Abstract
The finding that many chemotherapeutic agents have immunostimulatory effects has provided the impetus to combine chemotherapy and immunotherapy for synergistic antitumor effects. However, the critical determinants of effective antitumor immunity after chemotherapy have not been defined. Here we report that adoptive transfer of tumor-specific CD4+ T cells after chemotherapy with cyclophosphamide gave rise to polyfunctional CD4+ effector cells, which in turn intensified the inflammatory milieu and enhanced the activation of CD8+ T cells in the tumor microenvironment. Although this combined chemoimmunotherapy initially resulted in progressive regression of advanced B-cell lymphoma, its therapeutic efficacy was not durable and most mice succumbed to late relapse. Notably, relapse was associated with acquisition of a tolerized phenotype in tumor-specific CD4+ T cells, characterized by overexpression of program death-1 (PD-1). Remarkably, effective antitumor immunity was maintained and cure became prevalent when polyfunctional CD4+ effector cells were prevented from undergoing PD-1–mediated tolerization, either by antibody blockade of the PD-1–PD-L1 pathway, or targeted ablation of PD-1 in tumor-specific CD4+ T cells. Our study suggests that tumor-reactive CD4+ T cells act as the “gatekeepers” of the host antitumor immunity in the postchemotherapy setting, thereby their functional status governs the choice between eradication versus regrowth of residual tumors.
Introduction
Chemotherapy is a major treatment modality for many types of cancers. Chemotherapeutic drugs are chosen for their cytotoxicity toward cancerous cells. Nevertheless, increasing numbers of anticancer drugs have been found to exert immune enhancing effects through inducing various forms of immunogenic tumor cell death, which leads to enhanced antigen processing and presentation.1 In addition, some anticancer drugs can mitigate tumor-induced immunoregulatory mechanisms by depleting T regulatory cells (Tregs) or myeloid derived suppressor cells (MDSCs).2,3 Moreover, certain agents can cause transient lymphopenia,4 along with induction of a wealth of growth factors, resulting in robust expansion and survival of tumor-reactive immune cells.5 These findings have led to a growing recognition that elicitation of the endogenous host immunity may intrinsically contribute to the efficacy of some widely used antineoplastic agents.1,6 However, although standard chemotherapy can reduce the symptoms of cancer, in adult malignancies it is rarely curative. Tumor relapses often occur in patients after the initial treatment, suggesting that the endogenous antitumor immunity elicited by chemotherapy is not sufficient to provide durable therapeutic benefit.
It is generally believed that by somehow “resetting” the tumor microenvironment, chemotherapy creates a window of opportunity for therapeutic immune interventions. There is accumulating evidence that incorporation of chemotherapy with immunologic maneuvers, such as adoptive immunotherapy and therapeutic vaccines, can effectively treat established tumors.6 In this regard, cyclophosphamide (Cy), a prototypical alkylating agent widely used in cancer treatment, has been shown to be particularly effective in augmenting the effectiveness of adoptive T-cell therapy (ACT) in various animal models and some clinical studies.7-10 A great deal has been learned about the acute effects of Cy on potentiating CD8+ T cells, enhancing antigen presentation, and reducing/inactivating Tregs.11,12 However, to date the impact of Cy on tumor-reactive CD4+ effector T cells has received little attention. Understanding the role of CD4+ T cells in the postchemotherapy period has direct clinical relevance, because one consistent observation from human ACT trials is that beneficial clinical responses are often associated with the presence of tumor-reactive CD4+ T cells.10,13 Cy is a common component of the preconditioning chemotherapy regimens used in many ACT protocols, including recent clinical studies on treating B-cell malignancies with autologous T cells engineered to express a chimeric antigen receptor (CAR).14-16 Particularly, a phase 1 study conducted by Sadelain and colleagues in patients with chronic lymphocytic leukemia (CLL) demonstrated the therapeutic potential of the combination of Cy-treatment and CD4-dominant T-cell therapy.16 These studies achieved encouraging patient outcomes, although the long-term efficacy of this treatment strategy remains to be evaluated in a larger patient population.
In this study, we aimed to gain mechanistic insight into the role of CD4+ T cells in the postchemotherapy setting. Using a biologically relevant preclinical model in which mice with advanced B-cell lymphoma were treated with Cy followed by adoptive transfer of tumor-specific CD4+ T cells, we demonstrated that CD4+ T cells with a polyfunctional effector phenotype were not only critical for mounting robust antitumor immune responses that led to continuous tumor regression after chemotherapy, but also essential for maintaining a protective immunity against tumor recurrence.
Methods
Mice
Female BALB/c mice of 4 to 6 weeks old were purchased from the National Cancer Institute. TCR transgenic mice on a BALB/c background expressing an αβTCR-specific for amino acids 110 to 120 from influenza hemagglutinin (HA) presented by major histocompatibility complex (MHC) class II molecule IAd were originally generated in the laboratory of Dr H. von Boehmer (Harvard Medical School). Thy1.1+/+ HA-TCR Tg mice were generous gifts from Dr Hyam I. Levitsky (The Johns Hopkins University School of Medicine). The PD-1KO mice on a BALB/c background (PD-1-KO-N12[BALB/c]), generated in the laboratory of Dr Tasuku Honjo,17 were purchased from RIKEN BioResource Center. Thy1.1+/+ PD-1KOHA-TCR Tg mice were obtained by crossing the 2 transgenic strains. All mice were housed under specific pathogen-free conditions by Laboratory Animal Services (LAS) of the Georgia Health Sciences University (GHSU). Rag2−/− mice on BALB/c background were purchased from Tarconic. All animal experiments were approved by the Institutional Animal Care and Use Committee of GHSU.
Antibodies and reagents
Detailed descriptions of reagents are given in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Tumor cells and animal tumor models
The generation and maintenance of the wild-type (WT) murine B-cell lymphoma cell line A20 (A20WT) and its derivative cell lines A20HA were previously described.18 A20 cell line expressing granulocyte macrophage colony-stimulating factor (GM-CSF; A20GMCSF) was a gift from Dr Hyam I. Levitsky. Colorectal tumor cell line CT26, purchased from ATCC, was engineered to express HA (CT26HA). For T-cell adoptive transfer, spleens, and lymph nodes from specified Tg mice were harvested, homogenized, lysed by ACK lysing buffer (Invitrogen) to remove red blood cells. Cells were enriched for CD4+ T cells by magnetic-activated cell sorting (Miltenyi Biotec), and labeled with 2uM carboxyfluorescein diacetate succinimidyl ester (CFSE). The percentage of lymphocytes positive for CD4 and the clonotypic TCR (monoclonal antibody 6.5 for HA-specific CD4+ T cells) was determined by flow cytometry. A total of 2.5 ∼ 3 × 106 CD4+ TCR+ T cells were injected intravenously into each recipient.
A20HA tumor cells were inoculated to mice via 2 different routes as specified in study. When injected via tail vein (1 × 106 per mouse), A20HA tumors systemically disseminated to secondary lymphoid tissues, and infiltrated the liver and bone marrow at late stages, closely mimicking human B-cell malignancy.19 By contrast, subcutaneous injection of A20HA (5 × 106) on the hind flank led to localized tumor growth. CT26HA tumors (0.5 × 106) injected to mice via tail vein exclusively localized to the lungs. For subcutaneous A20HA tumors, tumor growth was monitored by caliper measurement of the tumor area every 3 days, and expressed as the product of 2 perpendicular diameters in square millimeters. Mice were euthanized when tumor size reached 400 mm2 or when tumor sites ulcerated. For systemic A20HA tumors, the experimental endpoints for survival studies were limb paralysis, appearance of ascites, and death. Long-term survival was defined as no sign of tumor presence and no symptom of sickness by day 40 after treatments. Cures were defined as no abnormalities on anatomical inspection of mice that have met the criteria for long-term survival.
Combinatorial chemoimmunotherapy
Details of the treatment procedures are given in supplemental Methods.
Immunofluorescence
Tumors were flash-frozen in optimal cutting temperature solution (Sakura). Sections (8 μm) were cut with a microcryotome (Leica), fixed for 10 minutes in cold acetone, and stained with rat anti-CD8 antibody (clone 53.67). After wash, sections were stained with Dylight 488–conjugated donkey anti–rat IgG (Jackson ImmunoResearch Laboratories) and DAPI, and analyzed on an Axio Observer inverted microscope (Zeiss) under a 20× objective.
Cell preparation and flow cytometry analysis
Detailed methods are given in supplemental Methods.
Quantitative real-time PCR
Detailed methods are given in supplemental Methods.
Statistical analysis
Data were analyzed using Prism 4.0 software (GraphPad). The statistical significance of the results was determined using the Student t test. Data for tumor survival were analyzed using a log-rank test. P values less than .05 were considered statistically significant.
Results
Chemotherapy with Cy drives a productive CD4+ T-cell response among adoptively transferred tumor-specific CD4+ T cells that otherwise would be tolerized
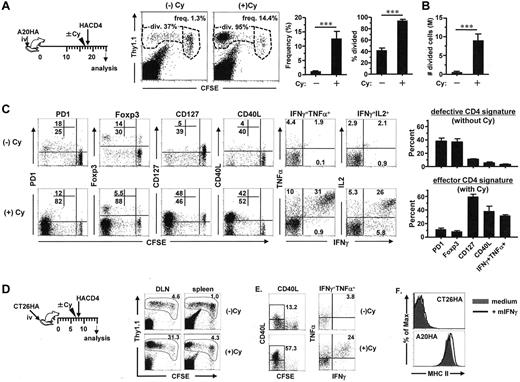
We recently reported that chemotherapy with Cy can potentiate antitumor CD4+ T-cell responses by driving the effector differentiation of adoptively transferred tumor-specific CD4+ T cells.9 In this study, we sought to identify the immunologic parameters that can reflect the functional status of tumor-specific CD4+ T cells in the postchemotherapy setting. Figure 1A shows that Cy-treatment of mice bearing systemic A20HA tumors led to a sharp increase in the frequency of the transferred HA-specific CD4+ T cells, accompanied by enhanced cell division. Correspondingly, there was substantial accumulation of highly divided tumor-specific CD4+ T cells in Cy-treated mice (Figure 1B). In addition, the divided CD4+ T cells in Cy-treated versus untreated mice showed marked differences in their phenotype and functionality as measured by a panel of selected phenotypic and functional markers. Among these markers, sustained PD-1 expression is associated with clonal exhaustion in CD8+ T cells,20 and Foxp3 is the hallmark of CD4+ regulatory T cells21 ; thus prominent expression of these molecules would be suggestive of a defective immune response. On the other hand, CD40L and Th1-type cytokines (such as IFNγ, TNFα, and IL2) are associated with CD4+ effector/helper activities,22,23 and CD127 (IL7R) expression correlates with effector to memory transition;24 thus expression of these molecules would imply a productive immune response. After adoptive transfer, tumor-specific CD4+ T cells in mice without chemotherapy exhibited a defective response signature, characterized by high levels of PD-1 and Foxp3, low levels of CD127 and CD40L, and a paucity of Th1-type cytokines [Figure 1C (−)Cy]. By contrast, pretreatment of the hosts with a single dose of Cy had profound beneficial effects on the transferred tumor-specific CD4+ T cells, resulting in reduced levels of PD-1 and Foxp3, but pronounced expression of CD127 and CD40L [Figure 1C (+)Cy]; and importantly, these cells acquired polyfunctionality—the ability to simultaneously produce multiple proinflammatory cytokines including IFNγ, TNFα, and IL2.
Tumor-specific CD4+ T cells transferred after Cy-treatment acquire a polyfunctional effector signature. Following the timeline depicted in the schema, mice were treated or not treated with Cy before receiving CFSE-labeled CD4+ T cells derived from HA-TCR Tg mice (Thy1.1 background). Seven days after T-cell transfer, spleen cells were harvested for analysis. (A) The frequency and cell division of the transferred CD4+ T cells are shown in representative dot plots. Results are summarized in bar graphs. (B) The absolute numbers of divided donor CD4+ T cells. The calculation formula is: total spleen cell number × frequency of transferred CD4+ T cells × fraction of divided cells (***P < .001). (C) Phenotypic and functional analyses of the transferred CD4+ T cells. PD1, Foxp3, CD127, and CD40L expression profiles relative to cell division of the transferred CD4+ T cells are shown. Cytokine expression in transferred CD4+ T cells was assayed by intracellular cytokine staining (ICS) after 4 hours of antigenic stimulation in vitro, and the plots shown are gated on the divided CD4+ T cells. Numbers in plots indicate the percentage of cells in the corresponding quadrant. The bar graphs summarize the percentages of divided donor CD4+ T cells positive for the indicated markers. Data shown in each bar graph are results from 2 independent experiments and represented as mean ± SD of at least 6 mice per group. (D) Similar experiments were conducted in CT26HA tumor model. The schema outlines the timeline of the procedures. Seven days after T-cell transfer, spleens and lung draining lymph nodes (DLNs) were recovered and analyzed. Dot plots are representative of the transferred CD4+ T cells in DLNs and spleen. Numbers represent the frequency of the gated donor CD4+ T cells. (E) Expression of CD40L in transferred CD4+ T cells in DLNs and ICS measurement of cytokines in divided donor CD4+ T cells. Numbers in the left panel indicate the percentages of CD40Lhigh cells in divided donor CD4+ T cells. Numbers in the right panel represent the percentages of IFNγ and TNFα double-positive cells in divided donor CD4+ T cells. Data shown are representative of 3 independent experiments with similar results. (F) CT26HA tumor cells remain MHC II negative even in the presence of IFNγ. CT26HA were cultured in the absence of presence of 100 ng/mL murine IFNγ for 2 days and then stained for MHC II. A20HA cells were used as a positive control for constitutive and IFNγ-inducible MHC II expressions.
Tumor-specific CD4+ T cells transferred after Cy-treatment acquire a polyfunctional effector signature. Following the timeline depicted in the schema, mice were treated or not treated with Cy before receiving CFSE-labeled CD4+ T cells derived from HA-TCR Tg mice (Thy1.1 background). Seven days after T-cell transfer, spleen cells were harvested for analysis. (A) The frequency and cell division of the transferred CD4+ T cells are shown in representative dot plots. Results are summarized in bar graphs. (B) The absolute numbers of divided donor CD4+ T cells. The calculation formula is: total spleen cell number × frequency of transferred CD4+ T cells × fraction of divided cells (***P < .001). (C) Phenotypic and functional analyses of the transferred CD4+ T cells. PD1, Foxp3, CD127, and CD40L expression profiles relative to cell division of the transferred CD4+ T cells are shown. Cytokine expression in transferred CD4+ T cells was assayed by intracellular cytokine staining (ICS) after 4 hours of antigenic stimulation in vitro, and the plots shown are gated on the divided CD4+ T cells. Numbers in plots indicate the percentage of cells in the corresponding quadrant. The bar graphs summarize the percentages of divided donor CD4+ T cells positive for the indicated markers. Data shown in each bar graph are results from 2 independent experiments and represented as mean ± SD of at least 6 mice per group. (D) Similar experiments were conducted in CT26HA tumor model. The schema outlines the timeline of the procedures. Seven days after T-cell transfer, spleens and lung draining lymph nodes (DLNs) were recovered and analyzed. Dot plots are representative of the transferred CD4+ T cells in DLNs and spleen. Numbers represent the frequency of the gated donor CD4+ T cells. (E) Expression of CD40L in transferred CD4+ T cells in DLNs and ICS measurement of cytokines in divided donor CD4+ T cells. Numbers in the left panel indicate the percentages of CD40Lhigh cells in divided donor CD4+ T cells. Numbers in the right panel represent the percentages of IFNγ and TNFα double-positive cells in divided donor CD4+ T cells. Data shown are representative of 3 independent experiments with similar results. (F) CT26HA tumor cells remain MHC II negative even in the presence of IFNγ. CT26HA were cultured in the absence of presence of 100 ng/mL murine IFNγ for 2 days and then stained for MHC II. A20HA cells were used as a positive control for constitutive and IFNγ-inducible MHC II expressions.
Of note, we have previously shown in this model that polyfunctional CD4+ effector cells do not arise if tumor cells do not express the cognate antigen,9 suggesting the importance of chemotherapy-induced antigen release. In addition to releasing tumor antigens by directly killing tumor cells, Cy used at certain doses can also cause lymphopenia, which can drive homeostatic proliferation of T cells.5,11 To dissect the relevance of antigen provision and lymphopenia, we conducted a series of experiments in which A20HA-bearing Rag2−/− mice that had received HA-specific CD4+ T cells were or were not subsequently immunized with irradiated A20HA tumor cells (supplemental Figure 1). The rationales for the experimental design were that Rag2−/− mice would provide a lymphopenic environment, and irradiated tumor cells would die in vivo and thus release antigens. The conclusion of this set of experiments was that the mere combination of lymphopenia and tumor cell death did not recapitulate the robust CD4-potentiating effects of Cy. Our results support the notion that the potent stimulatory effects of Cy on adoptive T-cell therapy is the combined result of its multiplex immunomodulating properties, including mitigation of immunosuppression and creation of an immunogenic milieu, in addition to inducing tumor cell death and lymphopenia.
The immune-potentiating effects of Cy on tumor-specific CD4+ T cells are applicable to MHC-II–negative tumors
Of B-cell origin, A20 tumor cells can potentially act as APCs for CD4+ T cells,25 raising the question whether the observed CD4-potentiating effects of Cy were unique to the lymphoma model. To address this, the same experimental procedures used in the A20HA lymphoma model were applied to a colorectal tumor model in which HA-expressing CT26 tumors (CT26HA) were inoculated to mice via tail vein. Analogous to the results seen in the A20HA tumor model, Cy significantly enhanced the expansion of the transferred CD4+ T cells in the draining lymph node and spleen (Figure 1D), and promoted the generation of polyfunctional CD4+ effector cells, marked by elevated CD40L expression and the ability to produce multiple proinflammatory cytokines (Figure 1E). Similar results were obtained in mice bearing HA-expressing renal cell or mammary carcinoma, RencaHA, or 4T1HA, respectively (data not shown). It is important to note that CT26 tumor cells, unlike A20 tumor cells, do not constitutively express MHC II molecules, and cannot be induced to express MHC II by IFNγ (Figure 1F). Collectively, the data indicate that the immune-potentiating effects of Cy on CD4+ T cells are not restricted to hematologic malignancy, but also applicable to solid tumors that cannot directly present antigens to CD4+ T cells.
CD4+ effector T cells significantly contribute to reprogramming of the immune milieu in the tumor microenvironment after chemotherapy
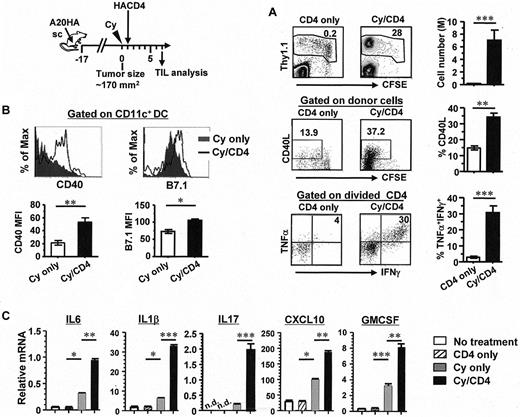
There is accumulating evidence that CD4+ T cells are critically involved in modulating the immune milieu in the tumor microenvironment.26-28 Given the robust CD4+ T-cell response after the combination of Cy-treatment and CD4+ T-cell transfer, we hypothesized that activated CD4+ effector cells might play a significant role in conditioning an immunogenic tumor milieu after chemotherapy. To facilitate the study of intratumoral milieu, A20HA tumors were subcutaneously inoculated to the flank of a mouse where they grew into a localized tumor mass. Mice with large (∼ 170 mm2) established A20HA tumors were treated or not treated with Cy, followed by adoptive transfer of HA-specific CD4+ T cells the next day (Figure 2 schema). Replicating the results seen in the systemic tumor model shown in Figure 1, Cy treatment led to vigorous expansion of the transferred tumor-specific CD4+ T cells in the tumor mass, and these infiltrating CD4+ T cells exhibited a polyfunctional effector phenotype with the signature expression of CD40L and proinflammatory cytokines including IFNγ and TNFα (Figure 2A). We reasoned that the prominent expression of CD40L and IFNγ by tumor-specific CD4+ T cells might lead to augmented activation and maturation of APCs. Supporting this notion, tumor-infiltrating CD11c+ dendritic cells (DC)s in mice receiving Cy plus CD4+ T-cell transfer had increased expression of CD40 and B7.1 compared with their counterparts in mice receiving Cy alone (Figure 2B). In mice not receiving chemotherapy (untreated and CD4+ T-cell transfer only), CD11c+ DCs were barely detectable in tumors (data not shown), suggesting that chemotherapy promotes tumor infiltration by DCs.
The presence of activated CD4+ T cells results in enhanced DC activation and heightened inflammation within the tumor microenvironment. The schema outlines the timeline of experimental procedures. Mice with subcutaneous A20HA tumors were divided into 4 groups when tumor size reached ∼ 170 mm2: no treatment, CD4+ T-cell transfer only, Cy only, and Cy plus CD4+ T-cell transfer. Seven days after T-cell transfer, tumor masses were excised and analyzed. (A) The immune status of tumor-infiltrating donor CD4+ T cells in mice with or without chemotherapy. Cells were stained as described in Figure 1. The frequency and cell division of the transferred CD4+ T cells, CD40L profile relative to cell division, and cytokine profile in divided cells are shown in representative plots. Numbers represent the percentages of cells in the given gate or quadrant. Bar graphs summarize results from 3 independent experiments with 3 mice per group in each experiment (mean ± SD). (B) Enhanced activation of intratumoral CD11c+ cells in mice receiving CD4+ T-cell transfer after Cy. Representative histograms show expression profiles of CD40 and B7.1 in gated CD11c+ cells in tumors resected from mice treated with Cy or Cy plus CD4+ T-cell transfer. Bar graphs summarize the median fluorescence intensity (MFI) of CD40 and B7.1 shown as mean ± SD (3 samples per group). (C) Cytokine/chemokine profile in tumor mass. Total RNA was extracted from resected tumor tissues and subjected to quantitative real-time PCR for the indicated genes. Each sample was measured in triplicate for each gene. Data represent the relative amount of target mRNA normalized to β-actin (mean ± SD). Data shown are results from 1 of 3 independent experiments with similar results (*P < .05, **P < .005, ***P < .001).
The presence of activated CD4+ T cells results in enhanced DC activation and heightened inflammation within the tumor microenvironment. The schema outlines the timeline of experimental procedures. Mice with subcutaneous A20HA tumors were divided into 4 groups when tumor size reached ∼ 170 mm2: no treatment, CD4+ T-cell transfer only, Cy only, and Cy plus CD4+ T-cell transfer. Seven days after T-cell transfer, tumor masses were excised and analyzed. (A) The immune status of tumor-infiltrating donor CD4+ T cells in mice with or without chemotherapy. Cells were stained as described in Figure 1. The frequency and cell division of the transferred CD4+ T cells, CD40L profile relative to cell division, and cytokine profile in divided cells are shown in representative plots. Numbers represent the percentages of cells in the given gate or quadrant. Bar graphs summarize results from 3 independent experiments with 3 mice per group in each experiment (mean ± SD). (B) Enhanced activation of intratumoral CD11c+ cells in mice receiving CD4+ T-cell transfer after Cy. Representative histograms show expression profiles of CD40 and B7.1 in gated CD11c+ cells in tumors resected from mice treated with Cy or Cy plus CD4+ T-cell transfer. Bar graphs summarize the median fluorescence intensity (MFI) of CD40 and B7.1 shown as mean ± SD (3 samples per group). (C) Cytokine/chemokine profile in tumor mass. Total RNA was extracted from resected tumor tissues and subjected to quantitative real-time PCR for the indicated genes. Each sample was measured in triplicate for each gene. Data represent the relative amount of target mRNA normalized to β-actin (mean ± SD). Data shown are results from 1 of 3 independent experiments with similar results (*P < .05, **P < .005, ***P < .001).
To assess the tumor milieu, tumor masses were resected from mice and assayed for the expression profiles of inflammatory cytokines and chemokines using quantitative real-time PCR. The transcripts of a panel of genes tested, including IL6, IL1β, IL17, CXCL10/IP10, and GMCSF, were low or undetectable in tumors recovered from untreated mice or mice that received CD4+ T-cell transfer only (Figure 2C). In line with published studies,29,30 Cy-treatment increased the transcripts of these inflammation-related genes (Figure 2C Cy only). Addition of tumor-specific CD4+ T cells after Cy further boosted the transcripts of these genes (Figure 2C Cy/CD4). Of note, IL17 protein was detected in a subset of donor CD4+ T cells on antigenic restimulation (supplemental Figure 2A), suggesting that the transferred tumor-specific CD4+ T cells contribute to elevated IL17 production in vivo. Taken together, these data suggest that Cy-treatment fosters a proinflammatory tumor milieu, and tumor-specific CD4+ effector cells can significantly reinforce this effect.
CD4+ effector T cells provoke functional maturation of intratumoral CD8+ T cells with which they collaborate to markedly enhance the efficacy of chemotherapy
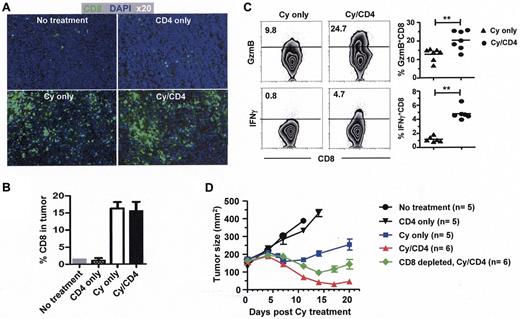
We next asked whether the presence of CD4+ effector cells would augment the recruitment and function of CD8+ T cells in tumor. In mice receiving no treatment or CD4+ T-cell transfer only, immunofluorescence (IF) staining of the tumors revealed that there was only scarce presence of endogenous CD8+ T cells; however, the tumors became heavily infiltrated by CD8+ T cells after Cy treatment, regardless of CD4+ T-cell transfer (Figure 3A). FACS analyses confirmed the observations of IF, and further demonstrated that the frequencies of tumor-infiltrating CD8+ T cells were comparable in mice that either received Cy only or Cy plus CD4+ T-cell transfer (Figure 3B). However, notably the intratumoral CD8+ T cells in mice that received Cy plus CD4+ T-cell transfer exhibited enhanced functional maturation, as reflected by the heightened levels of granzyme B and IFNγ, compared with their counterparts in mice that received Cy only (Figure 3C).
CD4+ effector T cells enable and collaborate with intratumoral CD8+ T cells to mediate enhanced antitumor effects after chemotherapy. Following the procedures depicted in Figure 2, tumors were excised 7 days after CD4+ T-cell transfer and subject to (A) immunofluorescence staining of host CD8+ T cells. The representative composite images show CD8+ cells (green) and DAPI (blue) staining in tumor sections (original magnification, ×20). (B) Frequency of tumor-infiltrating CD8+ T cells measured by flow cytometry analysis. Single-cell suspensions made from resected tumor masses were stained for CD8. Bar graph shows percentage of intratumoral CD8+ T cells in each group. Results shown are from 2 independent experiments with 6 mice per group (mean ± SD). (C) Functional analysis of intratumoral CD8+ T cells. Granzyme B level was evaluated ex vivo, IFNγ production was assayed by ICS after 4 hours stimulation of purified CD8+ T cells with PMA/ionomycin. Representative dot plots are shown, and the results are summarized in scatter plots (**P < .005). (D) Tumor growth kinetics in mice receiving the indicated treatments. Number of mice in each group is given.
CD4+ effector T cells enable and collaborate with intratumoral CD8+ T cells to mediate enhanced antitumor effects after chemotherapy. Following the procedures depicted in Figure 2, tumors were excised 7 days after CD4+ T-cell transfer and subject to (A) immunofluorescence staining of host CD8+ T cells. The representative composite images show CD8+ cells (green) and DAPI (blue) staining in tumor sections (original magnification, ×20). (B) Frequency of tumor-infiltrating CD8+ T cells measured by flow cytometry analysis. Single-cell suspensions made from resected tumor masses were stained for CD8. Bar graph shows percentage of intratumoral CD8+ T cells in each group. Results shown are from 2 independent experiments with 6 mice per group (mean ± SD). (C) Functional analysis of intratumoral CD8+ T cells. Granzyme B level was evaluated ex vivo, IFNγ production was assayed by ICS after 4 hours stimulation of purified CD8+ T cells with PMA/ionomycin. Representative dot plots are shown, and the results are summarized in scatter plots (**P < .005). (D) Tumor growth kinetics in mice receiving the indicated treatments. Number of mice in each group is given.
The copresence of the donor CD4+ T cells and the endogenous CD8+ T cells in the tumor lesion prompted us to determine their relative contribution to antitumor efficacy. Using tumor growth rate as a functional readout, Figure 3D shows that CD4+ T-cell transfer alone did not alter the tumor growth rate compared with untreated mice (CD4 only versus no treatment). When chemotherapy was given alone, tumor growth was briefly arrested but resumed shortly (Cy only). By contrast, the combination of Cy and CD4+ T-cell transfer (Cy/CD4) led to progressive regression of large established tumors over a period of 3 weeks. To evaluate the contribution of CD8+ T cells to tumor rejection, mice were injected with CD8-depleting antibody before receiving Cy plus CD4+ T-cell transfer. In the absence of the endogenous CD8+ T cells, the combination of Cy and CD4+ T-cell transfer was less effective in mediating tumor regression (Figure 3D CD8 depleted, Cy/CD4), suggesting the involvement of the host endogenous CD8+ T cells in tumor rejection. The results indicate that adoptive transfer of tumor-specific CD4+ T cells augments the efficacy of chemotherapy, and that the optimal antitumor effect of this chemoimmunotherapy requires the contribution of the endogenous CD8+ T cells.
Polyfunctional CD4+ T cells can be converted into a PD-1–dominant dysfunctional state in a tumor environment
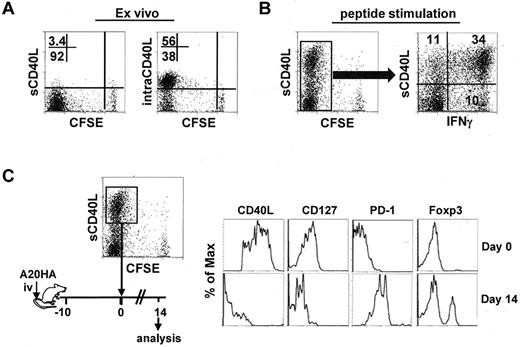
Chemotherapy can often reduce the symptoms of cancer, but it is rarely curative. Given the pivotal role of tumor-specific CD4+ effector cells in postchemotherapy hosts, it was important to know whether these cells could maintain their phenotype in the face of persistent residual tumors. To address this, we asked whether polyfunctional CD4+ effector cells were susceptible to tolerization after being re-exposed to tumors. We used CD40L as a marker for polyfunctional CD4+ effector cells. This was based on our observations that intracellular CD40L (intraCD40L) was abundant in highly divided tumor-specific CD4+ T cells recovered from tumor-bearing mice that received Cy plus CD4+ T-cell transfer (Figure 4A); and CD40L was rapidly mobilized to the surface (sCD40L) and primarily associated with IFNγ expression after a brief antigenic restimulation in vitro (Figure 4B). The highly activated CD4+ effector cells (CFSElow sCD40Lhigh) were isolated by FACS-sorting, and reinfused into mice bearing 10-day-old tumors (Figure 4C schema). Two weeks after T-cell transfer, the input cells lost expression of CD40L and CD127, became predominantly PD-1+ and acquired Foxp3 expression, manifesting a complete conversion from the effector cell signature to the defective cell signature (Figure 4C). The results demonstrate that highly activated CD4+ effector cells are at risk of being tolerized by residual tumors that persist after chemotherapy.
Polyfunctional CD4+ T cells acquire a PD1-dominant dysfunctional phenotype in a tumor environment. Following the same experimental procedures depicted in Figure 1, tumor-bearing mice were treated with Cy followed by adoptive transfer of HA-specific CD4+ T cells. Seven days after T-cell transfer, spleen cells were collected for analysis. (A) Ex vivo staining for surface (s) and intracellular (intra) expression of CD40L in the transferred CD4+ T cells. Numbers represent the percentages of cells in the given quadrant. (B) CD40L surface expression after antigenic stimulation and its correlation with IFNγ. Purified CD4+ T cells were stimulated with peptide/APC for 4 hours, then stained for surface CD40L and intracellular IFNγ. Plot showing the costaining of CD40L and IFNγ is gated on the divided cells. Data shown are representative of 3 indepen-dent experiments with similar results. (C) The CFSElowCD40Lhigh cells were collected by FACS-sorting. The phenotype of these cells was documented (top panel). The sorted cells were reinfused to mice bearing 10-day-old A20HA tumors. Two weeks after T-cell transfer, the transferred CD4+ T cells were recovered from the spleens and assayed for the expression of the indicated markers (bottom panel). Results shown are representative of 2 independent experiments with similar results.
Polyfunctional CD4+ T cells acquire a PD1-dominant dysfunctional phenotype in a tumor environment. Following the same experimental procedures depicted in Figure 1, tumor-bearing mice were treated with Cy followed by adoptive transfer of HA-specific CD4+ T cells. Seven days after T-cell transfer, spleen cells were collected for analysis. (A) Ex vivo staining for surface (s) and intracellular (intra) expression of CD40L in the transferred CD4+ T cells. Numbers represent the percentages of cells in the given quadrant. (B) CD40L surface expression after antigenic stimulation and its correlation with IFNγ. Purified CD4+ T cells were stimulated with peptide/APC for 4 hours, then stained for surface CD40L and intracellular IFNγ. Plot showing the costaining of CD40L and IFNγ is gated on the divided cells. Data shown are representative of 3 indepen-dent experiments with similar results. (C) The CFSElowCD40Lhigh cells were collected by FACS-sorting. The phenotype of these cells was documented (top panel). The sorted cells were reinfused to mice bearing 10-day-old A20HA tumors. Two weeks after T-cell transfer, the transferred CD4+ T cells were recovered from the spleens and assayed for the expression of the indicated markers (bottom panel). Results shown are representative of 2 independent experiments with similar results.
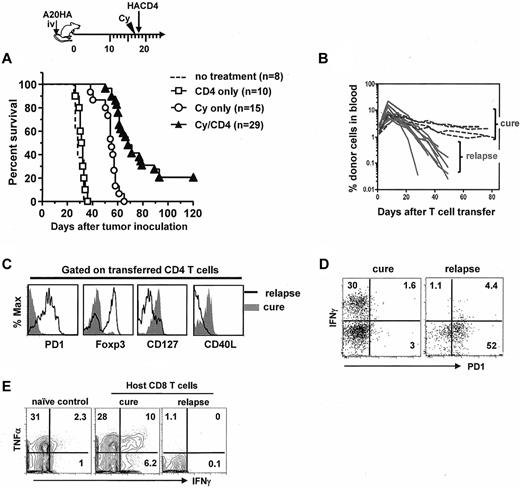
Tumor relapses after chemoimmunotherapy correlate with loss of the polyfunctional effector phenotype in tumor-specific CD4+ T cells
We next asked whether late relapse would occur in mice after receiving the combined chemoimmunotherapy, and if so, whether it would be associated with tolerization of the activated antitumor CD4+ T cells. We knew from Figure 1 that virtually all transferred CD4+ T cells initially became activated in Cy-treated tumor-bearing mice. When these mice were followed long-term after treatment, however, most mice eventually relapsed and died, and only a small cohort of mice (∼ 20%) achieved long-term survival or cure (Figure 5A). Serial analysis of peripheral blood revealed that tumor relapses correlated with a steep decrease in the frequencies of the transferred CD4+ T cells in circulation (Figure 5B). Furthermore, tumor-specific CD4+ T cells in relapsed mice lost the effector-cell signature and acquired the defective-cell signature (Figure 5C). Particularly, acquisition of PD-1 expression in these CD4+ T cells was accompanied by loss of cytokine-producing capability (Figure 5D). These changes in donor CD4+ T cells were also accompanied by loss of function in host CD8+ T cells in relapsed mice (Figure 5E). By contrast, mice that remained in remission (or cure) had a stable population of the transferred CD4+ T cells that continued to exhibit the effector cell signature (Figure 5B-D), and maintained a pool of responsive endogenous CD8+ T cells with memory attribute (more responsive than naive CD8+ T cells on challenge as shown in Figure 5E). Collectively, these data demonstrate that tumor relapses after chemoimmunotherapy correlate with acquisition of a PD-1–dominant dysfunctional phenotype in tumor-specific CD4+ T cells.
Tumor relapses after chemoimmunotherapy correlate with acquisition of the tolerized phenotype and loss of function in tumor-specific CD4+ T cells. The timeline of the procedures is outlined. Mice with established systemic A20HA tumors were not treated or treated as indicated. (A) The Kaplan-Meier plot depicts overall survival. The number of mice in each group is shown. (B) Retrospective correlation analysis of CD4+ T-cell frequency in peripheral blood and mouse survival. The frequencies of the transferred CD4+ T cells in peripheral blood were periodically monitored by FACS after mice received the combination treatment of Cy and CD4+ T-cell transfer. (C) Phenotype of the transferred CD4+ T cells in cured or relapsed mice. Spleen cells from cured mice or sick (relapsed) mice were analyzed as described in Figure 1C. (D) Costaining of PD-1 and IFNγ. Purified CD4+ T cells were stimulated with HA peptide-pulsed BALB/c splenocytes for 4 hours in the presence of GolgiStop. Plots shown are gated on divided donor CD4+ T cells. (E) IFNγ production by endogenous CD8+ T cells. Purified CD8+ T cells were assayed for IFNγ expression after 4 hours stimulation with PMA/ionomycin. CD8+ T cells from a naive mouse were used as control. Numbers represent the percentages of cells in the given quadrant. Results shown are representative of 3 independent experiments with similar results.
Tumor relapses after chemoimmunotherapy correlate with acquisition of the tolerized phenotype and loss of function in tumor-specific CD4+ T cells. The timeline of the procedures is outlined. Mice with established systemic A20HA tumors were not treated or treated as indicated. (A) The Kaplan-Meier plot depicts overall survival. The number of mice in each group is shown. (B) Retrospective correlation analysis of CD4+ T-cell frequency in peripheral blood and mouse survival. The frequencies of the transferred CD4+ T cells in peripheral blood were periodically monitored by FACS after mice received the combination treatment of Cy and CD4+ T-cell transfer. (C) Phenotype of the transferred CD4+ T cells in cured or relapsed mice. Spleen cells from cured mice or sick (relapsed) mice were analyzed as described in Figure 1C. (D) Costaining of PD-1 and IFNγ. Purified CD4+ T cells were stimulated with HA peptide-pulsed BALB/c splenocytes for 4 hours in the presence of GolgiStop. Plots shown are gated on divided donor CD4+ T cells. (E) IFNγ production by endogenous CD8+ T cells. Purified CD8+ T cells were assayed for IFNγ expression after 4 hours stimulation with PMA/ionomycin. CD8+ T cells from a naive mouse were used as control. Numbers represent the percentages of cells in the given quadrant. Results shown are representative of 3 independent experiments with similar results.
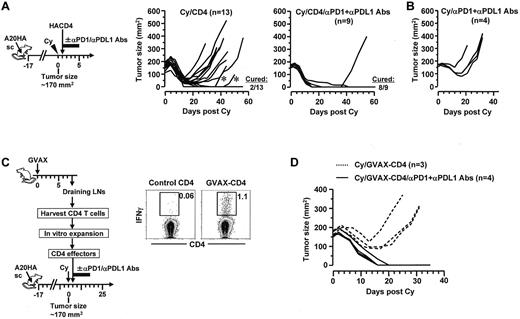
PD-1 blockade after chemoimmunotherapy markedly reduces the occurrence of relapse
Given the prominent expression of PD-1 in tolerized CD4+ effector cells, we hypothesized that disruption of the PD-1 pathway after chemoimmunotherapy can invigorate antitumor CD4+ T cells and consequently prevent tumor recurrence. To test this, mice with localized large A20HA tumors were treated with Cy plus CD4+ T-cell transfer, and then were injected or not injected with a mixture of blocking antibodies targeting PD-1 and PD-L1 (Figure 6A schema). Despite a period of progressive tumor regression, relapses were common (11 of 13) in mice receiving chemoimmunotherapy without subsequent PD-1/PD-L1 blockade (Figure 6A Cy/CD4). The relapsed tumors still expressed the target antigen HA (supplemental Figure 3), suggesting that relapses were not because of selective outgrowth of antigen-loss variants. By contrast, addition of PD-1/PD-L1 blockade after chemoimmunotherapy dramatically reduced relapse occurrence (1 of 9; Figure 6A Cy/CD4/αPD1+αPDL1 antibodies). As controls, mice treated with the combination of Cy and PD-1/PD-L1 blockade all had rapid tumor regrowth (Figure 6B Cy/αPD1+αPDL1 antibodies), indicating that PD-1/PD-L1 blockade by itself had minimal effects on residual tumors.
PD-1 blockade after chemoimmunotherapy markedly reduces the occurrence of relapse. (A) PD-1 blockade in combination with Cy and adoptive transfer of tumor-specific CD4+ T cells. Following the timeline of the procedures outlined, mice with localized large A20HA tumors were treated with Cy followed by HA-specific CD4+ T-cell transfer. Some mice were injected with a mixture of αPD-1 and αPD-L1 blocking antibodies (100 μg of each) for 5 consecutive days with the first injection given right after T-cell transfer. Tumor growth kinetics in mice was monitored. The results for mice receiving Cy plus CD4+ T-cell transfer without and with PD-1 blockade are depicted in the left graph and the right graph, respectively. Asterisks in the left graph mark 2 mice whose tumors initially regressed and became impalpable, but later regrew on the original location. (B) Tumor growth curves in control mice receiving Cy and PD-1 blockade. A group of tumor-bearing mice were treated with the combination of Cy and PD-1 blockade following the same administration schedule depicted in panel A schema. (C) PD-1 blockade in combination with Cy and adoptive transfer of vaccine-primed polyclonal CD4+ T cells. Following the procedures depicted in the schema, tumor-reactive CD4+ T cells were obtained from the DLNs of tumor-free mice that had been vaccinated with irradiated GMCSF-producing A20 tumors (GVAX). Tumor-reactivity of these CD4+ T cells were evaluated by incubating aliquot of vaccine-primed CD4+ T cells (GVAX-CD4) with irradiated A20 tumor cells overnight before conducting IFNγ ICS. Naive CD4+ T cells were cultured the same way and used as control. Dot plots shown represent the ICS results. Numbers represent the percentages of the gated cells. Purified vaccine-primed CD4+ T cells were expanded in vitro, and then adoptively transferred to Cy-treated tumor-bearing mice, with or without subsequent PD-1 blockade as described in panel A. Tumor growth curves in mice of each group are shown in panel D.
PD-1 blockade after chemoimmunotherapy markedly reduces the occurrence of relapse. (A) PD-1 blockade in combination with Cy and adoptive transfer of tumor-specific CD4+ T cells. Following the timeline of the procedures outlined, mice with localized large A20HA tumors were treated with Cy followed by HA-specific CD4+ T-cell transfer. Some mice were injected with a mixture of αPD-1 and αPD-L1 blocking antibodies (100 μg of each) for 5 consecutive days with the first injection given right after T-cell transfer. Tumor growth kinetics in mice was monitored. The results for mice receiving Cy plus CD4+ T-cell transfer without and with PD-1 blockade are depicted in the left graph and the right graph, respectively. Asterisks in the left graph mark 2 mice whose tumors initially regressed and became impalpable, but later regrew on the original location. (B) Tumor growth curves in control mice receiving Cy and PD-1 blockade. A group of tumor-bearing mice were treated with the combination of Cy and PD-1 blockade following the same administration schedule depicted in panel A schema. (C) PD-1 blockade in combination with Cy and adoptive transfer of vaccine-primed polyclonal CD4+ T cells. Following the procedures depicted in the schema, tumor-reactive CD4+ T cells were obtained from the DLNs of tumor-free mice that had been vaccinated with irradiated GMCSF-producing A20 tumors (GVAX). Tumor-reactivity of these CD4+ T cells were evaluated by incubating aliquot of vaccine-primed CD4+ T cells (GVAX-CD4) with irradiated A20 tumor cells overnight before conducting IFNγ ICS. Naive CD4+ T cells were cultured the same way and used as control. Dot plots shown represent the ICS results. Numbers represent the percentages of the gated cells. Purified vaccine-primed CD4+ T cells were expanded in vitro, and then adoptively transferred to Cy-treated tumor-bearing mice, with or without subsequent PD-1 blockade as described in panel A. Tumor growth curves in mice of each group are shown in panel D.
We next sought to confirm the findings of the preceding experiments, which employed surrogate tumor antigen and naive TCR Tg CD4+ T cells, in a more stringent experimental setting in which tumor-reactive, polyclonal CD4+ T cells derived from the endogenous repertoire were used to target physiologic tumor antigens. As shown in the Figure 6C schema, CD4+ T cells reactive to A20 tumor–derived endogenous antigens (not transgene HA) were obtained from the draining lymph nodes of tumor-free mice that had been vaccinated with irradiated GMCSF-producing A20 tumors (GVAX); these polyclonal CD4+ T cells were expanded to sufficient quantities in vitro, and then adoptively transferred to Cy-treated tumor-bearing mice, with or without subsequent PD-1/PD-L1 blockade. The tumor-reactivity of the vaccine-primed CD4+ T cells (GVAX-CD4) was documented by their capability to produce IFNγ on incubation with A20 tumors in vitro (Figure 6C dot plots). It is noteworthy that A20HA cells were still used as tumor inoculants in this set of experiment, thus the results can be compared with the relevant controls shown in preceding experiments. However, under this experimental setting HA was irrelevant to the antitumor immunity because it was not the target of vaccine-primed CD4+ T cells. Replicating the results seen in experiments using TCR Tg CD4+ T cells, chemoimmunotherapy using polyclonal CD4+ T cells followed by PD-1/PD-L1 blockade led to complete tumor regression and mice remained symptom-free by the time of analysis, whereas chemoimmunotherapy without PD-1/PD-L1 blockade resulted in only a transient remission (Figure 6D). Taken together, our data provide strong evidence that the PD-1/PD-L1 axis critically contributes to immune tolerance and tumor relapse after chemoimmunotherapy.
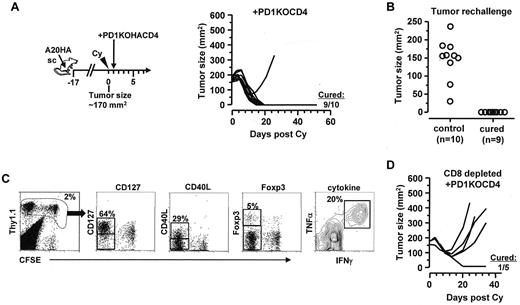
Targeted ablation of PD-1 in tumor-specific CD4+ T cells results in durable antitumor immunity after chemoimmunotherapy
Blocking antibodies specific for PD-1 or PD-L1 may act on multiple targets, including both CD4+ and CD8+ T cells. To definitively address the role of PD-1 specifically in the CD4 population, we bred our HA-specific TCR Tg mice onto a PD-1–deficient background. Figure 7A shows that adoptive transfer of HA-specific PD-1KOCD4+ T cells after chemotherapy led to dramatically improved therapeutic benefits, achieving a 90% cure rate (Figure 7A) that was comparable with the outcome of PD-1/PD-L1 blockade shown in Figure 6A. Moreover, the cured mice were all protected from subsequent tumor re-challenge (Figure 7B), suggesting the development of memory. In those protected mice, the transferred tumor-specific CD4+ T cells were readily detectable, and retained the polyfunctional effector phenotype (Figure 7C).We further showed that the survival benefit was lost in 4 of 5 mice when endogenous CD8+ T cells were depleted (Figure 7D), suggesting that the curative therapeutic effect conferred by PD1-deficient CD4+ T cells in postchemotherapy hosts still requires the involvement of endogenous CD8+ T cells. Collectively, these results indicate that tumor-specific CD4+ effector cells are key determinants of durable antitumor immunity in the postchemotherapy setting, and their functional status is dictated by cell-intrinsic PD-1 signaling.
Adoptive transfer of PD-1–deficient tumor-specific CD4+ T cells after Cy leads to durable antitumor effects. (A) Adoptive transfer of PD-1–deficient tumor-specific CD4+ T cells after chemotherapy. Following the depicted procedures, mice with large established subcutaneous A20HA tumors were treated with Cy, followed by adoptive transfer of PD-1–deficient HA-specific CD4+ T cells (PD1KOCD4). Tumor growth kinetics was monitored over time. The numbers indicate cured mice versus total mice in each group. (B) Protection from tumor rechallenge in cured mice. Cured mice were rechallenged with A20HA tumors on the flank opposite to the original tumor inoculation site. Ten naive BALB/c mice were also inoculated with tumors as controls. Graph depicts the tumor size of each mouse 20 days after tumor inoculation. (C) Persistence of transferred PD-1–deficient tumor-specific CD4+ T cells in cured mice. Mice protected from tumor rechallenge were killed and spleen cells were collected to examine the phenotype of the transferred CD4+ T cells. Representative plots show the expression profiles of the indicated markers. Numbers indicate the percentage of the gated population. (D) Requirement for endogenous CD8+ T cells for the curative outcome of chemoimmunotherapy. Tumor-bearing mice were injected with CD8-depleting antibody 1 day before receiving Cy-treatment and subsequent adoptive transfer of PD-1–deficient HA-specific CD4+ T cells. Tumor size was measured twice a week.
Adoptive transfer of PD-1–deficient tumor-specific CD4+ T cells after Cy leads to durable antitumor effects. (A) Adoptive transfer of PD-1–deficient tumor-specific CD4+ T cells after chemotherapy. Following the depicted procedures, mice with large established subcutaneous A20HA tumors were treated with Cy, followed by adoptive transfer of PD-1–deficient HA-specific CD4+ T cells (PD1KOCD4). Tumor growth kinetics was monitored over time. The numbers indicate cured mice versus total mice in each group. (B) Protection from tumor rechallenge in cured mice. Cured mice were rechallenged with A20HA tumors on the flank opposite to the original tumor inoculation site. Ten naive BALB/c mice were also inoculated with tumors as controls. Graph depicts the tumor size of each mouse 20 days after tumor inoculation. (C) Persistence of transferred PD-1–deficient tumor-specific CD4+ T cells in cured mice. Mice protected from tumor rechallenge were killed and spleen cells were collected to examine the phenotype of the transferred CD4+ T cells. Representative plots show the expression profiles of the indicated markers. Numbers indicate the percentage of the gated population. (D) Requirement for endogenous CD8+ T cells for the curative outcome of chemoimmunotherapy. Tumor-bearing mice were injected with CD8-depleting antibody 1 day before receiving Cy-treatment and subsequent adoptive transfer of PD-1–deficient HA-specific CD4+ T cells. Tumor size was measured twice a week.
Discussion
Although the concept of combined chemoimmunotherapy for cancer can be dated back to at least 3 decades ago,31 it was only recently that the mechanistic basis for the synergy between these 2 treatment modalities began to be elucidated at the cellular and molecular level.1,6 A successful chemoimmunotherapy should not only result in effective tumor destruction, but also prevent posttherapy relapse. Using a mouse model of posttherapy relapse, the current study provides novel insight into the dynamic interplay between tumors and antitumor CD4+ T cells in the context of chemoimmunotherapy. We showed that the mutually reinforcing effects between chemotherapy and tumor-reactive CD4+ T cells can forge an enhanced antitumor response. Importantly, we demonstrated that an effective and durable antitumor immunity can be achieved after chemotherapy if tumor-specific CD4+ T cells are maintained in the polyfunctional effector state by avoidance of PD-1–mediated tolerization.
It has been shown that activated CD4+ T cells, through CD40L-CD40 interaction, can mediate DC licensing, which is essential for priming and maintaining CD8+ effector cells in tumor microenvironment.32,33 Activated CD4+ T cells also enhance CD8+ T cell–mediated antitumor immunity by exerting important “postlicensing” roles, such as recruiting activated CD8+ T cells to tumor via the action of IFNγ, and promoting CD8+ T cell cytolytic function and proliferation through IL2.27,34 Moreover, CD4+ T cells may act on stromal cells to inhibit tumor angiogenesis through the effects of IFNγ and TNFα.35,36 Activated CD4+ T cells that can concurrently produce multiple proinflammatory cytokines (including IFNγ, TNFα, and IL2) are often referred as polyfunctional CD4+ T cells.23 The polyfunctional CD4+ T cells described in our study coexpressed CD40L and proinflammatory cytokines, endowing these cells with the potential to carry out diverse functions in a highly coordinated and synergistic fashion. Our demonstration of induction of polyfunctionality in transferred tumor-specific CD4+ T cells is potentially clinically relevant, because adoptive T-cell therapy using tumor antigen-specific CD4+ T cell clones has now become a viable treatment option for certain types of cancer.37 Our finding that the curative therapeutic effect conferred by CD4+ T cells in postchemotherapy hosts still required endogenous CD8+ T cells supports the concept that polyfunctional antitumor CD4+ T cells act as gatekeepers to maintain an effective host antitumor immunity, leading to durable remission or even cure.38
The present study confirms previous reports that Cy induces the release of an array of growth factors, inflammatory cytokines, and chemokines, including IL6, IL1β, IL17, CXCL10, and GMCSF, and so on.29,39 Here we showed that IL17 transcripts induced by Cy were further elevated by CD4+ T-cell transfer (Figure 2C), and the transferred tumor-specific CD4+ T cells may contribute to IL17 production in tumor (supplemental Figure 2A). Several recent studies demonstrate that Th17 cells can also be polyfunctional (coexpressing IL17 and IFNγ), exhibit certain memory cell attributes, and collaborate with CD8+ T cells to mediate long-term antitumor immunity.40-43 However, similar to other published studies using Cy treatment,29,39 we did not find significant population of CD4+ T cells that coexpressed IL17 and IFNγ (supplemental Figure 2B), suggesting that Cy may preferentially drive the development of Th1-type polyfunctional effector cells. In addition to IL17, we showed that the presence of activated CD4+ T cells also augmented the levels of IL1β, IL6, and GM-CSF. Intriguingly, these inflammatory molecules can have both beneficial and detrimental effects on antitumor immunity.38,44 Our results, in line with the report by Haabeth et al,28 suggest that tumor-specific CD4+ effector cells may play an essential role in driving therapy-induced inflammation toward tumor inhibition.
Given the importance of tumor-specific polyfunctional CD4+ effector cells, it is alarming that these cells are susceptible to tolerization by persistent residual tumors. Importantly, we identified the PD-1 pathway as a major mechanism mediating tolerization of CD4+ effector cells in the postchemotherapy setting. The therapeutic potential of PD-1/PD-L1 blockade has long been established in preclinical models,45,46 and has been validated in recent clinical trials.47,48 However, the therapeutic significance of PD-1 blockade on CD4+ T cells has been obscure. We showed that PD-1 blockade markedly improved the long-term efficacy of chemoimmunotherapy in the form of Cy-treatment plus adoptive transfer of monoclonal CD4+ T cells specific for a single tumor antigen (HA; Figure 6A). Moreover, the therapeutic benefit of PD-1 blockade also extended to chemoimmunotherapy using vaccine-primed polyclonal CD4+ T cells reactive to physiologic tumor antigens (epitopes undefined; Figure 6B-C). Our data suggest that PD-1 blockade immunotherapy, when used in combination with chemotherapy and adoptive immunotherapy, may sustain tumor-reactive CD4+ effector cells in addition to invigorating antitumor CD8+ T cells, and hence may greatly improve long-term patient outcomes. From an application standpoint, whereas the vaccine-primed CD4+ T cells used in our study were derived from immunized tumor-free donors, we believe that these cells can be obtained from vaccinated tumor-bearing hosts. This notion is supported by some recent clinical studies showing that polyfunctional CD4+ T cells can be induced in cancer patients after vaccination.49,50
We showed that targeted ablation of PD-1 in donor CD4+ T cells dramatically improved long-term survival after chemoimmunotherapy (Figure 7A), providing unequivocal evidence that the key relevant PD-1 expression was on tumor-specific CD4+ T cells. To our best knowledge, our study provides the first evidence that targeted disruption of PD-1 in tumor-specific CD4+ T cells is sufficient to result in sustained antitumor immunity and durable therapeutic outcome in postchemotherapy hosts. Interestingly, we previously showed in the same model that transfer of PD-1–deficient naive CD4+ T cells did not reject tumor in the absence of Cy-treatment, and the transferred cells still became tolerized.9 The results of this study suggest that PD-1 is not involved in the initial tolerance induction in naive CD4+ T cells, but is critical in tolerizing activated CD4+ T cells at the effector phase, thereby profoundly affecting the magnitude and durability of the antitumor immune responses elicited by chemoimmunotherapy.
In conclusion, our study unveils a previously unappreciated pivotal role of CD4+ T cells in controlling host antitumor immunity after chemotherapy, underscoring the importance of tumor-reactive CD4+ T cells in both initiating and maintaining an effective antitumor immunity in postchemotherapy hosts. The findings may have important implications for the design and implementation of more effective anticancer chemoimmunotherapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Hyam I. Levitsky and Dr Tasuku Honjo for transgenic mice; and Dr Yong Teng and Dr Lingqian Li for technical assistance.
This work was supported by a Research Scholar Grant (RSG-12-169-01-LIB) from the American Cancer Society and GHSU Special Funding Initiative to G.Z.; R01 CA72669 and P01 AI056299 to B.R.B.; AI065175, the JDRF and the Mason Trust to A.L.M.; and R01 CA096651 to D.H.M.
National Institutes of Health
Authorship
Contribution: Z.-C.D. performed research and analyzed results; L.H. performed research; H.Y. provided reagents; B.R.B., A.L.M., and D.H.M. contributed reagents, discussed results, and edited the paper; and G.Z. designed and performed research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gang Zhou, Cancer Immunology, Inflammation and Tolerance Program, Cancer Center, Georgia Health Sciences University, 1120 15th St, CN-4140, Augusta, GA 30912; e-mail: gzhou@georgiahealth.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal