Abstract
The clinical value of plasma Epstein-Barr virus (EBV) DNA has not been evaluated in patients with early-stage extranodal nasal-type NK/T-cell lymphoma (NKTCL) receiving primary radiotherapy. Fifty-eight patients with stage I disease and 11 with stage II disease were recruited. High pretreatment EBV-DNA concentrations were associated with B-symptoms, elevated lactate dehydrogenase levels, and a high International Prognostic Index score. EBV-DNA levels significantly decreased after treatment. The 3-year overall survival (OS) rate was 82.6% for all patients. Stage I or II patients with a pretreatment EBV-DNA level of ≤ 500 copies/mL had 3-year OS and progression-free survival (PFS) rates of 97.1% and 79.0%, respectively, compared with 66.3% (P = .002) and 52.2% (P = .045) in patients with EBV-DNA levels of > 500 copies/mL. The 3-year OS and PFS rates for patients with undetectable EBV-DNA after treatment was significantly higher than patients with detectable EBV-DNA (OS, 92.0% vs 69.8%, P = .031; PFS, 77.5% vs 50.7%, P = .028). Similar results were observed in stage I patients. EBV-DNA levels correlate with tumor load and a poorer prognosis in early-stage NKTCL. The circulating EBV-DNA level could serve both as a valuable biomarker of tumor load for the accurate classification of early-stage NKTCL and as a prognostic factor.
Introduction
Extranodal nasal-type NK/T-cell lymphoma (NKTCL) is a rare, endemic subtype of lymphoma that exhibits an aggressive, variable clinical course and is closely associated with Epstein-Barr virus (EBV) infection.1-16 NKTCL has distinct clinicopathologic features and is characterized by the expression of EBV and NK/T-cell markers (CD56/CD3ϵ and cytotoxic molecules) and predominantly occurs in younger patients with a high frequency of localized-stage disease and tendency for extranodal dissemination.2-10 Radiotherapy is the main treatment for early-stage NKTCL10-16 ; however, several studies have supported the use of combined radiotherapy and chemotherapy for primary tumors located in Waldeyer ring, in addition to high-risk or advanced-stage disease.6,16-19 In our previous work, we have shown that primary radiotherapy results in an excellent outcome, with 5-year overall survival (OS) rates of 70%-90% in patients with early-stage NKTCL of the upper aerodigestive tract.6,10,13,20,21
Routine pathologic and immunophenotypic evaluations are insufficient to predict the clinical outcome in NKTCL patients.8,22-24 Several clinical features, including age, primary location, stage, local invasiveness, and the international or Korea prognostic model, can predict the prognosis in patients with NKTCL.2-7,11-16,25,26 However, the variable outcomes of patients within heterogeneous subgroups suggest that clinical features alone cannot precisely predict the response to therapy or treatment outcome in NKTCL. In a previous study, we were unable to identify prognostic factors in patients with stage I NKTCL receiving radiotherapy, which was probably the result of the homogeneity of the cohort of patients who received a similar treatment regimen.21
Circulating cell-free EBV-DNA levels can be detected using real-time quantitative RT-PCR in the blood of patients with EBV-associated tumors, such as nasopharyngeal carcinoma and Hodgkin lymphoma.27-29 Previous studies have demonstrated that the EBV-DNA load correlates with clinical stage and can be used to monitor disease progression and predict prognosis.27-30 Because of the rarity of the disease, the correlation between EBV-DNA levels and prognosis has been explored in small cohorts (n < 40) of NKTCL patients with different pathologic subtypes, clinical stages, primary locations, and treatment regimens.31-35 The predictive value of potential biomarkers is dependent on the primary treatment, especially with the use of primary radiotherapy for early-stage NKTCL. Therefore, we performed a prospective study to ascertain the correlation between plasma EBV-DNA levels with clinical features and survival in early-stage NKTCL patients treated with primary radiotherapy.
Methods
Eligibility criteria
This observational study analyzed the prognostic value of plasma EBV-DNA copy number and was conducted at the Cancer Hospital and Institute of Chinese Academy of Medical Sciences and Peking Union Medical College in China. The study protocol and use of blood sample collection were approved by the Institutional Ethics Committees. In this prospective evaluation, a case report form was obtained from all patients. The eligibility criteria for inclusion in this study were as follows: (1) a diagnosis of NKTCL with histologic features and an immunophenotypic evaluation that included CD2, CD3ϵ, CD56, TIA-1, Gram-B, CD45RO, CD20/CD79α, and EBV-encoded RNA in situ hybridization, according to the World Health Organization classification for lymphomas9 ; (2) primary site localized in the upper aerodigestive tract; (3) stage I and II disease; (4) no prior therapy for lymphoma; and (5) patients with written informed consent in accordance with the Declaration of Helsinki. Between 2007 and 2009, 69 consecutive patients with newly diagnosed NKTCL of the upper aerodigestive tract were enrolled into this study.
Evaluation and treatment
All patients underwent a standard staging evaluation according to the Ann Arbor staging system, as described previously.10,16 The International Prognostic Index (IPI) was calculated for each patient according to the published criteria.36 All early-stage patients received radiotherapy (n = 36) or radiotherapy followed by chemotherapy (combined modality therapy, n = 33). Stage II patients and stage I patients with one or more adverse factors of IPI or paranasal extension received additional chemotherapy. Extended involved-field at a dose of 50 Gy was administered to patients with NKTCL.10,16,20 The majority of patients (91.3%) received intensity-modulated radiotherapy or 3-dimensional conformal radiotherapy. The chemotherapy regimen consisted of either CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or a CHOP-like regimen.
Specimen collection and DNA extraction
Blood was collected before radiotherapy and at the completion of radiotherapy (5-6 weeks from the start of radiotherapy) in all 69 patients with NKTCL. Blood was also collected from 45 healthy volunteers as negative controls. Plasma EBV-DNA was quantified using quantitative RT-PCR.
Approximately 4 mL venous blood was collected into tubes containing EDTA anticoagulant, incubated at 4°C for 30 minutes, centrifuged for 10 minutes at 1485g, 1.5 mL plasma was collected and frozen at −80°C before further processing. Total plasma cell-free DNA was isolated using the QIAamp Blood Mini Kit (QIAGEN) and eluted in 100-μL sterile, deionized-distilled water. Finally, 2-μL aliquots were used for the quantitative RT-PCR assay.
EBV-DNA plasmid construction and quantitative RT-PCR primer and probes
The BamHI-W assay was performed as previously described to generate an EBV DNA-containing plasmid.27 The primer and probe sequences were as follows: forward, 5′-CCCAACACTCCACCACACC-3′; reverse, 5′-TCTTAGGAGCTGTCCGAGGG-3′; probe, 5′-FAM-CACACACTACACACACCCACCCGTCTC-TAMRA-3′ (Invitrogen). The BamHI-W gene was amplified from plasma DNA isolated from 6 NKTCL patients diagnosed with EBV-positive tumor tissue. Each 25-μL PCR reaction contained 1 μL plasma DNA template, 2.5 μL Premix Gold buffer containing deoxynucleotide triphosphates (Takara), 10 pmol forward primer, 10 pmol reverse primer, 0.5 U TaqGold (Applied Biosystems), and deionized H2O. The PCR assay was performed at 93°C for 5 minutes, followed by 40 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds with a final extension of 72°C for 10 minutes. The PCR product was cloned into the pGM-T vector (Tiangen Biotech), purified using the QIAamp Blood Mini Kit (QIAGEN), and confirmed by sequencing using the ABI 3100 DNA Sequence Detector (Applied Biosystems).
EBV-DNA quantitative RT-PCR
Each 96-well plate included triplicate samples run in parallel with the EBV-DNA plasmid standard curve dilutions. The BamHI-W standard curve spanned 5-logs comprising serial 10-fold dilutions, containing 100-104 copies of EBV-DNA plasmid target per well. EBV-negative healthy volunteers were used as negative controls, and a no template control was run on each plate as a blank control.
The 25-μL real-time quantitative RT-PCR reaction mixture contained 2 μL EBV DNA plasmid or plasma DNA sample, 12.5 μL Premix Gold buffer containing deoxynucleotide triphosphates, 5 pmol forward primer, 5 pmol reverse primer, and 2.5 pmol of the probe, as previously described, in addition to 1.5 U TaqGold and deionized H2O. The quantitative RT-PCR assay was performed over 45 cycles of 93°C for 3 minutes, 93°C for 30 seconds, and 55°C for 45 seconds on the ABI 7500 Fast Real-Time PCR System (Applied Biosystems), according to the manufacturer's instructions. Fluorescence data were collected in real-time and analyzed using the Sequence Detection System Version 1.9 software. The results were expressed as the number of copies of EBV per milliliter of plasma. Samples showing fluorescence signals lower than the signal in the 101 standard were outside the linear range of the assay and considered to be negative.
Statistical analysis
Treatment response was evaluated based on the international response criteria for lymphoma.37 The number of copies of plasma EBV-DNA in the pretreatment and posttreatment samples was compared using the Wilcoxon test. The correlation between pretreatment EBV-DNA levels and clinical characteristics was analyzed using the Mann-Whitney test. The comparison of qualitative data was performed using the χ2 analysis. OS and progression-free survival (PFS) were defined as the time from initial therapy until the date of death from any cause or last follow-up, and as the time from initial therapy until the first event, including disease progression, relapse, or death. Survivals were estimated using the Kaplan-Meier method, and intergroup differences were calculated using the log-rank test. Multivariate analysis was performed using the Cox proportional hazard model to define the independent risk factors for survival rates.
Results
Patient characteristics and baseline of EBV-DNA
The clinical characteristics are listed in Table 1. The ratio of males to females was 1.88:1, with a median age of 39 years. Primary tumors were located in the nasal cavity (n = 58), nasopharynx (n = 6), larynx (n = 2), oropharynx (n = 1), tonsil (n = 1), or oral cavity (n = 1). The majority patients had stage I disease. All patients had a good performance status (Eastern Cooperative Oncology Group [ECOG] score, 0-1), and 65 patients (94.2%) were grouped in the low-risk group with an IPI score of 0 to 1. Encoded RNA in situ hybridization was positive in 86.2% of patients. The median follow-up time for all patients was 32 months (range, 1-58 months).
Clinical characteristics and plasma EBV-DNA levels
| Characteristics . | Patients . | |
|---|---|---|
| No. . | % . | |
| Age, y | ||
| Median (range) | 39 (15-71) | |
| Sex, male | 45 | 65.2 |
| Primary site | ||
| Nasal cavity | 58 | 84.1 |
| Others | 11 | 15.9 |
| B-symptoms | 26 | 37.7 |
| Elevated LDH level | 18 | 26.1 |
| Ann Arbor stage | ||
| I | 58 | 84.1 |
| II | 11 | 15.9 |
| ECOG | ||
| 0 | 39 | 43.5 |
| 1 | 30 | 56.5 |
| IPI | ||
| 0 | 46 | 66.7 |
| 1 | 19 | 27.5 |
| 2 | 4 | 5.8 |
| Pretreatment plasma EBV-DNA | ||
| Detectable | 58 | 84.1 |
| Median level, copies/mL (range) | 491 (0-453 125) | |
| Characteristics . | Patients . | |
|---|---|---|
| No. . | % . | |
| Age, y | ||
| Median (range) | 39 (15-71) | |
| Sex, male | 45 | 65.2 |
| Primary site | ||
| Nasal cavity | 58 | 84.1 |
| Others | 11 | 15.9 |
| B-symptoms | 26 | 37.7 |
| Elevated LDH level | 18 | 26.1 |
| Ann Arbor stage | ||
| I | 58 | 84.1 |
| II | 11 | 15.9 |
| ECOG | ||
| 0 | 39 | 43.5 |
| 1 | 30 | 56.5 |
| IPI | ||
| 0 | 46 | 66.7 |
| 1 | 19 | 27.5 |
| 2 | 4 | 5.8 |
| Pretreatment plasma EBV-DNA | ||
| Detectable | 58 | 84.1 |
| Median level, copies/mL (range) | 491 (0-453 125) | |
Plasma EBV-DNA was detected before treatment in 58 of 69 (84.1%) of patients with NKTCL, but not in any of the 45 healthy controls (P < .001). The median concentration of pretreatment EBV-DNA for all patients was 491 copies/mL (range, 0-453 125 copies/mL).
Correlation between clinical features and pretreatment EBV-DNA levels
To explore the correlation between plasma EBV-DNA load and prognostic factors, the pretreatment concentrations of EBV-DNA were compared according to the clinical features of all patients (Table 2). Patients with a pretreatment EBV-DNA level of > 500 copies/mL or ≤ 500 copies/mL were comparable with respect to age, sex, primary location, performance status, stage, and IPI. However, patients with a pretreatment EBV-DNA > 500 copies/mL were more likely to have B-symptoms (P = .002) and elevated lactate dehydrogenase (LDH) levels (P = .024), compared with those with a pretreatment EBV-DNA concentration of ≤ 500 copies/mL.
Correlation of clinical characteristics and pretreatment plasma EBV-DNA levels
| Characteristics . | Plasma EBV-DNA (copies/mL) . | ||||
|---|---|---|---|---|---|
| ≤ 500, n (%) . | > 500, n (%) . | P . | Median . | P . | |
| Age, y | .373 | .189 | |||
| ≤ 60 | 33 (94.3) | 30 (88.2) | 485 | ||
| > 60 | 2 (5.7) | 4 (11.8) | 5366 | ||
| Sex | .272 | .532 | |||
| Male | 25 (71.4) | 20 (58.8) | 271 | ||
| Female | 10 (28.6) | 14 (41.2) | 539 | ||
| B-symptoms | .002 | .002 | |||
| Presence | 7 (20.0) | 19 (55.9) | 1971 | ||
| Absence | 28 (80.0) | 15 (44.1) | 196 | ||
| Primary site | .703 | .416 | |||
| Nasal cavity | 30 (85.7) | 28 (82.4) | 485 | ||
| Others | 5 (14.3) | 6 (17.6) | 629 | ||
| LDH level | .024 | .003 | |||
| Elevated | 5 (14.3) | 13 (38.2) | 2312 | ||
| Normal | 30 (85.7) | 21 (61.8) | 196 | ||
| Ann Arbor stage | .090 | .089 | |||
| I | 32 (91.4) | 26 (76.5) | 271 | ||
| II | 3 (8.6) | 8 (23.5) | 3390 | ||
| ECOG score | .118 | .581 | |||
| 0 | 12 (34.3) | 18 (52.9) | 653 | ||
| 1 | 23 (65.7) | 16 (47.1) | 262 | ||
| IPI | .061 | .019 | |||
| 0 | 27 (77.1) | 19 (55.9) | 208 | ||
| 1-2 | 8 (22.9) | 15 (44.1) | 3898 | ||
| Characteristics . | Plasma EBV-DNA (copies/mL) . | ||||
|---|---|---|---|---|---|
| ≤ 500, n (%) . | > 500, n (%) . | P . | Median . | P . | |
| Age, y | .373 | .189 | |||
| ≤ 60 | 33 (94.3) | 30 (88.2) | 485 | ||
| > 60 | 2 (5.7) | 4 (11.8) | 5366 | ||
| Sex | .272 | .532 | |||
| Male | 25 (71.4) | 20 (58.8) | 271 | ||
| Female | 10 (28.6) | 14 (41.2) | 539 | ||
| B-symptoms | .002 | .002 | |||
| Presence | 7 (20.0) | 19 (55.9) | 1971 | ||
| Absence | 28 (80.0) | 15 (44.1) | 196 | ||
| Primary site | .703 | .416 | |||
| Nasal cavity | 30 (85.7) | 28 (82.4) | 485 | ||
| Others | 5 (14.3) | 6 (17.6) | 629 | ||
| LDH level | .024 | .003 | |||
| Elevated | 5 (14.3) | 13 (38.2) | 2312 | ||
| Normal | 30 (85.7) | 21 (61.8) | 196 | ||
| Ann Arbor stage | .090 | .089 | |||
| I | 32 (91.4) | 26 (76.5) | 271 | ||
| II | 3 (8.6) | 8 (23.5) | 3390 | ||
| ECOG score | .118 | .581 | |||
| 0 | 12 (34.3) | 18 (52.9) | 653 | ||
| 1 | 23 (65.7) | 16 (47.1) | 262 | ||
| IPI | .061 | .019 | |||
| 0 | 27 (77.1) | 19 (55.9) | 208 | ||
| 1-2 | 8 (22.9) | 15 (44.1) | 3898 | ||
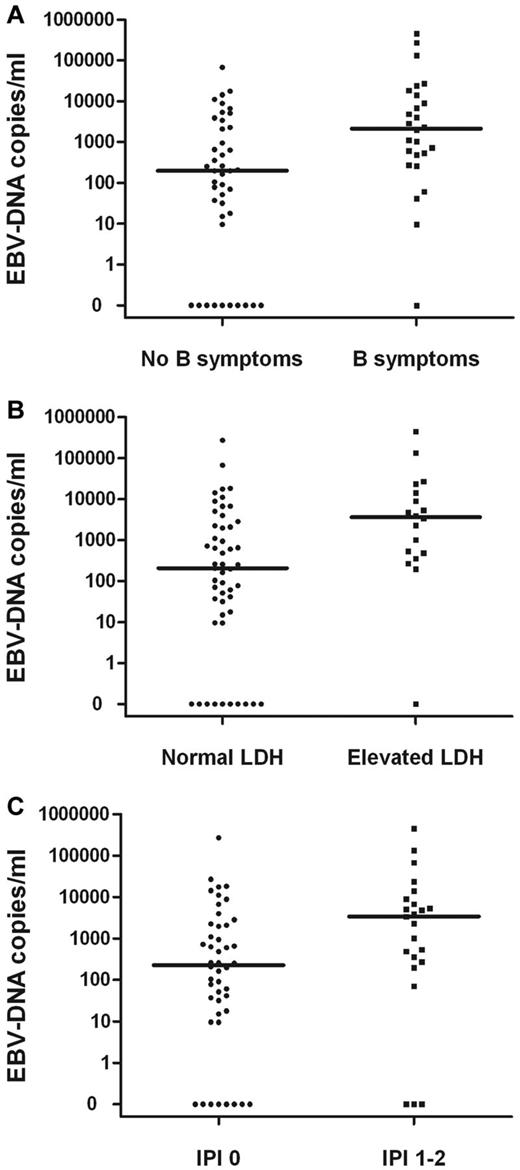
The pretreatment EBV-DNA concentrations for patients with B-symptoms, elevated LDH, or IPI score of 1-2 were significantly higher than those without B-symptoms (P = .002) or with a normal LDH level (P = .003) and an IPI score of 0 (P = .019; Figure 1). Correlations between the pretreatment median concentration of plasma EBV-DNA and clinical characteristics in patients with stage I or II disease are summarized in Table 2. In a subgroup analysis, similar results were observed in stage I patients (data not shown).
Correlations. Pretreatment plasma EBV-DNA copy number and B-symptoms (A), elevated LDH level (B), or IPI (C) in stage I and II patients.
Correlations. Pretreatment plasma EBV-DNA copy number and B-symptoms (A), elevated LDH level (B), or IPI (C) in stage I and II patients.
Changes in plasma EBV-DNA after treatment
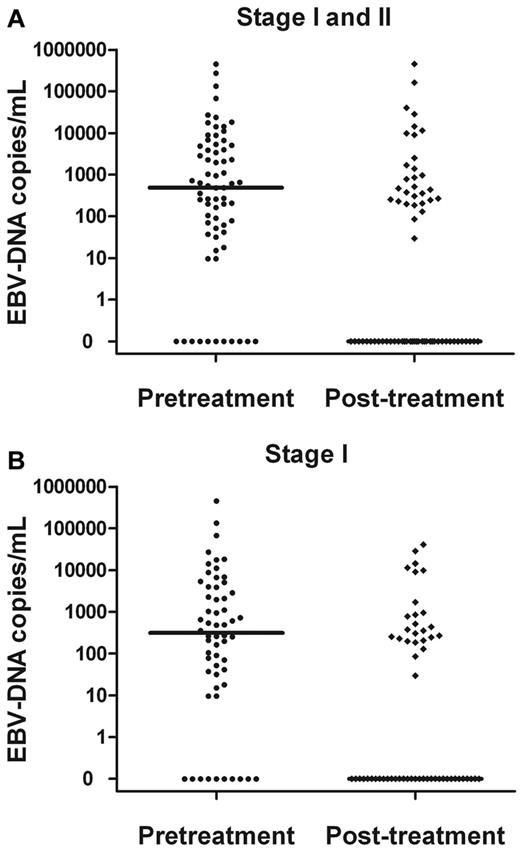
Plasma EBV-DNA levels significantly decreased after treatment (Figure 2). The median concentration of EBV-DNA in all patients was 0 copies/mL after treatment (vs 491 copies/mL pretreatment; P = .003). The median concentration of EBV-DNA for the stage I patients was 314 copies/mL pretreatment, which decreased to 0 copies/mL after treatment (P < .001).
Comparisons. Pretreatment and posttreatment plasma EBV-DNA copy number in stage I and II patients (A) and stage I patients alone (B).
Comparisons. Pretreatment and posttreatment plasma EBV-DNA copy number in stage I and II patients (A) and stage I patients alone (B).
Treatment response and survival
The treatment response was evaluated in each patient: 64 of 69 (92.8%) achieved complete response, 1 of 69 (1.4%) achieved partial response, and 4 patients (5.8%) showed progressive disease.
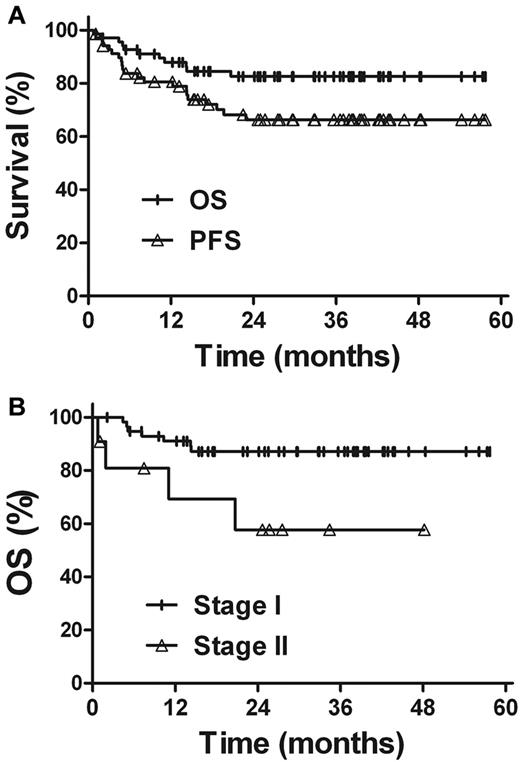
Within the median follow-up time of 32 months, the 3-year OS and PFS rates for all patients were 82.6% and 66.3%, respectively (Figure 3). Univariate analysis revealed that the clinical characteristics, such as sex, age, primary tumor location, B-symptoms, performance status, elevated LDH level, and IPI score, were not prognostic factors for OS and PFS (Table 3). Patients with stage I disease showed better survival rates. The 3-year OS and PFS rates were 87.1% and 69.5% for stage I patients, compared with 57.7% (P = .020) and 48.5% (P = .069) for stage II patients, respectively. The outcome of radiotherapy alone was comparable with that of combined modality therapy in patients with early-stage NKTCL. The 3-year OS and PFS rates were 88.5% and 71.9%, respectively, for radiotherapy alone and 76.6% (P = .313) and 60.3% (P = .344), respectively, for combined modality therapy.
Survival. (A) OS and PFS rates for all patients. (B) OS rate according to the Ann Arbor stage.
Survival. (A) OS and PFS rates for all patients. (B) OS rate according to the Ann Arbor stage.
Univariate analysis of prognostic factors and plasma EBV-DNA in terms of OS and PFS rates
| . | 3-y OS (%) . | P . | 3-y PFS (%) . | P . |
|---|---|---|---|---|
| Age, y | .940 | .904 | ||
| ≤ 60 | 82.6 | 66.7 | ||
| > 60 | 83.3 | 62.5 | ||
| Sex | .387 | .869 | ||
| Male | 85.4 | 65.0 | ||
| Female | 77.0 | 69.3 | ||
| B-symptoms | .995 | .436 | ||
| Presence | 82.6 | 59.6 | ||
| Absence | 83.0 | 70.4 | ||
| Primary site | .870 | .649 | ||
| Nasal cavity | 83.6 | 68.5 | ||
| Others | 74.1 | 49.2 | ||
| LDH level | .290 | .930 | ||
| Elevated | 0.713 | 65.4 | ||
| Normal | 0.855 | 66.4 | ||
| Ann Arbor stage | .020 | .069 | ||
| I | 87.1 | 69.5 | ||
| II | 57.7 | 48.5 | ||
| ECOG score | .202 | .129 | ||
| 0 | 76.0 | 55.8 | ||
| 1 | 88.2 | 77.0 | ||
| IPI | .813 | .430 | ||
| 0 | 81.8 | 63.7 | ||
| 1-2 | 84.0 | 73.0 | ||
| Pretreatment | .002 | .045 | ||
| ≤ 500 copies/mL | 97.1 | 79.0 | ||
| > 500 copies/mL | 66.3 | 52.2 | ||
| After treatment | .031 | .028 | ||
| Undetectable | 92.0 | 77.5 | ||
| Detectable | 69.8 | 50.7 |
| . | 3-y OS (%) . | P . | 3-y PFS (%) . | P . |
|---|---|---|---|---|
| Age, y | .940 | .904 | ||
| ≤ 60 | 82.6 | 66.7 | ||
| > 60 | 83.3 | 62.5 | ||
| Sex | .387 | .869 | ||
| Male | 85.4 | 65.0 | ||
| Female | 77.0 | 69.3 | ||
| B-symptoms | .995 | .436 | ||
| Presence | 82.6 | 59.6 | ||
| Absence | 83.0 | 70.4 | ||
| Primary site | .870 | .649 | ||
| Nasal cavity | 83.6 | 68.5 | ||
| Others | 74.1 | 49.2 | ||
| LDH level | .290 | .930 | ||
| Elevated | 0.713 | 65.4 | ||
| Normal | 0.855 | 66.4 | ||
| Ann Arbor stage | .020 | .069 | ||
| I | 87.1 | 69.5 | ||
| II | 57.7 | 48.5 | ||
| ECOG score | .202 | .129 | ||
| 0 | 76.0 | 55.8 | ||
| 1 | 88.2 | 77.0 | ||
| IPI | .813 | .430 | ||
| 0 | 81.8 | 63.7 | ||
| 1-2 | 84.0 | 73.0 | ||
| Pretreatment | .002 | .045 | ||
| ≤ 500 copies/mL | 97.1 | 79.0 | ||
| > 500 copies/mL | 66.3 | 52.2 | ||
| After treatment | .031 | .028 | ||
| Undetectable | 92.0 | 77.5 | ||
| Detectable | 69.8 | 50.7 |
Prognostic value of plasma EBV-DNA in stage I and II NKTCL
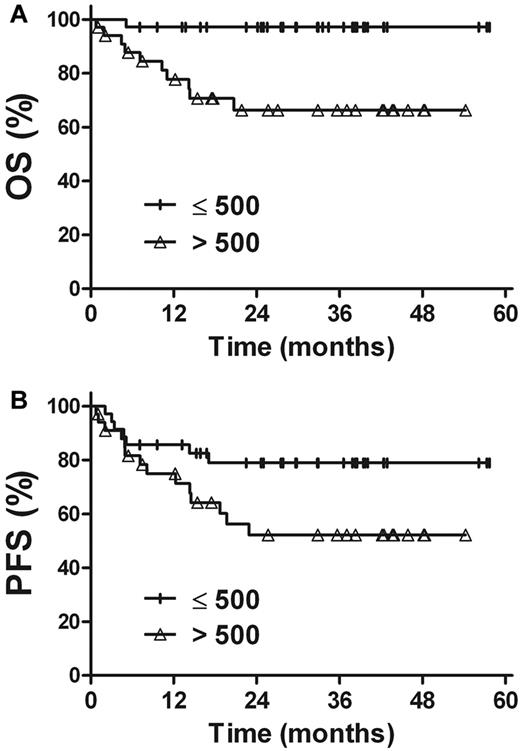
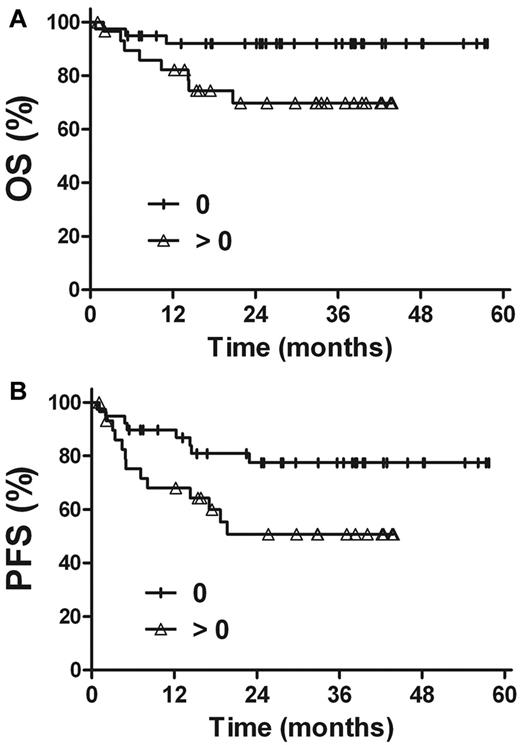
The prognostic value of EBV-DNA was evaluated in patients with stage I and II disease. For patients with a pretreatment EBV-DNA concentration of ≤ 500 copies/mL (n = 35), the 3-year OS and PFS rates were 97.1%, and 79.0%, respectively, compared with 66.3% (P = .002) and 52.2% (P = .045) in patients with a pretreatment EBV-DNA concentration of > 500 copies/mL (n = 34; Figure 4). Similarly, there were improved OS and PFS rates in patients with undetectable EBV-DNA after treatment (n = 39), compared with patients with detectable EBV-DNA after treatment (n = 30). The 3-year OS and PFS rates were 92.0% and 77.5%, respectively, for patients with undetectable EBV-DNA levels after treatment, compared with 69.8% (P = .028) and 50.7% (P = .031) for those with detectable levels of EBV-DNA after treatment (Figure 5).
Survival. OS (A) and PFS (B) rates for stage I and II patients in accordance with the pretreatment plasma EBV-DNA levels.
Survival. OS (A) and PFS (B) rates for stage I and II patients in accordance with the pretreatment plasma EBV-DNA levels.
Survival. OS (A) and PFS (B) rates for stage I and II patients according to the posttreatment plasma EBV-DNA levels.
Survival. OS (A) and PFS (B) rates for stage I and II patients according to the posttreatment plasma EBV-DNA levels.
In the multivariate analysis, the clinical stage (hazard ratio [HR] = 3.736; 95% CI, 1.091-12.795, P = .036) and post-treatment plasma EBV-DNA levels (HR = 3.769; 95% CI, 1.0-14.219, P = .049) were independent prognostic factors for OS, whereas only the posttreatment plasma EBV-DNA level (HR = 2.598; 95% CI, 1.075-6.28, P = .034) was an independent prognostic factor for PFS.
Prognostic value of plasma EBV-DNA in stage I NKTCL
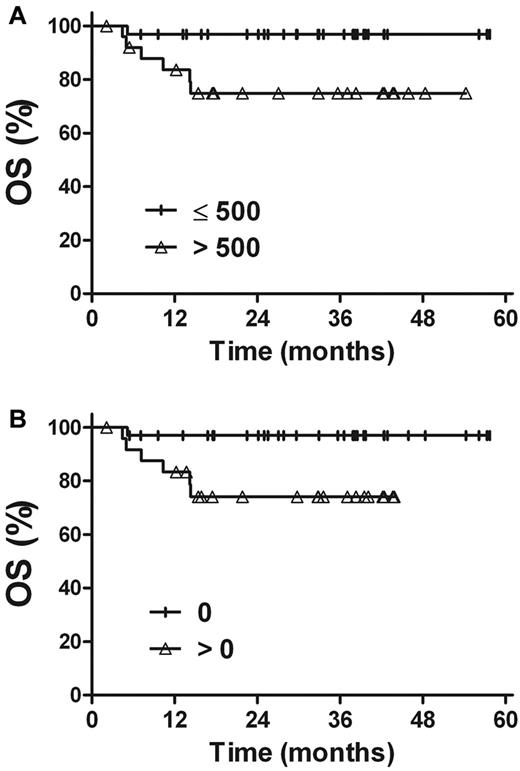
A similar analysis was performed in a large subgroup of stage I patients (Figure 6). The 3-year OS and PFS rates were 96.9% and 80.0%, respectively, for patients with a pretreatment plasma EBV-DNA concentration of ≤ 500 copies/mL (n = 32), compared with 74.8% (P = .019) and 56.1% (P = .103) for patients with pretreatment EBV-DNA levels of > 500 copies/mL (n = 26). The corresponding OS and PFS rates were 97.0% and 79.6% for patients with undetectable EBV-DNA after treatment (n = 33) and 74.1% (P = .015) and 55.7% (P = .044) for patients with detectable EBV-DNA after treatment (n = 25).
OS rate for stage I patients. (A) Pretreatment plasma EBV-DNA levels. (B) Posttreatment plasma EBV-DNA levels.
OS rate for stage I patients. (A) Pretreatment plasma EBV-DNA levels. (B) Posttreatment plasma EBV-DNA levels.
Discussion
NKTCL is rare in Western countries but more frequent in Asia. As in nasopharyngeal carcinoma, the higher incidence of NKTCL in the Chinese population may be the result of a higher prevalence of EBV infection.1,9,27,30,31 Because of the rarity of the disease, limited data exist on the prognostic importance of plasma EBV-DNA levels in NKTCL. The present study, which involved a large series of patients with early-stage NKTCL treated with primary radiotherapy, provides valuable data on treatment outcomes and the clinical value of EBV-DNA levels. We have shown that primary radiotherapy for early-stage NKTCL leads to a high complete response rate (> 90%), accompanied by a significant reduction in plasma EBV-DNA levels. Primary radiotherapy resulted in a favorable prognosis for early stage NKTCL (3-year OS 82.6%), especially in patients with low pretreatment and undetectable EBV-DNA loads after treatment (3-year OS > 90%), which indicated that both pretreatment and posttreatment EBV-DNA levels are important prognostic factors in early-stage NKTCL. In addition, high EBV-DNA levels of pretreatment correlated with B-symptoms, elevated LDH levels, and an IPI score of 1-2, reflecting a large tumor load and adverse prognostic factors.
Although many studies have explored the clinical prognostic factors in NKTCL, few have focused on the use of molecular biomarkers as prognostic predictors.22-24,38-41 Several small cohort of studies have demonstrated that a high EBV-DNA load is associated with advanced stage, poor clinical response, B-symptoms, and elevated LDH levels in NKTCL (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).31-35 Our results confirmed that pretreatment EBV-DNA levels correlate with B-symptoms, elevated LDH levels, and an IPI score of 1-2 in early-stage NKTCL. Interestingly, the median concentrations of EBV-DNA (491 copies/mL) in this series were similar to previous studies in low-risk patients who presented with early-stage disease, in which EBV-DNA was detected at relatively low levels (median, 349 or 659 copies/mL).31,33 In contrast, significantly higher EBV-DNA levels (median, 6.36 × 104 or 6.1 × 107 copies/mL) have been observed in high-risk patients with advanced-stage disease or tumors that are located in the extra-upper aerodigestive tract.32,34
NKTCL is closely associated with EBV infection. The positivity of EBV in the tumor tissue has been shown to be > 90%, and plasma EBV-DNA is detected in the majority (> 80%) of patients with NKTCL.31-33 A similar result was observed in our present study, and pretreatment plasma EBV-DNA was detected in 84.1% of patients with early-stage NKTCL. In contrast, Suzuki et al reported that pretreatment plasma EBV-DNA was detectable in only 43.8% (14 of 32) of patients,35 which was lower than both ours and other previous studies.31-33 Interestingly, in a recent study,42 EBV-DNA was detected in whole blood in 22 of 26 (84.6%) patients who received SMILE (steroid, methotrexate, ifosfamide, L-asparaginase, and etoposide) chemotherapy. The different percentages of detectable pretreatment EBV-DNA in previous studies are probably the result of small sample sizes, sample collection differences, and different experimental assay, in addition to other unknown factors.43 The creation of an international standard for EBV-DNA assay calibration would be an important step in quality assurance.
Generally, the prognosis and selection of the most appropriate treatment are assessed using both patient-related and standard tumor-related characteristics. We have shown that, in early-stage NKTCL patients treated with primary radiotherapy, the tumor stage, presence of paranasal extension, primary location, and IPI scores are the most significant prognostic factors and criteria for treatment decisions.6,10,16,20 Recent studies have revealed that the treatment outcome of primary radiotherapy in early-stage NKTCL is superior to doxorubicin-based chemotherapy.4,7,10,14,15,44 In this study of early-stage NKTCL patients who received radiotherapy with or without chemotherapy, the 3-year OS rate of 82.6% was similar to 2 previous phase 2 studies of concurrent chemoradiotherapy17,18 but was superior to similar EBV-DNA studies that used primary chemotherapy.31,33 Preliminary results in a small cohort of NKTCL patients have shown that patients with high concentrations of pretreatment EBV-DNA and detectable EBV-DNA levels after treatment have an extremely poor survival rate.31-35 In this large series of early-stage patients with a favorable prognosis, a survival difference in patients with various EBV-DNA loads was also observed. The pretreatment EBV-DNA level of ≤ 500 copies/mL and undetectable EBV-DNA levels after treatment predicted better survival rates in patients with early-stage NKTCL. Even in stage I disease, significantly inferior survival rates were associated with high EBV-DNA levels compared with patients with low EBV-DNA levels. In contrast to our series, which included a relatively homogeneous population of early-stage NKTCL patients treated with primary radiotherapy, other studies have demonstrated poorer prognoses in a cohort of chemotherapy-treated patients with heterogeneous histologic subtypes, tumor stages, primary locations, and chemotherapeutic regimens.19,31-35 However, it should be noted that patients with low EBV-DNA levels have demonstrated a favorable prognosis in all studies to date, regardless of disease and treatment heterogeneity, with an OS rate of approximately 95% in this study and 75%-80% in other studies (supplemental Table 1).19,31-35 Therefore, EBV-DNA appears to be a reliable prognostic factor in NKTCL, independent of the treatment strategy or stage; however, additional investigations with larger numbers of patients at all stages with longer follow-up durations are needed to confirm these findings.
Biomarker analysis in NKTCL is challenging as a result of disease heterogeneity, low sample availability, the various treatment modalities used, and the scarcity of validated assays.8,22-24,31-35 Our data suggest that EBV-DNA levels can predict prognosis in localized NKTCL. The clinical application of EBV-DNA as a biomarker will be particularly useful in early-stage NKTCL patients treated with radiotherapy because of the difficulty in postradiation evaluations, inflammatory changes after therapy, and the lack of clinically prognostic predictors in stage I disease.21 The available scope for additional improvement may be limited when the OS rate is already more than 90% in low-risk patients, such as pretreatment EBV-DNA ≤ 500 copies/mL and undetectable EBV-DNA loads after treatment. On the other hand, the OS and PFS were substantially lower in patients with advanced stage disease (stage II-IV) and high pretreatment or posttreatment EBV-DNA levels; therefore, the potential for benefit with experimental treatment strategies may be greater in these patients. Plasma EBV-DNA levels may be used as a stratification factor at presentation and during radiotherapy to identify high-risk patients who may benefit from early systemic therapy or EBV-targeted therapy.19,45,46 Recent studies have demonstrated that L-asparaginase or bortezomib show promising antitumor activity in T-cell lymphomas and EBV-related NKTCL.19,44,47-49 Thus, the integration of innovative systemic therapies as well as appropriate radiotherapy for patients with high baseline EBV-DNA levels or detectable EBV-DNA levels after treatment may further improve the long-term prognosis of these patients.
In conclusion, these results reveal that high plasma EBV-DNA levels correlate with adverse prognostic factors and are a significant predictor of poorer survival in patients with early-stage NKTCL treated with primary radiotherapy. The circulating EBV-DNA level could serve as a valuable biomarker of tumor load to supplement the Ann Arbor staging system for the accurate classification of early-stage NKTCL, in addition to acting as a prognostic factor.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Wei-Zhi Yang, Xue Gai, Jian-Wei Ye, and Yu Chen for technical assistance.
This work was supported by the National Natural Science Foundation of China (30870736 and 81071829).
Authorship
Contribution: L.Y.-X. designed the research, analyzed the data, and wrote the paper; W.Z.-Y. designed the research and analyzed the data; and L.Q.-F., W.H., J.J., W.W.-H., W.S.-L., S.Y.-W., L.Y.-P., F.H., R.H., W.R.-Y., C.B., Z.X.-M., L.N.-N., and Z.L.-Q. made the selection of the cases and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ye-Xiong Li, Department of Radiation Oncology, Cancer Hospital and Institute, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People's Republic of China; e-mail: yexiong@yahoo.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal