Abstract
Haploinsufficiency of ribosomal proteins (RPs) has been proposed to be the common basis for the anemia observed in Diamond-Blackfan anemia (DBA) and myelodysplastic syndrome with loss of chromosome 5q [del(5q) MDS]. We have modeled DBA and del(5q) MDS in zebrafish using antisense morpholinos to rps19 and rps14, respectively, and have demonstrated that, as in humans, haploinsufficient levels of these proteins lead to a profound anemia. To address the hypothesis that RP loss results in impaired mRNA translation, we treated Rps19 and Rps14-deficient embryos with the amino acid L-leucine, a known activator of mRNA translation. This resulted in a striking improvement of the anemia associated with RP loss. We confirmed our findings in primary human CD34+ cells, after shRNA knockdown of RPS19 and RPS14. Furthermore, we showed that loss of Rps19 or Rps14 activates the mTOR pathway, and this is accentuated by L-leucine in both Rps19 and Rps14 morphants. This effect could be abrogated by rapamycin suggesting that mTOR signaling may be responsible for the improvement in anemia associated with L-leucine. Our studies support the rationale for ongoing clinical trials of L-leucine as a therapeutic agent for DBA, and potentially for patients with del(5q) MDS.
Introduction
Diamond-Blackfan anemia (DBA; MIM# 105650) is a congenital bone marrow failure syndrome of childhood manifested as normochromic macrocytic anemia with absence or insufficient erythroid precursors in the bone marrow.1,2 Twenty-five percent of DBA patients have mutations in the RPS19 gene, which encodes a component of the 40S ribosomal subunit.3,4 A further 25% of DBA patients have been shown to have mutations in other ribosomal protein genes,5 supporting the hypothesis that DBA is a disease of altered ribosome assembly or function. DBA shares a number of its clinical features with several other congenital syndromes that also carry heterozygous mutations affecting ribosome biogenesis, such as Shwachman-Diamond syndrome (SDS), cartilage-hair hypoplasia syndrome, and dyskeratosis congenita (DC) suggesting that all of these conditions share at least some common pathogenic mechanisms; they have thus been termed “ribosomopathies.”6 In addition, evidence suggests that the anemia associated with the 5q minus (5q−) syndrome (or myelodysplastic syndrome with loss of all or part of chromosome 5q [del(5q) MDS]), a distinct subtype of myelodysplastic syndrome results from somatic heterozygous loss of the ribosomal protein gene RPS14 in hematopoietic stem cells.7,8 Efforts to understand why ribosomal protein haploinsufficiencies have such a specific and profound effect on erythroid development at the molecular level have focused on the activation and stabilization of p53 in response to ribosomal stress.9 However, the precise mechanisms governing how p53 stabilization occurs in response to ribosomal protein haploinsufficiency have not been clearly defined. Furthermore, not all bone marrow samples from patients with del(5q) MDS or DBA show evidence of p53 stabilization suggesting that other mechanisms also contribute to the pathogenesis of these conditions.9
The first line of therapy for patients with DBA is treatment with corticosteroids. However, only 80% of patients respond initially and less than one-half can be maintained on this therapy for extended periods.10 Nonresponders to corticosteroid therapy undergo regular blood transfusions and require additional iron-chelation therapy to prevent hemochromatosis. It has been suggested that DBA patients are more sensitive to iron-overload compared with patients with other transfusion-dependent anemias, such as thalassemia.11 The only definitive therapy for DBA is hematopoietic stem cell transplantation, however morbidity and mortality from infections and graft-versus-host disease is reason for concern with this line of therapy (reviewed by Boria et al4 ). The thalidomide derivative lenalidomide has been shown to have dramatic effects in patients with del(5q) MDS resulting in transfusion independence, suppression of the 5q− clone and improvement in hemoglobin levels and bone marrow morphologic features.12 However, not all patients respond to lenalidomide therapy. In addition to severe side effects, such as neutropenia, recent data have raised safety concerns for the use of lenalidomide in del(5q) MDS patients because of an increased risk of disease progression to acute myeloid leukemia (reviewed by Jädersten13 ). Thus there is an unmet clinical need for novel treatments for both of these conditions.
Given that RPs are critical components of the ribosome, several studies have addressed whether protein synthesis is affected as a result of haploinsufficient levels of RPs. Lymphoid cells from DBA patients show impaired protein synthesis.14 An increase in protein synthesis in DBA-derived cells after pretreatment with high concentrations of L-leucine was also observed. L-leucine is an essential amino acid that is unique among the branched chain amino acids in that it acts as a nutrient regulator of protein synthesis in skeletal muscle (reviewed by Kimball and Jefferson15 ). The results from such in vitro observations prompted investigators to assess whether there might be clinical benefit from administration of the amino acid L-leucine to DBA patients.16 In this case report, a marked improvement in anemia with reduced transfusion requirement was observed in a patient with DBA treated with L-leucine supplementation.
The mechanism by which L-leucine stimulates protein synthesis has been studied in a variety of cell types. In the skeletal muscle of amino acid deprived rats, L-leucine reexposure resulted in phosphorylation of p70 S6 (small ribosomal protein) kinase1 (S6K1) and 4EBP1 which in turn led to an increase in protein synthesis assessed by incorporation of radiolabeled amino acids. This effect was partially reversed by rapamycin suggesting it is mediated by the mammalian/mechanistic target of rapamycin (mTOR)/mTORC1 pathway (reviewed by Stipanuk17 ). However, to date there is no data linking activation of mTOR by L-leucine with increased protein synthesis in hematopoietic cells.
In this study, we report that L-leucine treatment results in improvement of anemia observed in zebrafish models of both DBA and del(5q) MDS. We also observed a marked improvement in developmental effects associated with RP loss, in the presence of L-leucine. We confirmed our findings in primary human CD34+ cells after shRNA knockdown of RPS19 and RPS14. We show, in addition, that L-leucine is able to activate the mTORC1 pathway via phosphorylation of S6K1 in the context of RP deficiency. Thus, we propose that L-leucine improves anemia in RP haploinsufficiency by promoting mRNA translation via the mTOR pathway.
Methods
Zebrafish husbandry and microinjections
Wild-type Ab stocks of Danio rerio and Tg(gata1:RFP) were maintained according to standard protocols at 28.5°C. Morpholinos (MOs) targeting the 5′UTR/ATG codon or slice donor sites of rps19 and rps14, respectively, were designed by Gene-Tools.18 MO concentrations were titrated to approximate haploinsufficient protein levels. MO sequences and doses are shown in Table 1. Embryos were raised in E3 solution (“egg water”; 5mM NaCl, 0.17mM KCl, 0.33mM CaCl2, 0.33mM MgSO4). All zebrafish experiments were approved by the Dana-Farber Cancer Institute ACUC.
Morpholino sequences and doses used in this study
| Target . | Sequence . | Amount (ng) . |
|---|---|---|
| Rps14 (exon2-intron2) | caggttttcaggatacatacTTGCC | 0.8 |
| Rps19 (5′UTR/ATG) | CACTGTTACACCACCTGGCATCTTG | 0.02 |
| Gata1(5′UTR) | CTGCAAGTGTAGGTATTGAAGATGTC | 4 |
| Gene-Tools standard control | CCTCTTACCTCAGTTACAATTTATA | 0.8 |
| Target . | Sequence . | Amount (ng) . |
|---|---|---|
| Rps14 (exon2-intron2) | caggttttcaggatacatacTTGCC | 0.8 |
| Rps19 (5′UTR/ATG) | CACTGTTACACCACCTGGCATCTTG | 0.02 |
| Gata1(5′UTR) | CTGCAAGTGTAGGTATTGAAGATGTC | 4 |
| Gene-Tools standard control | CCTCTTACCTCAGTTACAATTTATA | 0.8 |
Developmental staging of embryos was determined by somite number as described by Westerfield.19
Hemoglobin staining with O-dianisidine
Hemoglobin was detected using O-dianisidine as previously described at the timepoints stated.20 Semiquantitative analysis for the presence or absence of anemia was undertaken by 2 independent researchers for a minimum of 20 embryos per condition. Statistical analysis was performed using a 2-way analysis of variance (ANOVA) with Bonferroni posttest analysis to assess the effects of RP-knockdown and L-leucine.
Treatment of zebrafish with L-leucine, D-leucine, L-tryptophan, and rapamycin
Zebrafish injected with rps14, rps19, and control MOs were separated into 6-well plates at 24 hours postfertilization (hpf; 20-40 per well). One-half of the embryos were maintained in egg water, the other one-half were maintained in egg water with 100mM L-leucine, 100mM D-leucine, or 40mM of L-tryptophan. Morphants were assessed at 2, 3, and 4 days postfertilization (dpf). This dose of L-leucine was determined by dose titration until a robust effect was observed without detectable toxicity to the embryos. Embryos were treated with 1mM rapamycin as previously described.21 Rapamycin control embryos were treated with dimethylsulfoxide (DMSO).
Analysis of zebrafish by flow cytometry
rps14, rps19, and control morphants treated with L-leucine or egg water were analyzed by fluorescence-activated cell sorter (FACS) at 3 dpf. Individual embryos were rinsed once in cold phosphate-buffered saline (PBS). Embryos were then dissociated using the gentleMACS octo dissociator (Miltenyi Biotec), passed through a 40-μM filter, and washed once with cold PBS containing 5% fetal bovine serum. Data were acquired using a CyanADP (Beckman-Coulter). Dead cells were excluded using Hoesct 33 342 (Invitrogen). Absolute cell numbers were determined by adding 2.5 × 105 Flow-check fluorospheres (Beckman-Coulter) to a final volume of exactly 300μl per tube and calculating the red fluorescent protein (RFP)+ events compared with the number of beads. Data were analyzed with FlowJo Version 7.6 (TreeStar) and Prism 5 (GraphPad) software using a Student t test.
Immunohistochemistry
Immunohistochemistry was carried out as described.22 Briefly, rps14, rps19, and control morphants were fixed in paraformaldehyde (PFA) overnight, washed in PBS with 0.1% Tween 20 (PBST) followed by 150mM Tris-HCl at pH 9.0 for 5 minutes. Antigen retrieval was facilitated by subsequent incubation in 150mM Tris-HCl at pH 9 for 15 minutes at 70°C. Embryos were then blocked in 10% goat serum in PBS with 1% triton ×100 and 1% bovine serum albumin for 1 hour, followed by incubation in primary rabbit anti–phospho-S6 (Ser235/236) antibody (Cell Signaling) at 1:500, for 2 days at 4°C. After 3, 1 hour washes in PBS with 1% triton ×100, embryos were washed in methanol followed by blocking endogenous peroxidases in 0.3% hydrogen peroxide in 100% methanol. Secondary antibody staining with goat anti–rabbit horseradish peroxidase (Dako) was then carried out for 2 days at 4°C. Embryos were washed in PBST and developed using SigmaFast Diaminobenzidine with Metal Enhancer (Sigma-Aldrich).
Western blots
rps14, rps19, and control morpholino (MO) injected zebrafish embryos were treated with 100mM L-leucine and/or 1mM rapamycin as previously described. Forty-eight hours after treatment, the embryos were manually deyolked (20-30 per condition) and dissociated in protein lysis buffer containing 20mM HEPES, 150mM sodium chloride, 1% Triton-X-100, 10% glycerol, 1mM ethylenediaminetetraacetic acid, 100mM sodium fluoride, 1mM phenylmethylsulfonyl fluoride, and 17.5mM β-glycerophosphate. Western blot analysis was carried out as previously described.23 Antibodies used were: RPS14 (1:1000, Santa Cruz), RPS19 (1:1000, Abcam), and p-S6K1T389 (1:1000), S6K1 (1:1000), p-4E-BP1T37/46 (1:1000), and β-actin (1:5000) all purchased from Cell Signaling.
Imaging
Stained embryos were fixed in 4% PFA, and preserved and imaged in 90% glycerol. Live morphants were anesthetized in 0.4% tricaine in egg water for 3 minutes and immobilized in 1.5% methylcellulose. Embryos were visualized in Figures 1, 3, and 4 with a Nikon SMZ1500 zoom stereomicroscope (Nikon Instruments) and acquired with a Nikon camera and the NIS-Elements (Nikon Instruments) or ACT2U software (Nikon, Excel Technologies). In Figure 5 embryos were visualized with a Leica M205FA microscope (Leica Microsystems), a Leica DFC310FX camera and Leica application suite (Leica) software. Images were processed using Photoshop CS5 (Adobe).
Zebrafish body length and eye measurements
Pooled zebrafish morphants (20 per condition) were imaged at low magnification (3.75×) using a Nikon SMZ1500 zoom stereomicroscope. Measurements of body length or eye diameter were taken using Abode Photoshop CS3 (Adobe) ruler tool. To convert pixel measurements to millimeters, the pixel distance of 1mm distance from a 12-in ruler was analyzed using the same procedure. Measurements were analyzed in Prism using a 2-way ANOVA with Bonferronis posttest analysis to assess the effects of RP-knockdown, L-leucine, and time in combination (GraphPad).
Transduction of human CD34+ progenitor cells and growth in liquid culture
CD34+ hematopoietic progenitor cells purchased from Poietics were thawed and grown in serum-free expansion medium (SFEM) containing 100 ng/mL stem cell factor (Miltenyi Biotech), 10 ng/mL interleukin-3 (Miltenyi Biotech), 0.5 U/mL erythropoietin (EPO; Amgen) 40 mg/mL lipids (Sigma-Aldrich), 100 U/mL penicillin/streptomycin, and 2mM glutamine. Thawed cells were then targeted with short hairpin RNAs (shRNAs) targeting RPS19, RPS14, or the luciferase gene (control) as previously described.9 ShRNA sequences were published elsewhere.9 Transduced cells were next treated with 100μM or 1mM of L-leucine or no treatment and continuously selected with 1 μg/mL puromycin (Sigma-Aldrich) starting 24 hours after lentiviral transduction (day 2). Cells were transferred to high EPO containing medium (2 U/mL) on day 7 and harvested on day 10. Cell suspensions were labeled with a phycoerythrin-conjugated antibody to glycophorin-A (Gly-A; CD235a, Clone GA-R2; BD-Pharmingen) and CD71(lot 42 267, BD Pharmingen) examined by flow cytometry (FACSCalibur; BD Biosciences) and analyzed using Flowjo. In experiments using rapamycin (Sigma-Aldrich), 50nM of rapamycin was added to cell cultures at the same time of L-leucine addition.
Results
Defining a model of DBA with haploinsufficient levels of Rps19
Loss of Rps19 in zebrafish embryos has been shown to result in anemia and developmental defects,18,24 but the levels Rps19 protein were not previously examined in these studies. To model the degree of ribosomal haploinsufficiency more accurately, we titrated the dose of the MO targeting the rps19 ATG/5′UTR to obtain approximately 50% of the amount of Rps19 protein compared with control injected embryos (supplemental Figure 1A-I, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) thus recapitulating the human DBA genotype. We observed a severe anemia at doses as low as 0.02 ng, the dose resulting in a 50% decrease in Rps19 expression levels. Injection of MO doses lower than 0.02 ng resulted in embryos that appeared developmentally wild-type and were not anemic, suggesting there may be a threshold concentration of Rps19 required for normal development and hematopoiesis. For all subsequent experiments a dose of 0.02 ng rps19 MO was used.
L-leucine improves hemoglobinization in Rps19 deficient zebrafish embryos
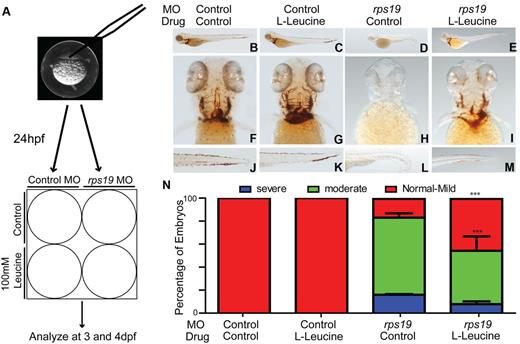
To determine whether the administration of L-leucine could rescue the phenotypic effects of Rps19 haploinsufficiency, 1 to 2 cell stage zebrafish embryos were injected with either control or rps19 morpholino and treated with 100mM L-leucine or egg water 24 hpf (Figure 1A). Embryos were monitored daily using a dissecting microscope and hemoglobinization was assessed using O-dianisidine staining at 4 dpf. rps19 morphants raised in egg water showed a profound anemia compared with control morphants (Figure 1B,F,J compared with D,H,L). rps19 morphants treated with 100mM L-leucine showed a striking improvement in hemoglobinization at 4 dpf compared with those raised in egg water alone (Figure 1E,I,M compared with D,H,L). In contrast, control morphants treated with egg water showed no differences compared with control morphants treated with L-leucine (Figure 1B,F,J compared with C,G,K). The improvement in hemoglobinization was quantified by 2 independent investigators and showed that the proportion of Rps19 deficient embryos demonstrating normal hemoglobinization or only mild anemia after exposure to 100mM of L-leucine for 3 days was 60% compared with 30% in rps19 morphants exposed to egg water alone (Figure 1N). We assessed the specificity of the effects of L-leucine on hematopoiesis by performing the same experiments using the inactive D-Leucine isomer. No improvement in the phenotype of D-Leucine treated embryos compared with those treated with egg water alone was observed (supplemental Figure 2A-F). Furthermore, to determine whether improvements in hemoglobinization could be induced by exposure to other essential amino acids, we treated embryos with L-tryptophan. An increase in developmental abnormalities affecting some embryos which resulted in “kinking” of the spinal cord were observed at high doses of L-tryptophan used (40mM) in both rps19 and control morphants. However, we did not observe any effect on hemoglobinization after L-tryptophan treatment (supplemental Figure 2G-L).
Treatment of Rps19 zebrafish morphants with L-leucine results in improved hemoglobinization. (A) Schematic of the experimental design. Embryos were injected at the single-cell stage with a morpholino targeting the translation initiation site of rps19, at 24 hours postfertilization (hpf) embryos were divided in half into 6-well plates and treated with 100mM L-leucine or egg water. (B-M) Representative images of zebrafish embryos at 4 days postfertilization (dpf) stained for hemoglobin with O-dianisidine. Lateral views with head to the left, dorsal upwards (B-E), ventral (F-I), and tail (J-M). Control morphants treated with egg water alone (B,F,J) showed normal hemoglobinization, as did the control morphants treated with 100mM L-leucine (C,G,K). rps19 morphants treated with egg water (D,H,L) were severely anemic and exhibited morphologic abnormalities including short body length, lack of bronchial arch development, and ventral tail kinking, all of which were alleviated with L-leucine treatment (E,I,M). (N) Quantification of the percentage of embryos exhibiting the phenotypes shown in panels B through M. There is a significant effect of L-leucine in the presence of Rps19 knockdown. The percentage of severely and moderately anemic rps19 morphants with leucine treatment are reduced, whereas those with normal hemoglobin or mild anemia are increased (***P < .001, 2-way ANOVA; error bars show SEM).
Treatment of Rps19 zebrafish morphants with L-leucine results in improved hemoglobinization. (A) Schematic of the experimental design. Embryos were injected at the single-cell stage with a morpholino targeting the translation initiation site of rps19, at 24 hours postfertilization (hpf) embryos were divided in half into 6-well plates and treated with 100mM L-leucine or egg water. (B-M) Representative images of zebrafish embryos at 4 days postfertilization (dpf) stained for hemoglobin with O-dianisidine. Lateral views with head to the left, dorsal upwards (B-E), ventral (F-I), and tail (J-M). Control morphants treated with egg water alone (B,F,J) showed normal hemoglobinization, as did the control morphants treated with 100mM L-leucine (C,G,K). rps19 morphants treated with egg water (D,H,L) were severely anemic and exhibited morphologic abnormalities including short body length, lack of bronchial arch development, and ventral tail kinking, all of which were alleviated with L-leucine treatment (E,I,M). (N) Quantification of the percentage of embryos exhibiting the phenotypes shown in panels B through M. There is a significant effect of L-leucine in the presence of Rps19 knockdown. The percentage of severely and moderately anemic rps19 morphants with leucine treatment are reduced, whereas those with normal hemoglobin or mild anemia are increased (***P < .001, 2-way ANOVA; error bars show SEM).
L-leucine increases red cell numbers in rps19-deficient zebrafish
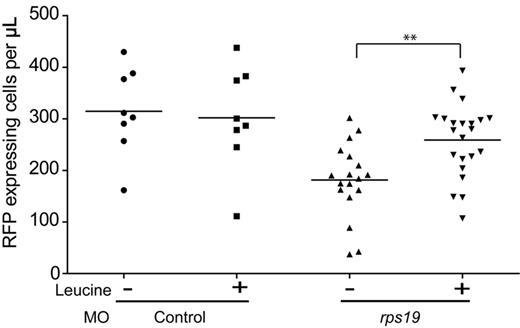
To determine whether the effects of L-leucine on rps19 morphants were confined to an increase in hemoglobinization or whether they also resulted in an increase in the number of erythroid cells, we used the transgenic zebrafish line Tg(gata-1:RFP). This line expresses red fluorescent protein (RFP) driven from the gata-1 promoter, and thus exhibits red fluorescence almost exclusively in erythroid cells at all stages of erythroid maturation.25 Rps19 and control morphant Tg(gata-1:RFP) embryos were treated with L-leucine or egg water and individual embryos were dissociated into single-cell suspensions at 3 dpf. The number of RFP-expressing cells per embryo was determined by flow cytometry. In rps19 morphants, the number of RFP-expressing cells was significantly lower than control morphants (Figure 2) when incubated in egg water alone. In contrast, rps19 morphants treated with L-leucine, showed a significant increase in the number of RFP-expressing erythroid cells. L-leucine treatment had no effect on the number of erythroid cells in control morphants. In contrast to our observations using hemoglobin staining, the majority of embryos exposed to L-leucine showed an increase in the number of RFP-expressing cells. Thus, although improvements in hemoglobin appear stochastic, the increase in erythroid cell numbers occurs in all embryos exposed to L-leucine. This may be because of the possible differential effects L-leucine may exert on erythroid cell proliferation and survival in comparison to cellular differentiation. Our results show that treatment of rps19 morphants with L-leucine results in an increase not only in the number of RFP-expressing erythroid cells but in increased hemoglobinization as well.
L-leucine increases the number of erythrocytes in Rps19 morphants. Scatter plot showing the percentage of RFP-expressing cells per embryo. Each data point is a single embryo. Embryos treated with egg water alone (−) show a statistically significant reduction in the number of erythroid cells in the presence of Rps19 knockdown (P < .001), and this could be rescued in the presence of 100mM L-leucine (**P < .005; Student t test).
L-leucine increases the number of erythrocytes in Rps19 morphants. Scatter plot showing the percentage of RFP-expressing cells per embryo. Each data point is a single embryo. Embryos treated with egg water alone (−) show a statistically significant reduction in the number of erythroid cells in the presence of Rps19 knockdown (P < .001), and this could be rescued in the presence of 100mM L-leucine (**P < .005; Student t test).
L-leucine rescues the developmental and growth defects in Rps19 deficient zebrafish embryos
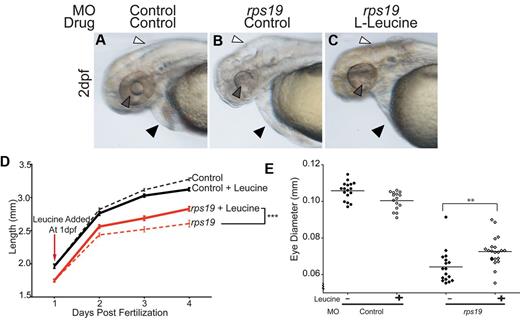
Rps19 haploinsufficient zebrafish exhibited a variety of developmental defects in addition to anemia. In particular eye, brain, and cardiac development were impaired in the majority of the rps19 morphants. Embryos also showed shortened body length, in keeping with the short stature observed in humans with DBA. Within 24 hours of commencing treatment with L-leucine, a marked improvement in all developmental abnormalities was seen in rps19 morphants (Figure 3B compared with C). rps19 morphants raised in egg water alone had small heads with cerebral edema (Figure 3B white arrow) and underdeveloped eyes (Figure 3B gray arrow) compared with controls (Figure 3A white and gray arrows). In addition, rps19 morphants also had cardiac edema (Figure 3B black arrows) compared with the control morphant (Figure 3A black arrow). The L-leucine treated rps19 morphants, on the other hand, had less cerebral and cardiac edema, and the eye and lens tissues were more clearly discernible (Figure 3C). To quantify these developmental defects, we measured the diameter of eyes in control or rps19 morphant embryos with and without L-leucine. L-leucine treatment resulted in a significant increase in eye diameter in rps19 morphants (Figure 3E). One of the most common extrahematopoietic manifestations of DBA in humans is short stature. Zebrafish with haploinsufficient levels of Rps19 show a marked growth defect and are significantly smaller than control morphants (Figure 3D red dashed line compared with black dashed line). With the addition of L-leucine, the rate of growth of rps19 morphants increased to a rate similar to that of control morphants treated with L-leucine for the first 24 hours (Figure 3D slope of the bold red compared with bold black line). By 2 dpf a statistically significant difference (P < .05) in body length between L-leucine treated and untreated Rps19 morphants was observed indicating that L-leucine therapy significantly improves overall growth in Rps19 deficiency. This effect became more significant at 3 and 4 dpf with the statistical values reaching P < .001 by 3 dpf.
Developmental abnormalities associated with Rps19 deficiency are alleviated with L-leucine treatment. (A-C) Brightfield images of live 2 dpf morphants, lateral view of head and yolk. rps19 morphants treated with control (B) displayed severe cranial (white arrow) and mild cardiac (black arrow) edema, as well as an underdeveloped eye, particularly the lack of a lens (gray arrow) compared with the control morphant (A). Cranial and cardiovascular edema was absent in rps19 morphants treated with L-leucine (white and black arrows, respectively) and eye development was markedly improved, most evident in the lens morphology (gray arrow). (D) Time course of body length measurements in rps19 morphants (red lines) and controls (black lines). L-leucine treated rps19 morphants (solid red line) had a significantly longer body length compared with rps19 morphants treated with control (dashed red line; ***P < .001, 2-way ANOVA). (E) Quantitation of eye defect in rps19 morphants using eye diameter. Filled shapes show eye diameter in embryos raised in egg water, open shapes show eye diameter in embryos raised in egg water with 100mM L-leucine (**P < .0001, 2-way ANOVA).
Developmental abnormalities associated with Rps19 deficiency are alleviated with L-leucine treatment. (A-C) Brightfield images of live 2 dpf morphants, lateral view of head and yolk. rps19 morphants treated with control (B) displayed severe cranial (white arrow) and mild cardiac (black arrow) edema, as well as an underdeveloped eye, particularly the lack of a lens (gray arrow) compared with the control morphant (A). Cranial and cardiovascular edema was absent in rps19 morphants treated with L-leucine (white and black arrows, respectively) and eye development was markedly improved, most evident in the lens morphology (gray arrow). (D) Time course of body length measurements in rps19 morphants (red lines) and controls (black lines). L-leucine treated rps19 morphants (solid red line) had a significantly longer body length compared with rps19 morphants treated with control (dashed red line; ***P < .001, 2-way ANOVA). (E) Quantitation of eye defect in rps19 morphants using eye diameter. Filled shapes show eye diameter in embryos raised in egg water, open shapes show eye diameter in embryos raised in egg water with 100mM L-leucine (**P < .0001, 2-way ANOVA).
Loss of Rps14 to haploinsufficient levels results in anemia, which is responsive to L-leucine therapy
Our results show that the addition of L-leucine to developing zebrafish embryos with approximately 50% wild-type levels of Rps19 results in improvements in the hemoglobinization, red cell numbers, and developmental defects. To assess whether this effect was specific to the loss of Rps19 alone, we used MOs directed against Rps14, targeting the exon2-intron2 boundary to knockdown expression to haploinsufficient levels (supplemental Figure 3I-J). Haploinsufficient levels of Rps14 are found in the bone marrow of patients with del(5q) MDS, but to date no germ line mutations in RPS14 have been identified in patients with Diamond-Blackfan anemia.4 Haploinsufficiency for Rps14 in zebrafish embryos resulted in a phenotype similar to that observed in Rps19 haploinsufficiency (supplemental Figure 3A-H). Rps14-deficient embryos were profoundly anemic and had short body length, abnormal bronchial arch development, along with brain and cardiac edema (supplemental Figure 3A-C,E-G). The level of knockdown of Rps14 was demonstrated by Western blot analysis (supplemental Figure 3J) and by the generation of aberrant splice variants generated by the Rps14 morpholino as measured by polymerase chain reaction analysis (supplemental Figure 3I). We also showed the phenotypes (anemia and developmental defects) specifically result from the knockdown of Rps14 by rescuing these effects after injection of RNA encoding zebrafish Rps14 (supplemental Figure 3D-H).
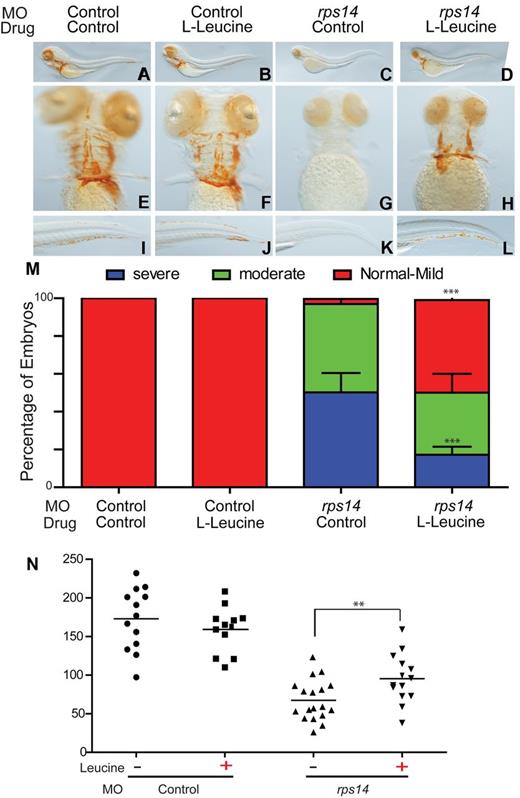
We assessed the effects of L-leucine on rps14 morphants as described for rps19 morphants. A striking improvement in the anemia observed in L-leucine treated rps14 morphants compared with rps14 morphants treated with egg water alone was observed (Figure 4 D,H,L compared with C,G,K). The addition of L-leucine to control morphants showed no obvious effect (Figure 4A,E,I vs B,F,J). The proportion of embryos showing an improvement in hemoglobinization was more marked for rps14 morphants than that observed for rps19 morphants. In rps14 morphants, L-leucine treatment resulted in only 10% of embryos with severe anemia at 4 dpf compared with 50% in the egg water treated rps14 morphant controls (Figure 4M). As with the L-leucine treated rps19 morphants, red cell numbers were also significantly increased in rps14 morphants treated with L-leucine compared with egg water alone as determined by FACS analysis of dissociated Tg(gata1:RFP) embryos injected with rps14 or control morpholino (Figure 4N). We also observed a marked improvement in the developmental effects associated with Rps14 haploinsufficiency similar to those observed with Rps19 (supplemental Figure 4); however, germ line mutations of RPS14 have not been described.4
L-leucine improved hemoglobinization and increases total numbers of erythrocytes in rps14 morphants. (A-L) Representative images of zebrafish embryos at 4 dpf stained for hemoglobin with O-dianisidine. Lateral views with head to the left, dorsal upwards (A-D), ventral (E-H), and tail (I-L). Control morphants treated with control (A,E,I) showed normal hemoglobinization, as did the control morphants treated with leucine (B,F,J). rps14 morphants treated with control (C-G-K) were severely anemic and exhibited morphologic abnormalities including short stature and lack of bronchial arch development similar to those observed in rps19 morphants. Developmental defects and anemia were alleviated after leucine treatment (D,H,L). (M) Quantification of the percentage of embryos exhibiting the phenotypes shown in panels A through L. The percentage of severely and moderately anemic rps14 morphants with leucine treatment were reduced, whereas those with normal hemoglobin/mild anemia increased (***P < .001, 2-way ANOVA with Bonferroni posttest analysis; error bars show SEM). (N) Scatter plot showing the absolute number of RFP-expressing cells per embryo. Each data point is a single embryo (**P < .01).
L-leucine improved hemoglobinization and increases total numbers of erythrocytes in rps14 morphants. (A-L) Representative images of zebrafish embryos at 4 dpf stained for hemoglobin with O-dianisidine. Lateral views with head to the left, dorsal upwards (A-D), ventral (E-H), and tail (I-L). Control morphants treated with control (A,E,I) showed normal hemoglobinization, as did the control morphants treated with leucine (B,F,J). rps14 morphants treated with control (C-G-K) were severely anemic and exhibited morphologic abnormalities including short stature and lack of bronchial arch development similar to those observed in rps19 morphants. Developmental defects and anemia were alleviated after leucine treatment (D,H,L). (M) Quantification of the percentage of embryos exhibiting the phenotypes shown in panels A through L. The percentage of severely and moderately anemic rps14 morphants with leucine treatment were reduced, whereas those with normal hemoglobin/mild anemia increased (***P < .001, 2-way ANOVA with Bonferroni posttest analysis; error bars show SEM). (N) Scatter plot showing the absolute number of RFP-expressing cells per embryo. Each data point is a single embryo (**P < .01).
L-leucine activates the mTORC1 pathway in rps19 and rps14 morphants
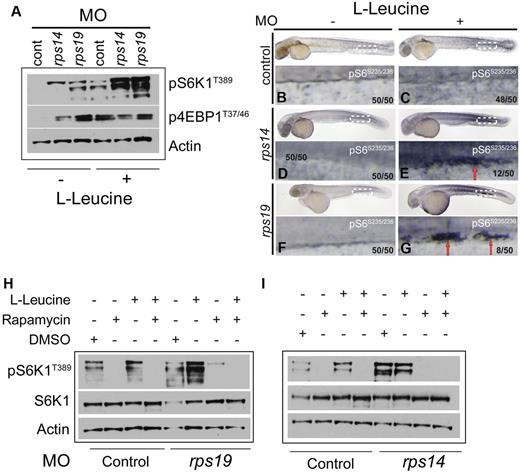
Signaling through the mammalian/mechanistic target of rapamycin (mTOR) pathway is known to up-regulate protein synthesis via multiple mechanisms involving phosphorylation and activation of a number of downstream targets involved in the docking of mRNA to the 40S ribosomal subunit during mRNA translation. Nutrients, such as amino acids, and in particular the branched-chain amino acids, such as L-leucine, are potent activators of the mTOR pathway.15 Phosphorylation (and thus activation) of one if its targets S6K1 by mTORC1 has been shown to promote a cascade of downstream signaling to substrates such as ribosomal protein S6 and eIF4B, which are useful surrogate markers for increased protein synthesis stimulated by mTORC1 activity. Thus, to determine whether L-leucine activates the mTORC1 pathway, we analyzed L-leucine treated rps14 and rps19 morphants using Western blotting at 48 hpf. As predicted, L-leucine led to an increase in phosphorylation of S6K1 in control morphants. Interestingly, an increase in phosphorylation of S6K1 was also observed in rps14 and rps19 morphants compared with the control morphants in the absence of L-leucine (Figure 5A). However, after L-leucine treatment, we observed a dramatic increase in phosphorylation of S6K1 in both rps14 and rps19 morphants compared with the L-leucine treated control morphants and morphants treated with egg water alone. No clear increase in phosphorylation levels of the second mTORC1 substrate, 4E-BP1, was observed in the L-leucine treated morphants (Figure 5A). These data indicate that L-leucine treatment in the context of Rps14 or Rps19 deficiency results in the activation of S6K1.
Haploinsufficiency of Rps14 or Rps19 in zebrafish result in phosphorylation of S6K1. (A) Zebrafish embryos were injected with rps14, rps19 and control MOs. Twenty-four hpf, embryos were treated with egg water (−) or with 100mM L-leucine (+) and incubated for an additional 24 hours. Embryos were then manually deyolked and the total protein isolated, subjected to Western blot analysis and probed sequentially with antibodies for p-S6K1T389, p-4E-BP1T37/46, and β-actin. (B-G) Whole mount staining of zebrafish embryos with p-S6 antibody. In control morphants L-leucine treatment results in a modest increase in pS6 which is apparent throughout the embryo. In rps14 (E) and rps19 (G) morphants, clusters of presumed hematopoietic progenitor cells expressing high levels of p-S6 are present in the caudal hematopoietic tissue (E-G red arrows) Number in brackets in the number of embryos showing the phenotype in the image. (H) Embryos were injected with control or rps19 MOs and treated with 1mM rapamycin or 1mM rapamycin + 100mM L-leucine at 24 hpf. Western blot analysis was performed 24 hours later. A DMSO control (diluent for rapamycin) was also included. The blot was sequentially probed with antibodies for p-S6K1T389, S6K1, and β-actin. (I) Embryos were injected with control or rps14 MO and treated with rapamycin and rapamycin+L-leucine as in panel B. The blot was sequentially probed with the same antibodies as in panel B.
Haploinsufficiency of Rps14 or Rps19 in zebrafish result in phosphorylation of S6K1. (A) Zebrafish embryos were injected with rps14, rps19 and control MOs. Twenty-four hpf, embryos were treated with egg water (−) or with 100mM L-leucine (+) and incubated for an additional 24 hours. Embryos were then manually deyolked and the total protein isolated, subjected to Western blot analysis and probed sequentially with antibodies for p-S6K1T389, p-4E-BP1T37/46, and β-actin. (B-G) Whole mount staining of zebrafish embryos with p-S6 antibody. In control morphants L-leucine treatment results in a modest increase in pS6 which is apparent throughout the embryo. In rps14 (E) and rps19 (G) morphants, clusters of presumed hematopoietic progenitor cells expressing high levels of p-S6 are present in the caudal hematopoietic tissue (E-G red arrows) Number in brackets in the number of embryos showing the phenotype in the image. (H) Embryos were injected with control or rps19 MOs and treated with 1mM rapamycin or 1mM rapamycin + 100mM L-leucine at 24 hpf. Western blot analysis was performed 24 hours later. A DMSO control (diluent for rapamycin) was also included. The blot was sequentially probed with antibodies for p-S6K1T389, S6K1, and β-actin. (I) Embryos were injected with control or rps14 MO and treated with rapamycin and rapamycin+L-leucine as in panel B. The blot was sequentially probed with the same antibodies as in panel B.
To determine whether downstream targets of mTORC1 signaling could be identified in hematopoietic cells, whole-mount antibody staining of rps19, rps14, and control morphants was performed using an antibody directed against phosphorylated ribosomal protein of the small subunit S6 (phospho-S6). A majority of embryos showed a global increased in expression levels of phospho-S6 in response to L-leucine treatment (Figure 5C,E,G compared with B,D,F). However, notably in rps14 and rps19 morphants, a proportion of embryos (24% and 16%, respectively) showed clusters of cells with a striking increase in the intensity of phospho-S6 expression in the caudal hematopoietic tissue (CHT; Figure 5D-G red arrows). This region is considered analogous to the fetal liver in mice and is the site of differentiation of definitive hematopoietic progenitors.26-28 The anatomic location of these cells suggests that they are developing hematopoietic progenitors expressing high levels of phospho-S6. These findings suggest a specific role for mTORC1 signaling in developing hematopoietic progenitors in response to L-leucine in RP morphants. To confirm that activation of S6K1 by L-leucine occurs via the mTORC1 pathway, we treated rps19 and rps14 morphants with the mTORC1-specific inhibitor rapamycin in the presence or absence of L-leucine. As expected, L-leucine alone activated the mTORC1 pathway as measured by phosphorylation of S6K1 (Figure 5H-I third lane). We also noted an increase in phosphorylation of S6K1 in the presence of the DMSO control (the diluent for rapamycin) which appeared to be abrogated in the presence of rapamycin (Figure 5H-I, supplemental Figure 5). Treatment of both rps14 and rps19 morphants with rapamycin blocked the phosphorylation of S6K1 (Figure 5B-C). In addition, treatment of the morphants with both rapamycin and L-leucine did not restore phosphorylated S6K1 expression (Figure 5H-I). These data indicate that haploinsufficient levels of Rps14 and Rps19 result in activation of the mTORC1 pathway. Treatment with L-leucine results in markedly increased levels of mTORC1 activation as measured by phosphorylation of S6K1. This suggests that high levels of activation of the mTORC1 pathway may overcome the effects of rps14 or rps19 deficiencies.
L-leucine rescues the anemia in human in vitro models of DBA and MDSdel(5q)
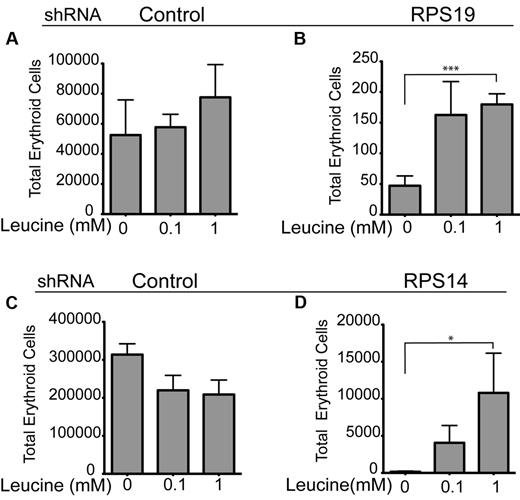
To address whether the marked improvement in the erythroid phenotype associated with Rps19 and Rps14 loss in zebrafish embryos was relevant to human RPS19 and Rps14-deficient diseases, we used lentiviral transduction of primary human CD34+ cells with shRNAs to recapitulate the haploinsufficiency of RPS14 or RPS19 in CD34+ cells.7,9 We have previously demonstrated that we can achieve haploinsufficient levels of RPS19 and RPS14 in these cells after transduction with short hairpins targeting RPS19 and RPS14, using reverse transcription-PCR and Western blot analysis.29 Analysis of CD34+ cells exposed to erythroid culture media with or without the addition of L-leucine was undertaken at 10 days of culture. Flow cytometry was used to measure surface expression of erythroid lineage markers (CD71+ and Glycophorin [Gly] A+). L-leucine treatment significantly increased the numbers of double positive (CD71+Gly A+) erythroid cells in RPS19-deficient cells treated with either 100μM or 1mM L-leucine, (Figure 6B; P < .001) and Rps14-deficient cells (Figure 6D; P < .02) treated with the same concentrations of L-leucine. Neither concentration of L-leucine had any significant effect on the control shRNA (Luciferase) treated CD34+ cells (Figure 6A-C). In summary, a significant increase in total erythroid cell numbers was achieved on treatment of RPS14 and RPS19-deficient CD34+ cells after L-leucine treatment, thereby validating our observations in the zebrafish models of DBA and del(5q) MDS.
L-leucine increases total number of erythroid (Gly A+ and CD71+) cells in RPS14 and RPS19 shRNA transduced CD34+ progenitor cells. (A-D) CD34+ hematopoietic progenitor cells were targeted with short hairpin RNAs (shRNAs) against RPS19 (B), RPS14 (D), and a control (luciferase; A-C) Transduced cells were treated with 100μM and 1mM L-leucine or no treatment and continuously selected with 1 μg/mL puromycin starting at day 2. Cells were transferred to high EPO containing medium (2 U/mL) on day 7 and harvested on day 10. The number of erythroid cells was determined by flow cytometry after staining for CD71 and glycophorin A. The experiments were performed in triplicate and repeated 3 times with similar results (***P < .001; *P < .02; Student t test).
L-leucine increases total number of erythroid (Gly A+ and CD71+) cells in RPS14 and RPS19 shRNA transduced CD34+ progenitor cells. (A-D) CD34+ hematopoietic progenitor cells were targeted with short hairpin RNAs (shRNAs) against RPS19 (B), RPS14 (D), and a control (luciferase; A-C) Transduced cells were treated with 100μM and 1mM L-leucine or no treatment and continuously selected with 1 μg/mL puromycin starting at day 2. Cells were transferred to high EPO containing medium (2 U/mL) on day 7 and harvested on day 10. The number of erythroid cells was determined by flow cytometry after staining for CD71 and glycophorin A. The experiments were performed in triplicate and repeated 3 times with similar results (***P < .001; *P < .02; Student t test).
Discussion
Several hypotheses have been proposed to explain the link between ribosomal protein haploinsufficiency and defective erythropoiesis. One hypothesis proposes that defects associated with RP haploinsufficiency result in defective ribosome biogenesis resulting in reduced levels of mRNA translation. In this study we sought to assess the efficacy of L-leucine as a stimulator of protein synthesis in alleviating defects associated with RP haploinsufficiency. Because both RPS19 and RPS14 play a role in the maturation of the 40S ribosomal subunit (reviewed by Narla and Ebert5 ) haploinsufficiency of these proteins in DBA and del(5q) MDS might result in reduced ribosome levels in all cells. However, because late stage erythroid cells exhibit rapid cell division coupled to an enormous translational demand for globin synthesis, such a decrease may be more acutely disruptive to the translational machinery in erythroid precursors, resulting in the observed anemia. In fact, DBA patients lacking an identified genetic defect also demonstrated reduced levels of 28 ribosomal protein mRNAs, including RPS19.30 Our observation that L-leucine, a modulator of 5′-cap–dependent mRNA translation, rescues the anemia associated with the 2 ribosomopathies in question, supports this hypothesis.
In this study, we show that administration of the amino acid L-leucine results in striking improvements in the erythroid and developmental defects resulting from haploinsufficient levels of Rps19 in a zebrafish model of DBA. We also show that L-leucine has a similar effect on zebrafish embryos deficient in Rps14, the probable genetic determinant of anemia in del(5q) MDS. Furthermore, we demonstrate that in primary human CD34+ cells after lentiviral knockdown of RPS19 or RPS14, L-leucine treatment significantly increases erythroid cell numbers on treatment with L-leucine, confirming the validity of our results in zebrafish in human cells. Our data represent the first animal model wherein L-leucine treatment demonstrates a marked improvement in the anemia and developmental defects observed in RP-deficiencies.
L-leucine is an essential amino acid that is unique among the branched-chain amino acids in that it acts as a nutrient regulator of protein synthesis in skeletal muscle and adipose tissue (reviewed by Kimball and Jefferson15 ). Experimental evidence showing impaired translation in DBA comes from analysis of primary lymphoid cells isolated from DBA patients. Incorporation of radiolabeled L-leucine showed a significant reduction in protein synthesis in cells derived from DBA patients.14 These findings were supported by the study conducted by Robledo et al who used siRNAs to knockdown ribosomal proteins individually, and showed a reduction in the proportion of actively translating mRNAs using polysome profiling in RP-deficient cells.31 To assess whether protein synthesis could be modulated in DBA patient cells, Pospisilova et al exposed primary DBA lymphocytes to various doses of L-leucine and demonstrated that protein synthesis could be increased using high doses of L-leucine. These investigators went on to publish a case report showing that L-leucine treatment resulted in clinical benefit to a patient with DBA.16
L-leucine is thought to modulate protein synthesis primarily via activation of cap-dependent translation through mechanistic/mammalian target of rapamycin complex 1 (mTORC1) signaling. mTORC1, a rapamycin-sensitive multiprotein complex, is a nutrient-sensitive serine/threonine kinase that acts as a master regulator of cell growth and survival (reviewed by Sengupta et al32 ). The mechanisms through which amino-acids, particularly L-leucine, regulate mTORC1 have only just begun to be addressed. The identification of the Rag (Ras-related GTPase) family of GTPases has provided a first step in the understanding of this mechanism. A recent study has shown that mTORC1 translocates to the lysosomal membrane in response to amino acid stimulation, where it interacts with a protein complex called Ragulator, which in turn brings the Rag GTPases in contact with the mTORC1 complex, a step that is necessary for its amino acid-dependent activation.33 Activation of mTORC1 then leads to an increase in the phosphorylation of 2 main target proteins; the first, eIF 4E-BP1 (eukaryotic initiation factor4E-binding protein 1) leads to the formation of the active eIF4E-eIF4G complex, which is critical for promoting cap-dependent mRNA translation (reviewed by Khanna-Gupta34 ). mTORC1 also phosphorylates S6 kinase-1, an activator of RPS6, which is thought to promote mRNA translation by regulating proteins involved in translation.35 We demonstrate a marked, rapamycin-sensitive increase in levels of phospho-S6K1 in L-leucine treated Rps14 and Rps19-deficient embryos, as well as a specific increase in the S6K1 downstream target, phospho-RPS6, in probable hematopoietic progenitors in the CHT in a proportion of embyros Rps14 and Rps19-deficient embryos.
It has been demonstrated by others that protein synthesis is reduced in cells derived from DBA patients. Paradoxically, our results show that S6K1 is phosphorylated in Rps14 and Rps19 deficient zebrafish even in the absence of L-leucine. Our observations suggest that activation of mTORC1 signaling in RP-deficient cells may be a response to stress where the cell up-regulates translation of a subset of mRNAs, the protein products of which are needed to overcome cellular distress. However, the addition of L-leucine to RP-deficient zebrafish morphants leads to increased activation of the mTORC1 pathway as measured by S6K1 phosphorylation. This suggests that the activation of mTORC1 pathway may need to be “hyperactivated” to meet the necessary translational demand for normal growth and erythropoiesis in the RP-deficient setting.
A second mechanism by which L-leucine may contribute to the improvement in anemia in RP-morphants and human cells is via specific up-regulation of translation of mRNAs carrying 5′ terminal oligopyrimidine tract (5′TOP) sequence in their 5′UTR adjacent to the 5′ m7Gppp cap.36 Such 5′TOPs confer the ability to rapidly shut down or activate translation of components of the ribosome machinery, and are found in a large portion of mRNAs involved in translation.37 5′TOP mRNAs are translationally repressed after amino acid withdrawal and conversely de-repressed on amino acid refeeding of cells. This mode of translational control is strictly dependent on the 5′TOP motif. Although it has been shown that 5′TOP mRNA translation occurs in an S6K1-independent manner, it is at least in part, sensitive to rapamycin and thus may be a component of the phenotype we observe in RP-deficient morphants.38 Interestingly a recent detailed study of the 5′UTR of the RPS19 gene identified a large number of novel 5′UTR variants in different tissues with only some possessing 5′TOPs. This study also addressed whether mutations in RPS19 affecting the 5′UTR altered the expression of RPS19 at the protein level and showed that variations in the 5′UTR significantly altered the expression level of RPS19.39 Thus, the effects that we observe on erythroid cells and development after administration of L-leucine occur via activation of the mTORC1 pathway and may also result from translational up-regulation of 5′TOP (or other as yet undefined) mRNAs.
One prominent feature we noticed in our zebrafish model is the apparent stochastic nature of the L-leucine rescue of anemia in RP-deficient embryos. Not only were there occasional morphants that appeared to show spontaneous improvement in the anemia, but in embryos exposed to L-leucine only 25% to 40% of embryos were rescued. A similar proportion of embryos showed no rescue at all. In contrast, the developmental changes seen in association with L-leucine administration showed more predictable, complete responses in all RP-deficient embryos. This may have occured because developmental processes are probably more sensitive to changes in translation associated with L-leucine treatment resulting in an improvement in growth and development. In addition it is clear that there is incomplete penetrance within families carrying DBA-associated mutations. There are now instances described where some family members carrying a mutation show minor skeletal or no defects, and others with the same genetic lesion have severe anemia. This may indicate that individual cell types may be exquisitely sensitive to gene dosage and that a threshold effect may be required for normal cellular function.
An accumulating body of evidence suggests that the tumor suppressor p53 pathway is activated in ribosomopathies (reviewed by Narla and Ebert5 ). In human hematopoietic progenitor cells haploinsufficient for RPS14 or RPS19, p53 accumulates selectively in erythroid progenitor cells, resulting in lineage-specific p53 target gene expression, cell-cycle arrest, and apoptosis. This effect can be reversed by pharmacologically inhibiting the activity of p53.9 Furthermore, elevated levels of p53 have been shown to be associated with a rapid decline in mRNA translation that is accompanied by inhibition of S6K1 phosphorylation and dephosphorylation of 4E-BP1.40 Although we have not measured protein levels of p53 in our L-leucine treated rps14 or rps19 zebrafish morphants, we did not observe any changes in p53 target gene expression after L-leucine treatment at the mRNA level (not shown), suggesting that L-leucine probably acts in a p53-independent manner.
Of note, the doses of L-leucine used in our zebrafish studies are in the millimolar range, and this level may not be achievable in DBA patients. However, we are not able to address at this time what final intracellular concentration of L-leucine is achieved in zebrafish embryos. During the developmental stages assessed, L-leucine is predominantly absorbed by diffusion in the developing zebrafish embryo because significant oral intake does not commence until after 5 days of development.41 The concentrations of L-leucine used in our study are in fact significantly lower than those used to assess the effects of L-leucine on protein synthesis in Sprague-Dawley rats (approximately 400mM).42 Furthermore, we observed an effect on human erythroid cells at concentrations greater than 1 log less than this, indicating that lower concentrations of L-leucine may be sufficient to have an effect on erythroid development in human cells.
In conclusion, we demonstrated for the first time in animal models of DBA and del(5q) MDS that L-leucine treatment of Rps19 and Rps14-deficient zebrafish embryos leads to alleviation of anemia and developmental defects. These observations have been confirmed in an in vitro human model for DBA and del(5q) using human hematopoietic progenitor CD34+ cells and shRNAs specific for RPS19 and RPS14 genes followed by L-leucine treatment. We demonstrate in addition, that L-leucine activates the mTORC1 pathway as measured by phosphorylation of its downstream target S6K1 in a rapamycin-sensitive manner and up-regulation of p-S6, a known substrate of S6K1. We conclude that L-leucine administration in Rps14 and Rps19-deficient states mitigates both the anemia and developmental defects and hypothesize that this may occur as a result of up-regulation of cap-dependent mRNA translation in RP-deficient cells. Our observations complement current ongoing clinical trials for DBA and strongly support the assessment of L-leucine as a therapeutic option in del(5q) MDS.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Greg Mollind, Lu Zhang, Carly Nicholls, Heather Callaway, and Jenna Hakkesteeg for zebrafish care, and Jee-Yeong Jeong for invaluable discussion and technical advice.
This work was supported by Leukemia & Lymphoma Research UK (GSK Greg Harper Clinical Research Training Fellowship E.M.P), the March of Dimes Foundation (B.H.P.), and the National Institutes of Health (NIH; B.H.P.), NIH 5R01 HL82945-06 (B.L.E.), and NIH 1K08 DK090145-01A1 (A.N.).
National Institutes of Health
Authorship
Contribution: E.M.P. designed, performed, and analyzed research and wrote the paper; M.V., A.N., and H.S. performed and analyzed research; M.L. performed research; B.H.P., N.B., A.T.L., and B.L.E. provided key reagents and input to the paper; and A.K.-G. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for E.M.P. is University College London Cancer Centre, London, United Kingdom.
Correspondence: Elspeth M. Payne, UCL Cancer Centre, 72 Huntley Street, London WC1E 6BT, United Kingdom; e-mail: beth.payne@cancer.ucl.ac.uk; or Arati Khanna-Gupta, Division of Hematology, Brigham and Women's Hospital, Harvard Medical School, Karp 5212, 1 Blackfan Circle, Boston, MA 02115; e-mail: akhanna-gupta@partners.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal