Abstract
Although B-cell chronic lymphocytic leukemia (B-CLL) clones with unmutated IGHV genes (U-CLL) exhibit greater telomerase activity than those with mutated IGHV genes (M-CLL), the extent to which B-cell receptor (BCR) triggering contributes to telomerase up-regulation is not known. Therefore, we studied the effect of BCR stimulation on modulating telomerase activity. The multivalent BCR ligand, dextran conjugated anti-μ mAb HB57 (HB57-dex), increased telomerase activity and promoted cell survival and proliferation preferentially in U-CLL cases, whereas the PI3K/Akt inhibitor LY294002 blocked HB57-dex induced telomerase activation. Although both U-CLL and M-CLL clones exhibited similar membrane proximal signaling responses to HB57-dex, telomerase activity and cell proliferation, when inducible in M-CLL, differed. B-CLL cells stimulated using bivalent F(ab′)2 -goat anti-μ antibody (goat anti-μ) exhibited higher membrane proximal response in U-CLL than M-CLL cells, whereas telomerase activity, cell survival, and proliferation were induced to lower levels than those induced by HB57-dex. In normal B lymphocytes, HB57-dex induced less protein phosphorylation but more cell proliferation and survival than goat anti-μ. Although both anti-BCR stimuli induced comparable telomerase activity, normal CD5+ B cells preferentially exhibited higher hTERT positivity than their CD5− counterparts. These findings provide an understanding of how BCR-mediated signals impact telomerase modulation in IGHV mutation-based subgroups of B-CLL and normal B cells.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) cases can be divided into 2 major clinically distinct subgroups1,2 by the degree of somatic mutations in the expressed immunoglobulin heavy variable (IGHV) genes.3 Based on such observations, the B-cell receptor (BCR) has become the subject of intense investigation in B-CLL.
Several groups have observed that B-CLL clones use a biased group of IGHV genes3,4 and associate these IGHVs nonrandomly with specific sets of IGHD and IGHJ segments (“stereotyped BCRs”),5,6 supporting a role for antigen drive in the promotion of CLL. Because the IGHVs in cases with stereotyped BCRs are usually not mutated (IGHV unmutated, U-CLL), these clones may have arisen from B cells triggered by an agent/antigen acting in a T cell–independent (T-I) fashion that did not effectively elicit or elicited in a nonclassic manner the somatic hypermutation process.7,8 To this effect, gene expression studies have identified BCR ligation-induced changes primarily in U-CLL cases.9,10 Surprisingly, B-CLL cells from these cases are more prone to spontaneous apoptosis and appear to be more dependent on environmental prosurvival signals than IGHV mutated (M-CLL) B-CLL cells.11 BCR signaling appears to play a pivotal role in the life of a B-CLL cell, in particular the U-CLL variety,12 as illustrated by the fact that sustained signaling through the BCR induces the antiapoptotic molecule Mcl-1 and promotes cell survival.13
Repeated cell activation (as may be elicited by autoantigen(s) encounter) can result in significant erosion of telomeric regions of the chromosome,14,15 and an association between short telomeres and genetic complexity, high-risk genomic aberrations, and short survival in B-CLL has been reported.16 Indeed, U-CLL cells exhibit shorter mean telomere lengths than M-CLL cells.17 Recent studies demonstrate similarity in telomere dynamics of CLL B cells and those observed in cells undergoing crisis in culture after abrogation of the p53 pathway,18 supporting the concept that telomere erosion and subsequent telomere fusion may be critical in the progression of CLL.
In normal B cells, BCR-mediated telomerase activation counteracts telomere erosion, which in turn can positively affect cellular longevity.19 Elevated telomerase activity has been found in premalignant lesions as well as overt malignancies of nonhematologic20,21 and hematologic nature.22 Indeed, telomerase activity and mean telomere lengths differ between the IGHV-based subgroups of B-CLL cases,17,23,24 and B-CLL cells with higher telomerase activity exhibit shorter lymphocyte doubling times (LDT < 12 months), an indicator of poor prognosis, have greater telomerase activity,17 and follow more aggressive clinical courses.23,25
Because to date the factors enhancing telomerase activity in B-CLL have not been completely deciphered, we examined whether engagement of the BCR on B-CLL clones cultured in vitro influences telomerase activity in U-CLL and M-CLL clones. Valency of antigenic epitopes and structure of the antigen can have strong influences on BCR signaling in normal B lymphocytes.26 For this reason, we compared U-CLL and M-CLL clones for their responsiveness to a multivalent, high molecular weight BCR ligand (high affinity HB57 anti-IgM mAb covalently linked to high MW dextran, HB57-dex) and to a bivalent F(ab′)2 preparation of polyclonal goat-anti–human IgM antibodies.
The multivalent BCR ligand and the bivalent BCR ligand elicited enhanced telomerase activity and augmented cell survival that was particularly evident with U-CLL. Although M-CLL cells did show membrane-proximal changes reflective of BCR-mediated activation, these did not translate into increased telomerase activity or prolonged cell survival.
Methods
Patients and healthy donors
The Institutional Review Board of the North Shore-LIJ Health System approved these studies, which were also conducted in accordance with the Declaration of Helsinki. After informed consent, heparinized venous blood was collected from 56 patients with B-CLL. Laboratory data and IGHV-D-J and IGK/LV-J DNA sequences were available on all cases and clinical information was available for most.1,3,17 Of the 56 B-CLL cases included in this study, 28 were chosen randomly from a group that expressed IGHV with < 2.0% difference from the most similar germline gene of both IGHV and IGK/LV (“unmutated IGHV cases”; U-CLL), and 28 from a group that had IGHV with > 2.0% difference from the most similar germline gene in either IGHV or IGK/LV (“mutated IGHV cases”; M-CLL). Cases included in this study comprised only those expressing sIgM and exhibiting allelic exclusion of IGHV. Leukocyte-enriched fractions from healthy persons were also used (Long Island Blood Services). These were provided by donors ≥ 60 years of age and were pretested and found negative for HIV and HBV antibodies.
Isolation of PBMCs
PBMCs were separated from the blood of CLL patients or from enriched leukocyte fractions from healthy donors by density gradient centrifugation using Ficoll-Paque (Pharmacia LKB Biotechnology) and were cryopreserved until further use using a programmable cell-freezing machine (Cryomed).
Isolation of B cells
B cells were isolated from PBMCs using the B-cell isolation kit (Miltenyi Biotec) or the Rosette-Sep kit (Stem Cell Technologies) according to the manufacturer's instructions. Purity of B cells was ascertained by exposing aliquots of cells to allophycocyanin-conjugated anti-CD19, PE-conjugated anti-CD3, PE-conjugated–anti-CD15, and FITC-conjugated anti-CD14 mAbs (BD Biosciences-PharMingen), fixing with 1% paraformaldehyde, and analyzing on a FACSCalibur flow cytometer (BD Biosciences).
Analysis of surface membrane phenotype
To study expression of BCR-associated molecules on B-CLL cells, FITC-conjugated anti-CD21, PE-conjugated anti-CD81, and anti-CD38 (all from BD Biosciences PharMingen), FITC-conjugated F(ab′)2 fragments of goat anti–human IgM (Southern Biotechnology Associates), PE-conjugated anti-CD79b (Immunotech Coulter), and FITC-conjugated anti–ZAP-70 (eBioscience) were used. Cryopreserved PBMCs were thawed rapidly, washed twice with RPMI 1640 medium, and suspended as 107/mL in PBS containing 1% BSA (Sigma-Aldrich) and 0.1% sodium azide. Cells were stained with antibody combinations that enabled quantification of percentages of B-CLL (CD19+CD5+) cells expressing CD21, CD79b, CD81, CD38, ZAP-70, or IgM.
Analysis of Ca2+ flux
Cryopreserved B cells were thawed and washed twice with RPMI 1640 containing 10% heat-inactivated FCS (Hyclone). Cells were suspended in dye-free RPMI 1640 supplemented with 2% FCS and loaded with Indo-1AM (1μM, Invitrogen) at 37°C for 30 minutes, washed twice, and suspended at 5 × 106 /mL. Cell aliquots (200 μL) were incubated for 15 minutes at room temperature for depolarization of Indo-1AM. Typically, baseline Ca2+ flux was assessed by acquiring data from unstimulated cells for 30 seconds and for a total of 5 minutes after stimulation. Data were acquired for a total of 5 minutes to evaluate the effect of the stimuli. In some initial experiments, Ca2+ flux was monitored for 15 minutes after stimulation using a FACS LSR-II machine (BD Biosciences). Data are represented as fold change in Ca2+ flux peak values relative to unstimulated controls.
Phosphoprotein analysis
Cryopreserved B cells were thawed and washed twice with RPMI 1640 containing 10% heat-inactivated FCS (Hyclone), suspended at 5 × 106/mL in the same medium, and incubated for 2 hours without any additives or in presence of pharmacologic inhibitors: p38 MAP kinase inhibitor (50nM; Biosource), MEK1/2 /Erk inhibitor-U 0126 (10μM; Cell Signaling Technology), PI-3 kinase/Akt inhibitor-LY 294002 (20μM; Calbiochem), or selective Akt inhibitor (at 5 or 20μM; Calbiochem). After incubation, cells were washed thoroughly and aliquoted for analysis of phosphoproteins and for evaluation of effects of pathway inhibitors on ability of various agents to stimulate telomerase activity. For phosphoprotein analysis, cells were stimulated for 5 minutes with dextran-conjugated murine antihuman IgM mAb (clone HB57 (intrinsic Fab′ affinity Ka of 5 × 108M−1), “HB57-dex”; 0.1 μg/mL)26 or with goat F(ab′)2 anti–human μ heavy chain (GAH-μ; 10 μg/mL; Southern Biotechnology Associates). To prevent dephosphorylation, stop buffer (2% methanol-free formaldehyde; Polysciences) + phosphatase inhibitor cocktail (5% volume/volume; EMD Chemicals/Calbiochem) were added and cells fixed at 37°C for 10 minutes as previously outlined.27 After washing, cells were permeabilized with chilled methanol (90% volume/volume) for 30 minutes at 4°C. Subsequent to washing twice, cells were stained for 30 minutes with AlexaFluor-488–conjugated mAbs to Akt, p38 MAP kinase, or Erk (all from Cell Signaling Technology). In some experiments, the effect of the selective Akt inhibitor on phosphorylation of hTERT in HB57-dex–treated cells was assessed using the polyclonal anti-phosphoTERT antibody. Cell lysates were also prepared from parallel cultures for assessment of changes in telomerase activity. Fold change in phosphorylation of proteins by clonal members was studied on the FACSCalibur as evidenced by an increase in staining for the phosphorylated forms of these proteins calculated as: mean fluorescence intensity of stimulated cells and mean fluorescence intensity of unstimulated cells
Cell cultures for assessment of cellular telomerase activity, proliferation, and survival
Purified B-CLL cells (∼ 99% pure) or normal B lymphocytes were suspended at a concentration of 1 × 106/mL in RPMI 1640 supplemented with antibiotics and 10% heat inactivated FCS (”complete culture medium”), and 3 × 106 cells were cultured in the following conditions: (1) unstimulated; (2) with HB57-dex (0.1 μg/mL); and (3) with goat F(ab′)2 antihuman μ at 10 μg/mL. Dextran-conjugated MOPC 21 mAb (Con-dex) served as control IgG1 mAb for initial experiments. For evaluation of response to a mitogenic stimulus, a mixture of phorbol 12-myristate 13-acetate (50 ng/mL; Sigma-Aldrich) and ionomycin (1μM; Sigma-Aldrich) was used. To assess cell proliferation, 2 × 105 cells were plated in triplicate in similar culture conditions (points 1-3) into 96-well microtiter flat-bottom plates and pulsed with tritiated thymidine (1 μCi/well) for the last 16 hours of a 64- to 68-hour incubation period. Subsequently, cultures were harvested and subjected to scintillation counting. Data are reported as stimulation indices. Bulk cultures were harvested 64-68 hours after initiation of culture and analyzed for telomerase induction and cell survival.
Preparation of cell extracts and estimation of telomerase activity
The detailed method for telomerase detection has been described.17 Cell lysates prepared from washed cells using cell lysis buffer provided in the TRAPeze telomerase detection kit (Millipore) that uses the telomere repeat amplification protocol28 were assessed for telomerase activity at ∼ 64-68 hours after stimulation. Autoradiograms were densitometrically scanned, and values for total product generated units calculated as per the manufacturer's instructions. Induction of hTERT protein was also studied in normal CD5-based B-cell subsets incubated with anti-BCR antibodies for 3 days. The polyclonal anti-hTERT antibody was used as outlined previously.29
Analysis of cell survival
Cultured cells were harvested at 64-68 hours and washed twice with cold PBS. Cells were then fixed for a minimum of 1 hour at 4°C in prechilled 75% ethanol, washed twice with cold PBS, and the pellet suspended in PBS. Fixed cells were exposed to propidium iodide (0.05 mg/mL; Roche Applied Biosciences) overnight in the presence of ribonuclease A (0.5 μg/mL; Boehringer Mannheim). At least 30 000 nuclei were analyzed for DNA content on the FACSCalibur. Results are presented as the percentage of nuclei showing subdiploid DNA content.
Statistical analyses
The paired t test was used to determine the significance of differences in stimulation indices in response to HB57-dex or GAH-μ F(ab′)2 within the same group of cases. The Mann-Whitney test was used when comparing average values from 2 parameters studied on 2 sets of cases.
Results
Constitutive expression of surface membrane BCR and associated molecules
PBMCs from 56 B-CLL cases were studied for surface expression of IgM, CD21, CD79b, and CD81. Although the percentage of CD21- and CD79b-expressing B cells was comparable between U-CLL and M-CLL cases, the percentages of B-CLL cells expressing sIgM and CD81 were significantly higher in U-CLL compared with M-CLL (P < .02 and P < .05, respectively; Table 1). As expected,1,30 the percentages of B-CLL cells expressing CD38 or ZAP-70 were also significantly higher among U-CLL cases (both P < .001).
Phenotypic analysis of U-CLL and M-CLL cases
| Marker . | U-CLL cells . | M-CLL cells . | P . |
|---|---|---|---|
| IgM | 78.9 ± 4 | 59.4 ± 6.6 | < .02 |
| CD21 | 84.8 ± 3.8 | 85.4 ± 2.7 | NS |
| CD79b | 72.5 ± 4 | 68.6 ± 4.4 | NS |
| CD81 | 67.6 ± 4 | 78.4 ± 3.6 | < .05 |
| CD38 | 44.1 ± 6.5 | 19.5 ± 4.4 | < .001 |
| ZAP-70 | 27.5 ± 5.6 | 7.1 ± 2.9 | < .001 |
| Marker . | U-CLL cells . | M-CLL cells . | P . |
|---|---|---|---|
| IgM | 78.9 ± 4 | 59.4 ± 6.6 | < .02 |
| CD21 | 84.8 ± 3.8 | 85.4 ± 2.7 | NS |
| CD79b | 72.5 ± 4 | 68.6 ± 4.4 | NS |
| CD81 | 67.6 ± 4 | 78.4 ± 3.6 | < .05 |
| CD38 | 44.1 ± 6.5 | 19.5 ± 4.4 | < .001 |
| ZAP-70 | 27.5 ± 5.6 | 7.1 ± 2.9 | < .001 |
Data are mean ± SE of the percentage of CD19+CD5+ cells from U-CLL or M-CLL cases expressing particular marker.
NS indicates not significant.
Analysis of membrane-proximal events elicited by multivalent anti-IgM mAb (HB57-dex)
BCR-mediated activation of B cells initiates a variety of events, such as mobilization of intracellular Ca2+ and phosphorylation of several key molecules involved in cell cycle- and survival-related pathways. A subgroup of 16 U-CLL cases and 15 M-CLL cases was analyzed for Ca2+ flux in response to BCR stimulation using HB57-dex. In preliminary kinetic studies, Ca2+ flux was monitored for up to 15 minutes after stimulation; in all subsequent experiments, Ca2+ flux was tracked for 5 minutes. As a positive control for cellular responsiveness, Ca2+ mobilization elicited by a mitogenic stimulus (PMA + ionomycin) was evaluated in every instance. Cells from all cases were efficiently loaded with Indo-1 and were found to be physiologically active because they responded to the mitogens. Figure 1A shows results from a representative experiment using U-CLL cells and Figure 1B summarizes values on maximum fold increases in Ca2+ flux in response to HB57-dex among subsets of CLL cases. Surprisingly, although mitogen-induced changes in Ca2+ flux were observed in every case, anti-IgM–induced changes in Ca2+ flux were low or absent.
Anti-IgM (HB57 dex)–mediated membrane proximal events. (A) Induction of Ca2+ flux by B-CLL cells. Ratiometric analysis of Indo-1 fluorescence in its Ca-bound to free state indicative of intracellular [Ca2+] was performed over a 15-minute period after activation with various reagents: PMA + ionomycin, goat anti–human-μ, or HB57dex. In all experiments, the ratio of Ca2+ mobilization in response to a signal to unstimulated was calculated over a 5-minute period (vertical dotted line). The figure shows representative tracings from 1 B-CLL case included in optimization studies. (B) Fold induction of Ca2+ flux by BCR stimulation using HB57-dex compared with baseline levels in 16 U-CLL and 15 M-CLL patients. Baseline levels were established by acquiring data from unstimulated cells for 30 seconds before addition of the stimulants. Statistical significance of the comparisons was evaluated using the paired t test. Differences were not significant. (C-E) BCR-mediated phosphorylation of Akt, Erk, and MAPK. Fold change in mean fluorescence intensity acquired by flow cytometry elicited by HB57-dex over unstimulated controls in all cases (2 scattergrams on the left); scattergrams on the right represent values obtained in cases segregated by IGHV gene mutations. (C) pAkt. (D) pErk. (E) p-p38MAPK. Statistical significance of the comparisons was evaluated using the paired t test. Differences were not significant.
Anti-IgM (HB57 dex)–mediated membrane proximal events. (A) Induction of Ca2+ flux by B-CLL cells. Ratiometric analysis of Indo-1 fluorescence in its Ca-bound to free state indicative of intracellular [Ca2+] was performed over a 15-minute period after activation with various reagents: PMA + ionomycin, goat anti–human-μ, or HB57dex. In all experiments, the ratio of Ca2+ mobilization in response to a signal to unstimulated was calculated over a 5-minute period (vertical dotted line). The figure shows representative tracings from 1 B-CLL case included in optimization studies. (B) Fold induction of Ca2+ flux by BCR stimulation using HB57-dex compared with baseline levels in 16 U-CLL and 15 M-CLL patients. Baseline levels were established by acquiring data from unstimulated cells for 30 seconds before addition of the stimulants. Statistical significance of the comparisons was evaluated using the paired t test. Differences were not significant. (C-E) BCR-mediated phosphorylation of Akt, Erk, and MAPK. Fold change in mean fluorescence intensity acquired by flow cytometry elicited by HB57-dex over unstimulated controls in all cases (2 scattergrams on the left); scattergrams on the right represent values obtained in cases segregated by IGHV gene mutations. (C) pAkt. (D) pErk. (E) p-p38MAPK. Statistical significance of the comparisons was evaluated using the paired t test. Differences were not significant.
Several previous studies have identified involvement of PI-3 kinase, Akt, p38 MAP kinase, and Erk in cell survival-related pathways in B-CLL.31-33 Therefore, we analyzed, using the phosphoflow technique, differences in phosphorylation induced by anti-IgM stimulation in CD19+CD5+ cells from 20 patients. Figure 1C-E illustrate phosphorylation of Akt, Erk, and p38 MAP kinase, respectively, observed in all cases and also in clones stratified by IGHV mutations. Notably, changes in phosphorylation of all 3 molecules induced by the dextran-conjugated anti-μ mAb were comparable between the U-CLL and M-CLL subgroups.
Telomerase induction by dextran-conjugated anti-IgM mAb
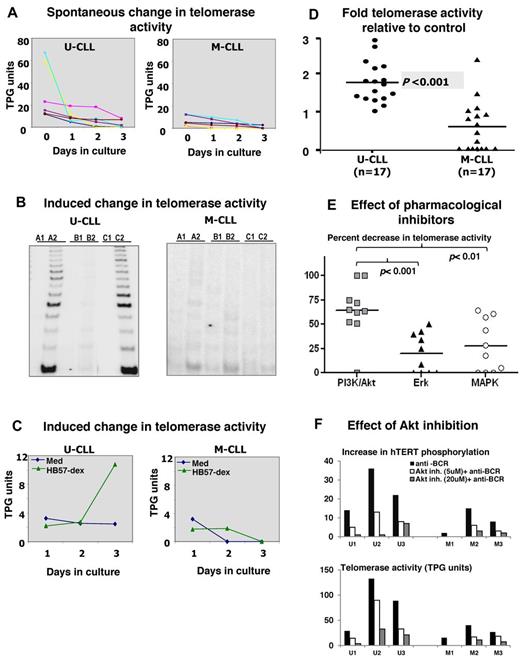
Ex vivo telomerase activity is lower in B cells isolated from the blood of M-CLL compared with U-CLL patients.17 To assess whether the 2 subsets differed in telomerase activity after BCR stimulation in vitro, B-CLL cells from 6 U-CLL and 6 M-CLL cases were cultured for 3 days in the absence or presence of HB57-dex. Cells from replicate cultures were harvested daily and cell lysates analyzed for telomerase activity. Although telomerase activity in the residual live cells from unstimulated cultures dwindled appreciably (to 17%-37% of that detected in freshly isolated cells in the U-CLL cases; Figure 2A), the HB57dex treatment up-regulated telomerase activity principally in U-CLL cells. Figure 2B (right panel) illustrates the autoradiograph from 1 representative case from each category. Figure 2C shows kinetics of telomerase induction in representative U-CLL and M-CLL clones cultured in the absence or presence of the anti-IgM stimulus. Finally, B-CLL cells from 17 U-CLL and 17 M-CLL patients were analyzed for induced changes in telomerase activity. Overall, 15 of 17 U-CLL cases exhibited HB57-dex–induced telomerase activity (Figure 2D); on the contrary, HB57-dex did not induce telomerase activity in 15 of 17 M-CLL cases.
Spontaneous and BCR-induced changes in telomerase activity in CLL cases. Purified B cells from 6 U-CLL and 6 M-CLL cases were cultured without or with BCR stimulation. (A) Spontaneous decrease in telomerase activity within residual live cells at day 1, 2, and 3 of culture in U-CLL (left) and M-CLL (right) cases. (B) Telomerase activity assessed in cell extracts prepared from uncultured cells (A1 and A2), cells cultured in medium alone (B1 and B2), or cells cultured with HB57-dex for 3 days (C1 and C2). A1, B1, and C1 represent heat-treated internal controls for A2, B2, and C2, respectively. The representative autoradiograph on the left (U-CLL case) shows induction/retention of telomerase activity, whereas the M-CLL case on the right does not. (C) Change in telomerase activity over time, elicited by anti-IgM stimulation, in 1 representative case each. Stimulation using HB57-dex induces telomerase activity in U-CLL cases, but not in M-CLL cases. (D) Pooled data for fold increase in telomerase activity within HB57-dex cultures, relative to control in 17 U-CLL (left) and 17 M-CLL cases (right). U-CLL showed a significantly higher fold change in telomerase activity than M-CLL cases (P < .001). (E) Pharmacologic inhibition of telomerase activity induced by BCR stimuli: B cells from 10 U-CLL cases were subjected to pretreatment with inhibitors of PI3K/Akt, Erk, or p38MAP kinase and then activated using HB57-dex for 3 days. Cells were harvested and cell lysates subjected to quantification of telomerase activity. Data represent percent inhibition of the HB57-dex-mediated telomerase activity as elicited by the inhibitors. (F) Effect of preincubation with Akt-specific inhibitor on HB57-dex-induced hTERT phosphorylation (upper panel) in 3 U-CLL cases (U1, U2, and U3) and 3 M-CLL cases (M1, M2, and M3). The inhibitor exerts a dose-dependent effect on phosphorylation hTERT. Lower panel: Functional telomerase activity in parallel cultures.
Spontaneous and BCR-induced changes in telomerase activity in CLL cases. Purified B cells from 6 U-CLL and 6 M-CLL cases were cultured without or with BCR stimulation. (A) Spontaneous decrease in telomerase activity within residual live cells at day 1, 2, and 3 of culture in U-CLL (left) and M-CLL (right) cases. (B) Telomerase activity assessed in cell extracts prepared from uncultured cells (A1 and A2), cells cultured in medium alone (B1 and B2), or cells cultured with HB57-dex for 3 days (C1 and C2). A1, B1, and C1 represent heat-treated internal controls for A2, B2, and C2, respectively. The representative autoradiograph on the left (U-CLL case) shows induction/retention of telomerase activity, whereas the M-CLL case on the right does not. (C) Change in telomerase activity over time, elicited by anti-IgM stimulation, in 1 representative case each. Stimulation using HB57-dex induces telomerase activity in U-CLL cases, but not in M-CLL cases. (D) Pooled data for fold increase in telomerase activity within HB57-dex cultures, relative to control in 17 U-CLL (left) and 17 M-CLL cases (right). U-CLL showed a significantly higher fold change in telomerase activity than M-CLL cases (P < .001). (E) Pharmacologic inhibition of telomerase activity induced by BCR stimuli: B cells from 10 U-CLL cases were subjected to pretreatment with inhibitors of PI3K/Akt, Erk, or p38MAP kinase and then activated using HB57-dex for 3 days. Cells were harvested and cell lysates subjected to quantification of telomerase activity. Data represent percent inhibition of the HB57-dex-mediated telomerase activity as elicited by the inhibitors. (F) Effect of preincubation with Akt-specific inhibitor on HB57-dex-induced hTERT phosphorylation (upper panel) in 3 U-CLL cases (U1, U2, and U3) and 3 M-CLL cases (M1, M2, and M3). The inhibitor exerts a dose-dependent effect on phosphorylation hTERT. Lower panel: Functional telomerase activity in parallel cultures.
Akt activity is involved in BCR-promoted hTERTphosphorylation and telomerase activity in U-CLL
To identify the relative contributions of Akt, Erk, and p38 MAP kinase to BCR-induced telomerase activity, we blocked the activities of these signaling intermediates with pharmacologic inhibitors. Doses were used that were found by phosphoflow to efficiently block phosphorylation of specific target molecules on stimulation through the BCR. Because BCR activation predominantly facilitated telomerase activation/retention in U-CLL cells, we studied the effect of these inhibitors on BCR-mediated telomerase activation in a set of 10 U-CLL cases. Erk inhibition effectively abrogated 5%-48% of the HB57-dex induced telomerase activity in 6 of 10 cases, whereas MAPK inhibition resulted in 3%-61% abrogation in 6 of 10 cases. On the other hand, PI3K/ Akt inhibitor-LY294002 abrogated 50%-100% of the HB57-dex induced telomerase activity in 9 of 10 cases and did not show any effect in 1 of 10 cases. The efficacy of PI3K/Akt inhibition differed significantly from that seen with Erk inhibition (P < .001) and MAPK inhibition (P < .01; Figure 2E). Furthermore, in a subset of 3 U-CLL cases and 3 M-CLL cases, the selective Akt inhibitor inhibited both phosphorylation of hTERT and functional telomerase activity in a dose-dependent manner (Figure 2F), suggesting that, among Akt, Erk, and p38MAPK, Akt plays the most prominent role in telomerase induction.
BCR activation induces proliferation and rescues U-CLL cells from apoptosis
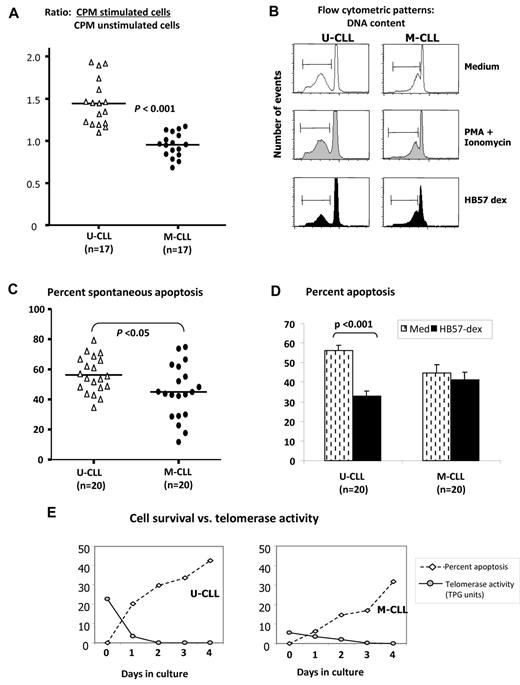
To determine the extent that enhanced telomerase activity in U-CLL was accompanied by enhanced viability and proliferation in response to BCR signals, B-CLL cells and B cells from normal subjects were cultured for 3 days with HB57-dex. Compared with the proliferative response elicited by HB57-dex in normal B cells (see Figure 6D) that observed in CLL cells is extremely low and U-CLL cells showed significantly greater stimulation indices compared with those observed for M-CLL cases (P < .001; Figure 3A).
Effects of HB57-dex on B-CLL cell proliferation and survival. (A) A total of 2 × 105 isolated B cells in triplicate from 17 U-CLL and 17 M-CLL cases were cultured for ∼ 68 hours with culture medium or with HB57-dex. Cultures were pulsed with tritiated thymidine for the last 16 hours and harvested to count incorporated radioactivity. Stimulation ratios were calculated for each case as the ratio of radioactivity incorporated in stimulated cells relative to unstimulated cells. HB57-dex elicited significantly higher proliferation in U-CLL compared with M-CLL clones (P < .001; Mann-Whitney test). Purified B-CLL cells from 20 cases each of the U-CLL and M-CLL subgroups were incubated without or with BCR stimulation or mitogenic stimulation with PMA + ionomycin for a period of 3 days. (B) DNA content indicative of sub G0 (apoptotic peak) measured by propidium iodide staining. (C) Spontaneous apoptosis in B cells from 20 U-CLL and 20 M-CLL cases was significantly higher in U-CLL. (D) HB57-dex mediated rescue from apoptosis. Bar graphs represent mean ± SE values for rescue from apoptosis 20 cases each. Significant rescue from apoptosis is observed only in HB57-dex–treated U-CLL cells. (E) Simultaneous analysis of cell survival and telomerase activity in cell extracts after 3 days in culture. There is an inverse association in representative U-CLL and M-CLL cases.
Effects of HB57-dex on B-CLL cell proliferation and survival. (A) A total of 2 × 105 isolated B cells in triplicate from 17 U-CLL and 17 M-CLL cases were cultured for ∼ 68 hours with culture medium or with HB57-dex. Cultures were pulsed with tritiated thymidine for the last 16 hours and harvested to count incorporated radioactivity. Stimulation ratios were calculated for each case as the ratio of radioactivity incorporated in stimulated cells relative to unstimulated cells. HB57-dex elicited significantly higher proliferation in U-CLL compared with M-CLL clones (P < .001; Mann-Whitney test). Purified B-CLL cells from 20 cases each of the U-CLL and M-CLL subgroups were incubated without or with BCR stimulation or mitogenic stimulation with PMA + ionomycin for a period of 3 days. (B) DNA content indicative of sub G0 (apoptotic peak) measured by propidium iodide staining. (C) Spontaneous apoptosis in B cells from 20 U-CLL and 20 M-CLL cases was significantly higher in U-CLL. (D) HB57-dex mediated rescue from apoptosis. Bar graphs represent mean ± SE values for rescue from apoptosis 20 cases each. Significant rescue from apoptosis is observed only in HB57-dex–treated U-CLL cells. (E) Simultaneous analysis of cell survival and telomerase activity in cell extracts after 3 days in culture. There is an inverse association in representative U-CLL and M-CLL cases.
Studies with B-CLL patients show that the size of a B-CLL clone in vivo is regulated by both the clonal growth and death rates.34 It was therefore of interest to examine whether U-CLL clones with heightened telomerase activity have diminished death rates. To address this, we first cultured purified B-CLL cells from 20 U-CLL cases and 20 M-CLL cases in the absence of any supplementary survival factors. Using propidium iodide fluorescence (Figure 3B), we observed higher levels of spontaneous apoptosis in U-CLL than M-CLL cases (P < .05; Figure 3C), consistent with other studies.11 Next, we analyzed the effect of BCR crosslinking by HB57-dex on levels of apoptosis within the U-CLL and M-CLL subsets over a 3-day period. Significant numbers of leukemic cells from U-CLL cases, but not M-CLL cases, were rescued from spontaneous apoptosis (P < .001; Figure 3D). To associate the activity of telomerase to the retention of viability of unstimulated cells in culture, we analyzed cell lysates taken from viable cells at incremental times over a 4-day period in culture. Figure 3E shows a representative case of the 6 analyzed from each U-CLL and M-CLL subgroup. Note the inverse association of cell survival and levels of telomerase activity.
Anti-BCR stimulation by polyclonal antibodies to IgM influences membrane-proximal and -distal events comparably in U-CLL and M-CLL cases
The form in which the antigen is displayed affects a B cell's ability to bind it via the BCR,26 and agonistic antibodies differ in their ability to bind to surface membrane IgM. The responsiveness of CLL B cells to F(ab′)2 fragments of goat anti-μ antibodies was compared with that elicited by HB57-dex by studying membrane-proximal Ca2+ flux. In both U-CLL and M-CLL cases, goat anti-μ elicited higher Ca2+ flux than that HB57-dex (both P < .01, Figure 4A, for comparison see Figure 1B). Changes in levels of phosphorylated Akt, Erk, and p38 MAP kinase elicited by goat anti-μ were also studied by phosphoflow; these antibodies elicited significantly greater phosphorylation of both Akt and Erk in U-CLL compared with M-CLL cases (Figure 4B-D).
Goat anti-μ F(ab′)2–mediated membrane-proximal events. (A) Ca2+ flux in response to goat anti-μ F(ab′)2 was assayed for 5 minutes. Scatter plots of fold change in Ca2+ flux relative to control in 16 U-CLL versus 16 M-CLL cases. (B) Scatterplots depicting fold change in phospho-Akt, phospho-Erk, and phospho-p38MAPK levels in 20 B-CLL cases divided into U-CLL and M-CLL subgroups. U-CLL and M-CLL exhibited significant differences in the extent of phospho-Akt and phospho-Erk up-regulation after exposure to goat anti-μ. (C) Comparison of efficacy of HB57-dex and goat anti-μ F(ab′)2 in eliciting protein phosphorylation of Akt, Erk, and p38 MAPK, in B cells from 20 CLL cases. Goat anti-μ elicited a stronger response in terms of Akt phosphorylations than HB57-dex did (P < .05).
Goat anti-μ F(ab′)2–mediated membrane-proximal events. (A) Ca2+ flux in response to goat anti-μ F(ab′)2 was assayed for 5 minutes. Scatter plots of fold change in Ca2+ flux relative to control in 16 U-CLL versus 16 M-CLL cases. (B) Scatterplots depicting fold change in phospho-Akt, phospho-Erk, and phospho-p38MAPK levels in 20 B-CLL cases divided into U-CLL and M-CLL subgroups. U-CLL and M-CLL exhibited significant differences in the extent of phospho-Akt and phospho-Erk up-regulation after exposure to goat anti-μ. (C) Comparison of efficacy of HB57-dex and goat anti-μ F(ab′)2 in eliciting protein phosphorylation of Akt, Erk, and p38 MAPK, in B cells from 20 CLL cases. Goat anti-μ elicited a stronger response in terms of Akt phosphorylations than HB57-dex did (P < .05).
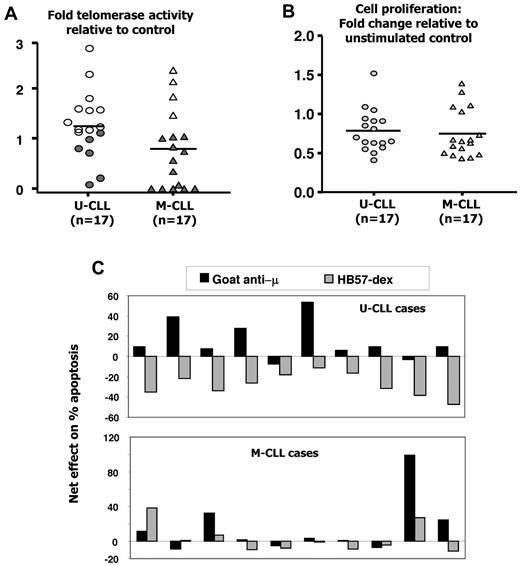
In the same CLL cases, telomerase activity increased between 1.2- and 2.8-fold in response to stimulation with goat anti-μ; however, a significantly greater proportion of U-CLL (11 of 17) cases responded than the M-CLL (4 of 17) cases (Figure 5A). Goat anti-μ induced lower proliferative responses than HB57-dex, and responsiveness did not differ between IGHV mutation-based subgroups (Figure 5B). Notably, HB57-dex rescued cells from apoptosis in 10 of 10 U-CLL cases examined, whereas the polyclonal goat anti-μ antibodies induced it in 8 of 10 instances (Figure 5C).
Effects of goat anti-μ F(ab′)2 on telomerase activity, proliferation, and survival in B-CLL cells. B-CLL cells from 17 cases each of the U-CLL and M-CLL subgroups were cultured alone or with F(ab′)2 fragments of goat anti-μ Ab. (A) Effect of BCR crosslinking on induction of telomerase activity. Four of 17 M-CLL clones (open symbols) show significant induction in telomerase activity. Fold increases in telomerase activity induced in U-CLL and M-CLL subgroups are comparable. (B) Goat anti-μ goat induced proliferative responses in 3 of 17 U-CLL and 5 of 17 M-CLL cases. (C) In a subset of 10 each of the cases studied for panel A, a comparison of the effects of the goat anti-μ F(ab′)2 with HB57-dex was performed. Note that, in 9 of 10 U-CLL cases, stimulation using HB57-dex resulted in rescue from spontaneous apoptosis, whereas 8 of 10 U-CLL cases showed an increase in apoptosis with the goat anti-μ F(ab′)2 Ab. Marginal (< 10%), insignificant rescue from apoptosis using HB57-dex was observed in 4 of 10 M-CLL cases.
Effects of goat anti-μ F(ab′)2 on telomerase activity, proliferation, and survival in B-CLL cells. B-CLL cells from 17 cases each of the U-CLL and M-CLL subgroups were cultured alone or with F(ab′)2 fragments of goat anti-μ Ab. (A) Effect of BCR crosslinking on induction of telomerase activity. Four of 17 M-CLL clones (open symbols) show significant induction in telomerase activity. Fold increases in telomerase activity induced in U-CLL and M-CLL subgroups are comparable. (B) Goat anti-μ goat induced proliferative responses in 3 of 17 U-CLL and 5 of 17 M-CLL cases. (C) In a subset of 10 each of the cases studied for panel A, a comparison of the effects of the goat anti-μ F(ab′)2 with HB57-dex was performed. Note that, in 9 of 10 U-CLL cases, stimulation using HB57-dex resulted in rescue from spontaneous apoptosis, whereas 8 of 10 U-CLL cases showed an increase in apoptosis with the goat anti-μ F(ab′)2 Ab. Marginal (< 10%), insignificant rescue from apoptosis using HB57-dex was observed in 4 of 10 M-CLL cases.
Effect of anti-BCR Abs on normal B cells
Next, we ascertained the ability of the anti-IgM reagents to elicit responses in purified B cells from normal elderly persons. Figure 6A shows increased phosphorylation of Akt, Erk, and p38 MAP kinase elicited by HB57-dex and goat anti-μ. Although both reagents were effective, in each instance, the goat anti-μ induced significantly greater phosphorylation of the 3 signaling intermediates than HB57-dex. Of note, goat anti-μ also led to phosphorylation of p38 MAP kinase, whereas, as noted for B-CLL cells, HB57-dex did not. Unlike with B-CLL cells, HB57-dex induced Ca2+ flux in normal B cells, as early as within 5 minutes of anti-BCR encounter (data not shown).
Anti-IgM–mediated outcomes in B cells from healthy donors. Comparison of efficacy of HB57-dex and goat anti-μ F(ab′)2 in eliciting protein phosphorylation of Akt, Erk, and p38 MAPK, in B cells from 6 elderly healthy donors. In each case, goat anti-μ elicited a stronger response. Differences were statistically significant as marked (A). Flow cytometric analysis of percentage of hTERT+ cells and density of hTERT expression in activated cells. B cells were cultured in the absence or presence of HB57-dex or goat anti-μ for 3 days. Comparison of fold change in percentage of hTERT+ cells indicates a higher positivity in CD5+ cells in 5 of 5 cases studied (B top panel). Each colored line connecting cell fractions indicates a single donor. Both anti-IgM agents elicited comparable changes in density of hTERT (B bottom panel). (C) Comparable induction of telomerase activity by both anti-IgM agents. (D) HB57-dex induced a significantly higher proliferative response in each of the 6 donors studied. (E) HB57-dex rescued cells from apoptosis, whereas goat anti-μ F(ab′)2 induced apoptosis in all cases studied (P < .001).
Anti-IgM–mediated outcomes in B cells from healthy donors. Comparison of efficacy of HB57-dex and goat anti-μ F(ab′)2 in eliciting protein phosphorylation of Akt, Erk, and p38 MAPK, in B cells from 6 elderly healthy donors. In each case, goat anti-μ elicited a stronger response. Differences were statistically significant as marked (A). Flow cytometric analysis of percentage of hTERT+ cells and density of hTERT expression in activated cells. B cells were cultured in the absence or presence of HB57-dex or goat anti-μ for 3 days. Comparison of fold change in percentage of hTERT+ cells indicates a higher positivity in CD5+ cells in 5 of 5 cases studied (B top panel). Each colored line connecting cell fractions indicates a single donor. Both anti-IgM agents elicited comparable changes in density of hTERT (B bottom panel). (C) Comparable induction of telomerase activity by both anti-IgM agents. (D) HB57-dex induced a significantly higher proliferative response in each of the 6 donors studied. (E) HB57-dex rescued cells from apoptosis, whereas goat anti-μ F(ab′)2 induced apoptosis in all cases studied (P < .001).
Normal B cells were also studied for induction of telomerase activity and cell proliferation and survival after 3 days of culture. Whereas both HB57-dex and goat anti-μ induced an increase in the number of hTERT+ cells in 4 of 5 normal persons (Figure 6B), neither stimulus brought about an increase in the density/amount of hTERT protein in hTERT+ normal B cells. Interestingly, among the cultured cells, CD5+ B cells from 4 of 5 healthy persons tested showed greater increase in hTERT+ cells than the CD5− B cells (Figure 6B). Finally, HB57-dex and goat anti-μ induced telomerase function to an equal degree (Figure 6C).
Although the 2 types of anti-BCR antibodies did not differ in induction of hTERT or telomerase activity, they did differ significantly in their abilities to induce cell proliferation and survival. In every case studied, HB57-dex elicited significantly higher proliferative responses than goat anti-μ (Figure 6D). In addition, HB57-dex rescued normal B cells from apoptosis in all people studied, whereas goat anti-μ induced significant levels of apoptosis in every instance (Figure 6E).
Comparative analyses reveal a positive cell survival-promoting trend in U-CLL cases stimulated by a crosslinking anti-BCR stimulus
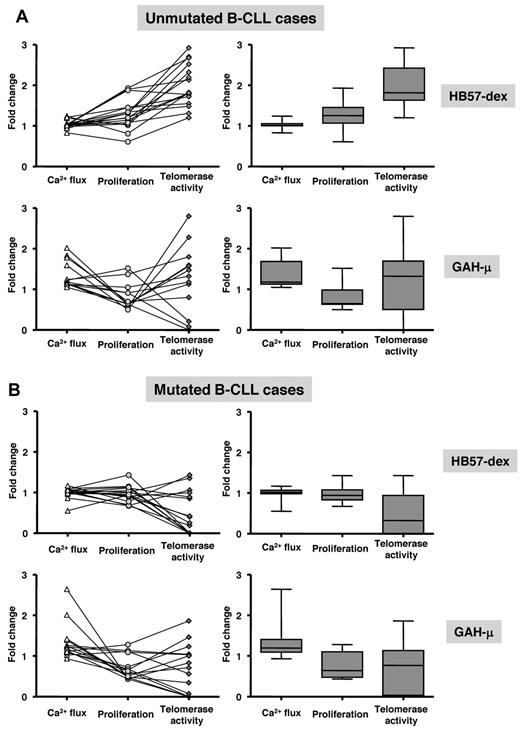
Interaction of the BCR with an agonist culminates in a series of events, which in turn govern distinct cell fates: proliferation or death. We focused on these outcomes by comparing fold changes in initial Ca2+ flux with fold change in DNA synthesis and telomerase activity. Such comparisons were made in 16 U-CLL and 16 M-CLL cases stimulated with HB57-dex and 28 cases, also divided equally between U-CLL and M-CLL, using goat anti-μ. Plots on the left side of Figure 7A and B represent data from individual cases, with lines connecting data points for the same cases; box-plots on the right represent the distribution within the sets of cases as a whole. U-CLL clones display a positive trend in responsiveness elicited by HB57-dex: the initial Ca2+ flux resulting in increased S phase entry accompanied a marked elevation in telomerase activity. On the other hand, although cell stimulation using goat anti-μ induced significant Ca2+ flux, this event did not lead to an increase in proliferation. Furthermore, induction of telomerase activity in the U-CLL cases by polyclonal anti-μ stimulation was very heterogeneous, with a marked elevation in 3 cases, marked suppression in 3, and no response in 8 of 14 cases studied (Figure 7A bottom left panel). For M-CLL, neither goat anti-μ nor HB57-dex elicited significant proliferation or telomerase activity in any of these cases (Figure 7B bottom right).
Comparison of Ca2+ flux, proliferation, and telomerase activity in individual cases. B-CLL cells from 16 U-CLL and 15 M-CLL cases were analyzed for responsiveness to HB57-dex and to goat anti-μ F(ab′)2. The left side of every panel depicts scattergrams representing fold change compared with unstimulated cells: Ca2+ flux, at 5 minutes after stimulation, and proliferation and telomerase activity after 3 days in culture. Each line connecting 3 points represents a single patient. The right half of every panel depicts box plots of the same data (on the left), indicating mean values (horizontal line inside box) and whiskers representing lowest and highest value for each parameter. The trend of responses is different for the U-CLL (A) and M-CLL (B) cases. B cells from unmutated cases show Ca2+ flux and proliferative response similar to those in the mutated cases, but their telomerase activity is significantly higher than in the mutated cases.
Comparison of Ca2+ flux, proliferation, and telomerase activity in individual cases. B-CLL cells from 16 U-CLL and 15 M-CLL cases were analyzed for responsiveness to HB57-dex and to goat anti-μ F(ab′)2. The left side of every panel depicts scattergrams representing fold change compared with unstimulated cells: Ca2+ flux, at 5 minutes after stimulation, and proliferation and telomerase activity after 3 days in culture. Each line connecting 3 points represents a single patient. The right half of every panel depicts box plots of the same data (on the left), indicating mean values (horizontal line inside box) and whiskers representing lowest and highest value for each parameter. The trend of responses is different for the U-CLL (A) and M-CLL (B) cases. B cells from unmutated cases show Ca2+ flux and proliferative response similar to those in the mutated cases, but their telomerase activity is significantly higher than in the mutated cases.
Discussion
In this study, we analyzed the effects of BCR crosslinking on induction of telomerase activity and related these findings to other more standard functional readouts of sIg signaling (survival and proliferation). The study was based on the hypotheses that a T-I antigen(s), which might not elicit a classic GC reaction, drive(s) B-CLL cells, especially in U-CLL cases,7,8 and that this drive is one of the factors accounting for clinical aggressiveness. We tested this possibility in a cohort of sIgM+ B-CLL cases (composing both U-CLL and M-CLL cases) stimulated by distinct anti-IgM Abs. For these experiments, we used surrogate antigens (anti-IgM Abs) that differ in affinity and valency. Because T-I antigens usually exhibit similar antigenic epitopes displayed repetitively along a linear backbone, we used an anti-μ mAb HB57 (Ka = 5 × 108M−1) conjugated to dextran (HB57-dex) to mimic multimeric binding of a native T-I antigen.35 Such responses were compared with those observed after stimulation with F(ab′)2 fragments of goat anti-μ antibodies (goat anti-μ), similar to those widely used as BCR agonists that activate B cells by bivalent binding. Various forms of anti-IgM reagents (soluble and bead-bound monoclonal and polyclonal Abs) have been used to study BCR responsiveness of B-CLL cells,13,36,37 although the extent that BCR stimulation contributes to the observed prolonged replicative history of the leukemic cells17 and eventually to aggressive clinical outcome remains unclear.
Initially, we ruled out the possibility that the dextran molecule used as a backbone for HB57 linkage itself delivered a signal by analyzing its ability to rescue B-CLL cells from apoptosis and promote cell proliferation (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This was not the case. Furthermore, although the number of sIgM+ cells was higher in the U-CLL cases studied (Table 1), a difference in surface receptor density was not found (supplemental Figure 2), thus differing from the findings of some.38,39
Telomerase, an enzyme that “repairs” short telomeres, acts during S-phase when these structures are replicated by conventional DNA duplication machinery.40 Among B-CLL subgroups, ex vivo telomerase activity is significantly higher in U-CLL cases.17 In addition, a recent quantitative study of hTERT transcript levels in CLL patients indicates a strong inverse correlation of levels of the full-length transcript with the percentage of IGHV mutations; thus, at both the mRNA and protein function levels, telomerase is elevated in U-CLL cases.41 We therefore sought to better understand how the efficacy of interaction with the BCR impacts telomerase activation and how downstream events are linked in B-CLL cells. Stimulation of U-CLL cells with HB57-dex consistently elevated telomerase activity and prolonged cell survival, whereas the same signal elicited minimal or no responses in M-CLL cells. This difference in responsiveness may not be solely explained by differential signaling capacities between U-CLL and M-CLL clones, as suggested previously.13,39 Indeed, using a multivalent ligand that probably simulates a higher affinity, repeated interaction of BCR with antigen the current study addresses the basis of the observed differences in BCR responsiveness in U-CLL and M-CLL cases.
Only certain biochemical inhibitors of key molecules in pathways downstream of BCR stimulation exhibited a marked effect on telomerase activity. The PI3K/Akt inhibitor LY294002 exerted a pronounced inhibitory effect, whereas the Erk inhibitor down-modulated HB57-dex–induced telomerase activity to a lesser extent (Figure 2E). A tight link between up-regulation of telomerase activity and increased cell survival was observed in individual cases; such a link provides indirect evidence for the “longevity-promoting” role played by telomerase in cell systems.42 However, this link was not as apparent for circulating B lymphocytes from normal persons. Thus, although B cells from normal donors stimulated with goat anti-μ showed greater phosphorylation of Akt, Erk, and p38 MAP kinase, rescue from spontaneous apoptosis and induction of cell proliferation were better achieved when cells were stimulated using HB57-dex. Notably, both anti-IgM agents induced comparable functional telomerase activity (Figure 7). Collectively, these data suggest that the multimeric nature of the HB57-dex stimulation is more relevant for rescue from apoptosis and induction of subsequent cell proliferation than for induction of telomerase, which is comparable to that of the goat anti-μ antibodies. Although CD5neg B cells constitute the majority of normal B cells, Tert protein expression was induced by anti-IgM cross linking in a greater proportion of CD5+ B cells in healthy persons.
Furthermore, at concentrations reported previously,39,43 goat anti-μ induced significant Ca2+ flux (Figure 4A) and phosphorylation of Akt and Erk in U-CLL cases (Figure 4B-C) but did not stimulate appreciable proliferation in most cases. Indeed, stimulation with goat anti-μ uniformly induced an increase in apoptosis of the stimulated U-CLL cells (Figure 5C). On the contrary, even at a low concentration of 0.1 μg/mL, HB57-dex elicited proliferation and rescue from apoptosis of the stimulated cells (Figure 3A-D) that originally exhibited features of G0/G1 blocked cells based on the tight G0/G1 peak in histograms of DNA content using propidium iodide (Figure 3B). Although the observed increases in cell proliferation seem negligible in vitro, such BCR-driven cell proliferation will impact noticeably in vivo on the bulk of the clone because relatively high lymphocyte counts are observed in CLL patients. In addition, if our data are representative of the level of proliferation induced in vivo, the findings suggest that the primary outcome of BCR stimulation is not proliferation but up-regulation of other processes that affect survival of CLL cells. However HB57-dex did not induce significant up-regulation of membrane proximal events. The superior efficacy of HB57-dex in providing survival and growth signals is probably the result of more prolonged receptor occupancy over the culture period, based on the multimeric structure provided by the dextran backbone. This is similar to the improved efficiency of polyclonal anti-IgM F(ab′)2 Abs to rescue B-CLL cells from apoptosis when displayed on beads.13 Comparative studies conducted to differentiate responsiveness of B-CLL stimulated with goat anti-μ in soluble versus immobilized forms have shown that the soluble form leads to decreased metabolic mitochondrial activity and therefore a proapoptotic process.43
BCR reactivity with autoantigens is a relatively common feature of B-CLL cells,44-48 consistent with some CLL clones, primarily of the U-CLL subgroup, deriving from progenitor cells evolutionarily adapted to recognize and react to particular (auto)antigenic epitopes.49 Cell proliferation without dangerous erosion of telomeres could occur if in vivo repeated T-I stimulations of B-CLL cells and their precursors by restricted sets of self-antigens in the microenvironment up-regulated telomerase; sustained signaling through the BCR would probably be especially effective at promoting survival and expansion of B-CLL cells, as occurs in vitro (Figure 3A,D).13 In murine B-1 cell systems, BCR signaling is influenced by locale,50 as self-antigen recognition in specific microenvironments can confer a form of anergy on activated B cells. In addition, partially or completely anergized B-CLL cells from either subset can recover both sIgM expression and signal-transducing capacity spontaneously in vitro or after capping/endocytosis, providing indirect evidence for engagement of putative antigen in vivo.39
Thus, this study indicates that T-I BCR-mediated signaling of B-CLL induces telomerase activity and promotes cell survival, apparently in the absence of major cell proliferation, preferentially in U-CLL cases. Up-regulated telomerase may link responsiveness to BCR stimulation and longevity of the clinically aggressive clonal cells from U-CLL. Such a connection between survival and T-I-induced telomerase activity was not found to exist in most M-CLL cases (Figures 2D and 3D); therefore, B cells from M-CLL cases might need other costimulatory (T cell–dependent?) interactions to promote cell survival and up-regulate telomerase's actions. Taken together, the present studies indicate that the quality (eg, valency, affinity) of antigen encounter with the BCR determines cell fate and longevity in CLL and may shed light on the observed differences in the pathophysiology of subgroups of CLL cases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Cancer Institute/National Institutes of Health (CA81554) and by philanthropic contributions from the Karches Foundation, Prince Family Foundation, Marks Foundation, Jerome Levy Foundation, Leon Levy Foundation, the Tebil Foundation Inc, Andrew and Mona Albert Fund, Inc, and Joseph Eletto Leukemia Research Fund.
National Institutes of Health
Authorship
Contribution: R.N.D. planned the project outline, designed and conducted experiments, analyzed data, and wrote the manuscript; S.T. and T.B. conducted experiments; S.P. provided dextran conjugated mAb; P.K.A.M. provided dextran-conjugated mAb and reviewed manuscript; S.L.A., J.E.K., and K.R.R. examined CLL patients, provided clinical material, and reviewed the manuscript; and N.C. discussed the project outline and suggested editorial changes to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.P. is Department of Botany, Gurudas College, Kolkata, West Bengal, India.
Correspondence: Rajendra N. Damle, Feinstein Institute for Medical Research, 350 Community Dr, Manhasset, NY 11030; e-mail: rdamle@nshs.edu.

![Figure 1. Anti-IgM (HB57 dex)–mediated membrane proximal events. (A) Induction of Ca2+ flux by B-CLL cells. Ratiometric analysis of Indo-1 fluorescence in its Ca-bound to free state indicative of intracellular [Ca2+] was performed over a 15-minute period after activation with various reagents: PMA + ionomycin, goat anti–human-μ, or HB57dex. In all experiments, the ratio of Ca2+ mobilization in response to a signal to unstimulated was calculated over a 5-minute period (vertical dotted line). The figure shows representative tracings from 1 B-CLL case included in optimization studies. (B) Fold induction of Ca2+ flux by BCR stimulation using HB57-dex compared with baseline levels in 16 U-CLL and 15 M-CLL patients. Baseline levels were established by acquiring data from unstimulated cells for 30 seconds before addition of the stimulants. Statistical significance of the comparisons was evaluated using the paired t test. Differences were not significant. (C-E) BCR-mediated phosphorylation of Akt, Erk, and MAPK. Fold change in mean fluorescence intensity acquired by flow cytometry elicited by HB57-dex over unstimulated controls in all cases (2 scattergrams on the left); scattergrams on the right represent values obtained in cases segregated by IGHV gene mutations. (C) pAkt. (D) pErk. (E) p-p38MAPK. Statistical significance of the comparisons was evaluated using the paired t test. Differences were not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/12/10.1182_blood-2012-02-409110/4/m_zh89991296720001.jpeg?Expires=1767827191&Signature=tknbSu9HfUtHNFfUqVW0kkuYYmrXrvLXcUvSlGrTrrAS3A8TdRaHTzi3iEGjPEjKVKS2l0BprAUW6e-zRbm2mnE8x930GXCEIN-Sf4uhOWTbV4PUCUOi9ghKJr1JJ31HMdoZfzO2EMembbVhzlHtKKG-xeEWuqk-ReOrhlJhONldX3Dun3cmpM~byfIB0F9-v6snV0wthPgvOMOuQA~8NbYdIC5h18-Ot~4JKZeOgdw-1wEN7c3HRsNBx-uhTM0qrA4gvaIcF2Vm4gLF-9AnjegjMLQHtOBe3O3cDUJ5aMckUDH5uOVU6qjEW-j2rIHRKf5YisYFOCDXYCJQaHo3RQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal