously.32 These CD34-enriched cell suspensions were next stained with anti-CD34, anti-CD1a, and anti-BMPRIA Abs, and CD34+CD1a−, CD34+CD1a−BMPRIA−, and CD34+CD1a−BMPRIA+ cell subpopulations were sorted on an FACSVantage SE (BD Biosciences) from the Centro de Citometría y Microscopía de Fluorescencia (Universidad Complutense de Madrid).
Lymph nodes were obtained from brain dead tissue donors through the Transplant Coordination Unit, Hospital Clínico San Carlos. Tissue cells were dispersed with a potter homogenizer followed by Ficoll centrifugation. Mononuclear cells were then used for flow cytometry analysis.
Flow cytometry and Abs
The following mAbs conjugated with FITC, PE, Pe-Cy5, PerCP, APC, or Alexa Fluor 647 were used for flow cytometric analysis: CD1a (HI149), CD3 (33-2A3 and UTH-C1), CD4 (RPA-T4), CD5 (UCHT2), CD7 (CD7-6B7), CD8 (RPA-T8), CD33 (WM53), CD34 (8G12 and 581), CD38 (HIT2), CD44 (G44-26), CD45 (2DI), CD45RA (HI100), CD56 (B159 and HCD56), CD94 (DX22), CD116 (MSB12), CD117 (YB5.B8), CD122 (MIK-β1), CD127 (R34.34), CD135 (BV10A4H2), CD161 (DX12), CD215 (JM7A4), CD335/NKp46 (9E2), IL-22 (142928), and RORC (AFKJS-9), from BD Biosciences, BioLegend, Beckman Coulter, Invitrogen, R&D Systems, Immunotools, and eBioscience. The extracellular domain of BMPRIA and BMP4 were detected with antibodies from R&D Systems and Santa Cruz Biotechnology, respectively. Immunofluorescence stainings for cell surface antigens, intracellular stainings, and annexin V stainings were performed as described previously.30 Analyses were conducted in an FACSCalibur flow cytometer (Centro de Citometría y Microscopía de Fluorescencia, Universidad Complutense de Madrid).
Quantitative RT-PCR
Real-time PCR was performed with specific TaqMan assays (Applied Biosystems) as described previously.32 GNB2L1 was used as endogenous control. Amplifications, detections, and analyses were performed in a 7.900HT Fast Real-time PCR System (Centro de Genómica, Universidad Complutense de Madrid). The Delta CT method was used for normalization to GNB2L1 mRNA.
Chimeric human-mouse FTOCs
Thymic lobes derived from 15-day-old embryos of SCID mice were recolonized with human CD34+CD1a−BMPRIA+ or CD34+CD1a−BMPRIA− thymic progenitors (1-2 × 104 cells/lobe) as described previously.27
NK cell differentiation assays
Thymic CD34+ progenitor subpopulations were cultured, as described previously,32 for different times in the presence of recombinant human (rh)SCF (100 ng/mL; ProSpec) and rhIL-15 (1-100 ng/mL; ProSpec). In some experiments rhBMP-4 (50 ng/mL; HumanZyme) or dorsomorphin (5μM; Merck) also were added to the culture medium.
Proliferation and cytotoxicity assays
To analyze cell proliferation, cultures were pulsed for 12 hours with 10μM BrdU. A BrdU Labeling and Detection Kit III (Roche Diagnostics) was used to measure BrdU incorporation into newly synthesized DNA, as described previously.30
Cytotoxicity was measured using the nonradioactive Cytotoxicity Detection Kit LDH (Roche Diagnostics), following the manufacturer's instructions. In brief, effector cells were cultured overnight in the presence of IL-12 (10 ng/mL) and IL-15 (100 ng/mL) and then cocultured with K562 cells in 96-well plates for 4 hours at different effector-to-target ratios. The percentage of specific lysis was determined from the amount of lactate dehydrogenase activity detected in culture supernatants.
BMP4 and cytokine measurements
Cells derived from FTOCs and NK cell differentiation assays were stimulated overnight with IL-12 plus IL-15, and the concentrations of IFN-γ, IL-10, and TNF-α were determined in the supernatants by ELISA (BioLegend). BMP4 levels were measured using an ELISA kit (R&D Systems).
Electron microscopy
Organ-cultured thymic lobes were fixed by immersion in 4% glutaraldehyde, buffered to pH 7.3 with Millonig's fluid, postfixed in 1% osmium tetroxide in the same buffer, and dehydrated in acetone for embedding in Araldite (Sigma-Aldrich). Sections were obtained with an OM-U3 ultramicrotome (Reichert). Ultrathin sections were double-stained with uranyl acetate and lead citrate and then examined in a JEM 1010 electron microscope (JEOL) at Centro de Citometría y Microscopía de Fluorescencia, Universidad Complutense de Madrid. Images were generated using a MegaView G2 camera (Zeiss) and the iTEM Imaging Platform software (Olympus).
Statistical analysis
The Student t test was used for statistical analysis. Values of P ≤ .05 (*), P ≤ .01 (**), and P ≤ .001 (***) were considered to be statistically significant.
Results
Phenotypic characterization and gene expression profile of human CD34+CD1a−BMPRIA+ intrathymic precursors
We reported previously the presence of a functional BMP2/4 signaling pathway in human thymic CD34+ cells. Intrathymic precursor cells expressed the factors needed for BMP signal transduction, BR-Smads, and Co-Smad proteins, as well as the type I BMP receptors, BMPRIB, ActRIA, and mainly BMPRIA.30
To better define the thymic progenitor cell subpopulation responding to BMP signals, we first conducted a flow cytometry analysis of CD34+CD1a−BMPRIA+ and CD34+CD1a−BMPRIA− cells, representing ∼ 20% and 80%, respectively, of the early intrathymic CD34+CD1a− precursor cell population. The phenotypic analysis showed that BMPRIA+ precursors mostly expressed the NK cell lineage–related marker CD161, as well as CD215/IL-15 receptor α and CD122/IL-2 receptor β. In contrast, the expression of these cell markers was markedly lower or even absent in BMPRIA− precursor cells (Figure 1A). Likewise, a much higher proportion of BMPRIA+ thymic progenitors expressed the cytokine receptors CD116/GM-CSF receptor α and CD135/Flt3. The expression of CD127/IL-7 receptor α and CD117/c-kit was also significantly higher in the CD34+CD1a−BMPRIA+ precursor cell subset (Figure 1A). Approximately 50% to 60% of BMPRIA+ progenitors were positive for CD5 antigen, and no expression of CD94 was found in BMPRIA+ and BMPRIA− precursor cell subpopulations (Figure 1A).
Surface marker expression pattern and gene expression profile of human CD34+CD1a−BMPRIA+ intrathymic precursors. (A) Purified thymic CD34+CD1a− cells were stained with anti-BMPRIA Abs and a range of mAbs against differentiation-specific antigens, and then they were analyzed by flow cytometry. Solid histograms represent the expression of the indicated cell surface markers on CD34+CD1a−BMPRIA− and CD34+CD1a−BMPRIA+ cell subsets. Open histograms represent background fluorescence using isotype-matched irrelevant mAbs. Similar staining patterns were obtained in 5 different experiments. (B) Gene expression in sorted CD34+CD1a−BMPRIA+ thymic precursors. Transcription levels of different genes were normalized for GNB2L1 mRNA content and are shown relative to GNB2L1-normalized transcription in the CD34+CD1a−BMPRIA− cell subset. (C) Percentage of BMPRIA+ cells in CD34loCD44hiCD5lo/− and CD34loCD44loCD5+ thymic precursor subpopulations. Horizontal bar represents the mean of 7 independent experiments (*P ≤ .05 by t test).
Surface marker expression pattern and gene expression profile of human CD34+CD1a−BMPRIA+ intrathymic precursors. (A) Purified thymic CD34+CD1a− cells were stained with anti-BMPRIA Abs and a range of mAbs against differentiation-specific antigens, and then they were analyzed by flow cytometry. Solid histograms represent the expression of the indicated cell surface markers on CD34+CD1a−BMPRIA− and CD34+CD1a−BMPRIA+ cell subsets. Open histograms represent background fluorescence using isotype-matched irrelevant mAbs. Similar staining patterns were obtained in 5 different experiments. (B) Gene expression in sorted CD34+CD1a−BMPRIA+ thymic precursors. Transcription levels of different genes were normalized for GNB2L1 mRNA content and are shown relative to GNB2L1-normalized transcription in the CD34+CD1a−BMPRIA− cell subset. (C) Percentage of BMPRIA+ cells in CD34loCD44hiCD5lo/− and CD34loCD44loCD5+ thymic precursor subpopulations. Horizontal bar represents the mean of 7 independent experiments (*P ≤ .05 by t test).
Remarkably, the expression of the transcription factor Nfil3/E4bp4, recently described to be essential for NK cell development,33,34 was 10 times higher in thymic BMPRIA+ precursors, as shown by the quantitative PCR results (Figure 1B). The expression of Id3, which also regulates the development of NK cells,13 was 3 to 4 times higher in the subpopulation of BMPRIA+ precursors, and GATA-2, PU.1, and SpiB mRNAs were also more abundant in BMPRIA+ progenitor cells (Figure 1B). Similar levels of GATA-3, Id2, Runx1, and Ets1 transcription factors were found in both CD34+ cell subsets (Figure 1B). On the contrary, Bcl11b, a key factor required for T-cell lineage,35,36 and Hes1 expression was lower in CD34+CD1a−BMPRIA+ than in CD34+CD1a−BMPRIA− cells (Figure 1B).
Because all these data suggested that the CD34+CD1a−BMPRIA+ subpopulation contained precursor cells committed toward the NK cell lineage, we analyzed the expression of BMPRIA in 2 distinct intrathymic precursor cell subpopulations that exhibit different lymphoid and myeloid precursor potential. Human CD34loCD44loCD5+ thymocytes are able to generate T cells but have lost the potential to generate DCs, whereas CD34loCD44hiCD5lo/− cells are progenitors with increased ability to generate myeloid cells but devoid of T-cell precursor activity. Although both subpopulations display NK cell precursor potential, CD34loCD44hi precursors are consistently more efficient than CD34loCD44lo thymocytes in generating NK cells.11 In correlation with these findings, we detected BMPRIA expression in a much higher proportion of CD34loCD44hi precursors (41%; range, 30%-66%), in comparison with CD34loCD44lo thymocytes (12%; range, 3%-25%; Figure 1C).
CD34+CD1a−BMPRIA+ intrathymic precursors mainly generate functional NK cells
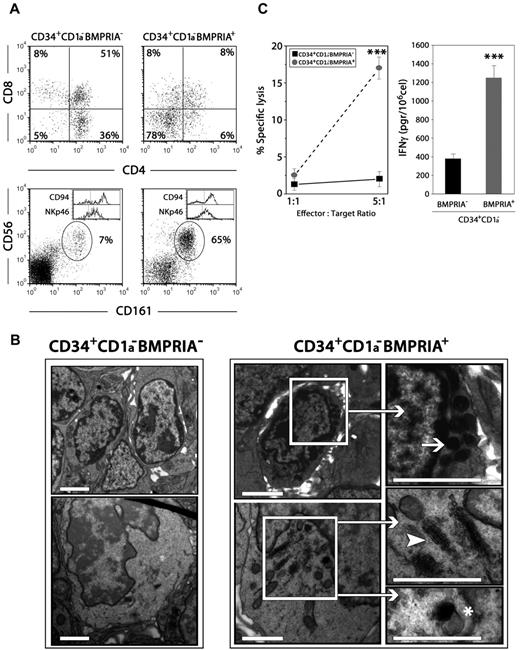
CD34+CD1a−BMPRIA+ and CD34+CD1a−BMPRIA− precursor cell subpopulations were next used to reconstitute alymphoid thymic lobes from fetal SCID mice. The differentiation potential of these two cell subpopulations was different; thus, when thymic lobes were reconstituted with BMPRIA− precursors, the percentage of CD3−CD161+CD56+ cells did not exceed 10%, and most cells exhibited a typical T-cell phenotype. On the contrary, when thymus lobes were reconstituted with BMPRIA+ precursors, the percentage of CD3−CD161+CD56+ NK cells may reach more than 60%, whereas the proportion of CD4+CD8+ thymocytes generated was minimal (Figure 2A). In both cultures, NK cells mostly expressed CD94 and NKp46 (Figure 2A).
Human CD34+CD1a−BMPRIA+ thymic precursors generate functional NK cells. Chimeric human-mouse FTOCs were performed with sorted human thymic CD34+CD1a−BMPRIA− or CD34+CD1a−BMPRIA+ cells and cultured for 9 days. (A) Cells recovered from hybrid FTOCs were labeled with anti–human CD45, anti-CD3, anti-CD4, and anti-CD8 or anti–human CD45, anti-CD3, and anti-CD56 combined with anti-CD161, anti-CD94, or anti-NKp46 mAbs. Dot plots show the expression of CD4/CD8 and CD56/CD161 on gated human CD45+ cells from hybrid FTOCs. Histograms show the expression of CD94 and NKp46 in human CD3−CD56+ cells. Data are representative of 4 independent experiments wherein a pool of at least 3 thymic lobes per experiment was analyzed. In the experiment shown, human cell recoveries were 4 × 104 and 1.4 × 104 for FTOCs reconstituted with CD34+CD1a−BMPRIA− or CD34+CD1a−BMPRIA+ cells, respectively. (B) Large numbers of NK cells develop in hybrid FTOCs reconstituted with CD34+CD1a−BMPRIA+ cell precursors. NK cells appear as round elements showing numerous electron-dense granules in the cytoplasm arranged close to the nucleus (arrow). Immature NK cells exhibit a well-developed Golgi complex (arrowhead) and moderately electron-dense secretory vesicles (asterisk). In contrast, thymocytes predominate in hybrid FTOCs reconstituted with CD34+CD1a−BMPRIA− cell precursors. Scale bars represent 2 μm. (C) Equal numbers of total human cells were used to assay the cytolytic capacity and IFN-γ production by NK cells generated in hybrid FTOCs reconstituted with CD34+CD1a−BMPRIA+ or CD34+CD1a−BMPRIA− precursors. The data represent the mean ± SD from 3 to 5 independent experiments (***P ≤ .001 by t test).
Human CD34+CD1a−BMPRIA+ thymic precursors generate functional NK cells. Chimeric human-mouse FTOCs were performed with sorted human thymic CD34+CD1a−BMPRIA− or CD34+CD1a−BMPRIA+ cells and cultured for 9 days. (A) Cells recovered from hybrid FTOCs were labeled with anti–human CD45, anti-CD3, anti-CD4, and anti-CD8 or anti–human CD45, anti-CD3, and anti-CD56 combined with anti-CD161, anti-CD94, or anti-NKp46 mAbs. Dot plots show the expression of CD4/CD8 and CD56/CD161 on gated human CD45+ cells from hybrid FTOCs. Histograms show the expression of CD94 and NKp46 in human CD3−CD56+ cells. Data are representative of 4 independent experiments wherein a pool of at least 3 thymic lobes per experiment was analyzed. In the experiment shown, human cell recoveries were 4 × 104 and 1.4 × 104 for FTOCs reconstituted with CD34+CD1a−BMPRIA− or CD34+CD1a−BMPRIA+ cells, respectively. (B) Large numbers of NK cells develop in hybrid FTOCs reconstituted with CD34+CD1a−BMPRIA+ cell precursors. NK cells appear as round elements showing numerous electron-dense granules in the cytoplasm arranged close to the nucleus (arrow). Immature NK cells exhibit a well-developed Golgi complex (arrowhead) and moderately electron-dense secretory vesicles (asterisk). In contrast, thymocytes predominate in hybrid FTOCs reconstituted with CD34+CD1a−BMPRIA− cell precursors. Scale bars represent 2 μm. (C) Equal numbers of total human cells were used to assay the cytolytic capacity and IFN-γ production by NK cells generated in hybrid FTOCs reconstituted with CD34+CD1a−BMPRIA+ or CD34+CD1a−BMPRIA− precursors. The data represent the mean ± SD from 3 to 5 independent experiments (***P ≤ .001 by t test).
The ultrastructural study of the chimeric human-mouse FTOCs demonstrated that in the thymus lobes reconstituted with BMPRIA+ precursors largely predominated cells containing abundant dense spherical granules, morphologically identifiable as NK cells (Figure 2B). Moreover, cells showing a dilated Golgi apparatus and immature secretory vesicles, presumably immature differentiating NK cells, also were observed in these thymus lobes (Figure 2B). In contrast, thymocytes were mainly present throughout the thymic parenchyma in FTOCs recolonized with BMPRIA− progenitors (Figure 2B).
To support the fact that CD34+CD1a−BMPRIA+ progenitors are able to generate higher numbers of NK cells, we assessed the cytotoxicity and cytokine production capacity of cells generated in both types of FTOC. As shown in Figure 2C, whereas cells recovered from the lobes reconstituted with BMPRIA− precursors were only able to respond minimally in a conventional cytotoxicity assay against a standard target cell line (K562), the cells generated in lobes reconstituted with BMPRIA+ precursors, which mostly corresponded to NK cells, were found to exhibit a significant cytotoxic capability. Concomitantly, the production of IFN-γ was notably higher in FTOC recolonized with CD34+CD1a−BMPRIA+ progenitors (Figure 2C).
These results show that the CD34+CD1a−BMPRIA+ cell subset gives rise mostly to functional NK cells in a thymic microenvironment and provide further support for the idea that a large number of the human thymic CD34+CD1a−BMPRIA+ progenitors represent NK cell precursors.
Thymic progenitors generate NK cells through a BMPRIA+ intermediate stage
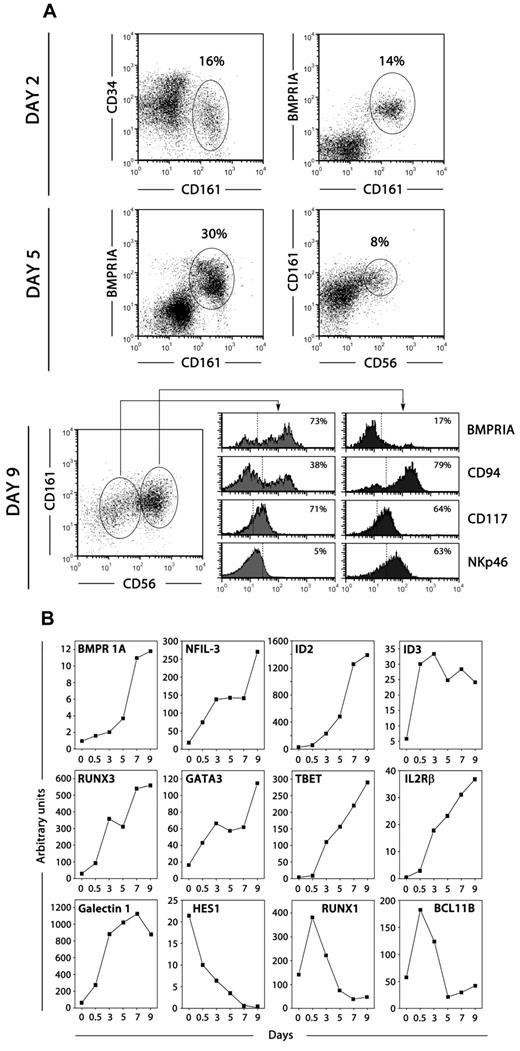
We investigated the differentiation of intrathymic CD34+CD1a−BMPRIA− progenitors in a differentiation assay specific to NK cell production. At various days after culture in the presence of SCF and IL-15, cells were harvested and flow cytometry and quantitative PCR analyses were performed. Approximately 15% to 20% of the recovered cells expressed the NK cell-associated marker CD161 after 48 hours of culture (Figure 3A). In addition, CD161+ cells mostly expressed BMPRIA, suggesting that the up-regulation of BMP signaling is an early event in human thymic NK cell development (Figure 3A). The frequency of CD161+BMPRIA+ cells increased in the next days of culture, but as cells acquired other NK cell markers, such as CD56, CD94, and NKp46, the expression of BMPRIA was down-regulated. Thus, the most mature population of CD94+CD161+CD56+ NK cells expressed low levels of BMPRIA (Figure 3A). In agreement, BMPRIA expression was detected in a higher proportion of freshly isolated immature thymic NK cells in comparison with mature NK cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
BMPRIA expression during NK cell development. (A) Thymic CD34+CD1a− BMPRIA− precursor cells were cultured with SCF and IL-15 for different days and then analyzed by flow cytometry for the expression of CD34, CD161, BMPRIA, CD56, CD94, CD117, and NKp46. Representative phenotypic analyses of total cells harvested at different days of culture are shown. Percentages of positive cells are indicated in dot plots and histograms. (B) Quantitative RT-PCR analysis of the expression of BMPRIA and genes relevant for NK cell development was performed on cells derived from thymic precursors cultured with SCF and IL-15 for different days. Gene expression levels were normalized to GNB2L-1 mRNA content. The data are representative of at least 3 separate experiments.
BMPRIA expression during NK cell development. (A) Thymic CD34+CD1a− BMPRIA− precursor cells were cultured with SCF and IL-15 for different days and then analyzed by flow cytometry for the expression of CD34, CD161, BMPRIA, CD56, CD94, CD117, and NKp46. Representative phenotypic analyses of total cells harvested at different days of culture are shown. Percentages of positive cells are indicated in dot plots and histograms. (B) Quantitative RT-PCR analysis of the expression of BMPRIA and genes relevant for NK cell development was performed on cells derived from thymic precursors cultured with SCF and IL-15 for different days. Gene expression levels were normalized to GNB2L-1 mRNA content. The data are representative of at least 3 separate experiments.
We also examined during the culture period the relative mRNA expression of genes relevant for NK cell development. As expected, under the influence of SCF and IL-15, the levels of transcription factors such as Nfil3, Id2, Id3, Runx3, Gata3, and T-bet as well as CD122 and Galectin-1 drastically increased throughout the culture (Figure 3B). Remarkably, the levels of transcripts for BMPRIA were increasing in parallel to those for transcription factors pivotal for NK cell development (Figure 3B). On the contrary, the expression of the Notch target gene Hes-1 was gradually decreasing during the whole culture period (Figure 3B); and Bcl11b, whose expression is also controlled by Notch signaling,35 and Runx1 were down-regulated from the first days of culture (Figure 3B).
BMP signaling is involved in NK cell development
We further evaluated whether BMP4 is autocrinally produced by developing NK cells. As shown in Figure 4A, BMP4 production started in the first days of culture and was initially associated with thymic precursors in progress toward NK cell commitment (Figure 3A). In the following days, BMP4 levels progressively augmented along with the appearance of increasing proportions of NK cells, and both CD161+CD56− and CD161+CD56+ NK cells produced BMP4 (Figures 3A and 4A). Therefore, thymus-derived NK cells up-regulate both BMPRIA expression and BMP4 secretion capacity from the early steps of the differentiation process.
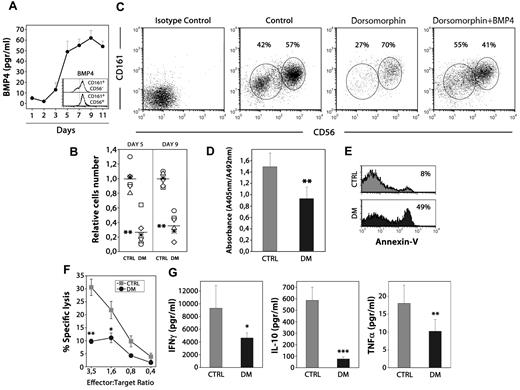
BMP signaling influences human NK differentiation. (A) The levels of BMP4 were determined by ELISA in the supernatants obtained culturing human thymic CD34+CD1a−BMPRIA− precursors with SCF and IL-15 for different days. Data represent the mean ± SD from 3 to 4 independent experiments. Histograms show intracellular staining for BMP4 on immature CD161+CD56− and mature CD161+CD56+ NK cells generated after 7 days of culture. (B) The scatter plots show the effects on cell recovery when the BMP inhibitor dorsomorphin was added to the cultures. The number of cells recovered in each experiment was divided by the mean number of cells recovered from the control cultures, to give the relative cell number from 6 individual experiments (**P ≤ .01 by t test). (C) Dot plots show CD161 versus CD56 expression from control and dorsomorphin-treated cultures after 9 days. As shown, the addition of BMP4 neutralized the inhibitory effect of dorsomorphin on human NK cell differentiation. (D) Determination of the proliferation rate of differentiating NK cells after 5 days of culture. Cells were pulsed for 12 hours with BrdU. A specific kit was used to measure BrdU incorporation into newly synthesized DNA. Results are the mean ± SD of 4 independent experiments, each with 2 cultures per point (**P ≤ .01 by t test). (E) Annexin V staining was measured by flow cytometry in NK cells harvested from control and dorsomorphin-treated cultures on day 5. Data are representative results from 3 independent experiments. (F) Equal numbers of NK cells generated in dorsomorphin-treated cultures (black circles) and untreated cultures (gray squares) were stimulated with IL-15 + IL-12 for 12 hours and assayed in a 4-hour standard cytotoxicity assay against K562 target cells at different effector-to-target ratios (E:T) in duplicate wells. The mean percentage of specific lysis ± SD is represented (n = 6 different individuals) for each E:T ratio (*P ≤ .05, **P ≤ .01 by t test). (G) NK cells (3 × 105 cells) recovered from control (gray bars) and dorsomorphin treated-cultures (black bars) were stimulated for 12 hours. Supernantants were collected and analyzed for the presence of IFN-γ, IL-10, and TNFα (*P ≤ .05, **P ≤ .01, and ***P ≤ .001 by t test).
BMP signaling influences human NK differentiation. (A) The levels of BMP4 were determined by ELISA in the supernatants obtained culturing human thymic CD34+CD1a−BMPRIA− precursors with SCF and IL-15 for different days. Data represent the mean ± SD from 3 to 4 independent experiments. Histograms show intracellular staining for BMP4 on immature CD161+CD56− and mature CD161+CD56+ NK cells generated after 7 days of culture. (B) The scatter plots show the effects on cell recovery when the BMP inhibitor dorsomorphin was added to the cultures. The number of cells recovered in each experiment was divided by the mean number of cells recovered from the control cultures, to give the relative cell number from 6 individual experiments (**P ≤ .01 by t test). (C) Dot plots show CD161 versus CD56 expression from control and dorsomorphin-treated cultures after 9 days. As shown, the addition of BMP4 neutralized the inhibitory effect of dorsomorphin on human NK cell differentiation. (D) Determination of the proliferation rate of differentiating NK cells after 5 days of culture. Cells were pulsed for 12 hours with BrdU. A specific kit was used to measure BrdU incorporation into newly synthesized DNA. Results are the mean ± SD of 4 independent experiments, each with 2 cultures per point (**P ≤ .01 by t test). (E) Annexin V staining was measured by flow cytometry in NK cells harvested from control and dorsomorphin-treated cultures on day 5. Data are representative results from 3 independent experiments. (F) Equal numbers of NK cells generated in dorsomorphin-treated cultures (black circles) and untreated cultures (gray squares) were stimulated with IL-15 + IL-12 for 12 hours and assayed in a 4-hour standard cytotoxicity assay against K562 target cells at different effector-to-target ratios (E:T) in duplicate wells. The mean percentage of specific lysis ± SD is represented (n = 6 different individuals) for each E:T ratio (*P ≤ .05, **P ≤ .01 by t test). (G) NK cells (3 × 105 cells) recovered from control (gray bars) and dorsomorphin treated-cultures (black bars) were stimulated for 12 hours. Supernantants were collected and analyzed for the presence of IFN-γ, IL-10, and TNFα (*P ≤ .05, **P ≤ .01, and ***P ≤ .001 by t test).
To explore the involvement of BMP signaling during NK cell differentiation, thymic precursors were cultured in the presence of SCF and IL-15 plus dorsomorphin, an inhibitor of BMP type I receptor serine-threonine kinase activity that blocks BMP ligand-induced phosphorylation of BR-Smads.37 The addition of dorsomorphin resulted in a profound decrease (60%-80% reduction) in the numbers of NK cells generated from thymic progenitors (Figure 4B), and the CD161+CD56− immature NK cell subpopulation was the most affected by the treatment with dorsomorphin (Figure 4C). The fact that BMP4 addition to the cultures counteracted the effects of dorsomorphin on NK development indicated that dorsomorphin was not nonspecifically toxic to developing NK cells but was inhibiting a biologic effect (Figure 4C).
BMP signaling regulates apoptosis and cell cycle progression in many developmental systems.19,38 Then, we analyzed the effects of dorsomorphin addition on the proliferation rate and apoptosis in the first days of culture, when most cells were NK precursors and immature NK cells. The blockade of BMP signaling pathway by dorsomorphin treatment reduced the IL-15–dependent expansion (Figure 4D) and also increased the percentage of apoptotic cells in cultures supplemented with dorsomorphin (range, 40%-55%) in comparison with control cultures (range, 4%-9%; Figure 4E). These results suggest that the autocrine production of BMP4 is necessary for the expansion and survival during thymic NK cell differentiation.
As shown in Figure 4B and C, some mature CD161+CD56+ NK cells could appear in the presence of dorsomorphin. However, the analysis of those cells showed that they exhibited an impaired cytotoxic capacity (Figure 4F) and a reduced ability to produce cytokines such as IFN-γ, IL-10, and TNF-α (Figure 4G), suggesting that BMP signaling also is required during NK cell differentiation for the acquisition of the functional capabilities of mature NK cells.
BMP4 and IL-15 cooperate in thymic NK cell differentiation
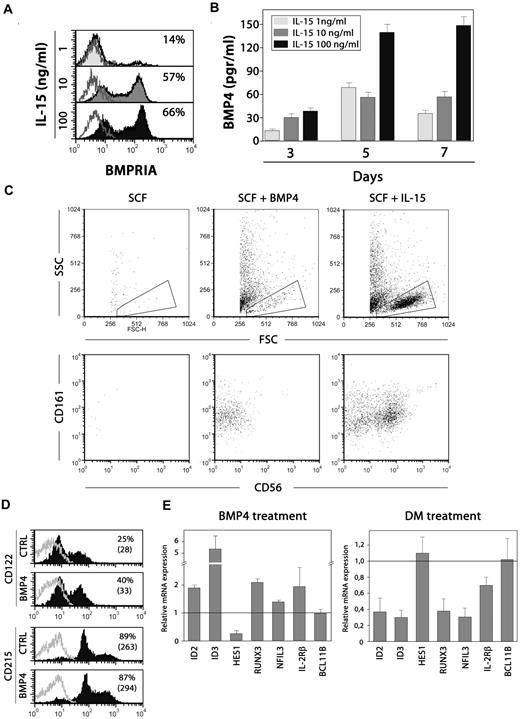
The above-mentioned results suggest that there is an interplay between IL-15 and BMP signaling during NK cell differentiation. To dissect this interaction, we first studied whether the IL-15–induced NK cell differentiation involves the up-regulation of BMP signaling. Using NK cell differentiation assays, the addition of increasing doses of IL-15 to the cultures, ranging from 1 to 100 ng/mL, showed that the expression of BMPRIA was highly induced in differentiating NK cells (Figure 5A), and the secretion of BMP4 also was increased in the culture medium, mainly when the highest dose of IL-15 was added (Figure 5B). Then, these results indicate that IL-15 promotes BMP signaling, in accordance with the fact that the inhibition of the BMP signaling pathway, using dorsomorphin, drastically inhibited the IL-15–induced expansion of thymic NK cell precursors.
Interplay between BMP4 and IL-15 in NK cell differentiation. (A) BMPRIA expression on NK cells generated after culturing CD34+CD1a−BMPRIA− cells for 7 days in the presence of SCF and different doses of IL-15. (B) The levels of BMP4 were determined in the supernatants of those cultures after 3, 5, and 7 days. Results are the mean ± SD of 2 independent experiments. (C) Dot plots show forward scatter (FSC) and side scatter (SSC) properties and CD161 and CD56 expression on cells derived from cultures supplemented with SCF alone (100 ng/mL), SCF + BMP4, or SCF + IL-15 (100 ng/mL) for 6 days. (D) Expression of CD122 and CD215 on thymic precursors after treatment with SCF and BMP4 for 36 hours. Control cells were cultured with SCF + IL-15. The percentages of positive cells (and their mean fluorescence intensities) are indicated in the histograms. One representative experiment of 3 is shown. (E) Expression of NK cell differentiation related genes after BMP4 or dorsomorphin treatment. Thymic CD34+CD1a−BMPRIA− precursors were cultured for 36 hours with SCF + IL-15 and then pulsed for 1 hour with BMP4. To inhibit endogenous BMP signaling, dorsomorphin was present during the whole culture period. Transcription levels of different genes in BMP4- or dorsomorphin-treated precursors were normalized for GNB2L1 mRNA content and are shown relative to GNB2L1-normalized transcription in the untreated cells. Data represent the mean ± SD from 2 to 3 independent experiments.
Interplay between BMP4 and IL-15 in NK cell differentiation. (A) BMPRIA expression on NK cells generated after culturing CD34+CD1a−BMPRIA− cells for 7 days in the presence of SCF and different doses of IL-15. (B) The levels of BMP4 were determined in the supernatants of those cultures after 3, 5, and 7 days. Results are the mean ± SD of 2 independent experiments. (C) Dot plots show forward scatter (FSC) and side scatter (SSC) properties and CD161 and CD56 expression on cells derived from cultures supplemented with SCF alone (100 ng/mL), SCF + BMP4, or SCF + IL-15 (100 ng/mL) for 6 days. (D) Expression of CD122 and CD215 on thymic precursors after treatment with SCF and BMP4 for 36 hours. Control cells were cultured with SCF + IL-15. The percentages of positive cells (and their mean fluorescence intensities) are indicated in the histograms. One representative experiment of 3 is shown. (E) Expression of NK cell differentiation related genes after BMP4 or dorsomorphin treatment. Thymic CD34+CD1a−BMPRIA− precursors were cultured for 36 hours with SCF + IL-15 and then pulsed for 1 hour with BMP4. To inhibit endogenous BMP signaling, dorsomorphin was present during the whole culture period. Transcription levels of different genes in BMP4- or dorsomorphin-treated precursors were normalized for GNB2L1 mRNA content and are shown relative to GNB2L1-normalized transcription in the untreated cells. Data represent the mean ± SD from 2 to 3 independent experiments.
Next, we determined whether BMP4 could support NK cell development in the absence of IL-15 signaling. After 6 days, no cells were recovered from cultures supplemented only with SCF (Figure 5C). In contrast, the presence of BMP4 allowed the differentiation of low numbers of immature CD161+ CD56− NK cells, in comparison with the cultures supplemented with SCF and IL-15 where high numbers of mature CD161+ CD56+ NK cells predominated (Figure 5C).
These data suggested that BMP signaling does not act only downstream but also upstream of IL-15. To address this issue, we analyzed the NK cell differentiation cultures at early time points, and we found that BMP4 was able to up-regulate the expression of CD122, thereby rendering higher numbers of IL-15–responsive cells (Figure 5D). The early effects of BMP signaling on NK cell development also were revealed when the expression of genes related to NK cell differentiation was analyzed after BMP4 addition. As can be seen in Figure 5E, Id2, Runx3 and mainly Id3 appeared up-regulated after a short-time exposure to BMP4. The expression of Hes1 was decreased and no significant changes were found in the levels of Nfil3, CD122, and Bcl11b (Figure 5E). Furthermore, when autocrine BMP signaling was inhibited with dorsomorphin and the expression of NK cell–related genes was analyzed, we observed that the expression of Nfil3 was notably reduced by 60% to 70%, compared with the expression levels reached in control cultures. Similarly, the expression of Id3, Id2, Runx3, and to a lesser extent CD122 also was diminished when BMP signaling was blocked, whereas the expression of Hes1 and Bcl11b remained unaffected (Figure 5E). Collectively, these data show that BMP signaling is involved in several stages of thymic NK cell development.
BMPRIA also is expressed by CD34+ progenitors in human lymph nodes
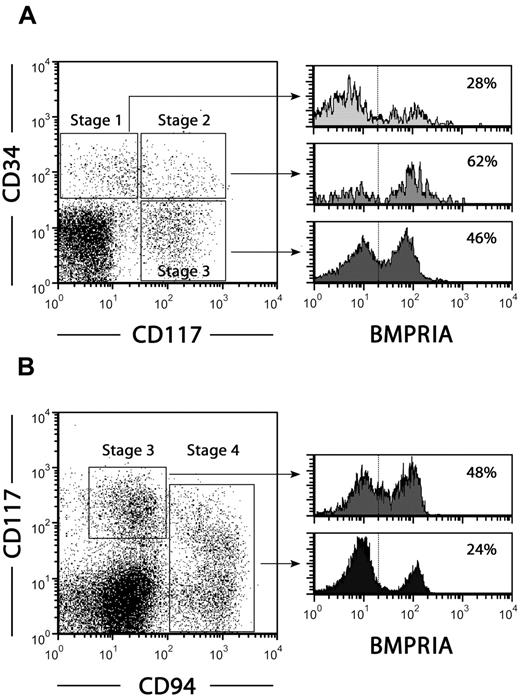
Freud et al used the combination of CD34, CD117, and CD94 antigens to define distinct NK cell developmental intermediates in human secondary lymphoid organs: stage 1, CD34+ CD117− CD94− pro-NK cells; stage 2, CD34+ CD117+ CD94− pre-NK cells; stage 3, CD34− CD117+ CD94− committed immature NK cells; and stage 4, CD34− CD117+/− CD94+ CD56+ NK cells.7
We investigated whether BMPRIA expression also is associated with NK cell precursors in human lymph nodes. Figure 6A shows that a high proportion (60%-70%) of stage 2 pre-NK cells expressed BMPRIA in comparison with stage 1 pro-NK cells (15%-30%). In addition, approximately half of the subpopulation of immature NK cells maintained BMPRIA expression, which almost disappeared among mature NK cells (Figure 6B).
Expression of BMPRIA in NK cell precursors from human lymph nodes. Lymph node mononuclear cells were used to analyze BMPRIA expression on stage 1-4 NK cell precursors. (A) Dot plot shows the expression of CD34 and CD117 in Lin− (CD3−, CD19−, CD14−, CD94−) cells. Histograms show BMPRIA expression on stage 1 CD34+ CD117− CD94−pro-NK cells, stage 2 CD34+ CD117+ CD94− pre-NK cells and stage 3 CD34− CD117+ CD94−committed immature NK cells. The percentages of positive cells are indicated in each histogram. (B) Dot plot shows the expression of CD117 and CD94 in Lin− (CD3−, CD19−, CD14−, CD34−) cells. Histograms show BMPRIA expression on stage 3 CD34− CD117+ CD94−immature NK cells and stage 4 CD34− CD117+/− CD94+ NK cells. The percentages of positive cells are indicated in each histogram. Data are representative of 3 independent experiments.
Expression of BMPRIA in NK cell precursors from human lymph nodes. Lymph node mononuclear cells were used to analyze BMPRIA expression on stage 1-4 NK cell precursors. (A) Dot plot shows the expression of CD34 and CD117 in Lin− (CD3−, CD19−, CD14−, CD94−) cells. Histograms show BMPRIA expression on stage 1 CD34+ CD117− CD94−pro-NK cells, stage 2 CD34+ CD117+ CD94− pre-NK cells and stage 3 CD34− CD117+ CD94−committed immature NK cells. The percentages of positive cells are indicated in each histogram. (B) Dot plot shows the expression of CD117 and CD94 in Lin− (CD3−, CD19−, CD14−, CD34−) cells. Histograms show BMPRIA expression on stage 3 CD34− CD117+ CD94−immature NK cells and stage 4 CD34− CD117+/− CD94+ NK cells. The percentages of positive cells are indicated in each histogram. Data are representative of 3 independent experiments.
Discussion
Our previous studies had shown that BMP ligands are produced in the human thymus and that human CD34+ precursors are one of the intrathymic cell subpopulations expressing BMP receptors, mainly BMPRIA.27,30 In the present report, we proposed to carry out a detailed phenotypic analysis of the subpopulation of CD34+ CD1a− early thymocyte progenitors that express BMPRIA and therefore are able to respond to BMP signals, and we found that these cells exhibit multiple phenotypic features typical of NK cell precursors. Moreover, these intrathymic progenitor cells mostly differentiate into functionally mature NK cells not only in the presence of suitable cytokines but also under the influence of the thymic stromal microenvironment, previously claimed to mainly promote T-cell maturation.
At present, information on NK cell differentiation in the human thymus is very limited, and most existing data only investigate the NK cell potential of different precursor cell subpopulations. In line with our results, it has been reported that among intrathymic CD34+ CD1a− cells, both CD5+ and CD5− cell subsets have NK cell as well as T-cell potential,9,15 and Márquez et al identified bipotential NK/DC progenitors characterized by expression of CD116 and CD33, and low levels of CD127.10,11 Likewise, the analysis of CD161 expression in immature thymocytes showed that a fraction of CD161-bearing cells coexpressed CD34 and CD117, lacked CD1a expression and that they were able to generate cytolytic cells on culture with IL-2.39
Recent studies have defined in human lymph nodes and tonsils a complete pathway of NK cell development starting with multipotent precursor cells (stage 1, CD34+ CD117− CD94− pro-NK cells and stage 2, CD34+ CD117+ CD94− pre-NK cells) proceeding through NK-committed cells (stage 3, CD34− CD117+ CD94− immature NK cells) into mature NK cells (stage 4, CD34− CD117+/− CD94+ CD56+ cells).1,7 According to our results from in vitro NK cell differentiation assays and ex vivo phenotypic analyses, we favor the idea that a similar progression of developmental stages may occur during NK cell differentiation in the human thymus. The intrathymic subpopulation of CD34+ BMPRIA+ cells that we have defined here could be equivalent to CD34+ CD117+ CD94− pre-NK cells. Supporting this notion, stage 2 pre-NK cells mostly express BMPRIA and share with thymic CD34+ BMPRIA+ precursors several phenotypic features, such as the expression of CD117, CD45RA, CD33, CD38, and CD44 antigens.2 More interesting is the fact that the only NK cell receptor consistently expressed in pre-NK cells is CD161, which is expressed by most CD34+ BMPRIA+ precursor cells. Likewise, pre-NK cells constitute the first developmental stage acquiring the ability to respond to IL-15, which agrees with the broad expression of CD122 and CD215 detected in intrathymic CD34+ BMPRIA+ cells.2,7 The analyses of lineage differentiation potentials have shown that stage 2 pre-NK cells can give rise to NK cells and, to a lesser extent, T cells and DCs, indicating that this cell subset is not fully committed to NK cell lineage.7 Similarly, although intrathymic CD34+ BMPRIA+ precursors can generate low numbers of T cells, they mainly differentiate into NK cells, even under the influence of thymic stromal cells in FTOC. It is also likely that CD34+ BMPRIA+ cells can produce DCs because some of the bipotent NK/DC progenitors described in the human thymus10,11 express BMPRIA and therefore must be included in the BMPRIA+ precursor cell subpopulation.
The comparative gene expression analysis of BMPRIA+ and BMPRIA− CD34+ cells also suggests the commitment of CD34+ BMPRIA+ precursors toward the NK cell lineage. Intrathymic CD34+ BMPRIA+ cells express higher levels of the transcription factor Nfil3 that constitutes a specific and essential requirement for the development of murine NK cells,33,34 and lower levels of Bcl11b, a zinc-finger transcription factor that has been demonstrated to be necessary for T-cell lineage commitment in mice, being specifically required for repression of NK cell–associated genes.35,36 In this context, Klein Wolterink et al have proposed that the relative expression levels of Nfil3 versus Bcl11b would be major determinants of NK versus T-cell fate decision at specific developmental cell stages.16 Id proteins, particularly Id2 and Id3, also have been shown to play an important role in the generation of NK cells.13,40,41 In humans, the enforced expression of Id2 or Id3 in thymic CD34+ CD1a− progenitor cells inhibits their differentiation into T cells and concomitantly promotes NK cell development,13,15 agreeing with the fact that CD34+ BMPRIA+ precursors express high levels of Id3, but not Id2, which is up-regulated later. Although it has been recently reported that Id2 would be the main factor that physiologically would control the differentiation of NK cells,15 our results suggest, in accordance to previous work in mice,42 that Id3 and Id2 may cooperate acting sequentially during NK cell development, with Id3 functioning in NK cell precursors and Id2 in the immature to mature NK cell transition. It is also interesting to emphasize that CD34+ BMPRIA+ thymic precursor cells express high levels of GATA-2, but not GATA-3. However, the levels of this latter increase notably during NK cell differentiation, confirming previous results showing that GATA-3 is required for the intrathymic differentiation of mouse NK cells.12 On the basis of all these results, we can therefore conclude that the subpopulation of intrathymic CD34+ BMPRIA+ progenitors includes the thymic NK cell precursors. In addition, we also propose that the expression of BMPRIA could be used to identify NK-committed CD34+ precursor cells in different organs. Although further experiments will be needed, we show the first evidence that BMPRIA expression is mainly associated with stage 2 CD34+ CD117+ pre-NK cells in comparison with stage 1 CD34+ CD117− pro-NK cells in human lymph nodes.
During the in vitro differentiation of thymic CD34+ cells into NK cells, BMP4 is secreted to the culture medium. This evidence, along with the expression of BMPRIA in the intrathymic subpopulation of NK cell lineage-committed CD34+ cells, points out the relevance of an autocrine BMP signaling in NK cell development. Our results show that the BMP signaling pathway functions at several stages, both before and after the acquisition of responsiveness to IL-15, a cytokine shown to be critical for NK cell development.43-45
In early stages, BMP pathway seem to promote NK cell differentiation, because the blockade of BMP signaling with the inhibitor dorsomorphin reduces the expression levels of CD122 as well as NK cell–associated transcription factors, mainly Nfil3, Id2, Id3, and Runx3. Likewise, in the absence of IL-15, the stimulation of BMP signaling up-regulates CD122, as well as Id3, Id2, and Runx3 expression in CD34+ cells, allowing their differentiation into immature NK cells that cannot further expand because of the lack of IL-15. Therefore, in the first steps of NK cell development, BMP signaling would be involved in producing a pool of IL-15–responsive NK cell precursors, probably by up-regulating the levels of Id2, Id3, and Runx3 transcription factors. Id proteins and Runx transcription factors are important gene targets of BMP pathway in several cell types,20,21,46 including human intrathymic CD34+ cells. Id2 and also Id3 proteins have been already involved in increasing a pool of thymic CD1a− CD5+ NK cell progenitors in humans through their ability to repress the function of E proteins, mainly HEB, that results in the inhibition of T-cell development.13,15 Runx3 has been reported to be dominantly expressed in NK cells from the NK precursor stage to mature NK cells and also has been shown to bind to CD122 promoter region and positively regulate CD122 expression in early stages of NK cell development.47 Nfil3 also is regulated by BMP signaling and its action has been described to be mediated via Id2.33
Different reports have pointed out that IL-15 plays an essential role promoting the survival, proliferation, and differentiation of committed NK precursors once they have been fully specified.15,16,43,48 In this context, our results show that the expression of BMPRIA is induced by IL-15 and the production of BMP-4 ligand also is increased by IL-15, indicating that IL-15 promotes autocrine BMP signaling during NK cell differentiation. Furthermore, the inhibition of the BMP pathway decreases the IL-15–induced proliferation and survival of developing NK cells, demonstrating that BMP signaling mediates, at least in part, the effects of IL-15. Id proteins, common targets for BMP signaling, also seem to be involved in these stages because Schotte et al have reported that Id2 controls the proliferative expansion of an IL-15–responsive thymic NK precursor cell subset.15
Our data show that the BMP signaling pathway also participates in the late stages of NK cell development because the treatment with the BMP inhibitor dorsomorphin generates very low numbers of mature NK cells exhibiting reduced cytotoxicity and diminished capacity to secrete cytokines. In agreement with these results and with the fact that BMP signaling is mediating IL-15 effects, the analysis of IL-15–deficient mice as well as CD122-deficient mice overexpressing the survival factor Bcl-2 has shown that, in late stages of NK cell development, IL-15 may participate in inducing the survival of mature NK cells and the acquisition of their effector functions.49,50
Taken together, our results show that the expression of BMPRIA identifies NK cell precursors in the human thymus and that unlike what has been described for T-cell development26-29,31 BMP signaling positively regulates the intrathymic differentiation of human NK cells.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Pediatric Cardiosurgery Units from Hospital Madrid-Montepríncipe and Hospital 12 de Octubre for thymus samples and the Transplant Coordination Unit from Hospital Clínico San Carlos for lymph node samples.
This work was supported by grants BFU2009-10315 and BFU2010-18250 (Ministerio de Ciencia e Innovación), RD06/0010/0003 (Instituto de Salud Carlos III), and GR35/10A-910552 (Universidad Complutense and Comunidad Autónoma de Madrid).
Authorship
Contribution: L.H. designed and performed the research and collected and analyzed data; V.G.M., J.V., C.H.-L., and M.N.V. performed research and collected data; J.R.N. provided essential materials; A.G.Z. and R.S. contributed to writing the paper; and A.Varas and A.Vicente designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angeles Vicente, Department of Cell Biology, Faculty of Medicine, Av Complutense s/n Complutense University, Cuidad Universitaria 28040 Madrid, Spain; e-mail: avicente@bio.ucm.es.
References
Author notes
A.Varas and A.Vicente contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal