Abstract
Despite improvements in first-line therapies, published results on the treatment of relapsed adult acute lymphoblastic leukemia (ALL) show that prognosis is still poor. The aim of the present retrospective analysis of the German Multicenter Study Group for Adult ALL was to identify prognostic factors and options for improvement. A total of 547 patients with a median age of 33 years (range, 15-55) experiencing their first relapse (406 vs 141 shorter or longer than 18 months from diagnosis) were evaluated. The aim of salvage therapy was to achieve a complete remission (CR) with subsequent a stem cell transplantation (SCT). The CR rate (assessed in Philadelphia chromosome– and BCR-ABL–negative ALL without CNS involvement) after the first salvage in relapse after chemotherapy (n = 224) was 42%. After failure of first salvage (n = 82), the CR rate after second salvage was 33%. In relapse after SCT (n = 48) the CR rate after first salvage was 23%. The median overall survival after relapse was 8.4 months and survival was 24% at 3 years. Prognostic factors for survival were relapse localization, response to salvage, performance of SCT, and age. Overall survival appeared superior compared with previously published studies, likely because of the high rate of SCT in the present study (75%). Further improvement may be achieved with earlier relapse detection and experimental approaches in early relapse. The study is registered at www.clinicaltrials.gov as NCT00199056 and NCT00198991.
Introduction
Treatment results for adult acute lymphoblastic leukemia (ALL) have improved considerably in the past decades: complete remission (CR) rates have increased to 85%-90% and overall survival (OS) rates to 40%-50%.1,2 Optimized risk stratification, the integration of stem cell transplantation (SCT), refined chemotherapy, the use of targeted therapies, and optimized supportive care, have all been important developments. However, despite all of these improvements in first-line therapies, at least one-third of standard-risk (SR) patients and up to two-thirds of high-risk (HR) patients eventually experience relapse, which is still a major therapeutic challenge. Several retrospective trials have demonstrated that remission rates after first salvage therapy range from 31%-44%.3-6 Clinical trials with newly registered drugs such as nelarabine or clofarabine in patients who were mostly refractory to first salvage have reported CR rates of 20%-23%.7-9 This is consistent with a retrospective analysis from the MD Anderson Cancer Center showing an 18% response rate in patients who underwent second salvage.10 In particular, patients with early relapse during intensive chemotherapy appear to be highly resistant to any chemotherapy approach. The reported OS rates after relapse, including outcomes after subsequent SCT, were 5%-8%.3-6 Allogeneic SCT is the only curative approach, but it has been performed in less than 50% of ALL patients.3-6 Furthermore, limited data are available on the response to salvage therapy during different stages of disease and in different subtypes of ALL.
In the present study, we analyzed data from a large series of adult patients with relapsed ALL who had been recruited into the consecutive German Multicenter Study Group for Adult ALL (GMALL) studies 06/99 and 07/03 for de novo ALL. In this series, in which a uniform first-line treatment was applied, the major aim was to analyze response and outcome data for subtypes of ALL, to identify prognostic factors for CR and OS, and to evaluate the impact of SCT after relapse. In addition, the analysis aimed to provide a reference for future trials pertaining to relapsed ALL, including pivotal trials with new drugs.
Methods
Study eligibility
The retrospective analysis included patients 15-55 years of age with ALL (patients with mature B-ALL were excluded) who were consecutively treated after first diagnosis within GMALL studies 06/99 and 07/03. A total of 547 patients with first relapse were included in the analysis. The GMALL studies were approved by the institutional review board of the University of Frankfurt (Frankfurt, Germany) and are registered at www.clinicaltrials.gov as NCT00199056 and NCT00198991. All patients gave written informed consent in accordance with the Declaration of Helsinki.
First-line treatment
The first-line treatment was risk adapted and based on intensive cyclical chemotherapy, as described previously.11 During first-line treatment, patients were allocated to risk groups based on conventional prognostic factors. High-risk factors were WBC above 30 000/μL at diagnosis in B-lineage ALL, pro-B-ALL, early or mature T-cell ALL, MLL-AF4/t(4;11) translocation, or no CR after induction I. Patients with any of these factors were allocated to the HR group. HR patients and those with Philadelphia chromosome–positive (Ph+) or BCR-ABL–positive ALL were candidates for allogeneic SCT after achievement of first CR. Myeloablative conditioning was recommended with VP16 and 12 Gy total body irradiation (TBI) for sibling and cyclophosphamide and 12 Gy TBI for matched unrelated SCT. The remaining patients were allocated to the SR group.
Patient cohorts
Different cohorts of patients were defined for the purpose of analysis. Based on first-line treatment, patients who relapsed during or after chemotherapy were analyzed separately from those patients who relapsed after SCT. Salvage therapy was different for patients with CNS involvement (intrathecal therapy), isolated extramedullary involvement, and Ph+/BCR-ABL+ ALL (tyrosine kinase inhibitors). Therefore, these 3 patient groups were excluded from the analysis of response to salvage therapy. The first and second approaches to salvage therapy were analyzed separately. The analysis of prognostic factors was focused on patients with relapse during or after chemotherapy without Ph+/BCR-ABL+ ALL without isolated extramedullary involvement and without CNS involvement to exclude confounding factors and to provide reference data in a well-defined patient population. Time to relapse, an important potential prognostic feature, was stratified at 18 months duration of first remission based on the results from a previous GMALL relapse trial.12 Patients with a remission duration shorter or longer than 18 months were categorized as early or late relapse, respectively.
Relapse treatment and data collection
Patients who relapsed after treatment in the initial study were followed, but the choice of salvage therapy was left to the discretion of the treating physicians. Limited data on therapy after relapse and performance of allogeneic SCT were collected, but the survival data were always recorded.
The choice of relapse treatment was mainly influenced by the duration of the first remission based on published data on its prognostic impact12 and on immunophenotype. In B-precursor ALL, the most frequently used regimens for early relapse were consolidation protocol I (high-dose cytarabine, high-dose methotrexate, etoposide, vindesine, and dexamethasone) or FLAG-IDA (high-dose cytarabine, fludarabine, and idarubicin). CLAEG (high-dose cytarabine, etoposide, and cladribine; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and nelarabine were most frequently used in T-lineage ALL. The most frequently used regimen for late relapse was a repetition of standard induction. For allogeneic SCT, a myeloablative conditioning regimen based on TBI was recommended, but details of regimens were not collected.
CR after salvage therapy was defined according to conventional criteria with a BM blast count below 5% and no peripheral blast cells or extramedullary manifestations. Recovery of peripheral blood counts may not have been reached in all cases, particularly if patients received SCT shortly after achievement of a marrow CR. Death during relapse therapy and failure to respond to relapse therapy were summarized as “failure.”
Statistical analysis
All patients were registered at the GMALL Study Center, where statistical analysis was performed with SAS Version 8.02 for PC. For all analyses, P ≤ .05 was considered statistically significant. Statistical analysis was mainly descriptive. Data are presented in tables, with percentage proportions for categorical variables and medians for continuous variables. Statistical comparisons were performed with the χ2 test for categorical variables and with the Wilcoxon test for continuous variables. The survival analysis was based on the Kaplan-Meier method. The survival time after relapse was calculated from the time point of relapse until death or the last follow-up. The median observation time in patients surviving after relapse was 23 months. Survival rates are given as probabilities of survival at 3 or 5 years, with a 95% confidence interval. The log-rank test was used to compare survival curves. Multivariate analysis was performed with the Cox logistical regression.
Results
Patients and patient characteristics
A total of 547 patients with first relapse were evaluable. The median age was 33 years (range, 15-55); 393 patients (72%) had B-lineage ALL; 150 (28%) had T-lineage ALL; and 94 patients had Ph+ ALL (17%). The median time to relapse was 162 days (range, 19-2838 days). A total of 432 (79%) patients had an early relapse, and 115 (21%) had a late relapse. The majority of patients (92%) had BM involvement at relapse; 6% had CNS involvement with or without other involvement, and 6% experienced isolated extramedullary relapse (Table 1).
Clinical characteristics
| Characteristic . | Relapse during/after chemotherapy . | Relapse after SCT . | P . | Total . |
|---|---|---|---|---|
| n* | 378 | 169 | 547 | |
| Age† | ||||
| Median age, y | 32 | 34 | > .05 | 33 |
| 15-25 | 129 (34%) | 50 (30%) | 179 (33%) | |
| 26-45 | 173 (46%) | 84 (50%) | 257 (47%) | |
| 46-55 | 76 (20%) | 35 (20%) | 111 (20%) | |
| Sex | ||||
| Male | 242 (64%) | 101 (60%) | > .05 | 343 (63%) |
| Female | 136 (36%) | 68 (40%) | 204 (37%) | |
| WBCs/μL† | ||||
| < 30 000 | 250 (69%) | 100 (62%) | > .05 | 350 (67%) |
| > 30 000 | 113 (31%) | 61 (38%) | 174 (33%) | |
| Immunophenotype† | ||||
| Pro-B | 18 (5%) | 18 (11%) | < .0001 | 36 (7%) |
| Pre-B/c | 253 (67%) | 104 (62%) | 357 (66%) | |
| Early T | 12 (3%) | 33 (20%) | 45 (8%) | |
| Mature T | 16 (4%) | 11 (7%) | 27 (5%) | |
| Thymic | 77 (20%) | 1 (1%) | 78 (14%) | |
| Risk group† | ||||
| Standard | 258 (68%) | 14 (8%) | < .0001 | 272 (50%) |
| High | 87 (23%) | 94 (56%) | 181 (33%) | |
| Very high (Ph+) | 33 (9%) | 61 (36%) | 94 (17%) | |
| Time from diagnosis to relapse, mo | ||||
| Median (range, d) | 288 (22-2838) | 122 (19-666) | < .0001 | 162 (19-2838) |
| < 18 | 268 (71%) | 164 (97%) | 432 (79%) | |
| > 18 | 110 (29%) | 5 (3%) | 115 (21%) | |
| Site of relapse | ||||
| BM only ± other | 317 (89%) | 111 (85%) | > .05 | 428 (88%) |
| BM ± CNS ± other | 12 (3%) | 6 (5%) | 18 (4%) | |
| Isolated CNS | 7 (2%) | 4 (3%) | 11 (2%) | |
| Isolated other extramedullary‡ | 22 (6%) | 10 (7%) | 32 (6%) |
| Characteristic . | Relapse during/after chemotherapy . | Relapse after SCT . | P . | Total . |
|---|---|---|---|---|
| n* | 378 | 169 | 547 | |
| Age† | ||||
| Median age, y | 32 | 34 | > .05 | 33 |
| 15-25 | 129 (34%) | 50 (30%) | 179 (33%) | |
| 26-45 | 173 (46%) | 84 (50%) | 257 (47%) | |
| 46-55 | 76 (20%) | 35 (20%) | 111 (20%) | |
| Sex | ||||
| Male | 242 (64%) | 101 (60%) | > .05 | 343 (63%) |
| Female | 136 (36%) | 68 (40%) | 204 (37%) | |
| WBCs/μL† | ||||
| < 30 000 | 250 (69%) | 100 (62%) | > .05 | 350 (67%) |
| > 30 000 | 113 (31%) | 61 (38%) | 174 (33%) | |
| Immunophenotype† | ||||
| Pro-B | 18 (5%) | 18 (11%) | < .0001 | 36 (7%) |
| Pre-B/c | 253 (67%) | 104 (62%) | 357 (66%) | |
| Early T | 12 (3%) | 33 (20%) | 45 (8%) | |
| Mature T | 16 (4%) | 11 (7%) | 27 (5%) | |
| Thymic | 77 (20%) | 1 (1%) | 78 (14%) | |
| Risk group† | ||||
| Standard | 258 (68%) | 14 (8%) | < .0001 | 272 (50%) |
| High | 87 (23%) | 94 (56%) | 181 (33%) | |
| Very high (Ph+) | 33 (9%) | 61 (36%) | 94 (17%) | |
| Time from diagnosis to relapse, mo | ||||
| Median (range, d) | 288 (22-2838) | 122 (19-666) | < .0001 | 162 (19-2838) |
| < 18 | 268 (71%) | 164 (97%) | 432 (79%) | |
| > 18 | 110 (29%) | 5 (3%) | 115 (21%) | |
| Site of relapse | ||||
| BM only ± other | 317 (89%) | 111 (85%) | > .05 | 428 (88%) |
| BM ± CNS ± other | 12 (3%) | 6 (5%) | 18 (4%) | |
| Isolated CNS | 7 (2%) | 4 (3%) | 11 (2%) | |
| Isolated other extramedullary‡ | 22 (6%) | 10 (7%) | 32 (6%) |
Total number of patients may vary for the different subgroups depending on the number of patients with evaluable data.
At first diagnosis.
Locations of extramedullary involvement were: mediastinum (n = 6), testis (n = 5), lymph nodes (n = 5), breast (n = 4), skin (n = 4), bone (n = 3), kidney (n = 2), liver (n = 2), nasopharynx/tonsils (n = 2), pleura/pericardium (n = 2), prostate (n = 1), paravertebral (n = 1), epidural (n = 1), parotis (n = 1), retina (n = 1), and lung (n = 1).
A total of 378 patients (69%) relapsed during or after first-line chemotherapy, whereas 169 patients (31%) relapsed after SCT performed during the first CR. A significantly higher proportion of patients with relapse after SCT had high-risk features because SCT was predominantly performed in HR or Ph+ patients (Table 1). This included patients with high-risk immunophenotypes such as pro-B-ALL (11% vs 5%) or early T-ALL (20% vs 3%; P < .0001), and patients allocated to the HR or very-high-risk group (92% vs 32%; P < .0001) for first-line treatment. The time to relapse was significantly shorter in patients who relapsed after SCT compared with those who relapsed during or after chemotherapy (122 vs 288 days; P < .0001). Few patients experienced late relapse after SCT compared with chemotherapy (3% vs 29%; P < .0001).
Response to salvage therapy
The response to salvage therapy was analyzed in patients with evaluable information about salvage type and who did not have CNS involvement, isolated extramedullary involvement, or Ph+ ALL. Data are reported separately for the first and second salvage approaches and for relapse after chemotherapy and relapse after SCT.
Response to first salvage in relapse during or after chemotherapy.
A total of 224 patients were evaluable; 160 (71%) had early relapse and 64 (29%) had late relapse. The overall CR rate was 42% after first salvage, 36% for early relapse, and 58% for late relapse (P = .003). The overall CR rate after first salvage was 46% for B-precursor ALL and 34% for T-ALL (P > .05). The CR rate was significantly lower for early relapse compared with late relapse (39% vs 64%; P = .003) in B-precursor ALL, whereas the difference was not significant in T-ALL (30% vs 42%; P > .05). Because of these differences and the fact that different salvage regimens were preferred, B-precursor and T-ALL were analyzed separately.
Early relapses in patients with B-precursor ALL were most frequently treated with the consolidation I regimen or with FLAG-IDA and CR could be obtained in 29% and 42%, respectively. This difference was not statistically significant. Early T-ALL relapse patients were most frequently treated with CLAEG, resulting in a 19% CR rate. For late relapse in B-precursor ALL, the most frequently used regimens were the GMALL standard induction regimen and FLAG-IDA. GMALL standard induction therapy induced a significantly higher CR rate (88% vs 29%; P < .0001). In late relapse of T-ALL, the most frequent regimen was CLAEG, which induced a 22% CR rate (Table 2).
Response to first salvage therapy in patients with relapse during/after chemotherapy
| . | Total . | B-lineage . | T-lineage . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n . | CR . | P† . | n . | CR . | P† . | n . | CR . | P† . | |
| 224 . | 95 (42%)* . | 159 . | 73 (46%)* . | 65 . | 22 (34%)* . | ||||
| Early relapse | 160 | 58 (36%) | > .05 | 114 | 44 (39%) | > .05 | 46 | 14 (30%) | > .05 |
| Consolidation I | 47 | 13 (28%) | 38 | 11 (29%) | 9 | 2 | |||
| FLAG-IDA | 39 | 16 (41%) | 38 | 16 (42%) | 1 | 0 | |||
| CLAEG | 16 | 3 (19%) | 0 | 0 | 16 | 3 (19%) | |||
| Standard induction | 9 | 3 | 8 | 2 | 1 | 1 | |||
| HDAC ± Mitox | 9 | 4 | 5 | 2 | 4 | 2 | |||
| HDMTX | 7 | 3 | 3 | 1 | 4 | 2 | |||
| Other chemotherapy | 15 | 6 (40%) | 8 | 4 | 7 | 2 | |||
| SCT in relapse‡ | 18 | 10 (56%) | 14 | 8 (57%) | 4 | 2 | |||
| Late relapse | 64 | 37 (58%) | < .0001 | 45 | 29 (64%) | .0003 | 19 | 8 (42%) | > .05 |
| CLAEG | 9 | 2 | 9 | 2 | |||||
| Standard induction | 30 | 27 (90%) | 27 | 24 (88%) | 3 | 3 | |||
| SCT in relapse | 1 | 1 | 1 | 1 | 0 | 0 | |||
| FLAG-IDA | 15 | 4 (27%) | 14 | 4 (29%) | 1 | 0 | |||
| HDAC ± Mitox | 1 | 0 | 1 | 0 | 0 | 0 | |||
| Other | 8 | 3 | 2 | 0 | 6 | 3 | |||
| . | Total . | B-lineage . | T-lineage . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n . | CR . | P† . | n . | CR . | P† . | n . | CR . | P† . | |
| 224 . | 95 (42%)* . | 159 . | 73 (46%)* . | 65 . | 22 (34%)* . | ||||
| Early relapse | 160 | 58 (36%) | > .05 | 114 | 44 (39%) | > .05 | 46 | 14 (30%) | > .05 |
| Consolidation I | 47 | 13 (28%) | 38 | 11 (29%) | 9 | 2 | |||
| FLAG-IDA | 39 | 16 (41%) | 38 | 16 (42%) | 1 | 0 | |||
| CLAEG | 16 | 3 (19%) | 0 | 0 | 16 | 3 (19%) | |||
| Standard induction | 9 | 3 | 8 | 2 | 1 | 1 | |||
| HDAC ± Mitox | 9 | 4 | 5 | 2 | 4 | 2 | |||
| HDMTX | 7 | 3 | 3 | 1 | 4 | 2 | |||
| Other chemotherapy | 15 | 6 (40%) | 8 | 4 | 7 | 2 | |||
| SCT in relapse‡ | 18 | 10 (56%) | 14 | 8 (57%) | 4 | 2 | |||
| Late relapse | 64 | 37 (58%) | < .0001 | 45 | 29 (64%) | .0003 | 19 | 8 (42%) | > .05 |
| CLAEG | 9 | 2 | 9 | 2 | |||||
| Standard induction | 30 | 27 (90%) | 27 | 24 (88%) | 3 | 3 | |||
| SCT in relapse | 1 | 1 | 1 | 1 | 0 | 0 | |||
| FLAG-IDA | 15 | 4 (27%) | 14 | 4 (29%) | 1 | 0 | |||
| HDAC ± Mitox | 1 | 0 | 1 | 0 | 0 | 0 | |||
| Other | 8 | 3 | 2 | 0 | 6 | 3 | |||
Patients with evaluable information on the type of salvage therapy, without CNS involvement and with Ph/BCR-ABL–negative ALL.
HDAC indicates high-dose cytarabine; HDMTX, high-dose methotrexate; and Mitox, mitoxantrone.
No percentage was calculated in subgroups with total number of cases less than 10.
χ2 test.
Patients received SCT as their salvage treatment; and CR rate indicates the remission rate after SCT.
Response to second salvage in relapse during or after chemotherapy.
In patients failing to achieve CR after first salvage, data for the response to second salvage therapy were available for 82 patients. The overall CR rate was 33% after second salvage, 25% in B-lineage ALL, and 44% in T-ALL (P = .07; Table 3).
Response to second salvage therapy in patients with relapse during/after chemotherapy
| . | Total . | B-lineage . | T-lineage . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n . | CR . | P . | n . | CR . | P . | n . | CR . | P . | |
| 82 . | 27 (33%)* . | 48 . | 12 (25%)* . | 34 . | 15 (44%)* . | ||||
| FLAG-IDA | 10 | 2 (20%) | > .05 | 9 | 1 | > .05 | 1 | 1 | > .05 |
| CLAEG | 4 | 1 | 0 | 0 | 4 | 1 | |||
| Nelarabine | 16 | 8 (50%) | 0 | 0 | 16 | 8 (50%) | |||
| HDAC ± Mitox | 4 | 0 | 3 | 0 | 1 | 0 | |||
| SCT in relapse† | 26 | 8 (31%) | 22 | 7 (32%) | 4 | 1 | |||
| Other | 22 | 8 (36%) | 14 | 4 (29%) | 8 | 4 | |||
| . | Total . | B-lineage . | T-lineage . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n . | CR . | P . | n . | CR . | P . | n . | CR . | P . | |
| 82 . | 27 (33%)* . | 48 . | 12 (25%)* . | 34 . | 15 (44%)* . | ||||
| FLAG-IDA | 10 | 2 (20%) | > .05 | 9 | 1 | > .05 | 1 | 1 | > .05 |
| CLAEG | 4 | 1 | 0 | 0 | 4 | 1 | |||
| Nelarabine | 16 | 8 (50%) | 0 | 0 | 16 | 8 (50%) | |||
| HDAC ± Mitox | 4 | 0 | 3 | 0 | 1 | 0 | |||
| SCT in relapse† | 26 | 8 (31%) | 22 | 7 (32%) | 4 | 1 | |||
| Other | 22 | 8 (36%) | 14 | 4 (29%) | 8 | 4 | |||
Patients with evaluable information about the type of salvage therapy, without CNS involvement and with Ph/BCR-ABL–negative ALL.
HDAC indicates high-dose cytarabine; and Mitox, mitoxantrone.
No percentage was calculated in subgroups with total number of cases less than 10.
Patients received SCT as their first salvage treatment; and CR rate indicates the remission rate after SCT.
Response to first salvage in relapse after SCT.
For 48 patients who had received allogeneic SCT in first remission, data for the response to first salvage therapy in relapse after SCT were available. The overall CR rate was 25%. The most frequently used regimens in B-precursor ALL were FLAG-IDA and repeated induction (29% vs 40% CR rate; P > .05). In T-ALL, nelarabine induced CRs in 3 of 8 patients (Table 4).
Response to first salvage therapy in patients with relapse after SCT
| . | Total . | B-lineage . | T-lineage . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n . | CR . | P . | n . | CR . | P . | n . | CR . | P . | |
| 48 . | 11 (23%)* . | 24 . | 6 (25%)* . | 24 . | 5 (21%)* . | ||||
| FLAG-IDA | 14 | 4 (29%) | > .05 | 10 | 4 (40%) | > .05 | 4 | 0 | > .05 |
| Induction | 10 | 4 (40%) | 6 | 2 | 4 | 2 | |||
| CLAEG | 3 | 0 | 3 | 0 | |||||
| Nelarabine | 8 | 3 | 8 | 3 | |||||
| HDAC ± Mitox | 1 | 0 | 1 | 0 | |||||
| Other | 9 | 0 | 8 | 0 | 1 | 0 | |||
| 2nd SCT in relapse† | 3 | 0 | 3 | 0 | |||||
| . | Total . | B-lineage . | T-lineage . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n . | CR . | P . | n . | CR . | P . | n . | CR . | P . | |
| 48 . | 11 (23%)* . | 24 . | 6 (25%)* . | 24 . | 5 (21%)* . | ||||
| FLAG-IDA | 14 | 4 (29%) | > .05 | 10 | 4 (40%) | > .05 | 4 | 0 | > .05 |
| Induction | 10 | 4 (40%) | 6 | 2 | 4 | 2 | |||
| CLAEG | 3 | 0 | 3 | 0 | |||||
| Nelarabine | 8 | 3 | 8 | 3 | |||||
| HDAC ± Mitox | 1 | 0 | 1 | 0 | |||||
| Other | 9 | 0 | 8 | 0 | 1 | 0 | |||
| 2nd SCT in relapse† | 3 | 0 | 3 | 0 | |||||
Patients with evaluable information about the type of salvage therapy, without CNS involvement and with Ph/BCR-ABL–negative ALL.
HDAC indicates high-dose cytarabine; and Mitox, mitoxantrone.
No percentage was calculated in subgroups with total number of cases less than 10.
Patient received SCT as their first salvage treatment; and CR rate indicates the remission rate after SCT.
Overall outcome after relapse
The median OS in 547 relapse patients was 8.6 months, with a survival probability of 24% ± 2% at 3 years (supplemental Figure 1A). The median survival after relapse during or after chemotherapy compared with relapse after SCT was 10 months versus 5.8 months, and the probability of survival was 28% ± 3% versus 15% ± 3% at 3 years (P < .0001; Figure 1). Patients with BM relapse with or without additional relapse localizations had an OS of 23% ± 2% at 3 years compared with patients with CNS involvement (27% ± 10%) and patients with other isolated extramedullary relapses (47% ± 10%; P = .02 for the 3 groups; supplemental Figure 1B).
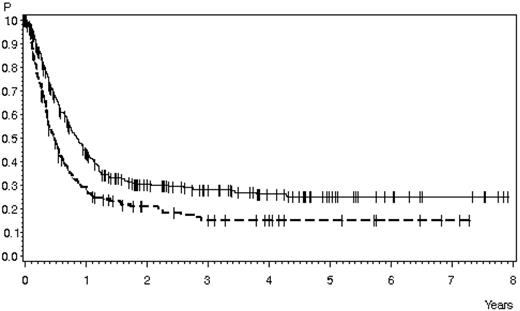
Survival in patients with relapsed ALL according to first-line therapy. Relapse during or after chemotherapy, n = 378 (solid line), 28% ± 3% after 3 years, 25% ± 3% after 5 years; median 10 months; relapse after SCT, n = 169 (dashed line), 15% ± 3% after 3 and 5 years, median 5.8 months (P < .0001).
Survival in patients with relapsed ALL according to first-line therapy. Relapse during or after chemotherapy, n = 378 (solid line), 28% ± 3% after 3 years, 25% ± 3% after 5 years; median 10 months; relapse after SCT, n = 169 (dashed line), 15% ± 3% after 3 and 5 years, median 5.8 months (P < .0001).
Prognostic factors
The prognostic factors for outcomes after relapse were analyzed in patients with relapse during or after chemotherapy and without CNS involvement, isolated extramedullary involvement, or Ph+ ALL.
Prognostic factors for CR.
CR after first salvage was influenced by age, with CR rates decreasing from 53% in patients 25 years of age or younger to 37% in patients older than 25 years (P = .02). CR rates were also significantly different for patients with early and late relapse (36% vs 58%; P = .003). Other factors had no significant impact on CR (Table 5).
Prognostic factors for achievement of CR and survival
| . | Total n . | CR* . | P . | n . | Survival† . | P . |
|---|---|---|---|---|---|---|
| Total | 224 | 94 (42%) | 291 | 29% ± 3% | ||
| Sex | ||||||
| Male | 141 | 59 (42%) | > .05 | 181 | 30% ± 4% | > .05 |
| Female | 83 | 36 (43%) | 110 | 26% ± 5% | ||
| Age, y | ||||||
| 15-25 | 75 | 40 (53%) | .06 | 107 | 38% ± 5% | < .0001 |
| 26-45 | 109 | 41 (38%) | .02§ | 129 | 28% ± 4% | |
| 46-55 | 49 | 14 (35%) | 55 | 12% ± 5% | ||
| WBCs/μL‡ | ||||||
| < 30 000 | 146 | 68 (47%) | > .05 | 201 | 29% ± 4% | > .05 |
| > 30 000 | 72 | 25 (35%) | 81 | 28% ± 5% | ||
| Risk group‡ | ||||||
| Standard | 164 | 74 (45%) | > .05 | 217 | 32% ± 4% | .01 |
| High | 60 | 21 (35%) | 74 | 20% ± 5% | ||
| Subtype | ||||||
| B-lineage | 159 | 73 (46%) | > .05 | 215 | 29% ± 3% | > .05 |
| T-lineage | 65 | 22 (34%) | 76 | 30% ± 6% | ||
| Subtype (detail) | ||||||
| C/pre-B | 150 | 72 (48%) | > .05 | 201 | 31% ± 4% | > .05 |
| Pro B | 9 | 1 (11%) | 14 | 0% | ||
| Early T | 9 | 3 (33%) | 11 | 9% ± 9% | ||
| Mature T | 11 | 2 (18%) | 12 | 17% ± 11% | ||
| Thymic T | 44 | 17 (39%) | 52 | 38% ± 7% | ||
| Time to relapse, mo | ||||||
| < 18 | 160 | 58 (36%) | .003 | 200 | 22% ± 3% | < .0001 |
| > 18 | 64 | 37 (58%) | 91 | 43% ± 6% | ||
| Response to first salvage | ||||||
| CR | 95 | 47% ± 6% | < .0001 | |||
| No CR | 129 | 13% ± 3% | ||||
| Response to second salvage‡ | ||||||
| CR | 27 | 35% ± 9% | .0003 | |||
| No CR | 55 | 8% ± 4% |
| . | Total n . | CR* . | P . | n . | Survival† . | P . |
|---|---|---|---|---|---|---|
| Total | 224 | 94 (42%) | 291 | 29% ± 3% | ||
| Sex | ||||||
| Male | 141 | 59 (42%) | > .05 | 181 | 30% ± 4% | > .05 |
| Female | 83 | 36 (43%) | 110 | 26% ± 5% | ||
| Age, y | ||||||
| 15-25 | 75 | 40 (53%) | .06 | 107 | 38% ± 5% | < .0001 |
| 26-45 | 109 | 41 (38%) | .02§ | 129 | 28% ± 4% | |
| 46-55 | 49 | 14 (35%) | 55 | 12% ± 5% | ||
| WBCs/μL‡ | ||||||
| < 30 000 | 146 | 68 (47%) | > .05 | 201 | 29% ± 4% | > .05 |
| > 30 000 | 72 | 25 (35%) | 81 | 28% ± 5% | ||
| Risk group‡ | ||||||
| Standard | 164 | 74 (45%) | > .05 | 217 | 32% ± 4% | .01 |
| High | 60 | 21 (35%) | 74 | 20% ± 5% | ||
| Subtype | ||||||
| B-lineage | 159 | 73 (46%) | > .05 | 215 | 29% ± 3% | > .05 |
| T-lineage | 65 | 22 (34%) | 76 | 30% ± 6% | ||
| Subtype (detail) | ||||||
| C/pre-B | 150 | 72 (48%) | > .05 | 201 | 31% ± 4% | > .05 |
| Pro B | 9 | 1 (11%) | 14 | 0% | ||
| Early T | 9 | 3 (33%) | 11 | 9% ± 9% | ||
| Mature T | 11 | 2 (18%) | 12 | 17% ± 11% | ||
| Thymic T | 44 | 17 (39%) | 52 | 38% ± 7% | ||
| Time to relapse, mo | ||||||
| < 18 | 160 | 58 (36%) | .003 | 200 | 22% ± 3% | < .0001 |
| > 18 | 64 | 37 (58%) | 91 | 43% ± 6% | ||
| Response to first salvage | ||||||
| CR | 95 | 47% ± 6% | < .0001 | |||
| No CR | 129 | 13% ± 3% | ||||
| Response to second salvage‡ | ||||||
| CR | 27 | 35% ± 9% | .0003 | |||
| No CR | 55 | 8% ± 4% |
Patients with relapse during or after chemotherapy and without Ph+/BCR-ABL+ ALL or CNS involvement.
CR rates were calculated for 224 patients with data on their response to first salvage therapy.
Survival rates are shown as probability of survival ± SD at 3 years.
At first diagnosis.
CR rates in patients younger and older than 25 years: 53% versus 37%, respectively.
¶Calculated for 82 patients with data on their response to second salvage therapy.
Prognostic factors for survival after relapse.
Survival 3 years after relapse was influenced by various factors. It significantly decreased with increasing age, from 38% ± 5% for patients 15-25 years of age to 28% ± 4% for patients 26-45 years of age to 12% ± 5% for patients 46-55 years of age (P < .0001; Figure 2A). Survival was also significantly poorer in patients with early relapse compared with later relapse (22% ± 3% vs 43% ± 6%; P < .0001; Figure 2B). Different cutoff points for time to relapse were analyzed, and all showed significant differences, with CR rates and survival rates decreasing with shorter remission duration and increasing with longer remission duration (supplemental Table 5A). The patient's risk group at the time of first diagnosis also influenced OS, whereas sex, WBC count, and ALL subtype were not significantly correlated with outcome (Table 5).
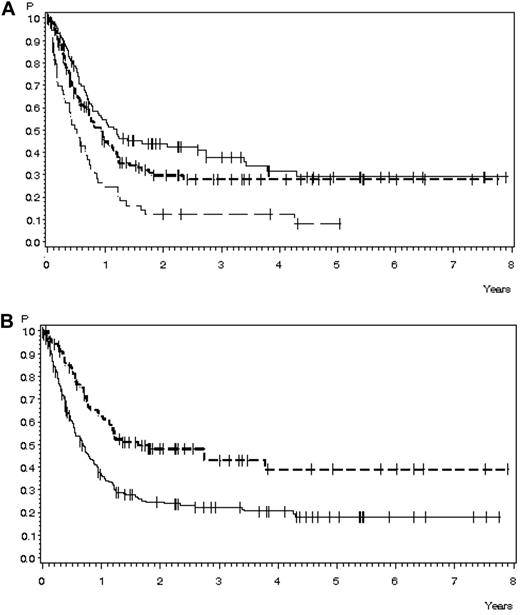
OS in 291 patients with relapse during or after chemotherapy, no Ph+ ALL, and no CNS involvement for whom data were available. (A) OS according to age: 15-25 years of age, n = 107 (solid line), 38% ± 2% after 3 years, median 14.4 months; 26-45 years of age, n = 129 (short dashed line), 28% ± 4% after 3 years, median 28.5 months; and 46-55 years of age, n = 55 (long dashed line), 12% ± 5% after 3 years, median 6.1 months (P < .0001). (B) OS according to time to relapse: < 18 months, n = 200 (solid line), 22% ± 3% after 3 years, median 8.3 months; > 18 months, n = 91 (dashed line), 43% ± 6% after 3 years, median 19.7 months (P < .0001).
OS in 291 patients with relapse during or after chemotherapy, no Ph+ ALL, and no CNS involvement for whom data were available. (A) OS according to age: 15-25 years of age, n = 107 (solid line), 38% ± 2% after 3 years, median 14.4 months; 26-45 years of age, n = 129 (short dashed line), 28% ± 4% after 3 years, median 28.5 months; and 46-55 years of age, n = 55 (long dashed line), 12% ± 5% after 3 years, median 6.1 months (P < .0001). (B) OS according to time to relapse: < 18 months, n = 200 (solid line), 22% ± 3% after 3 years, median 8.3 months; > 18 months, n = 91 (dashed line), 43% ± 6% after 3 years, median 19.7 months (P < .0001).
Response to salvage therapy as a prognostic factor was analyzed in subsets of patients for whom data were available. OS was 47% ± 6% for patients with CR after first salvage, compared with 13% ± 3% for failure patients (P < .0001; Figure 3A). For B-precursor ALL, survival was 46% ± 6% when CR was achieved after first salvage, compared with 8% ± 3% for failure patients (P < .0001). For T-ALL, survival rates were 50% ± 11% and 21% ± 6% (P = .003), respectively (supplemental Figure 3C).
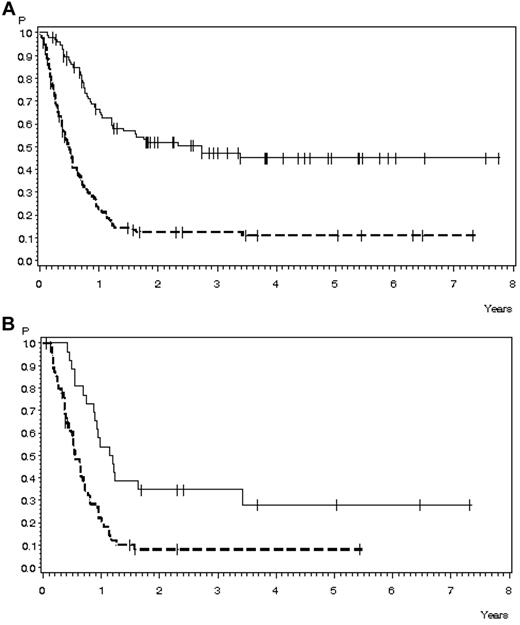
OS in 224 patients with relapse during or after chemotherapy, Ph−/BCR-ABL− ALL, and no CNS involvement for whom data on salvage therapy were available. (A) OS according to response to first salvage: CR, n = 95 (solid line), 47% ± 6% after 3 years, median 33.3 months; no CR, n = 129 (dashed line); 13% ± 3% after 3 years, median 5.8 months (P < .0001). (B) OS according to response to second salvage: CR, n = 27 (solid line), 35% ± 9% after 3 years, median 14.2 months; no CR, n = 55 (dashed line), 8% ± 4% after 3 years, median 6.6 months (P = .0003).
OS in 224 patients with relapse during or after chemotherapy, Ph−/BCR-ABL− ALL, and no CNS involvement for whom data on salvage therapy were available. (A) OS according to response to first salvage: CR, n = 95 (solid line), 47% ± 6% after 3 years, median 33.3 months; no CR, n = 129 (dashed line); 13% ± 3% after 3 years, median 5.8 months (P < .0001). (B) OS according to response to second salvage: CR, n = 27 (solid line), 35% ± 9% after 3 years, median 14.2 months; no CR, n = 55 (dashed line), 8% ± 4% after 3 years, median 6.6 months (P = .0003).
In patients with failure after first salvage, OS was 35% ± 9% when CR was achieved after second salvage and 8% ± 4% for failure cases (P = .0003; Figure 3B). For B-precursor ALL, survival was 36% ± 14% when CR was achieved after second salvage, compared with 3% ± 3% in failure cases (P = .001). For T-ALL, the corresponding survival rates were 33% ± 12% and 16% ± 8% (P > .05), respectively (supplemental Figure 3D).
In the multivariate analysis of prognostic factors for survival, 3 prognostic factors with significant effects in the univariate analysis (Table 5) were entered: age, time to relapse, and response to first salvage. In the 224 patients for whom data on all 3 factors were available, age (hazard ratio = 1.421; P = .004) and response to first salvage (hazard ratio = 3.186; P < .0001) remained statistically significant.
SCT after relapse
Performance of SCT.
Data on the performance of SCT were missing for 24 of the 224 patients for whom data on response to first salvage therapy were available. Further, this analysis was restricted to patients with relapse during or after chemotherapy and without CNS involvement, isolated extramedullary involvement, or Ph+ ALL, so the performance of SCT after relapse was analyzed in 200 patients. Overall, 75% (n = 149) of patients received SCT, of whom 32% were in CR after first salvage, 10% were in CR after later salvage, and 31% without CR (Table 6). A higher number of T-ALL patients received SCT in CR after later salvage compared with B-lineage ALL patients (25% vs 5%; P = .0003). Sixty-five of the 93 patients with CR after first salvage received SCT during a continuous first CR (70%). Overall, 86 of 93 patients with CR after first salvage received SCT in any phase of disease after salvage, compared with 63 of 107 patients with failure after first salvage (92% vs 59%; P < .0001).
Performance and outcome of SCT after relapse
| Evaluable . | Total (n = 200) . | B-lineage (n = 144) . | T-lineage (n = 56) . |
|---|---|---|---|
| Performance of SCT, n | |||
| Any SCT | 149 (75%) | 103 (72%) | 46 (82%) |
| SCT in CR after 1st salvage | 65 (32%) | 51 (35%) | 14 (25%) |
| SCT in later CR | 21 (10%) | 7 (5%) | 14 (25%) |
| SCT without CR | 63 (31%) | 45 (31%) | 18 (32%) |
| No SCT | 51 (25%) | 41 (28%) | 10 (18%) |
| Outcome according to SCT* | |||
| Any SCT | 38% ± 4% | 36% ± 5% | 43% ± 8% |
| SCT in CR after 1st salvage | 56% ± 7% | 50% ± 8% | 77% ± 12% |
| SCT in later CR | 39% ± 11% | 51% ± 20% | 34% ± 13% |
| SCT without CR | 20% ± 5% | 18% ± 6% | 25% ± 11% |
| No SCT | 0%† | 0%† | 0%† |
| Evaluable . | Total (n = 200) . | B-lineage (n = 144) . | T-lineage (n = 56) . |
|---|---|---|---|
| Performance of SCT, n | |||
| Any SCT | 149 (75%) | 103 (72%) | 46 (82%) |
| SCT in CR after 1st salvage | 65 (32%) | 51 (35%) | 14 (25%) |
| SCT in later CR | 21 (10%) | 7 (5%) | 14 (25%) |
| SCT without CR | 63 (31%) | 45 (31%) | 18 (32%) |
| No SCT | 51 (25%) | 41 (28%) | 10 (18%) |
| Outcome according to SCT* | |||
| Any SCT | 38% ± 4% | 36% ± 5% | 43% ± 8% |
| SCT in CR after 1st salvage | 56% ± 7% | 50% ± 8% | 77% ± 12% |
| SCT in later CR | 39% ± 11% | 51% ± 20% | 34% ± 13% |
| SCT without CR | 20% ± 5% | 18% ± 6% | 25% ± 11% |
| No SCT | 0%† | 0%† | 0%† |
Patients with relapse during or after chemotherapy and without Ph+/BCR-ABL+ ALL or CNS involvement.
Survival rates are shown as the probability of survival ± SD at 3 years.
Survival at 1 year.
The transplantation rate decreased with increasing age, from 88% of patients 15-25 years of age (n = 69) to 75% of patients 26-45 years of age (n = 93) and 47% of patients older than 45 years of age (n = 38; P < .0001 for all 3 groups). The SCT rate was also significantly lower in patients with early relapse (n = 143) compared with late relapse (n = 57; 70% vs 86%; P = .02).
Outcome according to performance of SCT.
No patient without SCT survived more than 1 year after relapse, compared with a 38% survival in patients with SCT at any time after first salvage (P < .0001; Figure 4A). Survival was significantly better when SCT was performed during CR after first salvage compared with SCT during later CR or SCT during relapse (56 ± 7% vs 39 ± 11% vs 20 ± 5%, respectively; P < .0001; Figure 4B). Similar results were observed for B- and T-lineage ALL (Table 6). No significant differences in outcomes were detected between B- and T-lineage ALL.
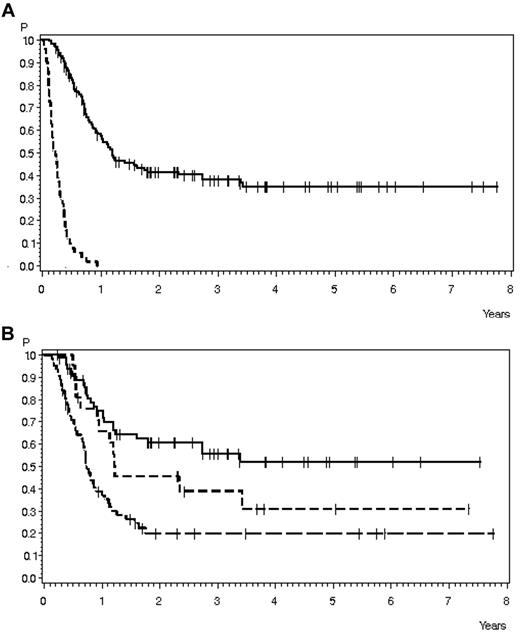
OS in 200 patients with relapse during or after chemotherapy, no Ph+ ALL, and no CNS involvement and for whom data on salvage therapy and SCT were available. (A) OS according to performance of SCT: SCT, n = 149 (solid line), 38% ± 4% after 3 years, median 14.7 months; no SCT, n = 51 (dashed line), 0% after 1 year, median 2.7 months (P < .0001). (B) OS according to SCT timing: SCT in CR after first salvage, n = 65 (solid line), 56% ± 7% after 3 years, median not reached; SCT in later CR, n = 21 (short dashed line), 39% ± 11% after 3 years, median 14.8 months; and SCT without CR, n = 63 (long dashed line), 20% ± 5% after 3 years, median 9 months (P < .0001).
OS in 200 patients with relapse during or after chemotherapy, no Ph+ ALL, and no CNS involvement and for whom data on salvage therapy and SCT were available. (A) OS according to performance of SCT: SCT, n = 149 (solid line), 38% ± 4% after 3 years, median 14.7 months; no SCT, n = 51 (dashed line), 0% after 1 year, median 2.7 months (P < .0001). (B) OS according to SCT timing: SCT in CR after first salvage, n = 65 (solid line), 56% ± 7% after 3 years, median not reached; SCT in later CR, n = 21 (short dashed line), 39% ± 11% after 3 years, median 14.8 months; and SCT without CR, n = 63 (long dashed line), 20% ± 5% after 3 years, median 9 months (P < .0001).
Discussion
The present study involved a large cohort of uniformly pretreated adult patients with relapsed ALL. In previously published studies, the long-term OS ranged from 5%-8%.3-6 The median age in these previous trials was 33-34 years, with age groups ranging between 15 and 81 years. The number of patients older than 55 years was low in all trials. In our present study, long-term survival was achieved in 24% of all patients (median age, 33 years) and in 28% of patients with relapse during or after chemotherapy. These data show that relapsed adult ALL is not an incurable disease.
One essential aim of this study was to provide data on the response to salvage therapy and long-term outcomes for well-defined subgroups of relapsed ALL with a focus on patients with Ph− ALL and without CNS involvement of isolated extramedullary involvement, or prior SCT. These data, including detailed results in immunologic subtypes and relapse stages, are helpful for clinical decision-making and could also serve as a reference for future trials exploring experimental drugs for subgroups of ALL.
Prospective studies in relapsed adult ALL have reported CR rates ranging from 50%-74% for a variety of treatment regimens.4,13-17 The overall CR rate of 42% for the first salvage approach in the present study was within the range reported from other retrospective studies (31%-44%).3-6 Clearly, remission rates depend on chemotherapy sensitivity, which is lower in early relapse compared with late relapse and lowest in patients with refractory disease after the first salvage approach. Oriol et al demonstrated previously that the CR rate was 38% in patients with a first remission duration of less than 1 year compared with 63% in patients with a remission duration longer than 2 years.6 In the present study, the CR rate in early relapse was 36% compared with 58% in late relapse. Definitions of early and late relapse were not uniform in the previously published studies. The most important conclusion is probably that early relapses during intensive chemotherapy indicate a pronounced resistance to conventional chemotherapy. In contrast, late relapses may have a completely different biologic mechanism because they arise during maintenance treatment or after therapy completion, probably from a small number of quiescent leukemia cells that did not acquire multiple drug resistance.
The increasing drug resistance after multiple treatment approaches is also evident from the fact that the response rates decreased to 33% in patients with failure after first salvage and to 25% in patients with relapse after SCT, which is the most intensive prior treatment approach available. Consistent with this, the MD Anderson Cancer Center reported CR rates of 31% in primary refractory disease and early relapse18 and 18% for second salvage.10
In the present study (as in all other retrospective studies of adult ALL), salvage therapies were chosen by local physicians, although some general rules for regimen selection were considered, including the use of short, intensive regimens to treat early relapses and the repetition of standard induction in late relapses. Overall, the approaches used herein represent clinical practice in more than 100 participating hospitals. Most importantly, repeated induction was the most favorable approach, with a 90% CR rate in late relapses. FLAG-IDA was a frequently used regimen in early relapse of B-precursor ALL, resulting in a CR rate of 42%. Before nelarabine became available, CLAEG was frequently used in relapsed T-ALL; however, remission rates with that treatment were only approximately 20%. Data reported for nelarabine are superior, with remission rates of 21%-36%9,19 in generally negatively selected groups of adults with relapsed T-ALL.
The OS after relapse in the present study was influenced by 4 major factors: age, duration of first remission, response to salvage therapy, and subsequent SCT. In patients younger than 25 years, survival rates of 38% were surprisingly favorable, whereas in patients older than 45 years, survival was very poor (12%). Oriol et al reported 15% survival in patients younger than 30 years compared with 10% in those above 30 years.6 Fielding et al described 12% survival in those younger than 20 years and 3%-4% in those older than 35 years.5 With increasing age, remission rates in response to salvage therapy decrease. In the present study, the CR rate was most favorable in patients younger than 25 years (53%), which was still inferior compared with the results of pediatric relapse trials showing remission rates as high as 84% with intensive prolonged cyclical chemotherapy.20 Children, and probably also young adults, tolerate intensive retreatment at relapse, which may increase the chance of achieving a second remission. Conversely, the performance of SCT is correlated with age. It remains unclear to what extent both effects are the result of disease biology, actual patient condition, and real toxicities or of physicians' decision to select less-toxic chemotherapies and avoid transplantation in older patients.
In a recently reported pediatric trial, the OS in patients younger than 19 years was 36%, although the number of early relapses within 18 months from diagnosis was only 26% compared with 71% in the present study. The rate of SCT was 34% in the pediatric trial compared with 88% in young adults in our trial.14 Although the limitations of a retrospective compared with a prospective trial have to be taken into account, these data show that outcome of relapsed ALL in adolescents and young adults may also depend strongly on performance of SCT.
In relapsed ALL, the response to first salvage therapy is probably the most important prognostic factor. In the present study, survival rates were 47% in patients with CR after first salvage, compared with 13% in failure cases. The only approach to improving outcome in patients with chemotherapy resistance is the use of targeted treatments with an alternative mechanism of action, such as antibody therapies.
Overall, 75% of the evaluable relapse patients without prior SCT in our analysis received SCT at some stage of disease after relapse. Seventy percent of patients with CR after first salvage were transferred to SCT in ongoing second CR. Allogeneic transplantation rates were higher compared with those in previously published studies, which ranged from 17%-58% in relation to patients achieving a second CR.3,4,6 Ph+ ALL (9%) and patients with CNS involvement (5%) were excluded from the present analysis. Furthermore, for proper comparison, it is important to consider the number of patients having a prior SCT in the different study populations: 13%-20% for prior allogeneic SCT and 26%-38% for autologous or allogeneic SCT in other retrospective analyses4-6 compared with 31% prior allogeneic SCT in the present study. In the largest retrospective analysis by Fielding et al, the overall transplantation rate after relapse was 24% in patients without prior SCT and 35% in those patients who also lived long enough after relapse to have the chance of a transplantation. The high transplantation rates in the patients in the present study were due to the availability of large donor banks representing the ethnic mix of the population and a health care system covering the costs for SCT. Rapidity of donor identification is extremely important because other trials have shown that the second remission only lasts for approximately 6 months.6
The results of the present study confirm that no long-term survival can be achieved without transplantation in relapsed adult ALL. The survival probability, including that of patients with extramedullary or CNS relapse, was 0% at 3 years without transplantation. The superior survival of transplantation patients after relapse compared with chemotherapy alone was also confirmed by Fielding et al in a landmark analysis that excluded chemotherapy patients with survival times shorter than 100 days.5
It is still debated whether attempts should be made to achieve CR before transplantation or if it is acceptable to perform transplantation in patients in partial remission without additional salvage therapy. In the present study, outcome was significantly poorer for patients receiving transplantation without CR compared with those with CR after first or later salvage. Furthermore, registry data from the European Group for Blood and Marrow Transplantation and the International Bone Marrow Transplant Registry show that the survival of allogeneic sibling SCT is superior if performed during the second remission (29%-34%) rather than during advanced disease (15%-18%).21,22 Data for matched, unrelated SCTs are similar,23 and Tavernier et al reported significantly better survival after SCT performed during the second CR (33%) compared with failure (12%) and immediate SCT at relapse (8%).4 These results support the strategy of offering relapse patients several lines of salvage therapy, thus increasing the chance to perform SCT during CR.
For future optimization, the detection of persistent or recurrent minimal residual disease should be considered as a trigger for initiation of salvage therapy because patients with molecular relapse have a high risk of hematologic relapse.24 The molecular response to salvage may be an essential prognostic factor because it influences the outcome of subsequent SCT.25 In addition, it is essential to improve SCT procedures. Specific conditioning regimens should be evaluated in relapsed ALL, and posttransplantation strategies, such as donor-lymphocyte infusions, maintenance therapy, or minimal residual disease–based targeted therapy to reduce relapse rate, should be explored.
The data presented herein are relevant for future evaluations of new experimental drugs. Patients with primary refractory disease, early relapse, refractory relapse, or relapse after SCT are candidates for experimental treatments because of their poor response to conventional approaches. Outcome is strongly correlated with the achievement of CR, which is therefore a suitable end point. However, long-term survival depends almost exclusively on performance of SCT.
The results of the present study provide reference data for future clinical trials in relapsed ALL subdivided according to disease stage and immunologic subtype. Our results emphasize that relapsed ALL could be cured in a considerable proportion of patients with optimized salvage therapy, including targeted, experimental drugs followed by SCT during a continuous second CR.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms Regina Reutzel for study coordination and data management and the GMALL study group for referring patients to the participating study centers and for follow-up documentation.
This study was supported by grants from Deutsche Krebshilfe (702657Ho2), the Federal Ministry of Education and Research (BMBF 01GI9971/8), and the German Carreras Foundation (DJCLS R10/11).
Authorship
Contribution: N.G. and D.H. designed the research; N.G. coordinated the study, performed the statistical analysis, and wrote the manuscript; and all authors recruited the study patients, performed the study procedures, collected and verified the data, and reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicola Gökbuget, Goethe University Hospital, Department of Medicine II, Theodor-Stern-Kai 7, 60590 Frankfurt, Germany; e-mail: goekbuget@em.uni-frankfurt.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal