Abstract
The platelet release reaction plays a critical role in thrombosis and contributes to the events that follow hemostasis. Previous studies have shown that platelet secretion is mediated by Soluble NSF Attachment Protein Receptor (SNARE) proteins from granule and plasma membranes. The SNAREs form transmembrane complexes that mediate membrane fusion and granule cargo release. Although VAMP-8 (v-SNARE) and SNAP-23 (a t-SNARE class) are important for platelet secretion, the identity of the functional syntaxin (another t-SNARE class) has been controversial. Previous studies using anti-syntaxin Abs in permeabilized platelets have suggested roles for both syntaxin-2 and syntaxin-4. In the present study, we tested these conclusions using platelets from syntaxin-knockout mouse strains and from a Familial Hemophagocytic Lymphohistiocytosis type 4 (FHL4) patient. Platelets from syntaxin-2 and syntaxin-4 single- or double-knockout mice had no secretion defect. Platelets from a FHL4 patient deficient in syntaxin-11 had a robust defect in agonist-induced secretion although their morphology, activation, and cargo levels appeared normal. Semiquantitative Western blotting showed that syntaxin-11 is the more abundant syntaxin in both human and murine platelets. Coimmunoprecipitation experiments showed that syntaxin-11 can form SNARE complexes with both VAMP-8 and SNAP-23. The results of the present study indicate that syntaxin-11, but not syntaxin-2 or syntaxin-4, is required for platelet exocytosis.
Introduction
Platelets are important for hemostasis because they respond to vascular damage by secreting components that promote thrombosis and its sequelae. Hypoactive secretion leads to bleeding,1-4 whereas hyperactive platelet secretion may be linked to increased thrombosis. Agonists exposed at a damaged site initiate platelet adhesion and activation. Platelet activation is marked by a rapid increase in the intracellular Ca2+ concentration ([Ca2+]i), which triggers release from 3 granular stores: dense granules, α-granules, and lysosomes.5,6 Dense granules contain small molecules and ions such as ADP, serotonin, and Ca2+, which are important for thrombogenesis.7 α-ganules are the most abundant granules in platelets (40-60/platelet) and their cargos are diverse, ranging from growth factors (eg, PDGF) and chemokines (eg, platelet factor 4 [PF4]) to adhesive molecules (eg, VWF and fibrinogen).8 These factors are not only important for clot stabilization, but also play roles in wound repair. Release of lysosomal cargo (eg, β-hexosaminidase) is thought to be involved in clot remodeling.5,6 Given its central role, the platelet secretory machinery could be a valuable target for antithrombotic therapy.
As with other regulated secretory systems, platelet exocytosis is mediated by Soluble NSF Attachment Protein Receptors (SNAREs) present on the granule/vesicle membranes (v-SNAREs) and on plasma/target membranes (t-SNAREs). Cognate v-SNAREs and t-SNAREs interact to form a trans-bilayer complex that juxtaposes granule and plasma membranes and is required for membrane fusion.9,10 Platelets express numerous v-SNAREs and t-SNAREs. The v-SNAREs VAMP-2, VAMP-3, VAMP-7, and VAMP-8 are readily detectible, but only VAMP-8 is required for granule release.11 Deletion of VAMP-3 and/or reduction of VAMP-2 has little effect on release. Deletion of VAMP-8 does not ablate release completely, but the combination of VAMP-8 deletion and inactivation of VAMP-2 and VAMP-3 eliminates secretion from permeabilized platelets completely. These data suggest that there is a hierarchy of VAMP usage in platelets, with VAMP-8 being the primary v-SNARE and VAMP-2, VAMP-3, or both mediating a less efficient fusion reaction in the absence of VAMP-8. Using VAMP-8–specific Fab fragments and recombinant tetanus toxin catalytic light chain, studies have shown that mouse and human platelets have similar patterns of VAMP usage (Q. Ren, S.W.W., unpublished data, 2008 and Schraw et al12 ). Two classes of t-SNAREs are present in platelets, SNAP-23/25 and syntaxins. SNAP-23 appears to be the dominant member of its family in platelets by mass.13,14 Studies in permeabilized platelets using inhibitory peptides and specific Fab Ab fragments have indicated that SNAP-23 is important for release from all 3 granule populations.13,15,16
Several syntaxins (syntaxin-2, syntaxin-4, syntaxin-7, and syntaxin-11) have been reported to be present in platelets.13,15,17,18 The identity of the functionally relevant syntaxin isoforms was initially defined using anti-syntaxin Abs as inhibitors in permeabilized platelets. Data from our laboratory and others suggested that syntaxin-2 is important for release from all 3 granule types, whereas syntaxin-4 plays a role in α-granule and lysosome release.13,15,16,19 A polyclonal Ab against syntaxin-2 (reexamined in the present study) inhibited release from all 3 stores when added to permeabilized human platelets.13,15,16 An anti–syntaxin-4 mAb only affected α-granule and lysosome release.15,16 Abs to syntaxin-7 were without effect.16 Whereas these data suggested the potential roles for syntaxin-2 and syntaxin-4 in platelet exocytosis, there were some inconsistencies. The specificity of the syntaxin-2 Ab was not defined completely. An additional anti–syntaxin-4 polyclonal Ab had no effect on release (P. Lemons, S.W.W., unpublished data, 2000). In the present study, we reexamined the conclusions from the original work by measuring platelet secretion from mouse strains in which syntaxins have been deleted and from human platelets that lack syntaxin-11. Deletion of syntaxin-2 or syntaxin-4 alone or in combination did not inhibit secretion from murine platelets. Platelet secretion from a patient with Familial Hemophagocytic Lymphohistiocytosis Type 4 (FHL4) lacking syntaxin-11 was severely defective. In contrast to previous studies,13,15,16,19 the results of the present study show that syntaxin-2 and syntaxin-4 are not required for release, but that syntaxin-11 is critical for platelet exocytosis.
Methods
Abs and reagents
Abs recognizing the indicated proteins were purchased from the following sources: syntaxin-11 and Munc18a were from SYnaptic SYstems; syntaxin-4 (clone 49) was from Transduction Laboratories; Munc18b was from Santa Cruz Biotechnology; LAMP-1 was from the Developmental Studies Hybridoma Bank (Iowa City, IA); PF4 was from R&D Systems; integrin β3 was from Cell Signaling Technology; and fibrinogen was from Innovative Research. Abs to syntaxin-2, syntaxin-4, syntaxin-7, SNAP-23, VAMP-8, Munc18c, Munc18a/b, RabGDI, synaptobrevin/VAMP-2, VAMP-7, endobrevin/VAMP-8, cellubrevin/VAMP-3, and Munc13-4 were as described previously.11,13,20 FITC-labeled anti–human P-selectin, anti-PECAM1, and anti–mouse P-selectin were from BD Pharmingen. FITC-conjugated PAC-1 was from BD Biosciences. FITC-conjugated anti–mouse GPVI, anti-GPIbβ, and PE-conjugated anti-GPIIb/IIIa were from Emfret Analytics. Alkaline phosphatase-conjugated anti-IgGs were from Sigma-Aldrich.
Transgenic mouse strains
Epimorphin/Syntaxin-2−/− mice on a C57/BL6 background were kindly provided by Dr Deborah C. Rubin (Washington University, St Louis, MO) and genotyped as described in Wang et al.21 Syntaxin-4flox/flox mice on a C57/BL6 background were generated by Ozgene by insertion of LoxP sites in introns 1 and 4, resulting in the deletion of the syntaxin-4 exons 2-4 by Cre-recombinase. The following primers were used for identification of the LoxP sites or the neomycin resistance gene, respectively: forward primer, 5′-GTTGAGGCAGGTGAGAAACC-3′ and reverse primer, 5′-ATCACCAAGAGGGTGAAGA-3′, forward primer, 5′-AGCCCAGTTGCTGGTGTATC-3′ and reverse primer, 5′-AGGAGGAAGAGGTGGAGGAG-3′. PF4-Cre+ mice were kindly provided by Dr Radek Skoda (University Hospital, Basel, Switzerland) and were genotyped as in Tiedt et al.22 Syntaxin-2/4 double-deletion mice were generated by crossing 3 strains: syntaxin-2 deletion mice, Syntaxin-4flox/flox mice, and PF4-Cre+ mice. All animal work was approved by the University of Kentucky Institutional Animal Care and Use Committee.
Syntaxin-11–deficient platelets from a FHL4 patient
The patient's genotype was determined by standard sequencing methods: syntaxin-11 allele 1-173T > C (D58R) and allele 2-173T > C (D58R). All procedures were approved by the institutional review boards at the Cincinnati Children's Hospital and the University of Kentucky.
Measurement of secretion from intact platelets
Blood collection, preparation of mouse or human platelets, and secretion assays were as described previously.11,13,15,16 Briefly, human platelets were prepared from blood drawn using ACD as an anticoagulant. Washed platelets were labeled with 0.4 μCi/mL [3H]-5-HT (serotonin; PerkinElmer Life Sciences) for 1 hour at 37°C. After washing with HEPES/Tyrode buffer (10mM HEPES/NaOH, pH 7.4, 5.56mM glucose, 137mM NaCl, 12mM NaHCO3, 2.7mM KCl, 0.36mM KH2PO4, and 1mM MgCl2) in the presence of 3 μg/mL of apyrase, the platelets were resuspended in HEPES/Tyrode buffer. Platelet concentrations were adjusted to 2.5 × 108/mL and a final concentration of 0.7mM CaCl2 was added before stimulation. For titration experiments, the indicated concentrations of thrombin (Chrono-Log) were added and the reactions were stopped with a 2-fold excess of hirudin (Sigma-Aldrich). For the time-course experiments, 0.05 U/mL of thrombin was added for the indicated time periods and the reactions were stopped with hirudin. Supernatants and pellets were recovered after centrifugation at 16 430g for 1 minute and the pellets were lysed with an equal volume of lysis buffer (PBS, pH 7.4, plus 1% Triton X-100) for 1 hour on ice. Equal volumes of the supernatant and the pellet were assayed for the 3 granule cargo markers: [3H]-5-HT for dense core granules, PF4 for α-granules, and β-hexosaminidase for lysosomes, as described previously.11,13,15,16
Flow cytometric analysis
Mouse or human platelets (2 × 106) were incubated with the indicated FITC-conjugated or PE-conjugated Abs (5 μL) for 15 minutes. Platelets were stimulated with thrombin (0.1 U/mL) for 1 minute and the reactions were stopped with a 2-fold excess of hirudin. Finally, the platelets were diluted 10-fold with HEPES/Tyrode buffer (pH 6.5). The samples were transferred to tubes and fluorescent intensities were measured using a FACScan flow cytometer and analyzed using CellQuest (BD Biosciences).
Platelet aggregation and ADP/ATP release
Mouse or human platelets (2.5 × 108/mL) were recalcified with 0.7mM CaCl2, placed into siliconized cuvettes, and stirred for 5 minutes at 37°C at 137g. Luciferin-luciferase substrate was added to the platelet samples followed by the indicated agonists and the percentage change in light transmission was measured; 100% refers to the transmittance of the blank. Aggregation and ADP/ATP secretion were monitored using a model 460VS Lumi-Dual Aggregometer and traces were acquired using a Model 810 Aggro/Link interface with Aggro/Link rev. 5.1.5 software (Chrono-Log).
Ultrastructure analysis
Washed human or mouse platelets were either kept resting or stimulated with 0.1 U/mL of thrombin for 3 minutes. The platelets were then processed for electron microscopy, as described previously with slight modifications.11,13 Briefly, equal volumes of 0.1% glutaraldehyde in White saline were added to the platelet suspension for 15 minutes at 37°C. The platelets were centrifuged and incubated in ice-cold 3% glutaraldehyde in White saline at 4°C for 1 hour. After 3 washes, the platelets were incubated with 1% OsO4. Osmicated platelets were washed twice and dehydrated with a series of ethanol solutions. The platelets were rinsed twice with propylene oxide and infiltrated overnight in a 1:1 mixture of propylene oxide and Spurr resin (10 g of vinyl cyclohexane dioxide, 6 g of DER epoxy resin, and 26 g of nonenyl succine anhydride with a final addition of 0.4 g of dimethylaminoethanol). After several washes in pure Spurr resin, samples were embedded in 150 μL of Spurr resin and polymerized at 60°C for 48 hours. Polymerized blocks were sectioned (70nM) and mounted on copper grids. Samples were examined using a Philips Tecnai 12 transmission electron microscope (FEI) and images were obtained with Gatan Digital Micrograph Version 1.83.842 software.
Quantification of platelet proteins
Platelet proteins were separated on SDS-PAGE gels and transferred electrophoretically to Immobilon-P membranes (Millipore). The blots were incubated with different dilutions of primary and secondary Abs. Ab binding was detected using enhanced chemifluorescence (ECF; GE Healthcare). Membranes were scanned with Typhoon 9400 Imager and quantified with ImageQuant Version 5.2 software (GE Healthcare). Recombinant proteins were used to generate the standard curves for quantification of the endogenous platelet proteins.
Immunoprecipitation of platelet proteins
Immunoprecipitations were carried out as described previously.23 Washed platelets were lysed by adding equal volumes of 2 × Lysis buffer (100mM HEPES/KOH, pH 7.4, 2% Triton X-100, 2mM EGTA, 2mM EDTA, 150mM NaCl, 2mM Na3VO4, phosphatase inhibitor cocktail, and protease inhibitor cocktail) on ice for 30 minutes. The lysates were clarified by centrifugation and the supernatants were precleared with rabbit IgG and protein A Sepharose (GE Healthcare). SNARE complexes were immunoprecipitated with anti–syntaxin-11 Ab, followed by incubation with protein A Sepharose. The immunoprecipitates were recovered by centrifugation, washed 3 times with lysis buffer, and the bound complexes were analyzed by SDS-PAGE and Western blotting.
Results
Syntaxin-2 and/or syntaxin-4 is not required for platelet secretion
Previous studies using anti-syntaxin Abs added to streptolysin O–permeabilized, human platelets suggested roles for syntaxin-2 and syntaxin-4 in platelet exocytosis. A polyclonal syntaxin-2 Ab inhibited all 3 granule release events.13,15,16 A mAb (clone 49) to syntaxin-4 blocked α-granule and lysosome secretion.13,15,19 Inconsistently, a polyclonal Ab against syntaxin-4 had no effect (P. Lemons, S.W.W., unpublished data, 2000). Given the ambiguities of using Abs (eg, cross-reactivity, nonspecific steric effects, and preparation contaminants), in the present study, we reexamined the conclusions of the previous work using genetic manipulation of syntaxin-2 and syntaxin-4 in murine platelets.
We first examined the secretion phenotype of platelets from mice lacking epimorphin/syntaxin-2.21 Immunoblotting confirmed the absence of syntaxin-2 and that none of the other 9 proteins examined was affected (Figure 1A; on longer exposures, a faint band was detected, see discussion of Ab specificity). There were no overt changes in any of the remaining syntaxins. Platelet counts and surface expression of GPIIb/IIIa, GPIbβ, GPVI, and PECAM-1 were normal compared with platelets from wild-type littermates (data not shown). Syntaxin-2−/− platelet morphology was unremarkable by electron microscopy (data not shown). To assess the efficiency of release, time-course experiments (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and thrombin dose-response experiments (Figure 1B) were performed and [3H]-serotonin, PF4, and β-hexosaminidase appearance in the releasate were measured. Release of these granule markers from the syntaxin-2−/− platelets was identical to that from wild-type platelets. Similarly, there was no deficit in ATP/ADP release as measured by lumi-aggregometry, P-selectin, or LAMP-1 exposure as measured by FACS (supplemental Figure 2A-D,F). Consistent with this phenotype, there was no defect in thrombin-induced aggregation or clot retraction (supplemental Figure 2E and data not shown). These data demonstrate that syntaxin-2 is not singularly required for platelet exocytosis.
Deletion of syntaxin-2 (Stx2) or syntaxin-4 (Stx4) has no effect on platelet secretion. Platelet extracts (5.0 × 107 platelets/lane) were prepared from wild type (Wt) and syntaxin-2–knockout (Stx2 KO; A) or syntaxin-4–knockout (Stx4 KO; C) mice and the indicated proteins were detected by Western blotting. [3H]-serotonin–labeled platelets from Stx2 KO (B) or Stx4 KO (D; gray circle symbols) or Wt (black square symbols) were prepared as described in “Methods” and were stimulated with the indicated thrombin concentrations for 1 minute. Release of [3H]-serotonin from dense granules, PF4 from α-granules, and β-hexosaminidase from lysosomes was measured and the percentage of secretion was calculated. The data are the averages of triplicate measurements with the SD indicated.
Deletion of syntaxin-2 (Stx2) or syntaxin-4 (Stx4) has no effect on platelet secretion. Platelet extracts (5.0 × 107 platelets/lane) were prepared from wild type (Wt) and syntaxin-2–knockout (Stx2 KO; A) or syntaxin-4–knockout (Stx4 KO; C) mice and the indicated proteins were detected by Western blotting. [3H]-serotonin–labeled platelets from Stx2 KO (B) or Stx4 KO (D; gray circle symbols) or Wt (black square symbols) were prepared as described in “Methods” and were stimulated with the indicated thrombin concentrations for 1 minute. Release of [3H]-serotonin from dense granules, PF4 from α-granules, and β-hexosaminidase from lysosomes was measured and the percentage of secretion was calculated. The data are the averages of triplicate measurements with the SD indicated.
Deletion of both syntaxin-2 and syntaxin-4 (Stx2/4) does not inhibit platelet secretion. (A) Platelet extracts (5.0 × 107 platelets/lane) were prepared from wild-type (Wt) and syntaxin-2 and syntaxin-4 double-knockout (Stx2/4 DKO) mice and the indicated proteins were detected by Western blotting. [3H]-serotonin–labeled platelets from Stx2/4 DKO (gray circle symbols) and Wt (black square symbols) were prepared as described in “Methods.” Release of [3H]-serotonin from dense granules, PF4 from α-granules, and β-hexosaminidase from lysosomes was measured as a thrombin-response curve (1-minute stimulation; B) or as a time course (0.05 U/mL of thrombin; C) and calculated as described in the legend to Figure 1.
Deletion of both syntaxin-2 and syntaxin-4 (Stx2/4) does not inhibit platelet secretion. (A) Platelet extracts (5.0 × 107 platelets/lane) were prepared from wild-type (Wt) and syntaxin-2 and syntaxin-4 double-knockout (Stx2/4 DKO) mice and the indicated proteins were detected by Western blotting. [3H]-serotonin–labeled platelets from Stx2/4 DKO (gray circle symbols) and Wt (black square symbols) were prepared as described in “Methods.” Release of [3H]-serotonin from dense granules, PF4 from α-granules, and β-hexosaminidase from lysosomes was measured as a thrombin-response curve (1-minute stimulation; B) or as a time course (0.05 U/mL of thrombin; C) and calculated as described in the legend to Figure 1.
We next examined the role of syntaxin-4. Global deletion of syntaxin-4 causes embryonic lethality24 ; therefore, a platelet-specific deletion strain was generated by crossing a strain containing a floxed syntaxin-4 gene with a strain carrying a Cre-recombinase transgene under the control of the PF4 promoter.22 The resulting syntaxin-4flox/flox/PF4-Cre+ strain (Stx4 KO) was used as a source of platelets for analysis. Immunoblotting confirmed the absence of syntaxin-4 and that there were no changes in the remaining syntaxins (Figure 1C). Of the secretory machinery examined, only the syntaxin-4 chaperone Munc18c was reduced. This coordinate expression has been noted for other syntaxin/Munc18 pairs.25 Platelet counts, surface expression of GPIIb/IIIa, GPIbβ, GPVI, and PECAM-1, and morphology were normal compared with non-Cre–expressing controls (data not shown). [3H]-serotonin, PF4, and β-hexosaminidase release were almost identical to that from non-Cre–expressing, control platelets (Figure 1D and supplemental Figure 1B). There were no defects in ATP/ADP release, P-selectin, or LAMP-1 exposure and no deficit in thrombin-induced aggregation or clot retraction (supplemental Figure 2). These data demonstrate that syntaxin-4 is not singularly required for platelet exocytosis.
These analyses suggested that neither syntaxin-2 nor syntaxin-4 alone is required for release. One possible explanation is that the 2 syntaxins are mutually compensatory. To address this point, we crossed the 2 strains to generate animals that lack syntaxin-2 and syntaxin-4 in their platelets. Immunoblotting confirmed the absence of both syntaxin-2 and syntaxin-4, whereas the other syntaxins appeared unchanged (Figure 2A). As expected, there was a decrease in Munc18c, but the other elements of the secretory machinery were unaffected. Platelet counts were significantly lower in these animals (approximately 25%), but surface expression of GPIIb/IIIa, GPIbβ, GPVI, and PECAM-1 were normal, as was their morphology (data not shown). Release of [3H]-serotonin, PF4, or β-hexosaminidase was not inhibited by the loss of the 2 syntaxins (Figure 2B-C); in fact, α-granule release was slightly enhanced. There were no defects in ATP/ADP release, P-selectin, or LAMP-1 exposure and no deficit in thrombin-induced aggregation (supplemental Figure 2). None of the 3 strains examined showed any defect in cargo sorting and all had normal levels of the 3 cargo molecules that are monitored in the secretion studies (data not shown). From this complete analysis of the 3 syntaxin-deficient strains, mouse platelet secretion does not require syntaxin-2 or syntaxin-4 either alone or in combination.
Syntaxin-11 is an abundant t-SNARE in human and murine platelets
The data described in the previous section clearly conflict with previous reports.13,15,16,19 To resolve these discrepancies, we undertook 2 approaches. The first was to reexamine some of the previously used Abs, specifically the polyclonal Ab directed to syntaxin-2. Second, because our previous data suggested that the more abundant platelet SNAREs are required for secretion,11 we measured the levels of the known syntaxins in platelets by quantitative Western blotting.
Previous studies showed that syntaxin-2, syntaxin-4, syntaxin-7, and syntaxin-11 were present in platelets,13,15,17,18 and the Western blots in the present study confirmed this. Recombinant syntaxins were used to assess Ab specificity and to generate standard curves for quantitative Western blotting using ECF. ECF coupled with the Typhoon Imager system offers a more linear response curve with a greater range than do other methods (eg, enhanced chemiluminescence). Increasing concentrations of purified recombinant syntaxins were subjected to Western blotting analysis using the “syntaxin-specific Abs” (Figure 3A). Whereas the anti–syntaxin-4, anti–syntaxin-7, and anti–syntaxin-11 reagents only detected the appropriate syntaxins, the anti–syntaxin-2 reagent showed detectible cross-reactivity with syntaxin-4 and syntaxin-11. Further analysis showed that this anti–syntaxin-2 Ab could immunoprecipitate syntaxin-11 (Figure 3B). Because this was the Ab used in the original studies,13,15,16 it seems plausible that its inhibitory effect could be a consequence of this cross-reactivity; this point was not addressed directly in the present study.
Syntaxin-11 is recognized by the syntaxin-2 polyclonal Ab. (A) Increasing amounts (2.5, 10, and 20 ng) of recombinant His6-tagged cytosolic domain of human syntaxin-2 (rStx2, lanes 1-3), human syntaxin-4 (rStx4, lanes 4-6), human syntaxin-7 (rStx7, lanes 7-9), and GST-tagged human syntaxin-11 (rStx11, lanes 10-12) were separated by SDS-PAGE and probed by Western blotting with the indicated Abs. (B) Human platelet extract was incubated with syntaxin-2–conjugated Sepharose beads. The specific bound proteins were eluted and subjected to Western blotting using anti–syntaxin-11 Ab. No Ab (−IgG) and nonspecific IgG (+IgG) controls were included.
Syntaxin-11 is recognized by the syntaxin-2 polyclonal Ab. (A) Increasing amounts (2.5, 10, and 20 ng) of recombinant His6-tagged cytosolic domain of human syntaxin-2 (rStx2, lanes 1-3), human syntaxin-4 (rStx4, lanes 4-6), human syntaxin-7 (rStx7, lanes 7-9), and GST-tagged human syntaxin-11 (rStx11, lanes 10-12) were separated by SDS-PAGE and probed by Western blotting with the indicated Abs. (B) Human platelet extract was incubated with syntaxin-2–conjugated Sepharose beads. The specific bound proteins were eluted and subjected to Western blotting using anti–syntaxin-11 Ab. No Ab (−IgG) and nonspecific IgG (+IgG) controls were included.
Using the Abs and the recombinant proteins, we used quantitative Western blotting analysis to measure the levels of the individual syntaxins. Given the specificity of the syntaxin-4, syntaxin-7, and syntaxin-11 reagents, these measurements should be valid and, in fact, because of the cross-reactivity of the anti–syntaxin-2 Ab, our measurements are likely an overestimation. Syntaxin-2 and syntaxin-7 levels were comparable in human and murine platelets (Table 1). Syntaxin-4 levels were higher in human platelets. The similar species-specific difference was seen for VAMP-2, which is higher in murine platelets.7 Syntaxin-11 levels were significantly higher than the other syntaxins in both human and murine platelets. Syntaxin-11 was approximately 6-fold more abundant than syntaxin-4 and approximately 3-fold more than SNAP-23 in human platelets. In murine platelets, syntaxin-11 was approximately 9-fold more abundant than any of the other t-SNAREs examined. Comparing the levels of syntaxin-11 and SNAP-23 with previously published measurements of the v-SNAREs,7 it is clear that t-SNAREs are in excess, even over the primary v-SNARE, VAMP-8. These data suggest that syntaxin-11 could be the functionally relevant syntaxin t-SNARE in platelets.
SNAREs in human and murine platelets
| SNARE . | Molecules/platelet . | |
|---|---|---|
| Human . | Murine . | |
| t-SNAREs | ||
| Syntaxin-2 | 1579 ± 429 | 1766 ± 121 |
| Syntaxin-4 | 9633 ± 75 | 1625 ± 184 |
| Syntaxin-7 | 3861 ± 564 | 3707 ± 372 |
| Syntaxin-11 | 51 717 ± 5459 | 46 966 ± 5516 |
| SNAP-23 | 19 374 ± 5384 | 5363 ± 798 |
| v-SNAREs | ||
| VAMP-2* | < 806 | 7629 ± 89 |
| VAMP-3* | 4588 ± 658 | 2197 ± 120 |
| VAMP-7* | 3766 ± 696 | 2712 ± 230 |
| VAMP-8* | 6590 ± 370 | 8360 ± 110 |
| SNARE . | Molecules/platelet . | |
|---|---|---|
| Human . | Murine . | |
| t-SNAREs | ||
| Syntaxin-2 | 1579 ± 429 | 1766 ± 121 |
| Syntaxin-4 | 9633 ± 75 | 1625 ± 184 |
| Syntaxin-7 | 3861 ± 564 | 3707 ± 372 |
| Syntaxin-11 | 51 717 ± 5459 | 46 966 ± 5516 |
| SNAP-23 | 19 374 ± 5384 | 5363 ± 798 |
| v-SNAREs | ||
| VAMP-2* | < 806 | 7629 ± 89 |
| VAMP-3* | 4588 ± 658 | 2197 ± 120 |
| VAMP-7* | 3766 ± 696 | 2712 ± 230 |
| VAMP-8* | 6590 ± 370 | 8360 ± 110 |
The number of molecules of the indicated SNAREs per platelet was determined by quantitative Western blotting using recombinant proteins to generate a standard curve. All measurements were made at least 3 times. The mass, in nanograms per 5 × 107 platelets, was determined based on comparison with the standard curves of recombinant proteins. This data were used to calculate the number of molecules per platelet using the molecular weight for SNAP-23 (23 kDa), Syntaxin-2 (33 kDa), Syntaxin-4 (34 kDa), Syntaxin-7 (30 kDa), and Syntaxin-11 (33 kDa).
Number of VAMP molecules per platelet from Graham et al.7
Syntaxin-11 is required for platelet secretion
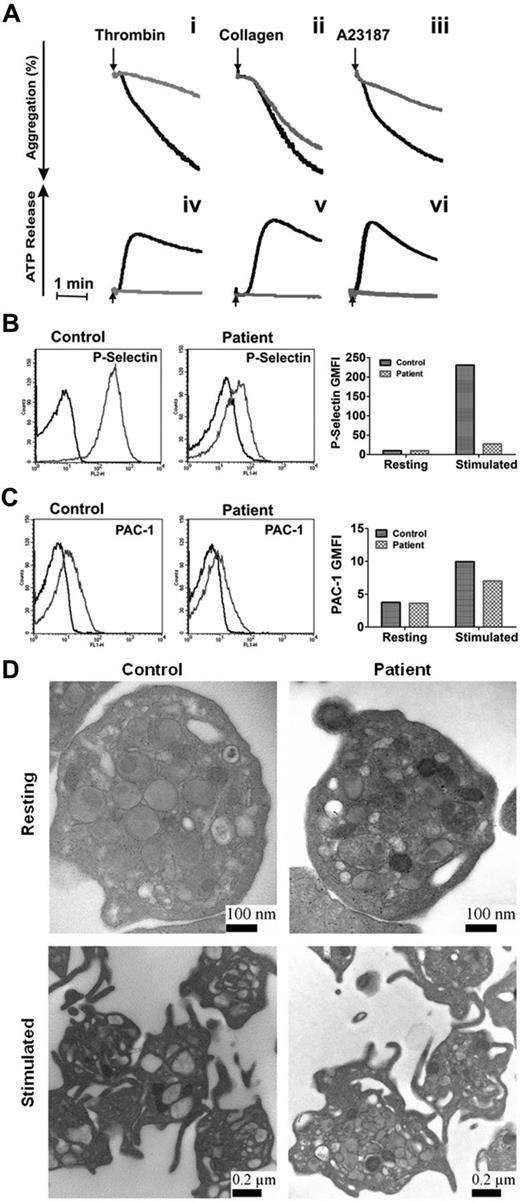
Our data showed that the loss of syntaxin-2 and syntaxin-4 does not affect platelet secretion, that syntaxin-11 is the most abundant syntaxin isoform, and that the anti–syntaxin-2 Ab that was previously shown to be inhibitory does cross-react with other syntaxins, notably syntaxin-11. These data make the case that syntaxin-11 is the relevant syntaxin for platelet exocytosis. To test this, we examined the secretion phenotype of platelets from a patient with FHL4. This rare patient has missense mutations in both copies of the syntaxin-11 gene, which result in the production of an unstable protein, as indicated by a lack of detectible protein in the platelets (Figure 4A). Other elements of the secretory machinery were not affected significantly, although there did appear to be a uniform decrease in all Munc18 isoforms. Thrombin-induced secretion for these platelets was affected severely (Figure 4B-C). Release of [3H]-serotonin and PF4 were significantly deficient and β-hexosaminidase release was attenuated. Consistently, ATP/ADP release in response to thrombin, collagen, and A23187 and thrombin-induced P-selectin exposure were greatly affected compared with platelets from a healthy donor (Figure 5A-B). Aggregation in response to thrombin and A23187 was also defective (Figure 5A). Aggregation in response to collagen was less affected (perhaps because of the high concentration of collagen used), although ATP release was ablated. Levels of the cargo molecules in platelets from the FHL4 patient were similar to those from a healthy control (PF4: 2331 ± 184 pg/2.5 × 108 control platelets, 2220 ± 86 pg/2.5 × 108 FHL4 platelets; β-hexosaminidase: 37 ± 5 U/2.5 × 108 control platelets, 30 ± 5 U/2.5 × 108 FHL4 platelets), indicating no defect in cargo sorting. Ultrastructure analysis of resting syntaxin-11–deficient platelets showed no remarkable defect (Figure 5D). When the platelets were activated, granules migrated to the center of the platelets, but were clearly visible and apparently unable to fuse. A similar phenotype was seen in platelets from VAMP-8−/− or Munc13-4−/− mice.4,11 These results show that the cytoskeletal rearrangements that occur on activation were normal in the deficient platelets, but that granule-plasma membrane fusion, and thus secretion, could not occur. In addition, integrin activation, as measured by PAC-1 binding, was only slightly affected by loss of syntaxin-11 (Figure 5C). These data indicate that the loss of syntaxin-11 causes a significant defect in platelet exocytosis.
Granule release is defective in syntaxin-11–deficient human platelets. Platelet extracts (5.0 × 107 platelets/lane) from control patients and an FHL4 patient were probed by Western blotting with the indicated Abs (A). [3H]-Serotonin labeled platelets from the FHL4 patient (gray circle symbols) and a normal control donor (black square symbols) were prepared as in “Methods” and stimulated with either the indicated thrombin concentration at room temperature for 1 minute (B) or 0.05 U/mL of thrombin for the indicated time points (C). Release of [3H]-serotonin from dense granules, PF4 from α-granules, and β-hexosaminidase from lysosomes was measured and calculated as described in the legend to Figure 1.
Granule release is defective in syntaxin-11–deficient human platelets. Platelet extracts (5.0 × 107 platelets/lane) from control patients and an FHL4 patient were probed by Western blotting with the indicated Abs (A). [3H]-Serotonin labeled platelets from the FHL4 patient (gray circle symbols) and a normal control donor (black square symbols) were prepared as in “Methods” and stimulated with either the indicated thrombin concentration at room temperature for 1 minute (B) or 0.05 U/mL of thrombin for the indicated time points (C). Release of [3H]-serotonin from dense granules, PF4 from α-granules, and β-hexosaminidase from lysosomes was measured and calculated as described in the legend to Figure 1.
Depletion of syntaxin-11 affects aggregation, ATP release, and P-selectin exposure, but not integrin activation or platelet ultrastructure. (A) Aggregation (i-iii) and ATP release (iv-vi) were monitored concurrently in a lumi-aggregometer. Washed platelets from a control donor (black traces) and FHL4 patient (gray traces) were stimulated with thrombin (0.1 U/mL; i, iv), collagen (10 μg/mL; ii, v), and A23187 (100nM; iii, vi) for 2-3 minutes; B-C) Washed platelets from a control donor (Control) and FHL4 patient (Patient) were stimulated with 0.1 U/mL of thrombin for 1 minute and then incubated with FITC-conjugated anti–P-selectin (B) or FITC-conjugated PAC-1 (C) Abs. The reactions were stopped with hirudin and the fluorescent intensities were measured by flow cytometry. The data were plotted as a histogram (left panels) and as the geometric mean fluorescence intensity (GMFI; right panels). Because of limited samples, the experiments in panels B and C were done only once. (D) Washed platelets from a control donor (Control) and FHL4 patient (Patient) were kept resting with 1 ng/mL of PGI2 (i-ii) or stimulated with 0.1 U/mL of thrombin (iii-iv) for 5 minutes. The platelets were fixed and processed for electron microscopic analysis as described in “Methods.” The samples were analyzed with a transmission electron microscope and images were obtained using Gatan software. The scale bars are indicated.
Depletion of syntaxin-11 affects aggregation, ATP release, and P-selectin exposure, but not integrin activation or platelet ultrastructure. (A) Aggregation (i-iii) and ATP release (iv-vi) were monitored concurrently in a lumi-aggregometer. Washed platelets from a control donor (black traces) and FHL4 patient (gray traces) were stimulated with thrombin (0.1 U/mL; i, iv), collagen (10 μg/mL; ii, v), and A23187 (100nM; iii, vi) for 2-3 minutes; B-C) Washed platelets from a control donor (Control) and FHL4 patient (Patient) were stimulated with 0.1 U/mL of thrombin for 1 minute and then incubated with FITC-conjugated anti–P-selectin (B) or FITC-conjugated PAC-1 (C) Abs. The reactions were stopped with hirudin and the fluorescent intensities were measured by flow cytometry. The data were plotted as a histogram (left panels) and as the geometric mean fluorescence intensity (GMFI; right panels). Because of limited samples, the experiments in panels B and C were done only once. (D) Washed platelets from a control donor (Control) and FHL4 patient (Patient) were kept resting with 1 ng/mL of PGI2 (i-ii) or stimulated with 0.1 U/mL of thrombin (iii-iv) for 5 minutes. The platelets were fixed and processed for electron microscopic analysis as described in “Methods.” The samples were analyzed with a transmission electron microscope and images were obtained using Gatan software. The scale bars are indicated.
Syntaxin-11 associates with other platelet SNAREs
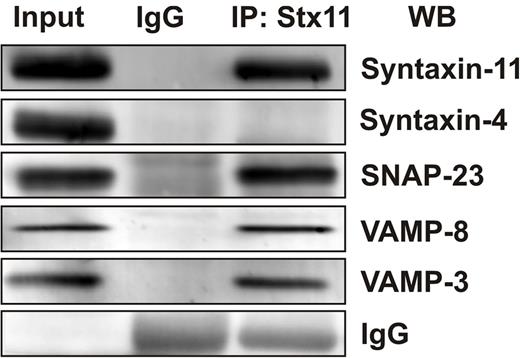
Our work and that of others has shown that secretion from human and mouse platelets requires VAMP-8 (Q. Ren, S.W.W., unpublished data, 2008 and Ren et al11 ) and SNAP-23.13,15,16,19 If syntaxin-11 is part of the platelet secretory machinery, one might expect that it would interact with some of the functionally relevant SNAREs. To test this, coimmunoprecipitation experiments were done using anti–syntaxin-11 Abs. As shown in Figure 6, anti–syntaxin-11 Ab coimmunoprecipitated both SNAP-23 and VAMP-8. In addition, some VAMP-3 was detected. Given that SNAREs can associate promiscuously when in solution, these data do not imply specificity, but do show that syntaxin-11 can function as a SNARE and can form complexes with other relevant platelet SNARE proteins.
Syntaxin-11 is associated with functionally relevant platelet SNAREs. Platelet extracts (Input) from resting human platelets were prepared by solubilization with 1% Triton X-100. After clarification, platelet extracts were incubated with syntaxin-11 polyclonal Ab or IgG control for 3 hours at 4°C. Immune complexes were recovered with protein A Sepharose. The bound proteins were eluted and separated by SDS-PAGE, followed by Western blotting with the indicated Abs.
Syntaxin-11 is associated with functionally relevant platelet SNAREs. Platelet extracts (Input) from resting human platelets were prepared by solubilization with 1% Triton X-100. After clarification, platelet extracts were incubated with syntaxin-11 polyclonal Ab or IgG control for 3 hours at 4°C. Immune complexes were recovered with protein A Sepharose. The bound proteins were eluted and separated by SDS-PAGE, followed by Western blotting with the indicated Abs.
Discussion
In the present study, we reexamined previous data suggesting specific roles for syntaxin-2 and syntaxin-4 in platelet secretion. Earlier studies had relied on “isoform-specific” Abs and permeabilized platelet secretion assays to define which t-SNAREs were required for dense granule, α-granule, and lysosome release. A polyclonal Ab to syntaxin-2 affected all 3 release processes and a mAb to syntaxin-4 affected α-granule and lysosome release.13,15,16,19 These results were attractive because they suggested that distinct SNAREs were used for each of the release events; however, there were caveats to the experimental approach. In the present study, we analyzed platelets from genetically modified mice to determine the effect that deletion of syntaxin-2 and syntaxin-4 had on platelet function. Deletion of either syntaxin alone or in combination had no significant effect on agonist-induced secretion from any of the 3 granule stores. No compensatory increases in other secretory machinery components were noted and no changes in platelet morphology or activation were detected in any of the platelets from the 3 mouse strains tested. To partially resolve the conflict between studies, we reexamined 2 of the reagents used previously (the anti–syntaxin-2 polyclonal Ab and the anti–syntaxin-4 mAb) and showed that the anti–syntaxin-2 Ab had significant cross-reactivity to syntaxin-11 (the presence of which was not known in the previous studies). The originally used syntaxin-4 Ab seems to be isoform specific, and was not characterized further because of insufficient quantities of the Ab. Additional data (Table 1) indicated that syntaxin-11 is one of the major t-SNAREs by number in both mouse and human platelets, which is consistent with its being the dominant functional syntaxin t-SNARE. Such an abundance/function relationship was shown previously for endobrevin/VAMP-8.7,11 With these insights, we examined the secretion phenotype of platelets from an FHL4 patient who lacked syntaxin-11. The platelets did not show any compensatory increases in the expression of other SNAREs, nor was there any obvious dysregulation of platelet activation. The platelets did show a profound defect in dense and α-granule cargo release and lysosome release was attenuated. Combining data from both murine and human platelets, it seems that syntaxin-11 is the functionally relevant syntaxin t-SNARE for platelet secretion. Consistently, syntaxin-11 can form a SNARE complex with the major, functionally relevant v-SNARE endobrevin/VAMP-8 and with SNAP-23.
The results of the present study are in contrast to those from previous studies. Work by us and others had used the pore-forming bacterial toxin streptolysin O to gain access to the platelet cytosol and thus manipulate the platelet secretory machinery by adding potentially inhibitory Abs. This approach was useful as an initial foray into the study of platelet secretion, but ambiguity in the effect of the inhibitors has made interpretation of results tenuous. Abs to syntaxin-2, syntaxin-4,13,15,16,19 VAMP-3,26,27 and Munc18c20,28 have been shown to affect secretion from permeabilized platelets; however, in each case, deletion of the proteins in mouse platelets caused no significant secretion defects. Conversely, deletions of VAMP-8, Munc18b, and syntaxin-11 cause clear release defects, as shown in Al Hawas et al29 and previously.11,12,20,30 Given the data presented here (Figure 3), it is possible that some of the reagents used in previous experiments cross-reacted with other SNARE isoforms that are required for platelet secretion. Because syntaxin-11 was not yet known to be present in platelets, it was hard for the original investigators to test the potential cross-reactivity of their reagents. It is also possible that there are steric effects when whole Abs are used. Whole Ab molecules could block access to specific SNAREs by binding to neighboring, nonessential SNAREs. Syntaxins have been shown to cluster and thus may be more sensitive to steric inhibition.31 Steric hindrance could be more significant if the SNARE proteins are concentrated into membrane microdomains such as rafts. In future studies, Fab fragments and specific peptide inhibitors, as was used to determine the role of SNAP-23,13,15 may be a more direct approach. Whereas it may never be completely clear why earlier studies showed inhibitory effects with the Abs used, the genetic studies presented herein demonstrate clearly that syntaxin-2 and syntaxin-4 are not required for platelet secretion.
Syntaxin-2 and syntaxin-4 were logical choices to mediate platelet secretion because they are present on the plasma membrane in several cell types.17,32,33 Syntaxin-7 and syntaxin-11 had largely been localized to internal membranes in nucleated cells.34-36 Syntaxin-11 was shown to colocalize with the calcium-dependent mannose-6-phosphate receptor, suggesting an endosomal distribution. Other data have shown a role for syntaxin-11 in regulated exocytosis events. Natural killer and cytotoxic T-lymphocyte cells lacking syntaxin-11 are unable to release their cytotoxic granules, leading to the dysregulation of the immune system seen in FHL4 patients.37-40 Other studies of mast cells suggest a role for syntaxin-11 in cargo release.36 Alternatively, syntaxin-11 has been proposed to be a negative regulator of release from macrophages, where it appears to sequester the relevant t-SNARE Vti1b. The studies presented herein show that syntaxin-11 is involved in platelet exocytosis and suggest that there may be mechanistic similarities between natural killer/cytotoxic T-lymphocyte cells and platelets. It should be noted that these data do not rule out a secondary role for either syntaxin-2 or syntaxin-4, as we have seen for VAMP-2 and VAMP-3. At present, sufficient reagents are not available to directly address this possibility.
These studies do raise an issue about how membrane fusion proceeds during platelet secretion. To mediate membrane fusion, the consensus held that the SNARE proteins used their coiled-coil domains and transmembrane domains (TMDs) to form a membrane-spanning complex that mediates fusion of granule and plasma membranes. Whereas the importance of the coiled-coil, SNARE motifs is clear, the relative importance of a classic TMD is less obvious. Several studies have suggested that traditional TMDs are not required (especially for syntaxins) if there is a sufficiently hydrophobic moiety attached (ie, a C45 prenyl group) or if other regulatory proteins are present and contribute to the fusion process.41 Syntaxin-11 does not have a classic TMD and is thought to be anchored in the membrane via some modification of the cluster of 6 cysteine residues at its C-terminus.34,36 Its partner t-SNARE, SNAP-23, is anchored by palmitoylation of a series of cysteines between its 2 SNARE domains,42 indicating that neither platelet t-SNARE has a classic TMD. Both SNAREs behave as integral membrane proteins and thus are reliant on hydrophobic modifications to anchor them in the membrane. Acylation (or prenylation) of these 2 SNAREs may have significant functional relevance, given that SNAP-23 (and presumably syntaxin-11) is recruited to lipid rafts on platelet activation.43 Future studies will be required to better understand whether differential modification of these platelet t-SNAREs might affect their function in platelets.
One final point raised by the results of the present study is the potential value of platelet secretion assays as diagnostic tools for identifying FHL patients. We (Al Hawas et al29 ) and others4,30 have shown platelet secretion defects associated with FHL type 3 (Munc13-4), type 4 (syntaxin-11), and type 5 (Munc18b/StxBP2). Platelet function assays, either FACS-based measurements of P-selectin or LAMP-1 exposure or lumi-aggregometry measuring ADP/ATP release, can be readily performed in most hematology laboratories. It is clear from the results of the present study that platelet dysfunction is a characteristic of at least 3 types of FHL. Therefore, platelet dysfunction could be considered as an additional criterion for the early diagnosis of patients with FHL.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mary Gail Engle and Jim Begley of the Chandler Medical Center Imaging Facility; Greg Bauman at the University of Kentucky Flow Cytometry and Cell Sorting Facility (which is supported by the Office of the Vice President for Research, the Markey Cancer Center, and a grant from the National Institutes of Health Share Instrument Program [S10 RR026827]) for flow cytometry; the Kentucky Blood Center; and members of the Whiteheart laboratory for their editorial comments during the preparation of this manuscript.
This work was supported by the National Institutes of Health (grants HL56652 and HL082193 to S.W.W. and AI079759 and AI076746 to A.H.F.) and by the Histiocytosis Association of America (to A.H.F.).
National Institutes of Health
Authorship
Contribution: S.Y., Z.A.K., and R.A.H. performed the experiments and prepared the figures; J.E.P. supplied the syntaxin-4flox/flox mice; A.H.F. recruited the FHL4 patient; S.W.W. analyzed the data; and S.Y. and S.W.W. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Sidney W. Whiteheart, PhD, Department of Molecular and Cellular Biochemistry, University of Kentucky College of Medicine, 741 South Limestone, Biomedical Biological Sciences Research Bldg, Lexington, KY 40536-0509; e-mail: whitehe@uky.edu.

![Figure 1. Deletion of syntaxin-2 (Stx2) or syntaxin-4 (Stx4) has no effect on platelet secretion. Platelet extracts (5.0 × 107 platelets/lane) were prepared from wild type (Wt) and syntaxin-2–knockout (Stx2 KO; A) or syntaxin-4–knockout (Stx4 KO; C) mice and the indicated proteins were detected by Western blotting. [3H]-serotonin–labeled platelets from Stx2 KO (B) or Stx4 KO (D; gray circle symbols) or Wt (black square symbols) were prepared as described in “Methods” and were stimulated with the indicated thrombin concentrations for 1 minute. Release of [3H]-serotonin from dense granules, PF4 from α-granules, and β-hexosaminidase from lysosomes was measured and the percentage of secretion was calculated. The data are the averages of triplicate measurements with the SD indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/12/10.1182_blood-2012-05-430603/4/m_zh89991295240001.jpeg?Expires=1767731279&Signature=WNkeQfvwqyUBh2V1~rsSV98Aj-5JUJRylOQXRnig959YoYAJmMN6M3hApikQITkvNgsZxKGuY6hjeEF2njVZeXO7KupVGeAdEM8mslC-mGKtq9clIZqJV3puT2d7NUXv6gqQOiwCbGsnekUDzlHNJxp4L4xPErNvEHzu4vr1g9A7NMu1HDogMIp32qiFifHet5uLB9hjmGfClZNmiFYYrhHewzKvBUO~bHAqXYiqc4bfnU2zrBd9Oa1tX8sDRRVLVvQt1NDL01r7BH1qYK6Ny3MPFzAWCJOhXio-g-7~RxaBdJNTc-6pL1QK9GtJmi7MVk5VTaWbHNQb2OqEhqYFwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Deletion of both syntaxin-2 and syntaxin-4 (Stx2/4) does not inhibit platelet secretion. (A) Platelet extracts (5.0 × 107 platelets/lane) were prepared from wild-type (Wt) and syntaxin-2 and syntaxin-4 double-knockout (Stx2/4 DKO) mice and the indicated proteins were detected by Western blotting. [3H]-serotonin–labeled platelets from Stx2/4 DKO (gray circle symbols) and Wt (black square symbols) were prepared as described in “Methods.” Release of [3H]-serotonin from dense granules, PF4 from α-granules, and β-hexosaminidase from lysosomes was measured as a thrombin-response curve (1-minute stimulation; B) or as a time course (0.05 U/mL of thrombin; C) and calculated as described in the legend to Figure 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/12/10.1182_blood-2012-05-430603/4/m_zh89991295240002.jpeg?Expires=1767731279&Signature=bHzAtw5F2aCG5Rxvf2LhS4-g4yWyzFDf6GNNdeIAnNIGX-i1WIeNkDR5VquEqqTI41g94-9lJJwiYWrKWc15AQ4GegqUfQHfR8oMGIIiMjmlrYqGo5FWRKADevnP8o7Z70IrY40svIA8~4xvOAjijWhOnnoO9dNn1BIeSQSVk3BNcdDkLoEpnZHswAbSgZYbbZ5UblLOJROtnhBiQIIw2p8HgoZvWJSQS9qkdpBYF0uwyCT9sQPPYGlOstJxawtDEuF3QuUfwTlGET5txHsqG9dOuWJ4ghrujr7vtsUHnOGpIqyVVJpryABth~iTHD45p1xLHw5NxhDMMDkGu5TZ6A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. Granule release is defective in syntaxin-11–deficient human platelets. Platelet extracts (5.0 × 107 platelets/lane) from control patients and an FHL4 patient were probed by Western blotting with the indicated Abs (A). [3H]-Serotonin labeled platelets from the FHL4 patient (gray circle symbols) and a normal control donor (black square symbols) were prepared as in “Methods” and stimulated with either the indicated thrombin concentration at room temperature for 1 minute (B) or 0.05 U/mL of thrombin for the indicated time points (C). Release of [3H]-serotonin from dense granules, PF4 from α-granules, and β-hexosaminidase from lysosomes was measured and calculated as described in the legend to Figure 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/12/10.1182_blood-2012-05-430603/4/m_zh89991295240004.jpeg?Expires=1767731279&Signature=0sXVqee4ApvSHtf0Ic3zcgeZ4dvTisBWGbDePfEMsnL1F3HzuBqdQj4ALFncs0Y7DiCmDmkVVbslYn3mblPz4koOWhPtCFKA7p3HYp1s1mhwBeIy~UOG3mJRCu7ctjfqfPQitLaF3M14XVb2jRIU~BCL8~bbBpePacLsRczf0q8hwW0FnN1gz2dLbaf-EdlU-LTqivkXR1SaN6B0Zy5dLKQRRIf-x7Z4rca81HNyaZ-v8GELcwYXsQtUB~ThNH4TQ855Go1-ErVxtkyRI2DXQNbHqCubDzUdsKNuivoKJzqsGXHxEreNkZ3rk8oTtm4ESwBVMK-NUpttzzcraQlGKg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal