Abstract
VEGF induces vascular permeability (VP) in ischemic diseases and cancer, leading to many pathophysiological consequences. The molecular mechanisms by which VEGF acts to induce hyperpermeability are poorly understood and in vivo models that easily facilitate real-time, genetic studies of VP do not exist. In the present study, we report a heat-inducible VEGF transgenic zebrafish (Danio rerio) model through which VP can be monitored in real time. Using this approach with morpholino-mediated gene knock-down and knockout mice, we describe a novel role of phospholipase Cβ3 as a negative regulator of VEGF-mediated VP by regulating intracellular Ca2+ release. Our results suggest an important effect of PLCβ3 on VP and provide a new model with which to identify genetic regulators of VP crucial to several disease processes.
Introduction
Vascular permeability (VP) plays an integral role in the pathology of cardiovascular disease, stroke, and cancer. VEGF was originally discovered as “vascular permeability factor” described as being a tumor-secreted factor that promotes microvascular permeability potently.1 It was later discovered separately as VEGF, an endothelial mitogen2 essential for the development of blood vessels.3-5 Ischemia resulting from cardiac infarction or stroke promotes VEGF expression, which leads to hyperpermeability, edema, and tissue damage.6,7 Angiogenesis is a key restorative mechanism in response to ischemia,8,9 creating the therapeutical challenge of regulating the negative and beneficial actions of VEGF temporally to reduce edema and improve ischemic tissue. In cancer, VEGF-mediated VP promotes tumor-cell extravasation through damaged endothelial cell junctions, often leading to widespread metastasis.10 In addition, VEGF expression induces tumor angiogenesis through extravasation of plasma proteins into the surrounding tissue to develop a provisional matrix capable of supporting vascular sprouting and tumor growth.1 Despite the prevalent role of VEGF-mediated VP in the pathophysiology of cardiac and cerebral diseases and cancer, the genetic regulation of VEGF-induced VP remains unclear because of the lack of an adequate, high-throughput in vivo model with which to assess quantitatively the cumulative contributions of receptors and transduction molecules to VEGF-mediated VP.
New methods are necessary to elucidate genetic regulators of VP. Present in vivo VP models, such as the Miles assay,11,12 the dual-isotope–modified Miles assay,13 the in vivo peripheral permeability assay,14 and intravital microscopy,15-17 have various disadvantages, including the requirement for expensive and time-intensive murine knockout models for genetic studies. The zebrafish (Danio rerio) is a vertebrate with an optically clear embryo that allows high-resolution live imaging and is amenable to high-throughput genetic manipulation, but has yet to be used as an in vivo model to study VEGF-induced VP. In the present study, we undertook the development of a heat shock–inducible zebrafish VEGF model through which VP can be visualized and quantitated in real-time using microangiography of fluorophore-conjugated dextrans. Protein translation-blocking morpholinos (MO) microinjected into VEGF-inducible zebrafish embryos represent a relatively inexpensive and high-throughput means of identifying regulators of VEGF-mediated VP. We demonstrate the utility of this newly developed approach by identifying phospholipase Cβ3 (PLCβ3) as a regulator of VEGF-mediated VP.
VEGF mediates its activities through 2 receptor tyrosine kinases, VEGFR2 and VEGFR1,18-20 with neuropilin acting as a coreceptor.21-23 Downstream signaling events induced by VEGF include the serine phosphorylation of PLCβ324 and tyrosine phosphorylation of PI3K and PLCγ.25 PLC isoforms mediate the hydrolysis of phosphatidylinositol 4,5-bisphosphate to diacylglycerol and inositol 1,4,5-triphosphate, all intracellular messengers that promote the activation of protein kinase C and intracellular Ca2+ release, respectively.26 We have shown that of the PLC isoforms expressed in endothelial cells, only PLCβ3 and PLCγ increase phosphatidylinositol 4,5-bisphosphate hydrolysis significantly after VEGF stimulation.27 Whereas PLCγ has been shown to promote VEGF-induced VP through an intracellular Ca2+-dependent mechanism,28 in the present study, we demonstrate that PLCβ3 acts as a novel negative regulator of VEGF-mediated hyperpermeability through a similar mechanism.
Methods
Creation of transgenic VEGF-inducible zebrafish
SWT zebrafish embryos at the 1-cell stage were coinjected with 2 nL of pkTol2-h70-mC-hVEGF-gcG plasmid (12.5 ng/μL) and transposase mRNA (12.5 ng/μL). Potential founders were selected by expression of enhanced green fluorescent protein (EGFP) in their eyes. Founders were raised to adulthood to produce F1 embryos and to identify true transgenics. An individual line with low background but high heat-shock induction of mCherry was selected for continued work. Zebrafish were maintained according to institutional animal care and use committee guidelines at Mayo Clinic, and all animal studies in this study were approved by the committee.
Heat-shock induction of the VEGF transgene
Heat shock was performed by transferring the zebrafish from 28.5°C to 37°C embryo water and incubating at 37°C for 30 minutes. We defined basal vascular permeability (BVP) as no exposure to heat shock, acute vascular hyperpermeability (AVH) as 1 heat-shock induction, and chronic vascular hyperpermeability (CVH) as three 30-minute heat-shock inductions of VEGF separated by 2 hours at 28.5°C.
mRNA injections
Cre mRNA was transcribed from pT3TS-Cre plasmid DNA29 linearized with XmaI using the mMessage mMachine T3 kit (Ambion). Embryos at the 1-cell stage were arrayed in an agarose microinjection template and 1.5 nL of mRNA (12.5 ng/μL) was microinjected into the cell of the embryo.
MO injections
A translation blocking c-src MO (TTTCTGGCTGACCTTTGGTTGACTG) was designed and a splice site blocking PLCβ3 MO30 was purchased from Gene Tools. Embryos at the 1- to 8-cell stage were arrayed in an agarose microinjection template and microinjected. Next, 4.5 nL of c-src MO (100μM) or 4.5 nL of nonspecific control MO (CATCATATTCAGGGTAGTCGAAGTT; 100μM) were microinjected. Finally, 9.25 ng of PLCβ3 (2.2 nL of a 500μM concentration) or 9.25 ng (2.2 nL of a 500μM concentration) of nonspecific control MO were microinjected.
Dextran injections
Microangiography was performed on anaesthetized, 3-days postfertilization (3-dpf) embryos placed in an agarose microinjection template by inserting a glass microneedle through the pericardium directly into the ventricle. FITC-dextran with a molecular weight of 2000 kDa and Texas Red-dextran with a molecular weight of 70 kDa were used. The dextran was solubilized in embryo medium at a 2 mg/mL concentration. Visualization and real-time imaging were performed using the previously described SCORE methodology31 on a Zeiss ApoTome and a Zeiss LSM 780 confocal microscope using standard FITC and dsRed filter sets.
Immunoblotting
Zebrafish embryos were lysed in RIPA buffer including protease inhibitors and centrifuged at 14 000g for 10 minutes at 4°C. Supernatant was collected and suspended in 4× sample buffer, boiled for 5 minutes, and run on a Tris-glycine SDS gel. The gel was transferred to an Immobilon-P membrane (Millipore) and a c-src polyclonal Ab (Santa Cruz Biotechnology) was used for immunodetection.
VEGF-induced microvascular permeability in mice
In vivo peripheral permeability assays were completed as described previously.14 Wild-type and PLCβ3–null mice were anesthetized with 2% Avertin (0.5 mL/20 g) and infused through the tail vein with 100 μL of FITC-dextran (5 mg/mL, 165 kDa). Animals were placed on a Kodak Multimodel Imager (2000MM) with warm water bottles to maintain body temperature and the central vessels in the ear were imaged. Control saline or mouse VEGF (10 ng/mL) was injected subdermally into the middle ear using a 30-gauge needle (30 μL). Ear vasculature was then imaged with fixed exposure times (30 seconds) in a stationary position from 2-45 minutes after injection. Fluorescence images were then quantified using Kodak MI Version 4 software.
Intracellular Ca2+ release
Human umbilical vein endothelial cells transfected with PLCβ3, PLCγ, or control shRNA were serum-starved overnight, loaded with Fura-2 AM, and then stimulated with VEGF (10 ng/mL). Intracellular Ca2+ concentrations were measured with the DeltaScan illumination system using Felix Version 1.1 software.
Quantitation of vascular permeability
To assess vascular permeability quantitatively, image segmentation programs using techniques in 2-dimensional digital signal processing were written in Matlab (MathWorks). For analysis of confocal time-lapse data, image noise due to decreased exposure time prompts for segmentation of the points of interest only. Noise removal is first performed through a median filter and a low-pass binomial filter. Local maxima are found through first- and second-order numerical differentiation. The points of interest are identified through threshold criteria and intensity data are collected. Corresponding graphical user interfaces are implemented for the ease of adaptation to different imaging qualities and modalities.
Statistics
All values are expressed as means ± SD. Statistical significance was determined using 2-sided Student t test and a value of P < .05 was considered significant.
For additional methods, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Creation of a VEGF-inducible zebrafish model of VP
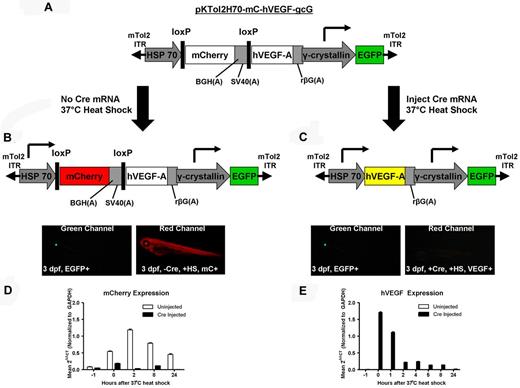
To generate the heat-shock–inducible VEGF zebrafish, we used the Tol2 transposon to integrate a single copy of our transgenic vector efficiently into germline cells.32 Within Tol2, the hVEGF transgene is driven by the heat-inducible HSP70 promoter and preceded by an upstream floxed mCherry gene (Figure 1A). Microinjection of cre recombinase mRNA into single cell embryos results in excision of the red fluorescent protein and expression of hVEGF on induction of the HSP70 promoter. The mini-Tol2 plasmid also contains an EGFP gene driven by the lens-specific γ-crystallin promoter, which enables simple identification of transgenic fish by visualizing EGFP in their eyes (Figure 1B-C). We microinjected transgenic single-cell embryos with cre mRNA to excise mCherry, heat shocked 3-dpf zebrafish at 37°C, and observed mCherry expression in noninjected fish (Figure 1B). mCherry was undetectable in fish that had been injected with cre mRNA (Figure 1C). Transcript levels of mCherry peaked around 2 hours after heat shock (Figure 1D) and VEGF expression was the highest immediately after heat shock (Figure 1E). This result indicates that VEGF-induced VP in the transgenic zebrafish needs to be assessed immediately after heat-shock induction because, as has been shown by others, VEGF mediates VP in a matter of minutes.1,11,33
Scheme of the transgenic heat-inducible VEGF zebrafish. (A) The pKTol2H70-mC-hVEGF-gcG transgene composed of a heat-inducible HSP70 promoter driving a floxed mCherry gene and hVEGF and a γ-crystallin promoter driving EGFP. mTol2 ITR indicates mini-Tol2 plasmid inverted terminal repeat; BGH(A), bovine growth hormone polyadenylation signal; SV40(A), simian virus 40 polyadenylation signal; rβG(A), rabbit β-globin polyadenylation signal. (B) The HSP70 promoter drives transcription of the mCherry gene, producing a red fluorescent protein in transgenic zebrafish. The lens-specific γ-crystallin promoter drives EGFP in the eyes. mC indicates mCherry; HS, heat shock. (C) Microinjection of cre recombinase mRNA into single-cell embryos results in the excision of the floxed mCherry gene, and subsequent heat-shock induction of the HSP70 promoter produces hVEGF. (D-E) Noninjected and cre recombinase–injected transgenic zebrafish were heat shocked at 37°C at 3 dpf to monitor the temporal expression of mCherry (D) and hVEGF (E) transcripts after induction of HSP70.
Scheme of the transgenic heat-inducible VEGF zebrafish. (A) The pKTol2H70-mC-hVEGF-gcG transgene composed of a heat-inducible HSP70 promoter driving a floxed mCherry gene and hVEGF and a γ-crystallin promoter driving EGFP. mTol2 ITR indicates mini-Tol2 plasmid inverted terminal repeat; BGH(A), bovine growth hormone polyadenylation signal; SV40(A), simian virus 40 polyadenylation signal; rβG(A), rabbit β-globin polyadenylation signal. (B) The HSP70 promoter drives transcription of the mCherry gene, producing a red fluorescent protein in transgenic zebrafish. The lens-specific γ-crystallin promoter drives EGFP in the eyes. mC indicates mCherry; HS, heat shock. (C) Microinjection of cre recombinase mRNA into single-cell embryos results in the excision of the floxed mCherry gene, and subsequent heat-shock induction of the HSP70 promoter produces hVEGF. (D-E) Noninjected and cre recombinase–injected transgenic zebrafish were heat shocked at 37°C at 3 dpf to monitor the temporal expression of mCherry (D) and hVEGF (E) transcripts after induction of HSP70.
Imaging VEGF-induced VP in zebrafish
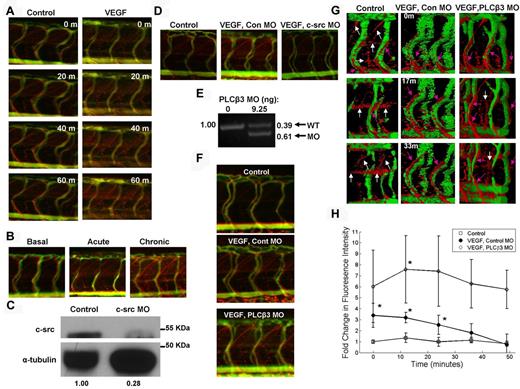
To investigate VP, microangiography was performed by coinjecting the ventricles of 3-dpf control and transgenic zebrafish with 70 kDa Texas Red-dextran as a permeabilizing tracer and 2000 kDa FITC-dextran as a marker of the veins. Control and transgenic zebrafish were heat shocked to induce VEGF in the transgenic zebrafish and imaged immediately using SCORE methodology.31 VP was monitored using time-lapse structured illumination fluorescence microscopy and steadily increasing VP was observed over the course of an hour in VEGF-induced zebrafish, as evident by greater leakage of Texas Red-dextran into the extravascular space compared with control zebrafish (Figure 2A). 3D rotating images showing greater Texas Red-dextran extravasation in VEGF-induced zebrafish than in controls were also captured (supplemental Videos 1 and 2). We sought to validate our observations of increased VP in VEGF-induced zebrafish through a pathologic assessment of vascular barrier integrity. To this end, control and VEGF-induced zebrafish were fixed and sectioned before electron microscopy analysis. Edema was observed in the VEGF-induced zebrafish, as evident by plasma proteins leaking into extravascular space through endothelial cells lining the lumen (supplemental Figure 1). These data indicate that VEGF induction causes extravasation of plasma proteins from zebrafish vessels during VP, and this leakiness can be visualized in real time using fluorophore-conjugated dextrans as tracers. The majority of the vascular leakiness induced by VEGF occurred through the intersegmental vessel (ISV) with minimal contributions from the posterior cardinal vein, caudal vein, dorsal longitudinal anastomotic vessel, caudal artery, and dorsal aorta.
VEGF induction promotes VP, which is increased by PLCβ3 knockdown. Microangiography was performed on 3-dpf zebrafish with red-permeabilizing tracer and green ISV marker (A,B,D,F-G). (A) Control and VEGF-induced zebrafish were imaged in real time at the indicated time points. Three-dimensional rotating images are shown in supplemental Videos 1 and 2. (B) Basal, acute, and chronic (0, 1, and 3 VEGF inductions, respectively) VP was assessed at 3 dpf and representative images are shown. (C) MO-mediated knockdown of c-src was confirmed by immunoblotting in 3-dpf zebrafish with α-tubulin as a loading control. Densitometric analysis revealed 72% knockdown of c-src. (D) Representative images of extravasated Texas Red-dextran are shown in control; VEGF-induced, control MO-injected; and VEGF-induced, c-src MO–injected 3-dpf zebrafish. (E) Control MO- and PLCβ3 MO–injected 3-dpf zebrafish cDNA was used for PCR to demonstrate a molar ratio of 39% wild-type (WT) to 61% PLCβ3 MO PCR product. (F) Control; VEGF-induced, control MO–injected; and VEGF-induced, PLCβ3 MO–injected zebrafish were imaged in real time and representative images are shown. (G) Surface projection representation of confocal live imaging performed at the indicated times on a set of similarly treated zebrafish. Pink arrows indicate tracer extravasated directly from ISVs; white arrows, tracer within diagonal and horizontal lymphatic vessels. See supplemental Videos 3 through 5 for live fluorescence microscopy and supplemental Videos 6 through 8 for real-time surface projection confocal imaging. (H) Quantitation of confocal time-lapse imaging. Open squares indicate controls (n = 4); closed circles, VEGF-induced, control MO (n = 4); and open diamonds, VEGF-induced, PLCβ3 MO (n = 5). *P < .05 for control versus VEGF-induced, control MO or VEGF-induced, control MO versus VEGF-induced, PLCβ3 MO. Error bars represent SD. Images depicted in panels A, B, D, and F were obtained using a Zeiss Apitome microscope equipped with a Fluar 5×, 0.25 numerical aperture lens at room temperature. Images shown in panel G were acquired using a Zeiss LSM 780 confocal microscope equipped with an LD Plan Neofluar 40×, 0.6 numerical aperture lens at room temperature.
VEGF induction promotes VP, which is increased by PLCβ3 knockdown. Microangiography was performed on 3-dpf zebrafish with red-permeabilizing tracer and green ISV marker (A,B,D,F-G). (A) Control and VEGF-induced zebrafish were imaged in real time at the indicated time points. Three-dimensional rotating images are shown in supplemental Videos 1 and 2. (B) Basal, acute, and chronic (0, 1, and 3 VEGF inductions, respectively) VP was assessed at 3 dpf and representative images are shown. (C) MO-mediated knockdown of c-src was confirmed by immunoblotting in 3-dpf zebrafish with α-tubulin as a loading control. Densitometric analysis revealed 72% knockdown of c-src. (D) Representative images of extravasated Texas Red-dextran are shown in control; VEGF-induced, control MO-injected; and VEGF-induced, c-src MO–injected 3-dpf zebrafish. (E) Control MO- and PLCβ3 MO–injected 3-dpf zebrafish cDNA was used for PCR to demonstrate a molar ratio of 39% wild-type (WT) to 61% PLCβ3 MO PCR product. (F) Control; VEGF-induced, control MO–injected; and VEGF-induced, PLCβ3 MO–injected zebrafish were imaged in real time and representative images are shown. (G) Surface projection representation of confocal live imaging performed at the indicated times on a set of similarly treated zebrafish. Pink arrows indicate tracer extravasated directly from ISVs; white arrows, tracer within diagonal and horizontal lymphatic vessels. See supplemental Videos 3 through 5 for live fluorescence microscopy and supplemental Videos 6 through 8 for real-time surface projection confocal imaging. (H) Quantitation of confocal time-lapse imaging. Open squares indicate controls (n = 4); closed circles, VEGF-induced, control MO (n = 4); and open diamonds, VEGF-induced, PLCβ3 MO (n = 5). *P < .05 for control versus VEGF-induced, control MO or VEGF-induced, control MO versus VEGF-induced, PLCβ3 MO. Error bars represent SD. Images depicted in panels A, B, D, and F were obtained using a Zeiss Apitome microscope equipped with a Fluar 5×, 0.25 numerical aperture lens at room temperature. Images shown in panel G were acquired using a Zeiss LSM 780 confocal microscope equipped with an LD Plan Neofluar 40×, 0.6 numerical aperture lens at room temperature.
Three distinct types of VP have been described: (1) BVP of normal tissues; (2) AVH, which occurs in response to a very short exposure to VEGF or other vascular permeabilizing agents; and (3) CVH, which characterizes pathologic angiogenesis.13 We observed BVP in the control zebrafish and sought to replicate all 3 types of VP. We defined BVP as no VEGF induction, AVH as 1 VEGF induction, and CVH as 3 VEGF inductions. VP increased as the quantity of VEGF-inductions increased (Figure 2B), demonstrating that the VEGF-inducible model can be used to determine how novel genetic regulators of VP may modulate distinct types of VP. Characteristics of pathologic angiogenesis, such as increased vessel formation,13 were observed in the CVH-induced zebrafish (supplemental Figure 2).
MO-mediated knockdown of c-src blocks VEGF-mediated VP
We sought to define genes that regulate VP using a reverse genetics approach of MO-mediated gene knockdown.34 To test the feasibility of this methodology, we knocked down c-src protein (Figure 2C), a gene known to be required for VEGF-induced VP.35 As expected, ablation of c-src in VEGF-induced zebrafish blocked VP, whereas a nonspecific, control MO had no effect on VEGF-induced VP (Figure 2D), demonstrating that MO-mediated gene knockdown can be used in the VEGF-inducible zebrafish model we created to identify potential genetic regulators of VEGF-induced VP.
PLCβ3 negatively regulates VEGF-mediated VP in zebrafish
PLCγ1 has been implicated in VP,28 and we have shown previously that PLCβ3 regulates VEGF-induced migration.24 Therefore, in the presents study, we sought to determine whether PLCβ3 regulates VEGF-mediated hyperpermeability. We induced VEGF in transgenic zebrafish that had been injected with a nonspecific control MO or the splice-blocking PLCβ3 MO30 (Figure 2E). Interestingly, VEGF-induced, splice-blocking PLCβ3 MO–injected embryos exhibited even greater VP than VEGF-induced, control MO–injected zebrafish, as shown by increased red tracer in the extravascular space (Figure 2F and supplemental Videos 3-5). Zebrafish not stimulated with VEGF but injected with PLCβ3 MO exhibited basal VP similar to that observed in controls (supplemental Figure 3). Surface projections of confocal real-time imaging (Figure 2G) and corresponding videos (supplemental Videos 6-8) demonstrated that the red extravasation tracer leaked directly from the ISV of VEGF-induced zebrafish administered control MO or PLCβ3 MO and accumulated along these vessels (Figure 2G pink arrows). In VEGF-unstimulated controls, the red tracer accumulated along diagonal and horizontal projections between the ISVs (Figure 2G white arrows), which is consistent with previously defined zebrafish lymphatic vessels.36 The small amount of tracer that extravasated from the ISVs was a consequence of basal VP. We quantitated red fluorescence intensity in the extravascular space at various time points throughout the live imaging series to measure VP and showed a significant increase in VP during the first 24 minutes after VEGF induction in the zebrafish (Figure 2H). Ablation of PLCβ3 caused a greater than 2-fold increase in VEGF-induced VP, as assessed by this quantitation method (Figure 2H). These findings suggest a novel role of PLCβ3 as a negative regulator of VEGF-mediated VP.
PLCβ3 negatively regulates VEGF-induced VP in mice
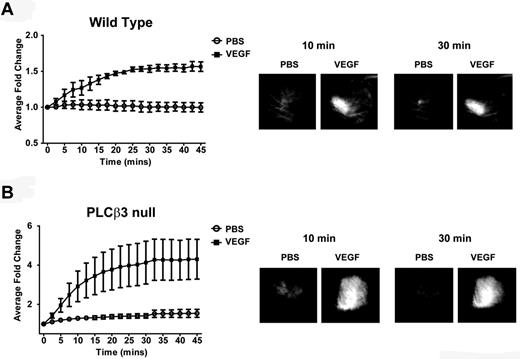
Given that PLCβ3 knockdown in zebrafish increased VEGF-induced VP, we sought to determine whether PLCβ3 regulates VP in a mammalian model. To this end, we performed in vivo permeability assays in wild-type and PLCβ3–deficient mice, as described previously.14 Briefly, mice were anesthetized, injected with FITC-dextran intravenously through the tail vein and with saline or VEGF intradermally in the ear skin and imaged. PLCβ3–knockout mice exhibited more than twice as much VEGF-induced permeability as wild-type mice (maximum induction mean for PLCβ3, 3.319 ± 0.7385 vs wild-type, 1.518 ± 0.1187; P = .0468; Figure 3), which corroborates the increased VEGF-mediated VP that we observed in PLCβ3 MO–injected zebrafish (Figure 2F-H) and supports our finding that PLCβ3 regulates VEGF-mediated VP negatively.
PLCβ3 null mice exhibit greater VEGF-induced VP than wild-type mice. (A-B) VEGF-induced VP was evaluated in wild-type and PLCβ3–null mice (n = 5/group). FITC signal, normalized to t0, is shown for subdermal PBS control and VEGF injections. The average fold change in signal after injection for wild-type (A) and PLCβ3 (B) mice. Representative ear images from 10 and 30 minutes after injection are shown in the panels on the right. Maximum induction mean for PLCβ3 was 3.319 ± 0.7385 versus wild-type, 1.518 ± 0.1187 (P = .0468). Error bars represent SD.
PLCβ3 null mice exhibit greater VEGF-induced VP than wild-type mice. (A-B) VEGF-induced VP was evaluated in wild-type and PLCβ3–null mice (n = 5/group). FITC signal, normalized to t0, is shown for subdermal PBS control and VEGF injections. The average fold change in signal after injection for wild-type (A) and PLCβ3 (B) mice. Representative ear images from 10 and 30 minutes after injection are shown in the panels on the right. Maximum induction mean for PLCβ3 was 3.319 ± 0.7385 versus wild-type, 1.518 ± 0.1187 (P = .0468). Error bars represent SD.
PLCβ3 regulates VEGF-mediated VP through a Ca2+-dependent mechanism
Because PLCγ induces Ca2+ release and thus promotes VP,28,37 we hypothesized that PLCβ3 may regulate calcium flux negatively, given our findings that ablation of PLCβ3 promotes increased VEGF-mediated VP. We performed an intracellular Ca2+-release assay and demonstrated that endothelial cells expressing PLCβ3 shRNA exhibited increased Ca2+ flux compared with control shRNA–transduced human umbilical vein endothelial cells (Figure 4A), indicating that PLCβ3 does indeed regulate Ca2+ flux negatively. As expected, shRNA-mediated knockdown of PLCγ caused a decrease in Ca2+ flux (Figure 4B). We also sought to determine whether PLCβ3 regulates Ca2+ release from the ER negatively. To ensure that only Ca2+ released from the ER was being monitored, EGTA was added to the buffer to chelate all extracellular Ca2+. After VEGF stimulation, no differences in Ca2+ flux were observed among the control shRNA and PLCβ3 shRNA cells, indicating that PLCβ3 has no effect on Ca2+ release from the ER (Figure 4C). To assess whether PLCβ3 modulates Ca2+ entry into the cell negatively, we introduced CaCl2 into the buffer after EGTA and VEGF treatment and found that knockdown of PLCβ3 greatly increased Ca2+ entry into the cell (Figure 4C). We validated this finding in vivo by demonstrating that chelation of intracellular Ca2+ with BAPTA-AM prevented the increased VP observed in VEGF-induced, PLCβ3 MO–treated zebrafish (Figure 4D). We propose a model in which PLCβ3 tightly regulates Ca2+ entry into the cell (Figure 4E). After down-regulation of PLCβ3, increased Ca2+ enters the cell, which leads to increased VEGF-mediated VP.
PLCβ3 regulates intracellular Ca2+ entry into the cell. (A-C) Human umbilical vein endothelial cells transfected with PLCβ3 (A,C), PLCγ (B), or control shRNA (A-C) were serum starved overnight, loaded with Fura-2 AM, and then stimulated with VEGF (10 ng/mL) at 100 seconds. (C) To distinguish intracellular Ca2+ release from the ER from Ca2+ entry into the cell, EGTA was added before VEGF stimulation at 50 seconds to measure ER release and CaCl2 was added at 750 seconds to assess cellular entry. (D) Microangiography using red-permeabilizing tracer and green ISV marker was performed on 3-dpf control (top panel); VEGF-induced, PLCβ3 MO–treated (middle panel); and VEGF-induced, PLCβ3 MO–treated zebrafish with 100μM BAPTA-AM added to the water 24 hours before VEGF induction (bottom panel). Representative images shown were obtained using a Zeiss Apitome microscope equipped with a Fluar 5×, 0.25 numerical aperture lens at room temperature. (E) Schematic of proposed model. VEGF binding to VEGF receptors causes increased intracellular Ca2+ (through Ca2+ entry into the cell and Ca2+ release from the endoplasmic reticulum). Elevated intracellular Ca2+ levels promote increased vascular permeability. Activated PLCβ3 inhibits Ca2+ entry into the cell, leading to a decrease in VEGF-induced vascular permeability.
PLCβ3 regulates intracellular Ca2+ entry into the cell. (A-C) Human umbilical vein endothelial cells transfected with PLCβ3 (A,C), PLCγ (B), or control shRNA (A-C) were serum starved overnight, loaded with Fura-2 AM, and then stimulated with VEGF (10 ng/mL) at 100 seconds. (C) To distinguish intracellular Ca2+ release from the ER from Ca2+ entry into the cell, EGTA was added before VEGF stimulation at 50 seconds to measure ER release and CaCl2 was added at 750 seconds to assess cellular entry. (D) Microangiography using red-permeabilizing tracer and green ISV marker was performed on 3-dpf control (top panel); VEGF-induced, PLCβ3 MO–treated (middle panel); and VEGF-induced, PLCβ3 MO–treated zebrafish with 100μM BAPTA-AM added to the water 24 hours before VEGF induction (bottom panel). Representative images shown were obtained using a Zeiss Apitome microscope equipped with a Fluar 5×, 0.25 numerical aperture lens at room temperature. (E) Schematic of proposed model. VEGF binding to VEGF receptors causes increased intracellular Ca2+ (through Ca2+ entry into the cell and Ca2+ release from the endoplasmic reticulum). Elevated intracellular Ca2+ levels promote increased vascular permeability. Activated PLCβ3 inhibits Ca2+ entry into the cell, leading to a decrease in VEGF-induced vascular permeability.
Discussion
Many components of signaling pathways have been linked qualitatively to VEGF-induced hyperpermeability using various models,37,38 but little is known about the quantitative contributions of these relative pathways. It has become increasingly difficult to synthesize a cumulative understanding of the molecular mechanisms that regulate VEGF-induced VP using data generated across incomparable systems. For example, in vitro permeability measurements correspond poorly with those obtained in vivo,39,40 and properties of endothelial cells, vascular beds, and vascular walls vary significantly among model organisms.37 A genetically accessible, high-throughput model is necessary to evaluate uniformly the quantitative contributions of signaling molecules that regulate VEGF-induced VP. In the present study, we describe a new VEGF-inducible zebrafish model for assessing VP in real-time. Optically clear zebrafish embryos facilitate high-resolution in vivo imaging of vascular formation, function, and integrity using fluorescently labeled tracers and transgenes. When used in conjunction with translation-blocking MOs that yield genetic phenotypes within 3-4 days of injection, the VEGF-inducible zebrafish model offers a high-throughput in vivo system for evaluating genetic regulation of VEGF-mediated hyperpermeability.
The VEGF-inducible zebrafish model presented herein has several advantages over current in vivo models of VP. We demonstrate the ability to monitor VEGF-mediated chronic VP in zebrafish, which is significant because tissue damage is exacerbated in ischemic disease and cancer by prolonged VEGF exposure.6,7 Elucidating distinct and overlapping molecular mechanisms that regulate VEGF-induced acute and chronic vascular hyperpermeability represents an important pursuit, because current murine-based permeability models have generally focused on acute VP. In addition, the ease of simultaneously knocking down multiple VP-regulatory proteins in the zebrafish model permits study of the interplay among effectors of VEGF-induced VP. Finally, the high-throughput nature of the zebrafish model of VP, when combined with MO technology, enables rapid screening for novel genetic regulators of VP.
In the present study, we exhibit the efficacy of the VEGF-inducible zebrafish model through the identification of PLCβ3 as a negative regulator of VEGF-induced VP. Previous work has shown that PLCβ3 and PLCγ1 have distinct roles in zebrafish development. PLCβ3 is required in neural crest cells for proper facial skeletal patterning,30 and PLCγ1 is necessary downstream of VEGF for arterial development.41 Given the unique functions of PLC isoforms in development and the defined role of PLCγ1 as a positive meditator of VEGF-induced VP, we sought to determine whether PLCβ3 is a novel regulator of VP. We have demonstrated herein that PLCβ3 opposes the function of PLCγ1 by regulating VEGF-mediated VP negatively, although these PLC isoforms regulate VEGF-induced hyperpermeability differentially through a similar intracellular Ca2+-dependent mechanism.28 We speculate that PLCβ3 may be activated by VEGFR2 based on ex vivo data implicating this receptor in PLCγ1 modulation of VP28 and our previous report demonstrating that VEGFR2 induced phosphorylation of serine residues on PLCβ3 to regulate endothelial cell migration.24 However, identification of the VEGF receptor(s) that activates PLCβ3 to regulate VEGF-induced VP negatively warrants further investigation beyond the scope of this study.
The integral role of VEGF-induced hyperpermeability in the pathologies of cancer and ischemic diseases underscores the clinical importance of understanding VP-regulatory pathways. For example, preclinical studies have demonstrated the efficacy of anticancer drugs that inhibit c-src,42 a gene required for VEGF-mediated VP. Our elucidation of a novel mechanism through which PLCβ3 modulates cellular and molecular regulation of VP has several important clinical implications. First, our results may be significant for patients receiving morphine because chronic exposure causes decreased PLCβ3 activity,43 which, in light of our present findings, may promote increased VP. Second, anticancer drugs developed to inhibit G-protein signaling44,45 may also stimulate VP through down-regulation of PLCβ3. Consequently, caution should be exercised when administering morphine or other drugs that inhibit PLCβ3 activity because they could promote edema and tissue damage through the induction of VP. The results of the present study exemplify the utility of the VEGF-inducible zebrafish model for the assessment of VP in real time and identify genetic regulators of VP that may translate into therapeutic targets for the treatment of cancer, heart disease, stroke, and other diseases.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Mayo Clinic zebrafish core facility and optical morphology facility for their assistance with this work.
This work was supported in part by the National Institutes of Health (HL70567, CA78383, and CA150190 to D.M.) and the Bruce and Martha Atwater Foundation (to D.M.). L.H.H. is a fellow of the National Cancer Institute, National Institutes of Health (NCI-T32 CA148073). D.M. is a visiting professor at King Saud University (Riyadh, Saudi Arabia).
National Institutes of Health
Authorship
Contribution: L.H.H. developed the zebrafish permeability model, performed all of the zebrafish experiments and imaging, and prepared the manuscript; L.H.H. and S.S. performed the immunoblotting; K.N.P. performed the murine permeability experiments; K.J.C. and P.V. created the VEGF-inducible zebrafish line; K.J.C. provided technical assistance with the zebrafish experiments and imaging; R.B. performed the Ca2+-release assays; X.G. developed the mathematical model; X.G. and S.B. analyzed the data for quantitation of permeability; T.E.S., A.M.D., and H.F.D. performed the EM studies; K.J.C., R.B., S.C.E, H.F.D., K.P.C., and D.M. revised the manuscript; S.C.E., H.F.D., K.P.C., and D.M supervised the project; and D.M. developed the original hypothesis and directed the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Debabrata Mukhopadhyay, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; e-mail: mukhopadhyay.debabrata@mayo.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal