Abstract
Chronic myeloid leukemia (CML) patients with the BCR-ABL T315I mutation do not benefit from therapy with currently approved tyrosine kinase inhibitors. Omacetaxine mepesuccinate is a protein synthesis inhibitor that has demonstrated activity in cells harboring the T315I mutation. This phase 2 trial assessed the efficacy of omacetaxine in CML patients with T315I and tyrosine kinase inhibitor failure. Patients received subcutaneous omacetaxine 1.25 mg/m2 twice daily, days 1-14, every 28 days until hematologic response or a maximum of 6 cycles, and then days 1-7 every 28 days as maintenance. Results for patients treated in chronic phase are reported here. Patients (n = 62) received a median of 7 (range, 1-41) cycles. Complete hematologic response was achieved in 48 patients (77%; 95% lower confidence limit, 65%); median response duration was 9.1 months. Fourteen patients (23%; 95% lower confidence limit, 13%) achieved major cytogenetic response, including complete cytogenetic response in 10 (16%). Median progression free-survival was 7.7 months. Grade 3/4 hematologic toxicity included thrombocytopenia (76%), neutropenia (44%), and anemia (39%) and was typically manageable by dose reduction. Nonhematologic adverse events were mostly grade 1/2 and included infection (42%), diarrhea (40%), and nausea (34%). Omacetaxine may provide a safe and effective treatment for CML patients with T315I mutation. This study is registered at www.clinicaltrials.gov as NCT00375219.
Introduction
The development of small-molecule inhibitors of the breakpoint cluster region–Abelson (BCR-ABL) tyrosine kinase has dramatically altered the therapeutic outcomes for most patients with chronic myeloid leukemia (CML). Before the development of the tyrosine kinase inhibitor (TKI) imatinib mesylate (Gleevec/Glivec; Novartis Pharmaceuticals) 10 years ago, patients with chronic-phase CML who were not eligible for allogeneic bone marrow transplantation had limited treatment options, and median survival was typically 4 to 5 years from diagnosis. For most patients with CML, therapy with imatinib and the second-generation TKIs dasatinib (Sprycel; Bristol-Myers Squibb) and nilotinib (Tasigna; Novartis Pharmaceuticals) holds the prospect of longer-term survival; current estimates of survival in responsive patients are similar to the life expectancy of the general population.1
The success of TKIs in the treatment of CML is tempered by reports that a significant proportion of patients (∼ 18%) do not achieve a complete cytogenetic response (CCyR) to imatinib, and a similar percentage of patients discontinue treatment because of unacceptable side effects or drug resistance.2 Early results from studies using dasatinib or nilotinib as initial therapy suggest that these results might be improved. Still, some patients do not respond optimally, and within 12 months, up to 16%-18% of patients have already been reported to discontinue frontline therapy with dasatinib or nilotinib because of toxicity, unacceptable response, or other reasons.3,4
Point mutations leading to amino acid substitutions in the ABL kinase domain of BCR-ABL that interfere with imatinib binding are the most commonly reported cause of imatinib resistance; they are detected in approximately 60% of patients who experience imatinib resistance.5 The second-generation compounds, dasatinib and nilotinib, have higher binding affinity to the ABL kinase domain than imatinib and consequently have activity against many of the imatinib-resistant kinase domain mutants. Notably, 1 BCR-ABL mutation, T315I, does not respond to any approved TKI in vitro or clinically. The prognosis for chronic-phase CML patients with this mutation is poor: reported median survival is approximately 22 months from the time of detection of the mutation.6 The emergence of the T315I mutation in CML patients treated with second-generation TKIs after failure of imatinib appears to be more common, with the T315I mutation detected in up to 40% of patients who acquire resistance to second-line TKI therapy.7,8 With the exception of stem cell transplantation (SCT),9,10 there are currently no effective therapies for CML patients with the T315I mutation.
Omacetaxine mepesuccinate (hereinafter omacetaxine) is a reversible protein translation inhibitor that was shown to have clinical efficacy in CML patients in the pre-TKI era.11,12 The antileukemic effect of omacetaxine is not affected by the presence of mutations in BCR-ABL. Omacetaxine has demonstrated activity against cells with the T315I mutation in preclinical studies,13 as well as preliminary efficacy in CML patients with this mutation.14,15 Here, we describe the results of a phase 2 study of omacetaxine in chronic-phase CML patients with a history of the T315I mutation who failed TKI therapy.
Methods
Study design
Study CGX-635-CML-202 was a prospective, multicenter, single-arm, open-label study designed to evaluate the safety and efficacy of subcutaneous administration of omacetaxine in patients with any phase of CML in whom 1 or more TKI therapies, including imatinib, had failed and who harbored the T315I BCR-ABL mutation.
Only results for patients treated in chronic phase are reported in this manuscript. Thirty centers in 10 countries participated in this trial. The clinical trial protocol was approved by the relevant institutional review boards. Informed written consent was obtained from each patient before enrollment in the study. The study was conducted in compliance with regulations of the Health Insurance Portability and Accountability Act and the Declaration of Helsinki.
Patient population
Male and female patients 18 years of age or older with Philadelphia chromosome–positive (Ph+) CML who had failed imatinib therapy and had a history of the T315I mutation were eligible. Patients with CML were assessed as being in chronic phase if they had less than 15% blasts in peripheral blood or bone marrow, less than 30% blasts plus promyelocytes in peripheral blood or bone marrow, less than 20% basophils in peripheral blood or bone marrow, and a platelet count greater than 100 × 109/L. Failure of imatinib therapy was defined as a lack of or loss of complete hematologic response (CHR) by 12 weeks, any cytogenetic response by 24 weeks, or major cytogenetic response by 52 weeks, or progressive leukocytosis. Patients with New York Heart Association class III or IV heart disease, active ischemia, or any other uncontrolled cardiac condition, or patients having had a myocardial infarction in the previous 12 weeks were excluded from the study. Other exclusion criteria included concurrent illnesses that would interfere with study conduct or assessments, including an active malignancy (other than CML), an uncontrolled active infection, or positive HIV or human T-lymphotropic virus I/II status. Patients who were eligible for SCT at the time of screening and patients in lymphoid blast crisis were also excluded from this study.
Patients had to have discontinued all previous antileukemic therapy for at least 2 weeks before initiation of omacetaxine therapy. In patients with rapidly proliferating disease, hydroxyurea could be administered immediately before and during the first 2 cycles of treatment.
Study treatment
Omacetaxine was provided in a single-use vial as a lyophilized powder containing 5 mg of active ingredient. Patients were trained by the nursing staff at each study center on the safe and accurate reconstitution and administration of omacetaxine in normal saline. The first dose of each cycle was administered under supervision of the study team. All subsequent treatments were administered at home by the patient or a caregiver, except for patients enrolled in France, where all doses of omacetaxine were administered by a health care professional because of local regulatory requirements. Patients were provided with a diary in which to record the time and location of injection and any reactions that occurred; compliance was assessed based on diary entries.
Dosing schedule
During the induction phase, patients received omacetaxine 1.25 mg/m2 twice daily subcutaneously for 14 days every 28 days. After achievement of a hematologic response, patients were transitioned to a reduced or maintenance dosing schedule consisting of omacetaxine 1.25 mg/m2 twice daily subcutaneously for 7 days every 28 days.
Patients received at least 1 induction cycle of therapy before beginning maintenance, and patients with no evidence of clinical response after 6 induction cycles were considered for removal from the study. The treatment goal as set by the protocol was to maintain the 28-day dosing cycle. The number of dosing days per cycle was reduced by 2 days in the subsequent cycle for patients who experienced an absolute neutrophil count (ANC) nadir of less than 0.5 × 109/L and/or platelet count of less than 50 × 109/L. In these instances, further treatment was delayed until ANC was greater than 1.0 × 109/L and platelet count was greater than 50 × 109/L. Administration of hematopoietic growth factors was permitted only in the event of febrile neutropenia. Erythropoietin, darbepoetin alfa, or blood transfusions were allowed at any time for treatment of anemia (any grade).
Assessments
Patients were evaluated every 7 days during induction cycles and every 14 days during maintenance cycles with complete blood counts and chemistry analyses. Preceding each induction and maintenance cycle, clinical evaluations, body surface area determinations, electrocardiograms, complete blood counts, and serum chemistry tests were performed. After every 3 months on study, patients underwent a bone marrow aspiration and cytogenetic analysis. An independent data monitoring committee was responsible for adjudicating the response of each patient. On discontinuation, patients were followed for survival by telephone survey.
Assessment of BCR-ABL transcript levels and BCR-ABL kinase domain mutations was performed at 1 of 2 central laboratories, in the United States or Germany, as appropriate to the location of the study site. Molecular response was assessed using Abl as a reference gene: BCR-ABL and ABL transcripts in peripheral blood were quantified by standard quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) and the BCR-ABL/ABL ratio was calculated. A major molecular response was defined as a BCR-ABL/ABL ratio of less than 0.1% according to the international scale.
The methodology used to detect the BCR-ABL T315I mutation varied between the 2 central laboratories. In the United States, the ABL kinase domain was amplified and sequenced. If the T315I mutation was detected, the amount of mutated T315I BCR-ABL transcript was quantified by a more sensitive technique, rapid pyrosequencing.16,17 In Europe, the T315I mutation was detected by denaturing high-performance liquid chromatography.18 If the sample was found to be positive for the T315I mutation, the amount of mutated T315I BCR-ABL transcript was quantified by qRT-PCR. The level of mutated T315I BCR-ABL transcript was compared with transcript unmutated at codon 315.
Efficacy end points
The primary efficacy end point was defined as the proportion of patients achieving either a CHR or major cytogenetic response (MCyR). A CHR was defined by a white cell count of no more than 10 × 109/L, a platelet count of less than 450 × 109/L, less than 20% basophils in peripheral blood, the absence of blasts or promyelocytes in peripheral blood, the presence of less than 5% myelocytes plus metamyelocytes in peripheral blood, and the absence of extramedullary involvement (including a normal-size liver and spleen on physical examination). Cytogenetic response was defined on the basis of the percentage of Ph+ metaphases in the bone marrow with at least 20 metaphases counted as follows: CCyR, 0; partial cytogenetic response (PCyR), 1%-35%; minor cytogenetic response, 36%-65%; and minimal cytogenetic response, 66%-95%. The rate of MCyR included patients with CCyR or PCyR (ie, 0%-35% Ph+ metaphases). For hematologic responses, only patients with a CHR that was sustained for 8 weeks were considered as responders in the efficacy analysis. In patients who received hydroxyurea during the first 2 cycles of treatment, response was considered CHR only if it was sustained for more than 8 weeks after interruption of hydroxyurea.
Secondary end points included minor and minimal cytogenetic responses, degree of suppression of BCR-ABL transcript levels (ie, molecular response), reduction of the proportion of T315I-mutated BCR-ABL, time to onset of response, duration of response, progression-free survival (PFS), and overall survival (OS).
Safety end points
The primary safety end point was the tolerance and toxicity of the omacetaxine regimen. The severity of adverse events occurring on study was graded by investigators according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0. Adverse events were classified by body system using the MedDRA thesaurus and categorized by system organ class and preferred term.
Statistical analysis
All analyses were performed in the intent-to-treat population, defined as all patients who provided written informed consent and received at least 1 dose of study drug. Summary statistics (number of patients, mean, median, range, SD) were used to describe continuous variables; for categorical variables, the number and percentage of total were tabulated.
Exact 1-sided lower 95% binomial confidence limits were calculated for primary response end points (ie, CHR and MCyR). For time-to-event variables (time to onset of response, duration of response, PFS, and OS), the median and 95% confidence interval (CI) were calculated using Kaplan-Meier product limit estimates. Time to onset of response was defined as the time from initiation of treatment until the date of first reported hematologic or cytogenetic response. Duration of response was defined as the time from the onset of hematologic or cytogenetic response until the date of objective evidence of disease progression, relapse, or death; patients with ongoing response and patients who discontinued treatment for reasons other than adverse events, progression, or death were censored at the last examination date. PFS was defined as the time from initiation of treatment until the date of death from any cause, development of accelerated-phase or blast-phase CML, loss of CHR or MCyR, or discontinuation because of toxicity or lack of efficacy; patients without progression and patients who discontinued treatment for reasons other than adverse events, progression, or death were censored at the last examination date. OS was defined as the time from initiation of treatment until death from any cause at any time, including long-term follow-up for survival by telephone survey after study discontinuation; patients alive at time of analysis were censored at the last recorded contact or evaluation.
Results
Patient demographics and treatments
From September 2006 to March 2010, 62 patients with chronic-phase CML with T315I mutation were enrolled in this study. The patients were heavily pretreated with conventional therapies for CML. All patients had failed therapy with imatinib and 46 (74%) had failed therapy with at least 2 TKIs (Table 1). Eighteen patients (29%) previously achieved MCyR with imatinib, including complete response in 12 patients (19%); minor response or minimal response to imatinib was reported in 5 additional patients (8%).
Patient demographics and baseline characteristics
| Demographic statistic/category . | Patients treated, N = 62 . |
|---|---|
| Median age, y (range) | 56 (26-83) |
| Male sex, n (%) | 42 (68) |
| Ethnicity, n (%) | |
| White | 48 (77) |
| Black | 4 (7) |
| Hispanic | 0 |
| Asian | 8 (13) |
| Other | 2 (3) |
| Median time since initial CML diagnosis, mo (range) | 50.0 (11-234) |
| ECOG performance status, n (%) | |
| 0 | 41 (66) |
| 1 | 20 (32) |
| 2 | 1 (2) |
| 3 | 0 |
| Type of TKIs previously received, n (%) | |
| Imatinib | 62 (100) |
| Dasatinib | 34 (55) |
| Nilotinib | 22 (36) |
| Other | 9 (15) |
| Number of TKIs previously received, n (%) | |
| 1 | 16 (26) |
| 2 | 30 (48) |
| ≥ 3 | 16 (26) |
| Other previous leukemia treatment, n (%) | |
| Hydroxyurea | 35 (56) |
| Interferon | 23 (37) |
| Cytarabine | 10 (16) |
| CHR status at study entry, n (%) | |
| CHR+ | 15 (24) |
| CHR− | 47 (76) |
| Demographic statistic/category . | Patients treated, N = 62 . |
|---|---|
| Median age, y (range) | 56 (26-83) |
| Male sex, n (%) | 42 (68) |
| Ethnicity, n (%) | |
| White | 48 (77) |
| Black | 4 (7) |
| Hispanic | 0 |
| Asian | 8 (13) |
| Other | 2 (3) |
| Median time since initial CML diagnosis, mo (range) | 50.0 (11-234) |
| ECOG performance status, n (%) | |
| 0 | 41 (66) |
| 1 | 20 (32) |
| 2 | 1 (2) |
| 3 | 0 |
| Type of TKIs previously received, n (%) | |
| Imatinib | 62 (100) |
| Dasatinib | 34 (55) |
| Nilotinib | 22 (36) |
| Other | 9 (15) |
| Number of TKIs previously received, n (%) | |
| 1 | 16 (26) |
| 2 | 30 (48) |
| ≥ 3 | 16 (26) |
| Other previous leukemia treatment, n (%) | |
| Hydroxyurea | 35 (56) |
| Interferon | 23 (37) |
| Cytarabine | 10 (16) |
| CHR status at study entry, n (%) | |
| CHR+ | 15 (24) |
| CHR− | 47 (76) |
CML indicates chronic myeloid leukemia; ECOG, Eastern Cooperative Oncology Group; TKI, tyrosine kinase inhibitor; and CHR, complete hematologic response.
Thirty-five patients (56%) were receiving hydroxyurea to control their white cell count immediately before commencing omacetaxine therapy. Fifteen patients (24%) were in CHR at time of enrollment in the study. Baseline cytogenetic assessments demonstrated no cytogenetic response (100% Ph+ cells) in 39 patients (63%), minimal response in 10 patients (16%), minor response in 3 patients (5%), and major response in 6 patients (10%); baseline cytogenetic status was unknown in 4 patients (7%). All patients had been diagnosed with the BCR-ABL T315I mutation before study enrollment; among 44 patients (71%) with baseline mutational analysis by a central laboratory, the presence of the mutation was confirmed in 38 patients (61%) and was not confirmed in 6 patients (10%).
Patients received a median of 7 treatment cycles (range, 1-41). At the time of data cutoff for this report (January 2011), 12 (19%) patients were still receiving study drug (Table 2). The most common causes for treatment discontinuation were disease progression or lack of response; 7 patients (11%) discontinued therapy because of adverse events and 3 patients discontinued to receive an allogeneic hematopoietic SCT. Treatment delays were common: 76% of patients had at least 1 cycle with a dose delay. The mean duration of dose delay was 16 days and the most common causes of a delay in treatment were thrombocytopenia (64%), neutropenia (30%), and pancytopenia (28%). Dose delays occurred most frequently after cycles 2 and 3 (71% and 77% of cycles were delayed, respectively).
Patient disposition
| Patient accounting . | Patients treated, n (%), N = 62 . |
|---|---|
| Ongoing | 12 (19) |
| Discontinued the study | 50 (81) |
| Primary reasons for discontinuation | |
| Disease progression | 15 (24) |
| Failure to achieve a response | 13 (21) |
| Adverse event | 7 (11) |
| Death | 4 (6) |
| Request of patient, primary investigator, sponsor, or regulatory agency | 4 (6) |
| Lost to follow-up | 1 (2) |
| Patient noncompliance | 1 (2) |
| Other* | 5 (8) |
| Patient accounting . | Patients treated, n (%), N = 62 . |
|---|---|
| Ongoing | 12 (19) |
| Discontinued the study | 50 (81) |
| Primary reasons for discontinuation | |
| Disease progression | 15 (24) |
| Failure to achieve a response | 13 (21) |
| Adverse event | 7 (11) |
| Death | 4 (6) |
| Request of patient, primary investigator, sponsor, or regulatory agency | 4 (6) |
| Lost to follow-up | 1 (2) |
| Patient noncompliance | 1 (2) |
| Other* | 5 (8) |
Includes allogeneic hematopoietic stem cell transplantation (n = 3), hematologic resistance (n = 1), and withdrawal of consent (n = 1).
Efficacy
Of the 62 patients enrolled in this study, 48 (77%; 95% lower confidence limit [LCL], 65%) achieved or maintained a CHR (Table 3). Response was maintained in all 15 patients who had a CHR at the start of omacetaxine therapy, 6 of whom (40%) had received hydroxyurea within 48 hours of study start. CHR rates were 88%, 77%, and 69% in patients who had previously received 1, 2, or 3 approved TKIs, respectively. Among patients with a T315I mutation confirmed by a central laboratory (n = 38), the CHR rate was 84%.
Response rates in chronic-phase CML patients treated with omacetaxine
| Response . | Patients treated, n (%), N = 62 . |
|---|---|
| Hematologic response categories | |
| Complete hematologic response | 48 (77%; 95% LCL, 65%) |
| No response | 12 (19) |
| Unevaluable | 2 (3) |
| Cytogenetic response categories | |
| Major | 14 (23%; 95% LCL, 13%) |
| Complete: 0% Ph+ cells* | 10 (16) |
| Partial: > 0%-35% Ph+ cells* | 4 (6) |
| Minor | 3 (5) |
| Minimal | 10 (16) |
| No response | 23 (37) |
| Unevaluable† | 12 (19) |
| Response . | Patients treated, n (%), N = 62 . |
|---|---|
| Hematologic response categories | |
| Complete hematologic response | 48 (77%; 95% LCL, 65%) |
| No response | 12 (19) |
| Unevaluable | 2 (3) |
| Cytogenetic response categories | |
| Major | 14 (23%; 95% LCL, 13%) |
| Complete: 0% Ph+ cells* | 10 (16) |
| Partial: > 0%-35% Ph+ cells* | 4 (6) |
| Minor | 3 (5) |
| Minimal | 10 (16) |
| No response | 23 (37) |
| Unevaluable† | 12 (19) |
CML indicates chronic myeloid leukemia; and LCL, lower confidence limit.
Includes both confirmed and unconfirmed response. Unconfirmed response is based on a single bone marrow cytogenetic evaluation for patients where a confirmatory evaluation is not available.
Patients with unevaluable cytogenetic responses are those with no postbaseline bone marrow assessment.
A cytogenetic response was achieved in 27 (44%) patients: 14 patients (23%; 95% LCL, 13%) achieved MCyR, including 10 (16%) with CCyR. The rate of MCyR was 60% (9 of 15) for those entering study in CHR and 11% (5 of 47) for those not entering in CHR. MCyR rates were 31%, 27%, and 6% in patients who had previously received 1, 2, or 3 approved TKIs, respectively, and 26% in patients with a confirmed T315I mutation (n = 38). Baseline hematologic and cytogenetic status for patients who achieved MCyR is shown in Table 4.
Baseline status and clinical course of patients achieving major cytogenetic response
| Patient . | Baseline hematologic/cytogenetic status . | Disposition . | No. of cycles received . | Best cytogenetic response on study . |
|---|---|---|---|---|
| 001-009 | CHR−/100% Ph+ | Ongoing | 14 | CCyR |
| 001-012 | CHR+/95% Ph+ | Ongoing | 17 | PCyR |
| 005-001 | CHR+/20% Ph+ | Ongoing | 32 | CCyR |
| 005-002 | CHR+/45% Ph+ | Ongoing | 5 | CCyR |
| 009-002 | CHR+/Not done | Ongoing | 21 | CCyR |
| 010-001 | CHR+/30% Ph+ | Ongoing | 16 | CCyR |
| 020-005 | CHR+/90% Ph+ | Ongoing | 15 | CCyR |
| 021-004 | CHR+/100% Ph+ | Discontinued (to receive allograft) | 3 | PCyR |
| 023-003 | CHR+/74% Ph+ | Discontinued (to receive cord blood transplant) | 10 | CCyR |
| 028-001 | CHR−/100% Ph+ | Discontinued (failure to achieve meaningful response) | 11 | PCyR |
| 028-002 | CHR−/91% Ph+ | Discontinued (failure to achieve meaningful response) | 9 | CCyR |
| 071-002 | CHR−/100% Ph+ | Discontinued (disease progression) | 10 | PCyR |
| 071-003 | CHR−/40% Ph+ | Discontinued (adverse event) | 5 | CCyR |
| 090-002 | CHR+/13% Ph+ | Discontinued (withdrawal by request) | 3 | CCyR |
| Patient . | Baseline hematologic/cytogenetic status . | Disposition . | No. of cycles received . | Best cytogenetic response on study . |
|---|---|---|---|---|
| 001-009 | CHR−/100% Ph+ | Ongoing | 14 | CCyR |
| 001-012 | CHR+/95% Ph+ | Ongoing | 17 | PCyR |
| 005-001 | CHR+/20% Ph+ | Ongoing | 32 | CCyR |
| 005-002 | CHR+/45% Ph+ | Ongoing | 5 | CCyR |
| 009-002 | CHR+/Not done | Ongoing | 21 | CCyR |
| 010-001 | CHR+/30% Ph+ | Ongoing | 16 | CCyR |
| 020-005 | CHR+/90% Ph+ | Ongoing | 15 | CCyR |
| 021-004 | CHR+/100% Ph+ | Discontinued (to receive allograft) | 3 | PCyR |
| 023-003 | CHR+/74% Ph+ | Discontinued (to receive cord blood transplant) | 10 | CCyR |
| 028-001 | CHR−/100% Ph+ | Discontinued (failure to achieve meaningful response) | 11 | PCyR |
| 028-002 | CHR−/91% Ph+ | Discontinued (failure to achieve meaningful response) | 9 | CCyR |
| 071-002 | CHR−/100% Ph+ | Discontinued (disease progression) | 10 | PCyR |
| 071-003 | CHR−/40% Ph+ | Discontinued (adverse event) | 5 | CCyR |
| 090-002 | CHR+/13% Ph+ | Discontinued (withdrawal by request) | 3 | CCyR |
CHR indicates complete hematologic response; CCyR, complete cytogenetic response; and PCyR, partial cytogenetic response.
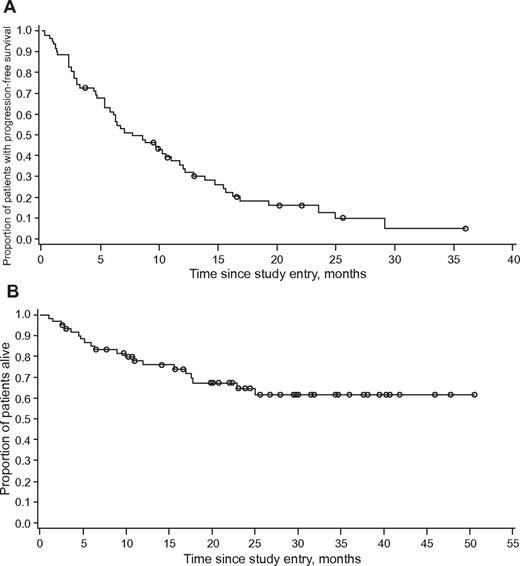
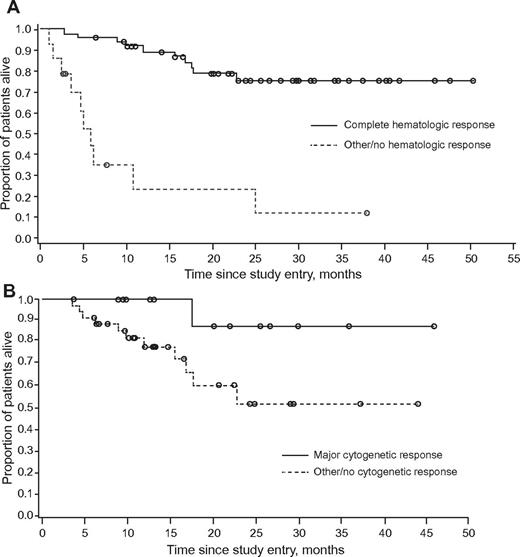
The median number of cycles administered to achieve CHR was 1 (range, 1-5), with a median time to response of 0.46 months (95% CI, 0.39-0.92 months). The median number of cycles to achieve MCyR was 2.5 (range, 2-5). The time to onset of MCyR could not be calculated for the population as a whole by the Kaplan-Meier method; considering only patients who achieved MCyR (n = 14), the arithmetic mean time to onset was 4.2 months (SD, 2.03 months). The median duration of CHR was 9.1 months (range, 2-46+), and the median duration of MCyR was 6.6 months (range, 2.1-30.0+). After a median follow-up of 19 months, median PFS was 7.7 months (95% CI, 5.8-11.0 months; Figure 1). At the time of data cutoff, 42 (68%) patients were still alive, and the median OS had not yet been reached (Figure 1). Achievement of MCyR and achievement or maintenance of CHR were both associated with longer OS (Figure 2).
Kaplan-Meier curve of patients with chronic-phase CML with T315I mutation treated with omacetaxine. (A) Progression-free survival and (B) overall survival.
Kaplan-Meier curve of patients with chronic-phase CML with T315I mutation treated with omacetaxine. (A) Progression-free survival and (B) overall survival.
Kaplan-Meier curve of overall survival in patients with chronic-phase CML with T315I mutation treated with omacetaxine. (A) Patients who achieved CHR compared to those who did not achieve CHR, and (B) patients who achieved MCyR compared to those who did not achieve MCyR.
Kaplan-Meier curve of overall survival in patients with chronic-phase CML with T315I mutation treated with omacetaxine. (A) Patients who achieved CHR compared to those who did not achieve CHR, and (B) patients who achieved MCyR compared to those who did not achieve MCyR.
Eight patients (17.0% of 47 evaluable patients; 95% CI, 7.7%-30.8%) achieved major molecular response. The proportion of the T315I-mutated clone as assessed by BCR-ABL kinase domain mutation analysis was reduced in 28 (61%) of 46 patients with available postbaseline assessments. Most of these patients (n = 17, 37.0%) had a 25%-74% reduction from baseline; 7 patients (15%) had a complete reduction (Table 5).
Largest reduction from baseline of proportion of T315I-mutated BCR-ABL
| Reduction of mutated clone, % . | Patients, n (%), N = 46 . | 95% CI . |
|---|---|---|
| 100 | 7 (15) | 8-34 |
| 75-99 | 1 (2) | 0.1-14 |
| 50-74 | 9 (20) | 11-40 |
| 25-49 | 8 (17) | 10-37 |
| 1-24 | 3 (7) | 2-21 |
| 0 | 10 (22) | 13-43 |
| Not assessable | 8 (17) | NA |
| Reduction of mutated clone, % . | Patients, n (%), N = 46 . | 95% CI . |
|---|---|---|
| 100 | 7 (15) | 8-34 |
| 75-99 | 1 (2) | 0.1-14 |
| 50-74 | 9 (20) | 11-40 |
| 25-49 | 8 (17) | 10-37 |
| 1-24 | 3 (7) | 2-21 |
| 0 | 10 (22) | 13-43 |
| Not assessable | 8 (17) | NA |
Data represent the best individual response assessment in patients who had available postbaseline assessments; 95% CIs calculated for binomial proportion of a level of response against all other levels of response.
CI indicates confidence interval; and NA, not assessable.
Adverse events
Therapy with omacetaxine was generally well tolerated in this study. However, hematologic events were common in the initial cycles of treatment. Grade 3 or 4 thrombocytopenia was observed in 76% of patients, neutropenia in 44%, and anemia in 39% (Table 6). Despite the frequency of neutropenia and thrombocytopenia, few patients developed neutropenic fever (8%) or major bleeding episodes (< 5%). Filgrastim was administered in 13% of patients and epoetin alfa/darbepoetin alfa in 21%. After treatment with omacetaxine, blood counts typically reached nadir values within 2 to 3 weeks after the first dose of each cycle and recovery of blood counts generally occurred within 1 to 3 weeks of the nadir. Myelosuppression was managed with reduction of the number of days of drug administration in subsequent cycles. After this regimen of dose reduction, the incidence of thrombocytopenia and neutropenia significantly decreased: by cycle 6, the reported incidence of grade 3/4 thrombocytopenia decreased from 39% to 10%, the incidence of grade 3/4 anemia decreased from 16% to 5%, and the incidence of grade 3/4 neutropenia decreased from 23% to 8%.
Most frequent (> 10%) adverse events associated with omacetaxine
| Adverse event . | Patients, n (%) . | ||
|---|---|---|---|
| All grades . | Grade 3/4 . | Grade 5 . | |
| Hematologic | |||
| Thrombocytopenia | 49 (79) | 47 (76) | 0 |
| Anemia | 41 (66) | 24 (39) | 0 |
| Neutropenia | 31 (50) | 27 (44) | 0 |
| Pancytopenia | 16 (26) | 13 (21) | 1 (2) |
| Leukopenia | 13 (21) | 11 (18) | 0 |
| Lymphopenia | 11 (18) | 10 (16) | 0 |
| Nonhematologic | |||
| Infection* | 26 (42) | 5 (8) | 1 (2) |
| Diarrhea | 25 (40) | 1 (2) | 0 |
| Nausea | 21 (34) | 1 (2) | 0 |
| Pyrexia | 18 (29) | 1 (2) | 0 |
| Fatigue | 18 (29) | 3 (5) | 0 |
| Asthenia | 17 (27) | 0 | 0 |
| Arthralgia | 14 (23) | 1 (2) | 0 |
| Injection site erythema | 13 (21) | 0 | 0 |
| Alopecia | 11 (18) | 0 | 0 |
| Constipation | 11 (18) | 0 | 0 |
| Headache | 11 (18) | 0 | 0 |
| Cough | 11 (18) | 0 | 0 |
| Upper abdominal pain | 10 (16) | 0 | 0 |
| Epistaxis | 9 (15) | 1 (2) | 0 |
| Insomnia | 8 (13) | 0 | 0 |
| Peripheral edema | 8 (13) | 0 | 0 |
| Back pain | 7 (11) | 1 (2) | 0 |
| Extremity pain | 7 (11) | 0 | 0 |
| Rash | 7 (11) | 0 | 0 |
| Myalgia | 7 (11) | 1 (2) | 0 |
| Adverse event . | Patients, n (%) . | ||
|---|---|---|---|
| All grades . | Grade 3/4 . | Grade 5 . | |
| Hematologic | |||
| Thrombocytopenia | 49 (79) | 47 (76) | 0 |
| Anemia | 41 (66) | 24 (39) | 0 |
| Neutropenia | 31 (50) | 27 (44) | 0 |
| Pancytopenia | 16 (26) | 13 (21) | 1 (2) |
| Leukopenia | 13 (21) | 11 (18) | 0 |
| Lymphopenia | 11 (18) | 10 (16) | 0 |
| Nonhematologic | |||
| Infection* | 26 (42) | 5 (8) | 1 (2) |
| Diarrhea | 25 (40) | 1 (2) | 0 |
| Nausea | 21 (34) | 1 (2) | 0 |
| Pyrexia | 18 (29) | 1 (2) | 0 |
| Fatigue | 18 (29) | 3 (5) | 0 |
| Asthenia | 17 (27) | 0 | 0 |
| Arthralgia | 14 (23) | 1 (2) | 0 |
| Injection site erythema | 13 (21) | 0 | 0 |
| Alopecia | 11 (18) | 0 | 0 |
| Constipation | 11 (18) | 0 | 0 |
| Headache | 11 (18) | 0 | 0 |
| Cough | 11 (18) | 0 | 0 |
| Upper abdominal pain | 10 (16) | 0 | 0 |
| Epistaxis | 9 (15) | 1 (2) | 0 |
| Insomnia | 8 (13) | 0 | 0 |
| Peripheral edema | 8 (13) | 0 | 0 |
| Back pain | 7 (11) | 1 (2) | 0 |
| Extremity pain | 7 (11) | 0 | 0 |
| Rash | 7 (11) | 0 | 0 |
| Myalgia | 7 (11) | 1 (2) | 0 |
Includes all preferred terms in system organ class “Infections and Infestations.”
Nonhematologic adverse events were generally mild to moderate in severity and were not dose-limiting. The most prevalent treatment-emergent events (any grade) were infection (42%), diarrhea (40%), nausea (34%), fever (29%), and fatigue (29%). Injection site conditions were common, with erythema reported in 13 patients (21%) and injection site pain and injection site reactions each reported in 6 patients (10%). Grade 3 or 4 nonhematologic events occurring in 2 or more patients were infection (n = 6 [10%]), fatigue (n = 3 [5%]), gastrointestinal hemorrhage (n = 2 [3%]), and increased alanine aminotransferase (n = 2 [3%]).
Nine deaths occurred on study or within the initial 30-day follow-up period. Two of these deaths were considered possibly or probably related to study drug by the investigators, one because of pancytopenia after cycle 3 and one because of pancytopenia with sepsis after cycle 6. In both cases, the patient had achieved a CHR. Cause of death was unknown in 1 patient, and other causes of death (all considered unrelated to study drug) were disease progression/death from disease (n = 4) and cerebral hemorrhage (n = 2). An additional 11 deaths were reported during long-term follow-up by telephone survey after completion of study therapy.
Discussion
Omacetaxine (formerly homoharringtonine) has been studied in the clinic for more than 30 years. There is now a significant body of data in the literature demonstrating that treatment with omacetaxine may be of clinical benefit to patients with CML and other hematologic malignancies.11,12,19-21 However, with the successful introduction of imatinib for the treatment of CML in 2001 and the second-generation TKIs shortly thereafter, interest in the development of omacetaxine in CML declined. Despite the success of TKI-based therapies in treating CML patients, TKI resistance, particularly that conferred by the T315I mutation, represents a growing and as yet unmet challenge in the treatment of CML. The poor prognosis for patients with CML in chronic phase with T315I (median survival of 22 months vs more than 10 years for TKI-responsive CML patients6 ) highlights the need to develop effective treatments for this subset of patients.
Omacetaxine is a first-in-class cephalotaxine that functions as a protein translation inhibitor. The mechanism of action of omacetaxine clearly differentiates it from the TKIs in that it provides clinical activity regardless of BCR-ABL mutation status. Intracellularly, omacetaxine-induced inhibition of protein synthesis results in inhibition of short-lived proteins that regulate proliferation and cell growth.22-24 One such protein is myeloid cell leukemia-1, an important regulator of lymphocytic and hematopoietic stem cell survival.13,24-26 In human primary CML stem cells (CD34+38lo), omacetaxine effectively induced apoptosis and impaired functionality of surviving stem cells as determined by colony-forming cell and long-term culture–initiating cell colony assays. However, both normal and non-CMLCD34+ cells were sensitive to inhibition by omacetaxine.26
The study presented here is the largest prospectively designed trial of CML patients with the T315I mutation reported to date. These patients were heavily pretreated and many had received multiple prior TKIs. In this setting, a CHR lasting at least 8 weeks was observed in 77% of patients. This includes patients who entered the study in CHR (24%), although 40% of those who entered in CHR did so by concomitant use of hydroxyurea. Among those not in CHR at the start of omacetaxine therapy, the rate of CHR was 70% without any other treatment. MCyR was achieved in 27% of patients (16% complete) and an additional 21% achieved a lesser cytogenetic response. These rates are significant considering the lack of treatment options available for this patient population. Furthermore, the overall survival of our patient population exceeded that reported in the literature for a similar patient population, suggesting clinical benefit.6 Patients who achieved CHR showed a clear survival benefit over patients who did not. This is in agreement with reports that suggest that in patients who have experienced failure with prior TKI therapy, CHR and minor cytogenetic responses correlate with improved survival, albeit not as meaningful as those who achieve CCyR.27
Omacetaxine was found to have a manageable safety profile that primarily comprised predictable and reversible myelosuppression. Myelosuppression is a common effect of antileukemic agents and is more frequent in patients who have been pretreated with multiple therapies. Myelosuppression was expected in such a cohort and planned for in the trial design. For example, grade 3/4 thrombocytopenia has been reported in 25%-30% of patients treated with nilotinib or dasatinib after imatinib failure, with neutropenia in 30%-35%, most of it in the first 2-3 months of therapy.3,4,28,29 Corresponding figures in the frontline setting are 10%-20% for thrombocytopenia and 20%-25% for neutropenia.3,4 Importantly, myelosuppression was usually reversible and only infrequently associated with serious infectious or bleeding complications. Adequate monitoring and proper dose adjustments (by reducing the number of days of omacetaxine administration) can alleviate this problem with continued therapy, although it must be considered that the observed reduction in myelosuppression in later cycles is also due in part to selection of patients who were tolerating therapy well.
The most common nonhematologic toxicities (diarrhea, fatigue, pyrexia, nausea, and asthenia) were generally mild to moderate in severity and typically not dose limiting. Earlier trials with intravenous forms of homoharringtonine had raised concerns about potential cardiac toxicity of omacetaxine.11,12 Importantly, a detailed analysis of safety data in the current study did not reveal cardiac toxicity with subcutaneous administration of omacetaxine. In addition, subcutaneous omacetaxine was safely self-administered by most patients at home without direct medical supervision.
Over the trial period, including follow-up time to collect survival information, there were a total of 20 deaths. Two were attributed by the investigators to drug, and both occurred in the context of pancytopenia. These findings underscore the general safety of omacetaxine therapy, but also indicate the need for adequate monitoring of patients, with routine complete blood cell counts (usually weekly during induction and every 1-2 weeks during maintenance, as performed in this study) and proper intervention with antibiotic therapy for any infectious complication. The use of filgrastim to alleviate the impact of neutropenia warrants further investigation.
In summary, this study represents the largest clinical trial of patients with the BCR-ABL T315I mutation. The results achieved with omacetaxine in this patient population suggest that there is a favorable benefit/risk profile for omacetaxine in adult CML patients who have the T315I mutation and have failed prior TKI therapy. Therefore, omacetaxine offers a new treatment alternative for this population of CML patients who otherwise have a poor prognosis and no proven treatment options.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the contributions of the Omacetaxine 202 Study Group investigators: Drs Sikander Ailawadhi, Luke Akard, Maria Baer, Michele Baccarani, Robert Emmons, Gabriel Etienne, Agnès Guerci, François Guilhot, Andrzej Hellmann, Françoise Huguet-Rigal, H. Jean Khoury, Pierre Laneuville, Philipp Le Coutre, Laurence Legros, Armin Leitner, Frederic Maloisel, David Marin, Tamas Masszi, Purvish Parikh, Candido Rivera, Philippe Rousselot, Thierry Facon, Richard Van Etten, Krzysztof Warzocha, Meir Wetzler, and Peter Wiernik.
This work was supported by ChemGenex Pharmaceuticals, a wholly owned subsidiary of Cephalon Inc. Medical writing assistance was provided by Powered 4 Significance LLC and supported by funding from Cephalon Inc.
Authorship
Contribution: J.C., A.-C.B., and H.K. designed the study; J.C., J.H.L., D.R., R.D., C.C., M.M., F.E.N., and H.K. contributed patients; J.C., C.C., N.N., A.R.C., M.M., F.E.N., and H.K. collected the data; J.C., N.N., A.-C.B., A.R.C., F.E.N., and H.K. analyzed the data; J.C., A.R.C., and H.K. wrote the manuscript; and all authors critically reviewed the manuscript and approved the final submitted version of the manuscript.
Conflict-of-interest disclosure: J.C. received research support from Novartis, BMS, Pfizer, Ariad, Deciphera, and ChemGenex, and served as consultant for Novartis, BMS, Pfizer, Ariad, and ChemGenex. C.C. received honoraria from Novartis and BMS. N.N., A.-C.B., and A.R.C. were employed by ChemGenex. M.M. served as speaker for ChemGenex, BMS, and MSD, and is a consultant for BMS, MSD, and Genzyme. F.E.N. served as speaker for ChemGenex, Novartis, and BMS, and is a consultant for Novartis, BMS, and Ariad. H.K. received research support from ChemGenex. The remaining authors declare no competing financial interests.
A complete list of Omacetaxine 202 Study Group investigators appears in the online supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Jorge Cortes, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0428, Houston, TX 77030; e-mail: jcortes@mdanderson.org.