Abstract
Pediatric follicular lymphoma (PFL) is a variant of follicular lymphoma (FL) presenting as localized lymphadenopathy in children. Unlike conventional adult FL, PFL typically does not recur or progress. Clear diagnostic criteria for PFL are lacking, and it is uncertain whether this indolent lymphoma is defined by age or may occur in adults. We analyzed 27 FL in patients < 40 years of age and found that all 21 cases that lacked a BCL2 gene abnormality (BCL2-N; P < .0001) and had > 30% Ki67 fraction (high proliferation index, HPI; P = .0007) were stage I and did not progress or recur; in comparison, all 6 cases with BCL2 rearrangement and/or PI < 30% were stage III/IV, and 5 of 6 recurred or progressed. In a separate cohort of 58 adult FL (≥ 18 years of age), all 13 BCL2-N/HPI cases were stage I, and none progressed or relapsed, whereas 11 of 15 stage I cases with BCL2 gene abnormality and/or LPI relapsed or progressed (P = .0001). The adult and pediatric BCL2-N/HPI FL cases had similar morphologic features. Our results confirm the highly indolent behavior of PFL and suggest that these are characterized by HPI and absence of BCL2 gene abnormality. PFL-like cases also occur in adults and are associated with indolent behavior in this patient population.
Introduction
Pediatric follicular lymphoma (PFL) is a variant of follicular lymphoma (FL) that occurs in children and typically presents as a clonal follicular proliferation resulting in localized lymphadenopathy or extranodal lesions.1 It has an excellent prognosis, with sustained complete remission achieved in most cases after systemic chemotherapy. Most reported cases of PFL lack the abnormal BCL2 protein expression and BCL2 gene rearrangements that characterize the majority of adult FL cases.2-4 In addition, PFL is reported to have distinctive morphologic features, including large expansile follicles with numerous centroblasts. The World Health Organization (WHO) Classification includes PFL as a provisional subtype of FL but does not provide a strict morphologic or immunophenotypic definition, nor does it provide criteria to distinguish it from conventional FL. Although the upper age limit defining “pediatric” is generally considered to be 18 years, it is not known whether tumors with the biologic features of PFL occur in adults. The distinction between conventional FL and PFL may be important because conventional FL generally is considered an indolent but incurable disease, usually associated with recurrence after systemic chemotherapy. In contrast, recurrence after therapy in PFL is rare, despite its frequently high histologic grade, and in some reports researchers suggest that PFL may not recur after excision, even without systemic treatment.5,6
In this study, we evaluated clonal nodal follicular B-cell proliferations occurring in patients younger than 40 years of age, to identify morphologic, immunophenotypic, and molecular genetic parameters that define follicular proliferations in children and young adults with low potential for progression or recurrence. We then applied these parameters to a cohort of adult patients with FL to determine whether they could identify similar cases with low potential for progression or recurrence, irrespective of patient age.
Methods
Patient specimens
This study was approved by the institutional review board of Massachusetts General Hospital (MGH). Clinical features of all cases, including staging, treatment, and outcome information, were obtained from patients' medical records.
Cohort 1.
Cohort 1 comprised 27 cases of nodal FL from patients < 40 years of age. Cases were identified from the MGH pathology archives (including both cases biopsied at MGH and outside referral cases) between 1999 and 2010, via electronic search using the term “follicular,” with original diagnoses of “follicular lymphoma” or “pediatric follicular lymphoma.” All identified lymph node biopsies with FL diagnosed between 1999 and 2010 from patients < 40 years of age were included, with the exception of cases with concurrent diffuse large B-cell lymphoma (DLBCL) or those from which diagnostic material could not be obtained. Cases lacking architectural effacement (eg, follicular hyperplasia with clonal B cells), and cases of extranodal FL were also excluded. In all cases, B-cell clonality had been confirmed by flow cytometric analysis and/or polymerase chain reaction–based analysis of immunoglobulin heavy chain gene rearrangements via the use of BIOMED-2 protocols and primer sets.7
Cohort 2.
Cohort 2 comprised 58 cases of nodal FL occurring in patients > 18 years of age. Thirty-nine were derived from a previously constructed tissue microarray (TMA) of unselected FL cases diagnosed between 1982 and 2004 at MGH. The TMA was generated from representative paraffin-embedded tissue blocks. Three 0.5-mm cores were obtained from 3 selected representative areas from each case, and the tissue cores were embedded into a single microarray paraffin block. Cases with concurrent DLBCL and cases in which the TMA material was unsuitable for analysis because of loss of tissue and/or failed immunostaining or FISH were excluded. Cases of extranodal FL were excluded from this cohort. Nineteen additional stage I FL cases, diagnosed between 1994 and 2012 in patients ≥ 18 years, also were retrieved from the MGH pathology archives. In all cases, B-cell clonality had been previously confirmed as described for cohort 1. No cases already selected for cohort 1 were included in cohort 2.
Morphologic analysis
All routine hematoxylin and eosin–stained sections, existing immunohistochemistry (IHC) stains, and pathology reports, flow cytometry reports, and any cytogenetic and/or molecular diagnostic reports were reviewed by 3 of the authors (cohort 1: A.L., N.L.H., and R.P.H.) or 2 of the authors (cohort 2: A.L. and R.P.H.), and the diagnosis of FL was confirmed for all cases. The following morphologic parameters were scored by consensus between 2 authors (A.L., and R.P.H.) for both cohort 1 and cohort 2: histologic grade according to WHO classification criteria; average greatest diameter of neoplastic follicles as determined by the measurement of 10 randomly selected follicles; a “starry-sky” appearance (numerous evenly dispersed phagocytic histiocytes) in > 50% of follicles; and the presence of marginal zone differentiation. In addition, the presence of diffuse areas was recorded for cohort 2 cases. Histologic parameters were scored on the corresponding whole slide sections for all TMA cases.
IHC stains
IHC stains were performed on formalin-fixed, paraffin-embedded tissue sections (cohort 1 and a subset of cohort 2) and TMA paraffin sections (subset of cohort 2) with the Ventana Benchmark Autostainer (Ventana Medical Systems) using the Ventana 3, 3′ diaminobenzidine tetrahydrochloride kit according to the manufacturer's instructions. All cases were stained with primary antibodies for CD20, CD3, BCL6, BCL2, MUM1/IRF4, and Ki-67 (Ventana Benchmark). In addition, cases in cohort 1 were stained with antibodies to CD10 and CD21. IHC for BCL2 was evaluated as the proportion of intrafollicular cells (scored as 0 for negative, and 1+, 2+, 3+, or 4+ for < 26%, 26%-50%, 51%-75%, or > 75% of intrafollicular cells, respectively). In addition, the intensity of BCL2 staining was scored with a semiquantitative scale of staining intensity: intensity similar to T cells was scored as 3+, staining stronger than T cells was scored as 4+, distinct staining weaker than T cells was scored as 2+, and faint staining was scored as 1+. Estimation of the proliferation index (PI) score was based on the percentage of Ki67-positive B cells within neoplastic follicles (on whole sections for all TMA cases). A cutoff of > 30% PI to define high-proliferation index (HPI) was based on a previous outlier analysis of a large series of grade 1-2 FL cases.8 The scoring of BCL2 and Ki67 PI was performed by consensus of 2 of the authors (A.L. and R.P.H.).
FISH analysis
FISH was performed for detection of gene rearrangements involving BCL2 and BCL6 (cohorts 1 and 2), MYC (cohort 2), and IRF4 (subset of cohort 1 cases showing MUM-1/IRF4 protein expression).
BCL2, BCL6, MYC, IRF4/BAP.
Detection of breakpoints affecting BCL2, BCL6, MYC, and IRF4 loci was performed on formalin-fixed, paraffin-embedded sections. The probes and protocols used are described in the supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article. The assays were considered positive if 1 red and 1 green signal were split-apart by more than 1 probe length. To account for unbalanced translocations, the loss of a single green or of a single red signal also was considered positive. Cases were scored as BCL2-rearranged (BCL2-R) on the basis of detecting unambiguous probe separation or splitting in at least 15% of observed cells (50-cell count).
IRF4.
Detection of breakpoints affecting the IRF4 locus was performed on formalin-fixed, paraffin-embedded sections using probes and protocols as previously described.9
Statistics
All statistical analysis was performed with the use of Prism 4 software (GraphPad). Patient clinical characteristics were summarized as numbers and percentages for categorical values, and median and range for continuous variables. Progression-free survival was defined as time from pathologic diagnosis of lymphoma to the date of lymphoma progression, relapse (for treated patients), or death from any cause. Comparisons and associations between categorical variables were analyzed by the Fisher exact test or Mann-Whitney U test. Median survivals were calculated with the Kaplan-Meier product-limit method. Log-rank test was used to test the equality of survivor functions.
Results
We initially reviewed cases of nodal FL in a pediatric and young adult patient population (cohort 1) to identify pathologic features associated with indolent behavior in this population. We subsequently used these features to evaluate cases of adult nodal FL (cohort 2) to determine whether cases similar to “pediatric-type” FL occurred in adults and whether these cases also exhibited indolent behavior.
Cohort 1: FLs in children, adolescents, and young adults < 40 years of age
Clinical features.
The 27 FL cases in cohort 1 occurred in patients ranging from 8 to 36 years of age (median, 18 years; Table 1) with a median follow-up time of 57 months. The majority (21/27; 78%) were Ann Arbor stage I, of which most (17/21, 81%) were localized to the head and neck (cervical, submandibular, submental, postauricular, or periparotid lymph nodes), with the remainder localized to inguinal lymph nodes. There were no stage II cases. Six cases presented with advanced stage disease (stage III or IV), with involvement of peripheral lymph nodes in all cases and bone marrow in 4 of 6 cases.
Clinicopathologic features of patients < 40 years with follicular lymphoma (cohort 1)
| . | All patients (n = 27) . | Stage I (n = 21) . | Stage III-IV (n = 6) . | P . |
|---|---|---|---|---|
| Clinical features | ||||
| Median age, y (range) | 18 (8-36) | 17 (8-36) | 23 (17-34) | .03 |
| Age of patient ≥ 18 y | 14/27 (52) | 9/21 (43) | 5/6 (83) | .16 |
| M:F | 22:5 | 18:3 | 4:2 | .58 |
| Histologic features | ||||
| Complete nodal effacement | 18/27 (67) | 14/21 (67) | 4/6 (67) | 1.0 |
| Marginal zone differentiation | 3/27 (11) | 3/21 (14) | 0/6 (0) | 1.0 |
| Follicle size > 2 mm diameter | 15/27 (56) | 14/21 (67) | 1/6 (17) | .06 |
| > 50% “Starry-sky” follicles | 14/27 (52) | 13/21 (62) | 1/6 (17) | .08 |
| Histologic grade 3A | 10/27 (37) | 9/21 (43) | 1/6 (17) | .36 |
| Immunophenotypic and genetic features | ||||
| BCL2+ tumor cells (≥ 2+ intensity) | 14/27 (52) | 8/21 (38) | 6/6 (100) | .02 |
| BCL2 gene rearrangement | 5/27 (19) | 0/21 (0) | 5/6 (83) | < .0001 |
| Median Ki67 PI (range) | 65% (5%-95%) | 70% (40%-95%) | 25% (10%-70%) | .008 |
| Ki67 PI < 30% | 3/27 (11) | 0/21 (0) | 3/6 (50) | .007 |
| Ki67 PI < 30% or BCL2 gene rearrangement | 6/27 (22) | 0/21 (0) | 6/6 (100) | < .0001 |
| Treatment | ||||
| Excision only | 14/27 (52) | 14/21 (67) | 0/6 (0) | ND |
| Radiation therapy only | 1/27 (4) | 1/21 (5) | 0/6 (0) | |
| Chemotherapy | 12/27 (44) | 6/21 (28) | 6/6 (100)* | |
| Outcome | ||||
| Relapse or progression | 5/27 (19) | 0/21 (0) | 5/6 (83) | < .0001 |
| Persistent disease at latest follow-up | 3/27 (11) | 0/21 (0) | 3/6 (50) | .007 |
| Died from disease | 1/27 (4) | 0/21 (0) | 1/6 (17) | .22 |
| . | All patients (n = 27) . | Stage I (n = 21) . | Stage III-IV (n = 6) . | P . |
|---|---|---|---|---|
| Clinical features | ||||
| Median age, y (range) | 18 (8-36) | 17 (8-36) | 23 (17-34) | .03 |
| Age of patient ≥ 18 y | 14/27 (52) | 9/21 (43) | 5/6 (83) | .16 |
| M:F | 22:5 | 18:3 | 4:2 | .58 |
| Histologic features | ||||
| Complete nodal effacement | 18/27 (67) | 14/21 (67) | 4/6 (67) | 1.0 |
| Marginal zone differentiation | 3/27 (11) | 3/21 (14) | 0/6 (0) | 1.0 |
| Follicle size > 2 mm diameter | 15/27 (56) | 14/21 (67) | 1/6 (17) | .06 |
| > 50% “Starry-sky” follicles | 14/27 (52) | 13/21 (62) | 1/6 (17) | .08 |
| Histologic grade 3A | 10/27 (37) | 9/21 (43) | 1/6 (17) | .36 |
| Immunophenotypic and genetic features | ||||
| BCL2+ tumor cells (≥ 2+ intensity) | 14/27 (52) | 8/21 (38) | 6/6 (100) | .02 |
| BCL2 gene rearrangement | 5/27 (19) | 0/21 (0) | 5/6 (83) | < .0001 |
| Median Ki67 PI (range) | 65% (5%-95%) | 70% (40%-95%) | 25% (10%-70%) | .008 |
| Ki67 PI < 30% | 3/27 (11) | 0/21 (0) | 3/6 (50) | .007 |
| Ki67 PI < 30% or BCL2 gene rearrangement | 6/27 (22) | 0/21 (0) | 6/6 (100) | < .0001 |
| Treatment | ||||
| Excision only | 14/27 (52) | 14/21 (67) | 0/6 (0) | ND |
| Radiation therapy only | 1/27 (4) | 1/21 (5) | 0/6 (0) | |
| Chemotherapy | 12/27 (44) | 6/21 (28) | 6/6 (100)* | |
| Outcome | ||||
| Relapse or progression | 5/27 (19) | 0/21 (0) | 5/6 (83) | < .0001 |
| Persistent disease at latest follow-up | 3/27 (11) | 0/21 (0) | 3/6 (50) | .007 |
| Died from disease | 1/27 (4) | 0/21 (0) | 1/6 (17) | .22 |
Values are no. (%) unless otherwise noted.
ND indicates not done; and PI, proliferation index.
Followed by an initial period of observation (10 and 32 mo) in 2 patients.
Histologic features.
The histologic features of the cohort 1 FL cases are summarized in Table 1. All cases were characterized by a follicular proliferation with partial (9/27, 33%) or subtotal-to-complete (18/27, 67%) effacement of the underlying lymph node architecture (Figure 1). Clonal B cells comprised 20%-96% (median, 72%) of all the B cells in the 13 cases with available flow cytometry data. Twenty-four of the cases had intact follicles whose size, shape, and cellular composition were variable. In 3 cases, the follicles had poorly defined borders with prominent marginal zone differentiation at the follicle periphery, extending into interfollicular areas. The presence of BCL6+ and/or CD10+ expression within the interfollicular neoplastic component (3/3 cases) and the identification of CD10+ clonal B cells by flow cytometry (2/3 cases) excluded the possibility of nodal marginal zone lymphoma in these cases. In the majority of the cases (15/27, 56%) the follicles were large and expansile (> 2 mm in average greatest diameter), including a subset of cases (8/27, 30%) with markedly enlarged and/or coalescent follicles (> 3 mm in average greatest diameter; Figure 1A).
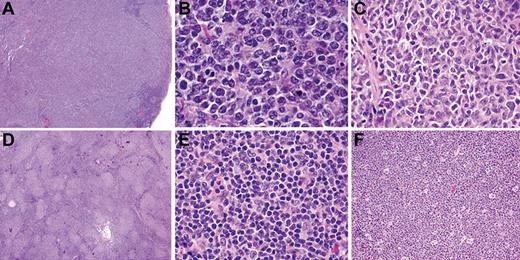
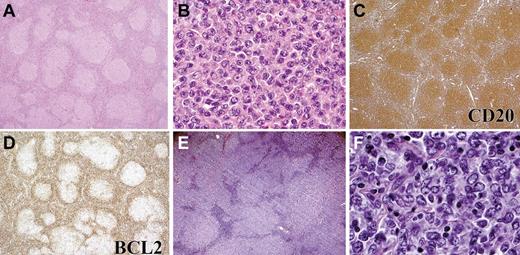
Histologic features of FL in children and young adults (cohort 1). All cases showed at least partial architectural effacement by a follicular proliferation. In 1 representative case, there are large, expansile, and coalescent follicles (A) composed of small- to medium-sized centrocytes and numerous medium- to large-sized centroblasts (B), consistent with histologic grade 3A (B). In a second case (C), medium-sized centrocytes predominate and centroblasts are uncommon. The neoplastic infiltrate of a third case is composed of relatively small follicles (D), composed predominantly of small centrocytes (E). “Starry-sky” appearance, as illustrated in a fourth case (F), was frequent. Images were captured at ×40 magnification (A,D); at ×100 magnification (F); and at ×400 magnification (B,C,E) with the use of an Olympus, BX53 microscope, Olympus DP25 digital camera.
Histologic features of FL in children and young adults (cohort 1). All cases showed at least partial architectural effacement by a follicular proliferation. In 1 representative case, there are large, expansile, and coalescent follicles (A) composed of small- to medium-sized centrocytes and numerous medium- to large-sized centroblasts (B), consistent with histologic grade 3A (B). In a second case (C), medium-sized centrocytes predominate and centroblasts are uncommon. The neoplastic infiltrate of a third case is composed of relatively small follicles (D), composed predominantly of small centrocytes (E). “Starry-sky” appearance, as illustrated in a fourth case (F), was frequent. Images were captured at ×40 magnification (A,D); at ×100 magnification (F); and at ×400 magnification (B,C,E) with the use of an Olympus, BX53 microscope, Olympus DP25 digital camera.
The follicles usually were composed of a heterogeneous admixture of variably sized centrocytes, tingible body macrophages, follicular dendritic cells, and centroblasts. The centroblasts were often medium in size with vesicular chromatin and multiple nucleoli (Figure 1B) and distinction between centrocytes and small centroblasts was often difficult. The centroblast count was > 15 per high-power field in 10 of 27 cases (37%), consistent with grade 3A histology according to WHO classification criteria. In other cases, the large cells present within follicles mostly had centrocytic morphology, and centroblasts were not sufficiently increased to warrant a grade 3 designation (Figure 1C). There were no cases with a monomorphic proliferation of centroblasts (grade 3B histology). Among the 12 cases with an average follicle diameter of < 2 mm, 9 showed a proliferation of relatively uniform, small follicles, of which 7 had a predominant population of small, irregular centrocytes resembling “conventional” low-grade FL (Figure 1D-E).
BCL2 and MUM1/IRF4 IHC and PI
The IHC features of the cohort 1 cases are summarized in Table 1. There was strong, uniform BCL2 staining (3+ or 4+ intensity in > 50% of neoplastic cells) in 5 of 27 cases (19%), with 9 additional cases having 2+ intensity BCL2 expression in 26%-50% of the neoplastic cells (Figure 2Av-vi and 2Bv-vi). Five of 25 cases (20%) evaluated showed MUM1/IRF4 staining in a subset (10%-30%) of neoplastic cells (supplemental Figure 1). The Ki67 PI in the neoplastic follicles ranged from 5% to 95% (median 65%; Figure 2Ai-ii and 2Bi-ii). Twenty-four of 27 cases (89%) had a PI of > 30%, including 14 of 17 (82%) of the histologic grade 1-2 cases, which is within the spectrum of “low-grade follicular lymphoma with high proliferation index.”8 The 3 cases with < 30% PI had PI values of 5%, 10%, and 10%; there were no cases with PI > 10% and < 40%.
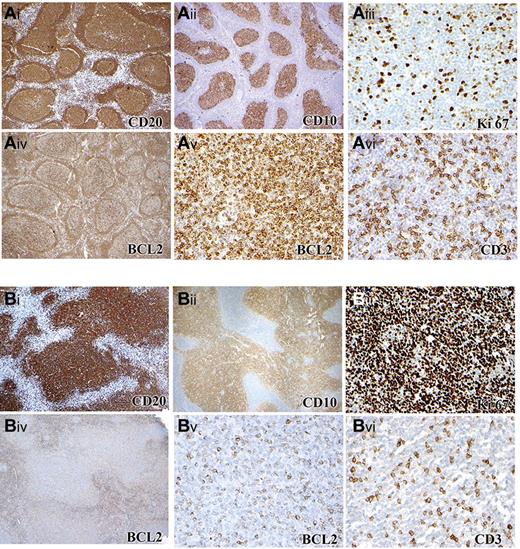
Immunophenotype of FL in children and young adults (cohort 1). (A) Case with BCL2 rearrangement and low PI composed of CD20+ (Ai), CD10+ (Aii) follicles with a low Ki67 PI (Aiii) that are BCL2 positive (Aiv). Higher-power image of BCL2 (Av) and CD3 (Avi) staining of the same area shows 4+ BCL2 staining intensity. (B) Case with no BCL2 rearrangement and with HPI composed of an expansile follicular proliferation of CD20+ (Bi), CD10+ (Bii) follicles with a high Ki67 PI (Biii) Ki-67 and with some expression of BCL2 (Biv). Higher-power image of BCL2 (Bv) and CD3 (Bvi) staining of the same area, showing 2+ BCL2 staining intensity. Panels Ai-ii, Aiv, Bi-ii, and Biv were captured at ×40 magnification and Aiii, Av-vi, Biii, and Bv-vi at ×200 magnification with the use of an Olympus, BX53 microscope, Olympus DP25 digital camera.
Immunophenotype of FL in children and young adults (cohort 1). (A) Case with BCL2 rearrangement and low PI composed of CD20+ (Ai), CD10+ (Aii) follicles with a low Ki67 PI (Aiii) that are BCL2 positive (Aiv). Higher-power image of BCL2 (Av) and CD3 (Avi) staining of the same area shows 4+ BCL2 staining intensity. (B) Case with no BCL2 rearrangement and with HPI composed of an expansile follicular proliferation of CD20+ (Bi), CD10+ (Bii) follicles with a high Ki67 PI (Biii) Ki-67 and with some expression of BCL2 (Biv). Higher-power image of BCL2 (Bv) and CD3 (Bvi) staining of the same area, showing 2+ BCL2 staining intensity. Panels Ai-ii, Aiv, Bi-ii, and Biv were captured at ×40 magnification and Aiii, Av-vi, Biii, and Bv-vi at ×200 magnification with the use of an Olympus, BX53 microscope, Olympus DP25 digital camera.
BCL2, BCL6, and IRF4 FISH
None of the 25 cases evaluated had a BCL6 translocation or gain by FISH. All 5 cases with MUM1/IRF4 staining in a subset of neoplastic B cells were negative for IRF4 gene rearrangement by FISH. Five of 27 cases had BCL2 rearrangement by FISH; there were 2 normal fused red/green signals in the remaining 22 cases, without evidence of gain or loss. All 5 BCL2-rearranged cases had strong (3+ or 4+ intensity) BCL2 staining by immunohistochemistry. One case that was BCL2 strongly positive by immunohistochemistry (4+ intensity) did not show BCL2 rearrangement by FISH. None of the 10 cases with 2+ or less intensity BCL2 staining had a BCL2 rearrangement.
Correlation of clinical and pathologic features with stage and outcome of cohort 1 cases
Among clinical and histologic parameters assessed (Table 1), only older age was significantly associated with stage III/IV disease (P = .03). There was a borderline association of both large follicles (average follicle diameter > 2 mm; P = .06) and prominent “starry-sky” appearance of follicles (P = .08) with stage I disease. There was no association of histologic grade with stage. Both the presence of BCL2 expression by immunohistochemistry (P = .02) and the presence of a BCL2 gene rearrangement by FISH (P < .0001) were associated with stage III/IV disease. Patients with stage III/IV disease had a lower PI (P = .008) and a PI of < 30% was associated with stage III/IV disease (P = .007). The combination of an absent BCL2 gene rearrangement and high (> 30%) PI was present in all 21 stage I cases and in none of the 6 stage III/IV cases (P < .0001).
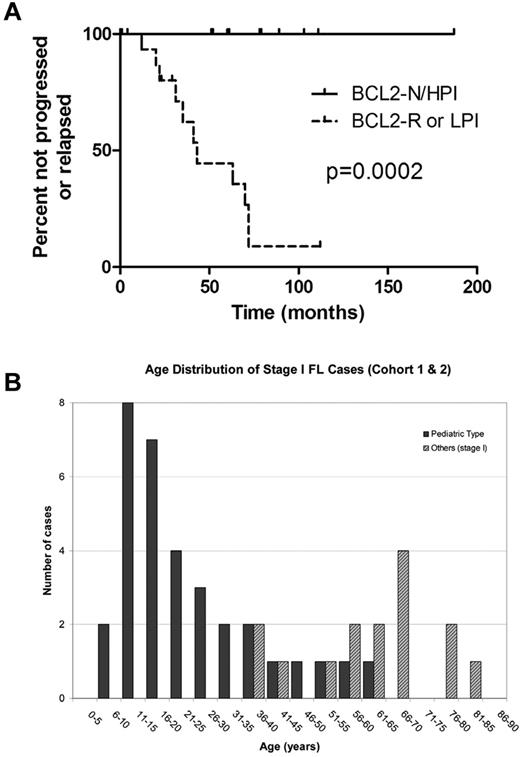
The majority of patients with stage I disease (14/21, 67%) were treated with excision only, whereas all 6 of the patients with stage III/IV disease received cytotoxic chemotherapy, either at initial diagnosis (4/6) or after progression after an initial period of observation; 3 patients experienced multiple relapses and were treated with allogeneic bone marrow transplantation. At latest follow-up (median, 57 months), none of the 21 stage I cases had progressed or relapsed, including the 14 patients treated with excision only, whereas 5 of 6 stage III/IV patients progressed or relapsed. At the latest follow-up, only 1 patient (with stage III/IV disease) had died of lymphoma. Patients with FL lacking any BCL2 gene abnormality and with > 30% PI (BCL2-N/HPI), all of whom had stage I disease, had superior progression-free survival compared with patients whose FL either had BCL2 rearrangement or PI < 30% (P < .0001, Figure 3A). High PI alone (P = .002) and absence of BCL2 gene abnormality alone (P = .002) also were associated with superior progression-free survival (data not shown).
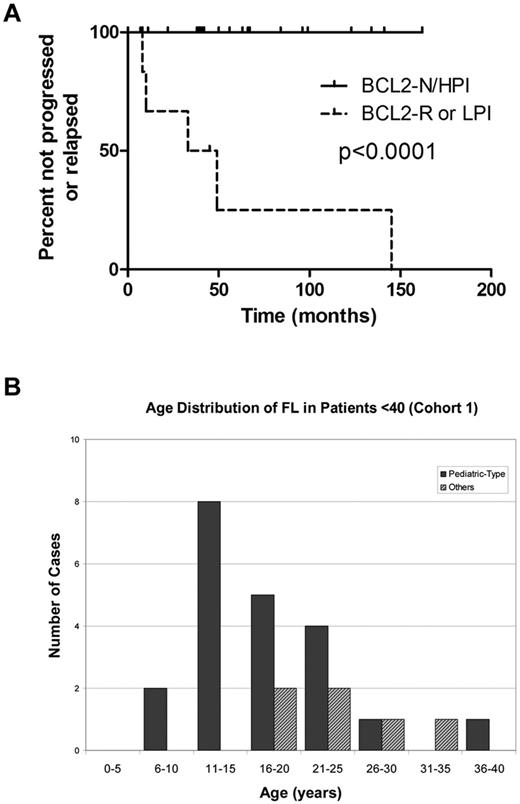
Outcome and age distribution of cohort 1 FL patients (< 40 years of age). (A) Outcome of 27 cohort 1 FL patients (< 40 years of age). Patients with FL lacking BCL2 gene abnormality and with elevated Ki67 proliferation index (BCL2-N/HPI) demonstrated superior freedom from progression or relapse (median time not reached) compared with patients with FL bearing BCL2 gene rearrangement (BCL2-R) or nonelevated proliferation index (LPI; median, 41 months). (B) Age distribution of FL in patients < 40 (cohort 1).
Outcome and age distribution of cohort 1 FL patients (< 40 years of age). (A) Outcome of 27 cohort 1 FL patients (< 40 years of age). Patients with FL lacking BCL2 gene abnormality and with elevated Ki67 proliferation index (BCL2-N/HPI) demonstrated superior freedom from progression or relapse (median time not reached) compared with patients with FL bearing BCL2 gene rearrangement (BCL2-R) or nonelevated proliferation index (LPI; median, 41 months). (B) Age distribution of FL in patients < 40 (cohort 1).
Clinicopathologic features of BCL2-N/HPI FL cases
The 21 BCL2-N/HPI FL cases occurred in patients between 8 and 36 years of age (median, 17 years), with 43% of patients ≥ 18 years of age (Figure 3B). The majority of BCL2-N/HPI cases (14/21, 67%) were characterized by large, expansile follicles, frequently showing a “starry-sky” appearance (Figure 1A-B,F). Despite the high PI, 12 of 21 (57%) BCL2-N/HPI cases were histologic grade 1-2, and 3 of these cases were morphologically indistinguishable from low-grade conventional FL, with numerous closely packed, similarly sized small follicles composed of small to medium-sized centrocytes (Figure 4).
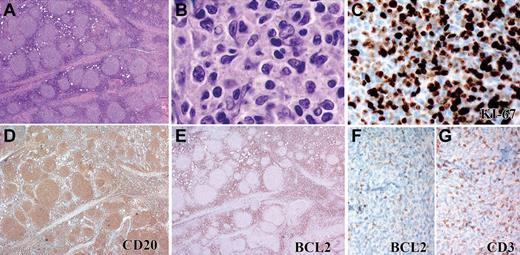
Pediatric-type FL (cohort 1) with unusual morphologic features. Some FL cases lacking BCL2 rearrangement and with HPI were composed of small follicles (A) containing predominantly small centrocytes and few centroblasts (B). The Ki67 proliferation index is high (C), and the CD20+cells (D) lack expression of BCL2 protein (E). Higher-power image of BCL2 (F) and CD3 (G) staining of same field shows no expression of BCL2. Panels A, D, and E were captured at ×40 magnification; F and G at ×100 magnification; B at ×1000, and C at ×400 magnification with the use of an Olympus, BX53 microscope, Olympus DP25 digital camera.
Pediatric-type FL (cohort 1) with unusual morphologic features. Some FL cases lacking BCL2 rearrangement and with HPI were composed of small follicles (A) containing predominantly small centrocytes and few centroblasts (B). The Ki67 proliferation index is high (C), and the CD20+cells (D) lack expression of BCL2 protein (E). Higher-power image of BCL2 (F) and CD3 (G) staining of same field shows no expression of BCL2. Panels A, D, and E were captured at ×40 magnification; F and G at ×100 magnification; B at ×1000, and C at ×400 magnification with the use of an Olympus, BX53 microscope, Olympus DP25 digital camera.
Cohort 2: clinicopathologic analysis of FL cases in adults ≥18 years
The 58 FL patients in cohort 2 ranged from 18 to 85 years of age (median, 57 years), with a median follow-up of 91 months. There were 28 stage I patients, 0 stage II patients, 11 stage III patients, 15 stage IV patients, and 4 patients of unknown stage. These FL cases were evaluated by BCL2 FISH and Ki67 staining and divided into 4 categories: FL lacking BCL2 gene abnormality and with PI > 30% (BCL2-N/HPI); with BCL2 gene rearrangement or gain and PI > 30% (BCL2-R/HPI); lacking BCL2 gene abnormality and with Ki67 < 30% (BCL2-N/LPI); and with BCL2 gene rearrangement or gain and PI < 30% (BCL2-R/LPI). Thirty-two cases had a BCL2 gene rearrangement, and 1 patient had an extra copy of the BCL2 gene. This latter case had strong (4+) BCL2 staining by immunohistochemistry in > 75% of the neoplastic cells and PI > 30% and was included in the BCL2-R/HPI group. Twenty-five cases had 2 copies of the intact BCL2 gene; the lack of BCL2 rearrangement was confirmed on the donor blocks for the 13 cohort 2 cases derived from the TMA. The clinical and outcome data of the 4 BCL2/PI groups are shown in Table 2. There was no difference in the proportion of patients in each group treated with chemotherapy versus radiotherapy or observation only. Although 0 of 13 patients in the BCL2-N/HPI group experienced relapse or disease progression, the majority of the patients in all other groups relapsed or progressed (P < .0001) and had persistent FL at latest follow-up (P = .0002).
BCL2 gene abnormality and PI status of adults with follicular lymphomas (cohort 2)
| . | BCL2-N HPI (n = 13) . | BCL2-R HPI* (n = 8) . | BCL2-N LPI (n = 12) . | BCL2-R LPI (n = 25) . | P . |
|---|---|---|---|---|---|
| Median age, y (range) | 37 (18-61) | 60 (32-81) | 65 (39-82) | 61 (31-80) | .002 |
| Stage I | 13/13 (100) | 2/7 (28) | 5/12 (42) | 8/22 (36) | .001 |
| Treated with chemotherapy | 7/9 (78) | 5/7 (71) | 8/12 (67) | 16/22 (73) | .96 |
| Relapse or progression | 0/13 (0) | 5/8 (63) | 10/12 (83) | 21/24 (88) | < .0001 |
| Persistent disease at latest follow-up | 0/13 (0) | 5/8 (63) | 8/12 (67) | 18/25 (72) | .0002 |
| Median follow-up, mo (range) | 61 (1-187) | 36 (6-242) | 107 (52-217) | 119 (31-218) | .05 |
| . | BCL2-N HPI (n = 13) . | BCL2-R HPI* (n = 8) . | BCL2-N LPI (n = 12) . | BCL2-R LPI (n = 25) . | P . |
|---|---|---|---|---|---|
| Median age, y (range) | 37 (18-61) | 60 (32-81) | 65 (39-82) | 61 (31-80) | .002 |
| Stage I | 13/13 (100) | 2/7 (28) | 5/12 (42) | 8/22 (36) | .001 |
| Treated with chemotherapy | 7/9 (78) | 5/7 (71) | 8/12 (67) | 16/22 (73) | .96 |
| Relapse or progression | 0/13 (0) | 5/8 (63) | 10/12 (83) | 21/24 (88) | < .0001 |
| Persistent disease at latest follow-up | 0/13 (0) | 5/8 (63) | 8/12 (67) | 18/25 (72) | .0002 |
| Median follow-up, mo (range) | 61 (1-187) | 36 (6-242) | 107 (52-217) | 119 (31-218) | .05 |
Values are no. (%) unless otherwise noted.
BCL2-N indicates no BCL2 gene abnormality by FISH; BCL2-R, BCL2 gene rearrangement or gain by FISH; HPI, high (> 30%) Ki67 proliferation index; LPI: low (< 30%) Ki67 proliferation index.
Includes 1 case with 1 extra copy of BCL2 gene by FISH and strong BCL2 expression, as demonstrated by IHC.
The 13 BCL2-N/HPI cases occurred in patients ages 18-61 years (median age, 37 years) and all were stage I. The patients presented with isolated cervical (7/13), periparotid (1/13), or inguinal (5/13) lymphadenopathy. All cases were characterized by a follicular proliferation with partial (1/13, 8%) or subtotal-to-complete (12/13, 92%) effacement of the underlying lymph node architecture. Clonal B cells comprised 40%-73% (median, 50%) of all B cells in the 7 cases with available flow cytometry. Five cases were grade 1-2, 7 were grade 3A, and 1 was grade 3B (Figure 5). The Ki67 PI ranged from 40% to 90% (median, 68%) within the neoplastic follicles. The clinicopathologic features of the BCL2-N/HPI cases are compared with the 15 other cohort 2 stage I FL cases in Table 3. The BCL2-N/HPI cases occurred in younger patients (P = .0005), with no predilection for sex or site (Figure 6B; Table 3). They were more likely to display follicles with prominent “starry-sky” appearance (P = .001), be histologic grade 3 (P = .004), and lack diffuse areas (P = .04).
Morphologic and IHC features of pediatric-type FL in adults. Representative example of a pediatric-type FL from an adult (cohort 2). The follicular proliferation is composed of variably sized follicles (A) with increased centroblasts, consistent with grade 3A (B). The follicles are composed of CD20+ B cells (C) that do not express BCL2 protein (D). (E, F) One patient with FL in cohort 2 had grade 3B histology. This lymphoma contained large, expansile follicles (E) composed of a uniform population of large centroblasts (F). Panels A, C, D, and E were captured at ×40 magnification; and B and F at ×400 magnification with the use of an Olympus, BX53 microscope, Olympus DP25 digital camera.
Morphologic and IHC features of pediatric-type FL in adults. Representative example of a pediatric-type FL from an adult (cohort 2). The follicular proliferation is composed of variably sized follicles (A) with increased centroblasts, consistent with grade 3A (B). The follicles are composed of CD20+ B cells (C) that do not express BCL2 protein (D). (E, F) One patient with FL in cohort 2 had grade 3B histology. This lymphoma contained large, expansile follicles (E) composed of a uniform population of large centroblasts (F). Panels A, C, D, and E were captured at ×40 magnification; and B and F at ×400 magnification with the use of an Olympus, BX53 microscope, Olympus DP25 digital camera.
Clinicopathologic features of adults with stage I follicular lymphomas (cohort 2)
| . | BCL2-N/HPI (n = 13) . | Other stage I (n = 15) . | P . |
|---|---|---|---|
| Clinical features | |||
| Age, y (median) | 37 (18-61) | 62 (39-82) | .0005 |
| M:F | 8:5 | 7:8 | .47 |
| Head and neck site | 8/13 (62) | 6/15 (40) | .27 |
| Histologic features | |||
| Diffuse areas | 1/13 (8) | 7/15 (47) | .04 |
| Marginal zone differentiation | 1/13 (8) | 0/15 (0) | .46 |
| Follicle size > 2 mm diameter | 5/12 (42) | 3/15 (20) | .40 |
| > 50% “Starry-sky” follicles | 7/12 (58) | 0/15 (0) | .001 |
| Histologic grade 3* | 8/13 (62) | 1/15 (7) | .004 |
| Treatment† | |||
| Excision only | 0/9 (0) | 1/15 (7) | ND |
| Radiation therapy only | 2/9 (22) | 7/15 (47)‡ | |
| Chemotherapy (at initial diagnosis or after radiation therapy) | 7/9 (78) | 7/15 (47) | |
| Outcome | |||
| Relapse or progression | 0/13 (0) | 11/15 (73) | < .0001 |
| Persistent disease at latest follow-up | 0/13 (0) | 6/15 (40) | .02 |
| Died from disease | 0/13 (0) | 1/15 (7) | 1.0 |
| . | BCL2-N/HPI (n = 13) . | Other stage I (n = 15) . | P . |
|---|---|---|---|
| Clinical features | |||
| Age, y (median) | 37 (18-61) | 62 (39-82) | .0005 |
| M:F | 8:5 | 7:8 | .47 |
| Head and neck site | 8/13 (62) | 6/15 (40) | .27 |
| Histologic features | |||
| Diffuse areas | 1/13 (8) | 7/15 (47) | .04 |
| Marginal zone differentiation | 1/13 (8) | 0/15 (0) | .46 |
| Follicle size > 2 mm diameter | 5/12 (42) | 3/15 (20) | .40 |
| > 50% “Starry-sky” follicles | 7/12 (58) | 0/15 (0) | .001 |
| Histologic grade 3* | 8/13 (62) | 1/15 (7) | .004 |
| Treatment† | |||
| Excision only | 0/9 (0) | 1/15 (7) | ND |
| Radiation therapy only | 2/9 (22) | 7/15 (47)‡ | |
| Chemotherapy (at initial diagnosis or after radiation therapy) | 7/9 (78) | 7/15 (47) | |
| Outcome | |||
| Relapse or progression | 0/13 (0) | 11/15 (73) | < .0001 |
| Persistent disease at latest follow-up | 0/13 (0) | 6/15 (40) | .02 |
| Died from disease | 0/13 (0) | 1/15 (7) | 1.0 |
BCL2-N indicates no BCL2 gene rearrangement or gain by FISH; F, female; HPI, high (> 30%) Ki67 proliferation index; M, male; and ND, not done.
Includes 1 grade 3B case.
Treatment modality unknown for 4 patients in the BCL2-N/HPI group.
One patient received rituximab monotherapy at relapse.
Outcome and age distribution of Stage I FL cases. (A) Outcome of 28 cohort 2 Stage I FL patients (≥ 18 years of age). Patients with FL lacking BCL2 gene abnormality and with elevated Ki67 proliferation index (BCL2-N/HPI) demonstrated superior freedom from progression or relapse (median time not reached) compared with patients with FL bearing BCL2 gene rearrangement or gain (BCL2-R) or nonelevated proliferation index (LPI; median, 43 months). (B) Age distribution of stage I FL cases (cohorts 1 and 2).
Outcome and age distribution of Stage I FL cases. (A) Outcome of 28 cohort 2 Stage I FL patients (≥ 18 years of age). Patients with FL lacking BCL2 gene abnormality and with elevated Ki67 proliferation index (BCL2-N/HPI) demonstrated superior freedom from progression or relapse (median time not reached) compared with patients with FL bearing BCL2 gene rearrangement or gain (BCL2-R) or nonelevated proliferation index (LPI; median, 43 months). (B) Age distribution of stage I FL cases (cohorts 1 and 2).
Among all 28 cohort 2 stage I FL cases, BCL2-N/HPI status was associated with lack of relapse/progression (P < .0001) or persistent disease at latest follow-up (P = .02; Table 3). Median follow-up time was 61 months for BCL2-N/HPI cases and 92 months for the other cohort 2 stage I cases. The time to progression or relapse was not reached for the BCL2-N/HPI cases and 43 months for the other stage I cases (P = .0002, log rank test; Figure 6A). Considering only the stage I patients treated with chemotherapy, the time to progression or relapse was not reached for the BCL2-N/HPI cases and 63 months for the other stage I cases (P = .01, log rank test). Among all stage I patients, grade 3 histology (P = .008) and prominent “starry-sky” follicles (P = .06), but not follicle size or presence/absence of diffuse areas, were associated with longer freedom from progression or relapse (log rank test, data not shown). Neither patient age nor sex was associated with freedom from progression or relapse (data not shown).
Evaluation of BCL2-N/HPI cases in both pediatric and adult FL (cohorts 1 and 2)
On the basis of our finding that the BCL2-N/HPI status was associated with favorable outcome in FL in both cohorts 1 and 2 and often also was associated with histologic features traditionally attributed to “pediatric FL” (expansile follicles, “starry-sky” appearance, and grade 3 histology), we compared the clinical and pathologic features and outcome of all BCL2-N/HPI FL cases (combined cohorts 1 and 2) in patients ages < 18 years versus those in patients ≥ 18 years of age (Table 4). Compared with patients ≥18 years, the BCL2-N/HPI cases in children and adolescents occurred more often in males (P = .03) and had a borderline significantly greater PI (P = .06); there were no differences in histologic parameters. Although none of the BCL2-N/HPI FL patients in either age group progressed or recurred, the majority (10/18, 56%) of the patients ≥ 18 years of age were treated with chemotherapy, whereas 9 of 12 (75%) of the children and adolescents < 18 years of age were treated with excision only. Progression-free and overall survival of combined cohort 1 and 2 patients (all stages) according to BCL2-N/HPI status are provided in supplemental Figure 2.
Clinicopathologic features of “pediatric-type” (BCL2-N/HPI) follicular lymphomas
| . | Age < 18 years (n = 12) . | Age ≥ 18 years (n = 22) . | P . |
|---|---|---|---|
| Clinical features | |||
| M:F | 12:0 | 14:8 | .03 |
| Head and neck site | 10/12 (83) | 15/22 (68) | .44 |
| Stage I | 12/12 (100) | 22/22 (100) | 1.0 |
| Histologic features | |||
| Marginal zone differentiation | 2/12 (17) | 2/22 (9) | .60 |
| Follicle size > 2 mm diameter | 9/12 (75) | 10/21 (48) | .15 |
| > 50% “Starry-sky” follicles | 9/12 (75) | 11/22 (50) | .27 |
| Histologic grade 3 | 6/12 (50) | 11/22* (50) | 1.0 |
| Immunophenotypic features | |||
| BCL2+ tumor cells ( ≥ 2+ intensity) | 3/12 (25) | 3/22 (14) | .64 |
| Ki67 PI (median) | 90% (40-95%) | 65% (40-95%) | .06 |
| Treatment† | |||
| Excision only | 9/12 (75) | 5/18 (28) | ND |
| Radiation therapy only | 0/12 (0) | 3/18 (17) | |
| Chemotherapy (at initial diagnosis or after radiation therapy) | 3/12 (25) | 10/18 (56) | |
| Median follow-up, mo | 53 | 60 | .84 |
| . | Age < 18 years (n = 12) . | Age ≥ 18 years (n = 22) . | P . |
|---|---|---|---|
| Clinical features | |||
| M:F | 12:0 | 14:8 | .03 |
| Head and neck site | 10/12 (83) | 15/22 (68) | .44 |
| Stage I | 12/12 (100) | 22/22 (100) | 1.0 |
| Histologic features | |||
| Marginal zone differentiation | 2/12 (17) | 2/22 (9) | .60 |
| Follicle size > 2 mm diameter | 9/12 (75) | 10/21 (48) | .15 |
| > 50% “Starry-sky” follicles | 9/12 (75) | 11/22 (50) | .27 |
| Histologic grade 3 | 6/12 (50) | 11/22* (50) | 1.0 |
| Immunophenotypic features | |||
| BCL2+ tumor cells ( ≥ 2+ intensity) | 3/12 (25) | 3/22 (14) | .64 |
| Ki67 PI (median) | 90% (40-95%) | 65% (40-95%) | .06 |
| Treatment† | |||
| Excision only | 9/12 (75) | 5/18 (28) | ND |
| Radiation therapy only | 0/12 (0) | 3/18 (17) | |
| Chemotherapy (at initial diagnosis or after radiation therapy) | 3/12 (25) | 10/18 (56) | |
| Median follow-up, mo | 53 | 60 | .84 |
Values are no. (%) unless otherwise noted.
BCL2-N indicates no BCL2 gene rearrangement or gain by FISH; F, female; HPI, high (> 30%) Ki67 proliferation index; M, male; and ND, not done.
Includes 1 grade 3B case.
Treatment modality unknown for 4 of the age ≥ 18 years patients.
Discussion
We analyzed clonal follicular proliferations in children and young adults in an attempt to better define PFL. We found that cases that lacked a BCL2 gene abnormality by FISH (BCL2-N) and had a HPI (> 30%) uniformly had stage I disease and did not progress or relapse, even when treated with excision alone. The majority of these BCL2-N/HPI cases had histologic features consistent with PFL, such as large, expansile follicles, as previously described in the literature.2-4,10,11 However, this group also included some cases with small, round follicles composed predominantly of small centrocytes, morphologically resembling conventional grade 1-2 FL rather than PFL. In contrast to the BCL2-N/HPI cases, follicular proliferations in children and young adults with BCL2 gene rearrangement or a low PI tended to present with advanced-stage disease and demonstrated a natural history similar to that of conventional FL occurring in adults. We found that BCL2-N/HPI cases actually occurred beyond childhood into the 4th decade. Conversely, conventional FL with BCL2 gene rearrangement was exceedingly rare in patients < 18 years, with only a single 17-year-old patient with BCL2-R in our series (Figure 6B) and 2 patients (13 and 17 years of age) in a previous series.2
To assess the frequency and clinicopathologic features of cases of BCL2-N/HPI FL in adults, we analyzed a separate cohort of FL patients ≥ 18 years. We found that BCL2-N/HPI nodal FL occurred in adulthood into the 7th decade, and similar to PFL, did not progress or recur (Figure 6B). Most of the BCL2-N/HPI cases identified in this cohort had classic morphologic features of PFL, including large expansive follicles, “starry-sky” pattern, and a predominance of centroblasts or large centrocytes; all were stage I, and none progressed or relapsed. In contrast, the majority of the adult nodal Stage I FL that had BCL2 gene rearrangement and/or a nonelevated PI demonstrated morphology and behavior similar to conventional FL and either progressed or relapsed. Thus, within a cohort of adult patients with FL, the BCL2-N/HPI phenotype was strongly associated with stage I disease and, among all stage I adult patients with FL, this “pediatric-type” phenotype was strongly associated with freedom from progression or relapse. Unlike the younger patients with PFL, who were mostly treated with excision alone, most adults with pediatric-type FL with available treatment information received chemotherapy or radiotherapy; thus the contribution of chemotherapy to good outcomes in this older age group is uncertain. However, by comparison, 5 of 7 stage I adult non-PFL type lymphomas treated with chemotherapy relapsed, suggesting that the BCL2-N/HPI status was an important determinant of this indolent behavior in adults.
It is possible that BCL2-N/HPI FL cases may be related to a broad group of indolent extranodal clonal FLs that lack BCL2 gene abnormalities, such as primary cutaneous follicle center lymphoma,12 which shares some features with pediatric-type FL, including follicles often composed of large centrocytes and variable proportions of centroblasts, negative (or sometimes weak) expression of BCL2 by neoplastic cells, and lack of BCL2 gene rearrangement.13-22 This group of indolent clonal follicular proliferations may also include primary FL of the testis (in both adults and children), which also are characteristically localized and have CD10+, BCL6+, and BCL2− neoplastic cells but lack BCL2 gene rearrangements and do not generally progress or recur after orchiectomy.23-25 More recently, PFL-like proliferations of the ovary with low-stage, grade 3A histology and lacking BCL2 rearrangement have been described that have more favorable outcome compared with conventional FL of the ovary with respect to progression and recurrence.26 These indolent types of FL are characterized by architectural effacement, which distinguishes them from previously described cases of follicular hyperplasia with clonal B cells.27
PI has been shown to have prognostic significance in FL and tends correlate with the number of large cells and histologic grade.28 In one series, cases of FL with low grade and discordantly high proliferation index (LGHPI) were found to behave more aggressively than their non-HPI counterparts and had inferior disease-specific survival more akin to grade 3 FL.8 In contrast to the LGHPI cases in our study, BCL2 protein expression was present in 20 of 24 of the LGHPI cases in that study, and BCL2 gene rearrangement status was not assessed. These data suggest that HPI may be associated with more aggressive behavior in the setting of FL with BCL2 gene abnormalities but not in the setting of BCL2 nonrearranged clonal follicular proliferations. This idea is supported by a recent study demonstrating significant genetic alteration and gene expression profile differences between t(14;18)–positive and t(14;18)–negative FL cases, the latter of which mostly were of low stage and had an elevated PI.29 Although overall survival did not differ between FL with and without t(14;18) in that series, freedom from progression or relapse were not reported. In addition, patients < 23 years of age were excluded, and a subset of the t(14;18)-negative cases had BCL6 translocations, which were not present in the pediatric-type FL cases in our series.
We have demonstrated the prognostic value of simultaneous evaluation of PI status and BCL2 gene abnormalities by FISH in FL, irrespective of age. In one pediatric FL series, BCL2 protein expression and BCL2 gene rearrangement was noted in the 2 cases (of 13) which showed progression or relapse after combination chemotherapy. This finding led to the suggestion that BCL2 protein expression in PFL identifies a subset of patients whose disease is aggressive and/or more refractory to chemotherapy (although these data did not reach statistical significance in that study).2 Interestingly, 3 additional cases in that series with abnormal expression of BCL2 protein, but no BCL2 gene rearrangement, did not progress or relapse. We also found some cases in both cohorts with BCL2 expression by immunohistochemistry despite lacking BCL2 gene rearrangement or gain; these cases exhibited a favorable outcome and low stage similar to the other BCL2-N cases. Although strong BCL2 expression correlated closely with an unfavorable outcome, there are drawbacks to assessing BCL2 protein expression for prognostication, including the subjectivity of interpreting weak immunostaining intensity and the occurrence of BCL2 gene mutations that may abrogate immunostaining in some cases.30,31 For these reasons, we suggest that evaluation of BCL2 gene rearrangement by FISH may be a better approach for defining the BCL2-N/HPI group. One case in our series had an extra copy of the BCL2 gene, without rearrangement; this case showed strong expression of BCL2 protein and recurred 35 months after treatment with chemotherapy. This finding suggests that gain of BCL2 accompanied by strong protein expression may be equivalent to BCL2 gene rearrangement in terms of association with more aggressive behavior, but further study is required to evaluate the clinical behavior of such cases.
High-grade conventional FL (grade 3A/3B) can occasionally be associated with both HPI and an absence of BCL2 gene rearrangement.32-36 As with low-grade FL, lack of BCL2 rearrangement in grade 3A FL is uncommon and occurs in < 15% of cases.35 The majority of these cases harbor alternative aberrations, such as BCL6 translocation or amplification or BCL2 gene amplification.33-35,37,38 BCL2-N status is more frequent in FL grade 3B (up to 90% of cases); however, pure FL grade 3B is extremely rare (most are associated with concurrent DLBCL) and is usually negative for CD10, with strong MUM1/IRF4 expression and frequent BCL6 gene rearrangements.36 In contrast, the FL in this study were CD10+, with minimal or no MUM1/IRF4 expression and absent BCL6 gene rearrangement. Only 1 of all pediatric-type FL cases in our series had morphologic features of FL grade 3B; this case was stage I and exhibited indolent clinical behavior, with no evidence of disease 78 months after chemotherapy administration.
Salaverria et al recently described a subset of high-grade FL (3/24) and DLBCL (20/24) occurring in children and young adults that are characterized by a novel IG-IRF4 translocation, and strong MUM1/IRF4 expression, often presenting with limited stage disease.9 Several cases had concurrent BCL6 or MYC rearrangement. None of the cases in the current study had IG-IRF4 translocation or strong IRF4 expression, indicating that these IG-IRF4 cases appear to be biologically distinct from the pediatric-type FL described in our series and may represent a spectrum of DLBCL in childhood, rather than a FL subtype. Oschlies et al also described a series of high-grade FL and DLBCL cases in children that had long event-free survival after treatment with chemotherapy. The majority of these patients presented with advanced-stage disease and tumors were mostly negative for BCL6, BCL2, and MYC translocations; a small subset demonstrated IGH translocations for which the partner was not identified.39 These cases may also fall within the spectrum of DLBCL in children and are unlike the cases of pediatric-type FL described in this report, which were uniformly localized and show excellent event-free survival even in the absence of treatment with chemotherapy or radiotherapy.
In summary, by using FISH for BCL2 rearrangement and the Ki67 proliferation index, we have shown that the combination of a normal BCL2 gene and HPI (BCL2-N/HPI phenotype) identifies a type of nodal FL in both children and adults that is localized and displays highly indolent clinical behavior. Although most cases have morphologic features similar to those previously described in PFL, some are morphologically indistinguishable from conventional low-grade adult FL. These proliferations occur most frequently in the head and neck and have a male predominance. Importantly, although these cases occur predominantly in young patients, they can also occur in adult patients and appear to have the same indolent behavior. We propose the term “pediatric-type” FL to encompass cases of stage I nodal FL that both lack BCL2 gene abnormalities by FISH and have high PI by Ki67 immunohistochemistry. The localized nature and benign clinical course of these lesions raises the question of whether these are truly malignant or actually represent a benign clonal proliferation with low malignant potential. However, in contrast with florid follicular hyperplasia with clonal CD10+ B cells, pediatric-type FL shows partial or complete effacement of the nodal architecture, and such cases are typically diagnosed as lymphoma by pathologists.27 Our data suggest that the diagnosis of pediatric-type FL can be applied irrespective of age to identify low-stage FLs that do not recur or progress. Further study is required to determine whether pediatric-type FL occurring in adults may be effectively managed by excision alone, similar to pediatric-type FL occurring in children.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thanks Drs Atul Butala, Anjulika Chawla, Matthew Hajduk, Marlene Magrini-Greyson, Elizabeth Manaloor, Walter Lamar, and Howard Meyerson for their assistance in obtaining clinical information. They also thank Steven Conley and Michelle F. Lee for assistance with figures.
A.L. is supported by AMA Foundation Seed Grant Research Award, MGH Physician-Scientist Development Award, and Dana Farber/Harvard Cancer Center New Faculty Recruitment Fund. R.S. is supported by KinderKrebs Initiative Buchholz/Holm-Seppensen. I.S. received a scholarship of the Alexander-von-Humboldt Foundation.
Authorship
Contribution: A.L. and R.P.H. designed research, collected, analyzed, and interpreted data, and wrote the manuscript; J.A.F. and N.L.H. designed research and analyzed data; A.M.A., D.D.-S., A.J.I., D.O.L., and G.S.P. performed and analyzed data; E.P.H., M.S.H., H.J.W., and L.R.Z. assisted with interpretation of data; Z.S. and L.R.Z. collected data; I.S. performed IRF4 FISH; and I.S. and R.S. analyzed and interpreted data.
Conflict-of-interest disclosure: D.D.-S. serves as a consultant for BioReference Laboratories Inc. E.P.H. serves as a consultant for Sigma Tau, Millenium, Biogen-Idec, Pfizer, Seattle Genetics, and Genentech. The remaining authors declare no competing financial interests.
Correspondence: Abner Louissaint Jr, MD, PhD, Massachusetts General Hospital, 55 Fruit St, Warren 2, Boston, MA 02114; e-mail: alouissaint@partners.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal