Abstract
Neutropenia is a common side effect of cytotoxic chemotherapy and radiation, increasing the risk of infection in these patients. Here we examined the impact of body temperature on neutrophil recovery in the blood and bone marrow after total body irradiation (TBI). Mice were exposed to either 3 or 6 Gy TBI followed by a mild heat treatment that temporarily raised core body temperature to approximately 39.5°C. Neutrophil recovery was then compared with control mice that received either TBI alone heat treatment alone. Mice that received both TBI and heat treatment exhibited a significant increase in the rate of neutrophil recovery in the blood and an increase in the number of marrow hematopoietic stem cells and neutrophil progenitors compared with that seen in mice that received either TBI or heat alone. The combination treatment also increased G-CSF concentrations in the serum, bone marrow, and intestinal tissue and IL-17, IL-1β, and IL-1α concentrations in the intestinal tissue after TBI. Neutralizing G-CSF or inhibiting IL-17 or IL-1 signaling significantly blocked the thermally mediated increase in neutrophil numbers. These findings suggest that a physiologically relevant increase in body temperature can accelerate recovery from neutropenia after TBI through a G-CSF–, IL-17–, and IL-1–dependent mechanism.
Introduction
Neutropenia is a major dose-limiting toxicity of cancer treatments, including chemotherapy and radiation therapy. Patients undergoing these treatments are often at an increased risk of infection until neutrophil numbers recover. Moreover, because both chemotherapy and total body irradiation (TBI) can damage mucosal and skin barriers, patients are at a greater risk for bacterial, viral, and fungal infections,1,2 the severity of which has been strongly related to the degree and duration of the neutropenia.3 Therefore, it is often critical that steps be taken to speed neutrophil recovery in patients who have received cytotoxic antineoplastic regiments. Currently, recombinant G-CSF is often administered to patients to accelerate neutrophil recovery.4
Under normal physiologic conditions, G-CSF is a powerful regulator of granulopoiesis. G-CSF stimulates the proliferation of granulocytic precursors by inducing the commitment of multipotental progenitor cells to the myeloid lineage5 and by increasing the rate of neutrophil production at the expense of other cell lineages.6 In addition, G-CSF induces neutrophils to egress into the blood7,8 by down-regulating surface expression of the chemokine receptor, CXCR4,9,10 and the production of its ligand, CXCL12 (also known as stromal cell–derived factor-1 or SDF-1), by osteoblasts.11 Despite the value of recombinant G-CSF in recapitulating many of the marrow-stimulating functions of endogenous G-CSF, its use as a single agent in recombinant form is often associated with significant toxicities not normally seen during physiologic production of this cytokine. Rare but serious complications include: splenic rupture, allergic reactions, and vascular events.12 These problems, combined with the high cost of using recombinant growth factors, have led to recommendations to limit the use of these agents for patients at highest risk for infectious complications.3
To identify new strategies that could safely augment neutrophil numbers in patients with treatment-induced neutropenia, we have considered biologic events associated with the physiologic induction of G-CSF activity and increased neutrophil production in response to infection or injury. Recognizing that inflammation and infection often trigger a sustained elevation in body temperature (ie, fever), we wondered whether body temperature elevation itself affects neutrophil production. Indeed, previous research from our laboratory and that of others has revealed the ability of mild, systemic heat treatment to increase neutrophil numbers in the peripheral blood or in tissues of mice given lipopolysaccharide or after bacterial infections.13-16 In view of these data, we tested the possibility that heat treatment given after TBI might accelerate neutrophil recovery in a mouse model of radiation-induced neutropenia. We also explored whether cytokines known to have an involvement in homeostatic regulation of neutrophils could be impacted by elevated body temperature.
Methods
Mice, TBI administration, and heat treatment
Female, 8- to 10-week-old C57BL/6, BALB/c, and C3H/HeJ mice were obtained from the National Cancer Institute and The Jackson Laboratory. Female, 8- to 10-week-old B6.129-IL17RtmlN10 mice (Amgen) were backcrossed into the C57BL/6 background for 9 generations. Mice were maintained in specific pathogen-free facilities and were treated in accordance with the guidelines established by the Animal Care and Use Committee at Roswell Park Cancer Institute (Buffalo, NY) with approval from the Institutional Animal Care and Use Committee. For TBI administration, mice were placed into sterilized Plexiglas pie cages with filtered tops (Braintree Scientific) and secured into a Mark I-68 Cs137 Irradiator (JL Shepherd and Associates) with a rotating raised platform. Mice underwent TBI at 0.6593 Gy/min for a total dose of either 3 or 6 Gy. Mild, systemic heat treatment was given 2 hours after TBI and was administered simply by placing mice in a warmer ambient temperature, as previously described.17,18 To prevent dehydration, mice were given 1 mL of sterile saline intraperitoneally immediately before being placed into preheated microisolator cages in a gravity convection oven with preheated incoming fresh air (Memmert, Model BE500). During heat treatment, the animals were regularly observed through the glass door of the chamber, and body temperature was monitored every 20-30 minutes. Body temperature determinations were made using the BioMedic Data Acquisition System (Model DAS 50001), and mouse temperatures were maintained at 39.5 ± 0.3°C for 6 hours. Nonheated control (normothermic) mice were kept at room temperature and subjected to the same manipulations as that of the heated mice.
Recovery assays and cell counts in the peripheral blood and bone marrow
To determine leukocyte numbers in the peripheral blood of mice, retro-orbital eye bleeds were performed at the indicated time points, and blood was collected into heparin-coated tubes. To determine overall cellular composition in the bone marrow, femurs were removed at indicated time points after treatment and bone marrow cells were flushed out of the femoral stalk with 0.1% BSA in PBS. For both the peripheral blood and the bone marrow, erythrocytes were lysed using ACK lysis buffer, and cells were counted using a hemocytometer. To determine leukocyte recovery in the blood after TBI, leukocytes were isolated and processed 1 to 2 weeks before treatment (to establish baseline counts) and 3, 7, 14, 21, and 28 days after treatment with no animal being bled more than once every 2 weeks or more than 2 times to ensure that excess blood loss did not influence experimental results. Cells were then stained with Ly6G (FITC, clone 1A8; BD Biosciences PharMingen), Ly6C (FITC, clone AL-21; BD Biosciences PharMingen), CD11b (PE, clone M1/70; BD Biosciences PharMingen), CD3ϵ (PE, clone 145.2C11; BD Biosciences PharMingen), B220 (FITC, clone RA3-6B2; BD Biosciences PharMingen), and/or NK1.1 (FITC, clone PK136; BD Biosciences PharMingen) monoclonal antibody and analyzed by flow cytometry using a FACSCalibur flow cytometer. Analysis of data was performed with FCS Express (De Novo Software). The total number of the various leukocyte populations was calculated using the percentage of that population and the total number of leukocytes counted. Percent recovery was determined using the following formula: (number of cells at time point/number of cells at baseline) × 100.
Flow cytometry analysis of HSCs and granulocytic progenitor cells
Analysis of progenitor and hematopoietic stem cell (HSC) numbers by flow cytometry was performed on bone marrow cells harvested from the femurs and tibiae of mice 12 and 48 hours after treatment. Cells were depleted of erythrocytes through lysis in ACK buffer. Using a flow panel similar to Pronk et al,19 cells were stained with primary antibodies to the lineage markers CD3ϵ (biotin, clone 145-2C11; BD Biosciences PharMingen), B220 (biotin, clone RA3-6B2, BD Biosciences PharMingen), GR1 (biotin, clone RB6-8C5; BD Biosciences PharMingen), MAC1 (biotin, clone M1/70, BD Biosciences PharMingen), and Ter119 (biotin, clone TER-119; BD Biosciences PharMingen) and then the secondary antibody streptavidin SA-AF700 (eBioscience) to determine the lineage positive/negative populations. To determine hematopoietic stem and progenitor cell numbers, the following antibodies were used: Sca-1 (PE-Cy7, clone D7; eBioscience), c-kit (allophycocyanin-EFlour780, clone 2B8; eBioscience), CD150 (PE or peridinin chlorophyll protein-Cy505, clone mShad150; eBioscience), CD105 (PE, MJ7/18; eBioscience), and/or CD16/CD32 (allophycocyanin, clone 93; eBioscience). All samples were run on the LSR II flow cytometer (BD Biosciences PharMingen) and analyzed using FlowJo Version 9.3.2 software.
Bone marrow CFU assays
Femurs of mice were removed 1, 2, and 4 days after 3 or 6 Gy TBI. Bone marrow was flushed out of the bone shaft using IMDM, and erythrocytes were lysed using ACK buffer. Mouse colony-forming unit (CFU) assays were performed according to directions (MethoCult; StemCell Technologies) by mixing 2 × 104 bone marrow cells in methylcellulose containing rmSCF, rmIL-3, and rhIL-6. Colonies were counted and scored on coded plates on day 12 to ensure for unbiased counts. The number of CFU per femur was calculated. Colony types were determined by morphologic examination using an inverted microscope.
Determining cytokine concentrations
Blood, spleen, liver, bone marrow, mesenteric lymph nodes, and the intestine was collected from mice 3, 6, 9, 12, 16, 20, and 24 hours after treatment. Blood was allowed to clot for 30 minutes at room temperature then spun down at 1500g for 10 minutes and serum was collected. Spleen, liver, bone barrow, mesenteric lymph nodes, and intestine were placed in CellLytic MT Mammalian Tissue Lysis with protease inhibitor cocktail (Sigma-Aldrich) on ice and were lysed. Samples were then centrifuged at 12 000g for 10 minutes at 4°C, supernatants were collected, and protein concentrations were determined using a Bio-Rad Protein Assay (Bio-Rad). G-CSF (Invitrogen), IL-17 (R&D Systems), TNF-α (BioLegend), IL-6 (BD Biosciences PharMingen), IL-1β (BioLegend), IL-1α (R&D Systems), TGF-β (R&D Systems), KC/CXCL1 (R&D Systems), and IL-23 (R&D Systems) ELISAs were performed according to the manufacturer's instructions using serum or protein lysates.
In vivo G-CSF neutralization and IL-1 receptor blockade
To block G-CSF function, 250 μg of anti–mouse G-CSF antibodies (clone 9B4CSF; eBioscience) was injected intraperitoneally immediately after TBI and again after heat treatment. Control animals were treated with rat IgG2a, κ antibodies (eBioscience) in the same manner. To block IL-1RI, mice were injected intraperitoneally with 10 mg/kg recombinant mouse IL-1Ra (ProSpec) starting immediately before TBI and again every 12 hours for 3 days. Control animals were injected intraperitoneally with saline. Neutrophil counts were performed on days 7 and 14 after neutralization or receptor blockade.
Apoptosis detection
The intestines of mice that were left untreated or treated with heat alone, 3 Gy TBI alone, or TBI followed 2 hours later with a heat treatment were resected and flushed with cold PBS 12 and 24 hours after treatment then fixed in 10% (volume/volume) neutral buffered formalin and paraffin-embedded. ApopTag staining was performed on tissue cut into 5-μm-thick paraffin-embedded tissue sections and were stained according to the manufacturer's directions (ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit; Millipore).
Statistics
Student t test was used for comparing the means of 2 separate groups. ANOVA comparison tests were used when comparing the means of multiple treatment groups to the untreated group. Statistically significant differences were defined as a P ≤ .05.
Results
Heat treatment significantly enhances neutrophil recovery in the blood after TBI
To examine the effect of elevated body temperature on leukocyte recovery in the peripheral blood after TBI, C57BL/6 mice were exposed to 3 Gy TBI and then were either externally warmed, resulting in an increase in core body temperature from ∼ 37°C to ∼ 39.5°C or were maintained under normothermic conditions. Peripheral blood leukocyte numbers were evaluated on days 3, 7, and 14 after TBI (Figure 1A). As expected, at 3 days after TBI, overall leukocyte numbers were significantly reduced in the peripheral blood of mice exposed to TBI alone compared with counts from nonirradiated control animals. However, by day 7 and 14 in mice given heat treatment after TBI, we observed a significant increase in leukocyte numbers compared with those given TBI alone (∼ 1.7-fold increase on day 7 and ∼ 1.6-fold increase on day 14). Heat treatment alone had no effect on leukocyte numbers at the time points examined (Figure 1A).
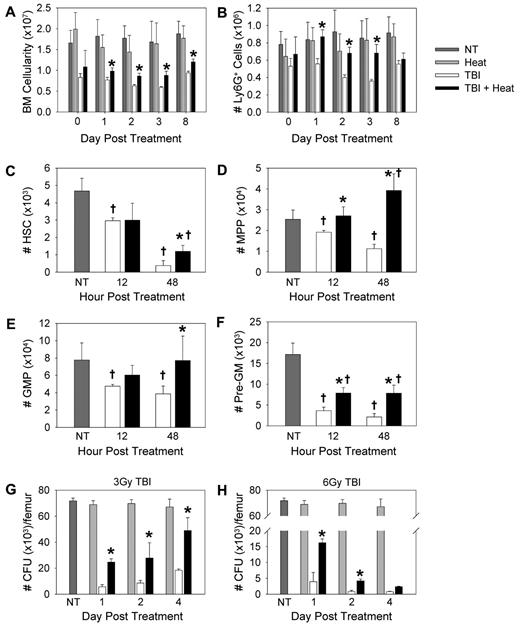
Neutrophil numbers in the peripheral blood are increased when TBI is followed by heat treatment. Mice were left untreated (NT), treated with heat alone, with TBI alone, or with TBI followed 2 hours later with mild heating sufficient to raise their body temperature to approximately 39.5°C for 6 hours. The total number of leukocytes (A) and the total number of Ly6G+ cells (B-D) were calculated in C57BL/6 (A-B), BALB/c (C), and C3H/HeJ (D) mice treated with 3 Gy TBI with or without heat treatment. Neutrophil numbers were calculated using the percentage of Ly6G+ cells and the total leukocyte cell counts. (E) Total number of Ly6G+ cells was calculated in C57BL/6 mice treated with 6 Gy TBI with or without heat treatment. Each graph is representative of at least 3 separate experiments. n = 5 mice per group. Statistical analysis comparing TBI alone and TBI followed by heat treatment was performed: *P < .02 (Student t test).
Neutrophil numbers in the peripheral blood are increased when TBI is followed by heat treatment. Mice were left untreated (NT), treated with heat alone, with TBI alone, or with TBI followed 2 hours later with mild heating sufficient to raise their body temperature to approximately 39.5°C for 6 hours. The total number of leukocytes (A) and the total number of Ly6G+ cells (B-D) were calculated in C57BL/6 (A-B), BALB/c (C), and C3H/HeJ (D) mice treated with 3 Gy TBI with or without heat treatment. Neutrophil numbers were calculated using the percentage of Ly6G+ cells and the total leukocyte cell counts. (E) Total number of Ly6G+ cells was calculated in C57BL/6 mice treated with 6 Gy TBI with or without heat treatment. Each graph is representative of at least 3 separate experiments. n = 5 mice per group. Statistical analysis comparing TBI alone and TBI followed by heat treatment was performed: *P < .02 (Student t test).
To determine which leukocyte populations were altered by heat treatment, the recovery of neutrophil (Ly6G+), B-cell (B220+), T-cell (CD3+), monocyte (Ly6C+, CD11b+), and NK-cell (NK1.1+) populations after 3 Gy TBI was determined by flow cytometry. The recovery of B-cell, T-cell, monocyte, and NK-cell populations was unaffected by heat treatment after TBI (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In contrast, neutrophil numbers in the peripheral blood were significantly increased in animals heated after TBI compared with TBI alone (∼ 6.1-fold increase as early as 3 days after treatment; Figure 1B). This finding was reflected in an increase in neutrophil recovery seen starting as early as 3 days after TBI exposure (supplemental Figure 2A). To ensure that the thermally mediated enhancement in neutrophil recovery was not confined to the C57BL/6 mouse model, neutrophil recovery assays were performed in BALB/c and C3H/HeJ mice. These 3 strains of mice are known to exhibit variations in their sensitivities to TBI with C57BL/6 mice being the least sensitive and BALB/c mice being the most sensitive because of a double-strand DNA relative repair defect.20,21 Similar to that seen in C57BL/6 mice, both BALB/c and C3H/HeJ mouse models exhibited enhanced neutrophil recovery in the peripheral blood when 3 Gy TBI was followed with heat treatment (Figure 1C-D; supplemental Figure 2B-C).
To determine whether heat treatment can impact neutrophil numbers after a higher dose of radiation, C57BL/6 mice were exposed to 6 Gy TBI. At the 2- and 4-week time point, heat treatment was again associated with significantly increased neutrophil numbers in the blood after TBI compared with animals treated with TBI alone (Figure 1E). This was also demonstrated by a significant enhancement in neutrophil recovery (∼ 3.7-fold increase) seen 2 weeks after TBI and heat treatment (supplemental Figure 2D).
Heat treatment increases the number of granulocytic progenitors in the bone marrow after TBI
Because overall cellularity and neutrophil numbers increased in the bone marrow when 3 Gy TBI was followed with heat treatment (Figure 2A-B), we next examined HSC and granulocytic progenitor cell numbers phenotypically in the bone marrow. HSCs (Lin−, Sca-1HI, c-kitHI [LSK], CD150+, CD105HI; Figure 2C), multipotent progenitor (LSK, CD150−, CD105LO; Figure 2D), granulocyte-macrophage progenitor (Lin−, Sca-1−, c-kitHI, FcγHI, CD150−; Figure 2E), and pre-granulocyte-macrophage progenitor (Lin−, Sca-1−, c-kitHI, Fcγ−, CD150−, CD105−/LO; Figure 2F) numbers were increased in the bone marrow of mice that received heat treatment after TBI exposure compared with those given TBI alone.
Mild heating increased the number of neutrophils and granulocytic progenitors in the bone marrow after TBI. (A) Bone marrow was isolated from the femur and tibia of C57BL/6 mice that received 3 Gy TBI with or without heat treatment, and then erythrocytes were lysed and total number of bone marrow cells was quantified. (B) The percentage of Ly6G+ cells was determined by flow cytometry. Neutrophil numbers were calculated using the percentage of Ly6G+ cells and the total bone marrow cell counts. (C-F) C57BL/6 mice were left untreated (NT), treated with TBI alone, or with 3 Gy TBI followed 2 hours later with a heat treatment. At 12 and 48 hours after treatment, bone marrow was isolated from one femur and tibia from each mouse and filtered. Erythrocytes were lysed and total number of cells quantified. Number of HSCs (C), multipotent progenitor (D), granulocyte-macrophage progenitor (E), and pre-granulocyte-macrophage progenitor (F) cells were determined using the percentage of each cell population as determined by flow cytometry and the overall bone marrow cell count. (G-H) CFU assays were performed using 2 × 104 bone marrow cells from mice given either 3 Gy (G) or 6 Gy (H) TBI with or without heat treatment. The bone marrow cells were mixed in methylcellulose with rmSCF, rmIL-3, and rhIL-6. On day 12, colonies were scored on coded plates for unbiased counts. Colony types were identified on a morphologic basis. The number of CFUs per femur was calculated. Each graph is representative of at least 2 separate experiments. n = 3-5 mice per group. †P < .04, compared with untreated mice. *P < .04, TBI alone mice versus TBI followed by heat treatment.
Mild heating increased the number of neutrophils and granulocytic progenitors in the bone marrow after TBI. (A) Bone marrow was isolated from the femur and tibia of C57BL/6 mice that received 3 Gy TBI with or without heat treatment, and then erythrocytes were lysed and total number of bone marrow cells was quantified. (B) The percentage of Ly6G+ cells was determined by flow cytometry. Neutrophil numbers were calculated using the percentage of Ly6G+ cells and the total bone marrow cell counts. (C-F) C57BL/6 mice were left untreated (NT), treated with TBI alone, or with 3 Gy TBI followed 2 hours later with a heat treatment. At 12 and 48 hours after treatment, bone marrow was isolated from one femur and tibia from each mouse and filtered. Erythrocytes were lysed and total number of cells quantified. Number of HSCs (C), multipotent progenitor (D), granulocyte-macrophage progenitor (E), and pre-granulocyte-macrophage progenitor (F) cells were determined using the percentage of each cell population as determined by flow cytometry and the overall bone marrow cell count. (G-H) CFU assays were performed using 2 × 104 bone marrow cells from mice given either 3 Gy (G) or 6 Gy (H) TBI with or without heat treatment. The bone marrow cells were mixed in methylcellulose with rmSCF, rmIL-3, and rhIL-6. On day 12, colonies were scored on coded plates for unbiased counts. Colony types were identified on a morphologic basis. The number of CFUs per femur was calculated. Each graph is representative of at least 2 separate experiments. n = 3-5 mice per group. †P < .04, compared with untreated mice. *P < .04, TBI alone mice versus TBI followed by heat treatment.
To determine the effect of heating on the ability of stem and progenitor cells to proliferate and differentiate into granulocytes or monocytes, we performed clonogenic assays. Bone marrow cells from control mice, mice treated with heat alone, mice treated with 3 or 6 Gy TBI alone, and mice treated with TBI followed by heat treatment were cultured with methylcellulose supplemented with rmSCF, rmIL-3, and rhIL-6 (Figure 2G-H). Heat treatment after 3 or 6 Gy TBI resulted in a significant increase in the total number of bone marrow-derived hematopoietic colonies compared with TBI alone. This increase appeared to be primarily from an enhancement in CFU-G and CFU-GM numbers (supplemental Figure 3). Because G-CSF is a potent stimulator of granulopoiesis and neutrophil release from the bone marrow to the periphery, we next examined whether heat treatment altered the concentration of G-CSF after TBI.
G-CSF is required for the thermally mediated enhancement in neutrophil recovery after TBI
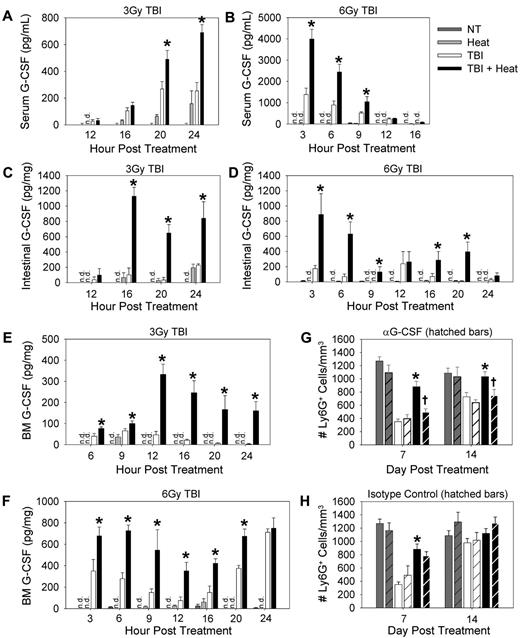
To assess the impact of heat treatment on G-CSF concentrations, C57BL/6 mice were treated with 3 or 6 Gy TBI followed with heat treatment, and ELISAs were performed on serum, bone marrow, and intestinal tissue lysates at various time points after TBI exposure. In all sites tested, heating after TBI significantly increased G-CSF concentrations compared with TBI alone (Figure 3A-F). To test the role of G-CSF in the thermally mediated enhancement of neutrophil recovery seen after 3 Gy TBI, we examined neutrophil numbers in the blood of mice treated with or without neutralizing antibodies against G-CSF given immediately after TBI and again 2 hours after heat treatment. As shown previously (Figure 1B), heat treatment when given after TBI significantly enhanced neutrophil numbers compared with mice that received TBI alone (Figure 3G-H). However, when G-CSF was neutralized, neutrophil numbers were equivalent between the mice that received heat treatment or TBI alone (Figure 3G), suggesting that the thermal-mediated increase in neutrophil numbers after TBI is G-CSF–dependent. Control isotype antibody had no effect on neutrophil numbers (Figure 3H). These observations led us to ask the question of what may be driving the thermal-mediated increase in G-CSF concentrations in our experimental model. Given the fact that IL-17 signaling is known to stimulate an increase in systemic G-CSF levels, thus increasing neutrophil numbers in the periphery,22 we turned our attention to determining whether IL-17 was mechanistically involved in mediating the thermal effect on neutrophil numbers after TBI.
G-CSF is required for the thermally mediated acceleration of neutrophil recovery after TBI. G-CSF concentrations were determined by ELISA in the serum (A-B), intestinal lysates (50 μg; C-D), and bone marrow lysates (40 μg; E-F) of C57BL/6 mice given either 3 Gy (A,C,E) or 6 Gy (B,D,F) TBI. (G-H) Ly6G+ cells counts were performed in mice that have been given anti–G-SF neutralizing antibody (G) or isotype control antibody (H) given immediately after 3 Gy TBI and 2 hours after heat treatment. Hatched bars indicate mice that received antibody treatment. Each graph is a representative of at least 3 separate experiments. n = 3-5 mice per group. *P < .03, TBI alone group versus TBI followed by heat. †P < .04, TBI + heat + anti–G-SF group versus the TBI + heat group. n.d. indicates not detected.
G-CSF is required for the thermally mediated acceleration of neutrophil recovery after TBI. G-CSF concentrations were determined by ELISA in the serum (A-B), intestinal lysates (50 μg; C-D), and bone marrow lysates (40 μg; E-F) of C57BL/6 mice given either 3 Gy (A,C,E) or 6 Gy (B,D,F) TBI. (G-H) Ly6G+ cells counts were performed in mice that have been given anti–G-SF neutralizing antibody (G) or isotype control antibody (H) given immediately after 3 Gy TBI and 2 hours after heat treatment. Hatched bars indicate mice that received antibody treatment. Each graph is a representative of at least 3 separate experiments. n = 3-5 mice per group. *P < .03, TBI alone group versus TBI followed by heat. †P < .04, TBI + heat + anti–G-SF group versus the TBI + heat group. n.d. indicates not detected.
IL-17 drives the G-CSF–mediated increase in neutrophil recovery seen when heat treatment is given after TBI
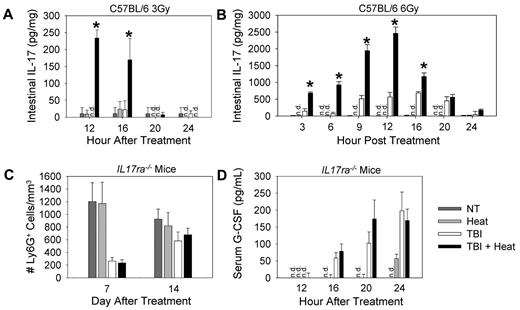
To determine whether IL-17 concentrations are being altered by heat treatment, ELISAs were performed on serum, spleen, liver, intestine, mesenteric lymph node, and bone marrow lysates from mice that received no treatment, heat treatment alone, 3 or 6 Gy TBI alone, or TBI followed by heat treatment. In the intestine, heat or TBI treatment alone resulted either in undetectable or low concentrations of IL-17 (Figure 4A-B). However, when TBI was followed by heat treatment, there was a significant increase in IL-17 concentrations in the intestine. IL-17 was not detectable in the serum, spleen, liver, mesenteric lymph nodes, and bone marrow (data not shown), suggesting that the thermally enhanced IL-17 response was restricted to the intestinal microenvironment out of the tissue sites examined.
Increased neutrophil recovery after heat treatment is dependent on IL-17. C57BL/6 or IL17ra−/− mice were left untreated (NT), treated with heat treatment alone, TBI alone, or TBI followed 2 hours later with a 6-hour heat treatment. (A-B) Cell lysates from intestine of C57BL/6 mice that received either 3 Gy (A) or 6 Gy (B) TBI with or without heat treatment were collected, and ELISAs were performed using 50 μg lysate per well to determine concentrations of IL-17. (C) Ly6G+ cell counts were performed in IL17ra−/− mice 7 and 14 days after TBI. (D) Serum from IL17ra−/− mice that received 3 Gy TBI with or without heat treatment was collected, and ELISAs were performed to determine G-CSF concentrations. Each graph is a representative of at least 2 separate experiments. n = 5 mice per group. *P < .01, TBI alone group vs TBI followed by mild heating. n.d. indicates not detected.
Increased neutrophil recovery after heat treatment is dependent on IL-17. C57BL/6 or IL17ra−/− mice were left untreated (NT), treated with heat treatment alone, TBI alone, or TBI followed 2 hours later with a 6-hour heat treatment. (A-B) Cell lysates from intestine of C57BL/6 mice that received either 3 Gy (A) or 6 Gy (B) TBI with or without heat treatment were collected, and ELISAs were performed using 50 μg lysate per well to determine concentrations of IL-17. (C) Ly6G+ cell counts were performed in IL17ra−/− mice 7 and 14 days after TBI. (D) Serum from IL17ra−/− mice that received 3 Gy TBI with or without heat treatment was collected, and ELISAs were performed to determine G-CSF concentrations. Each graph is a representative of at least 2 separate experiments. n = 5 mice per group. *P < .01, TBI alone group vs TBI followed by mild heating. n.d. indicates not detected.
To determine whether the heat-mediated enhancement of neutrophil recovery after TBI is IL-17 dependent, we compared neutrophil numbers in IL17ra−/− mice subjected to TBI with or without heat treatment. Heat treatment had no effect on neutrophil numbers in the peripheral blood (Figure 4C) after 3 Gy TBI in IL17ra−/− mice, indicating that IL-17 signaling was required for the thermal enhancement in neutrophil recovery. Because G-CSF was also required to observe the effect of heat treatment after TBI and because IL-17 is a potent regulator of G-CSF concentrations, we next examined G-CSF levels in the IL17ra−/− mice and observed no difference in G-CSF concentrations in the serum of mice receiving 3 Gy TBI alone or TBI followed by heat treatment (Figure 4D). These data suggest that the thermal-mediated increase in G-CSF concentrations is IL-17 dependent.
G-CSF is produced by both mesenchymal and myeloid cells, and its concentration is increased by IL-17.23-25 To examine whether IL-17 signaling in cells of mesenchymal and/or hematopoietic origin was required for the thermal-mediated enhancement of neutrophil recovery after TBI, we generated chimeras in which donor bone marrow cells (1 × 106) from C57BL/6 or IL17ra−/− mice were transplanted into lethally irradiated C57BL/6 or IL17ra−/− recipient mice. Neutrophil numbers in the peripheral blood were then examined 12 weeks after transplantation on mice that were left untreated, treated with 3 Gy TBI alone, or treated with TBI followed by heat treatment. Heat treatment significantly increased the number of neutrophils after TBI when host mice (both IL17ra−/− and C57BL/6) received C57BL/6 bone marrow (supplemental Figure 4A,C). However, when IL17ra−/− bone marrow was transplanted into C57BL/6 or IL17ra−/− hosts, no effect from heat treatment was seen (supplemental Figure 4B,D). These findings suggest that the G-CSF–mediated increase in neutrophil numbers seen when heat treatment is given after TBI requires IL-17 signaling in hematopoietic cells.
Thermally enhanced neutrophil recovery after TBI is dependent on IL-1
Several cytokines can stimulate IL-17 production by immune or epithelial cells, including IL-23, TGF-β, IL-6, TNF-α, and IL-1.26-32 To determine whether the concentration of any of these cytokines is altered when heat treatment is given after TBI, ELISAs were performed using intestine tissue lysates harvested from mice that received TBI alone, heat treatment alone, or TBI followed by heat treatment. IL-23 and TGF-β were not detected in any of the samples tested (data not shown). After heat treatment, IL-6 levels were significantly decreased after TBI compared with animals that received TBI alone (supplemental Figure 5A). TNF-α was detected in both the TBI alone and the TBI followed by heat treatment groups; however, there was no significant difference between the 2 groups (supplemental Figure 5B). Intestinal IL-1β and IL-1α concentrations in mice that received heat treatment after 3 or 6 Gy TBI were greater than mice that were given TBI alone (Figure 5A-D).
From these observations, we next chose to investigate whether IL-1 contributed to the thermally mediated enhancement in neutrophil recovery by inhibiting IL-1α and IL-1β signaling by blocking IL-1RI using rmIL-1Ra.33 Neutrophil numbers were examined in mice that received rmIL-1Ra immediately before TBI and again every 12 hours for 3 days. As seen earlier (Figure 1B), mice that received heat treatment after TBI had increased numbers of neutrophils compared with mice that received TBI alone (Figure 5E compare solid bars). However, when IL-1 signaling was blocked (Figure 5E hatched bars), thermally enhanced neutrophil production was significantly reduced, suggesting that IL-1 signaling is required for the thermal-mediated increase in neutrophil recovery. Neutralization of IL-1β by treating mice with anti–IL-1β antibodies immediately after TBI and again after heat treatment only partially inhibited neutrophil recovery when heat treatment was given after TBI (supplemental Figure 6A-B). In addition, the neutralization of IL-1β also partially inhibited the thermally mediated increase in IL-17 concentrations after TBI (supplemental Figure 6C). These findings suggest that IL-1α along with IL-1β may be necessary to mediate the thermal enhancement in neutrophil recovery.
IL-1 is important for the thermally mediated increase in neutrophil numbers after TBI. Cell lysates from the intestine of C57BL/6 mice that received either 3 Gy (A,C) or 6 Gy (B,D) TBI with or without heat treatment were collected, and ELISAs were performed using 50 μg lysate per well to determine concentrations of IL-1β (A-B) or IL-1α (C-D). (E) Blocking IL-1 activity with rmIL-1Ra (given immediately before 3 Gy TBI and again every 12 hours for 3 days) reduces the effect of heating. Ly6G+ cell counts were performed on mice that received saline (solid bars) or rmIL-1Ra (hatched bars) in no treatment, TBI alone and TBI + heat groups. Each graph is a representative of at least 2 Ly6G+ cell counts were performed on mice that received rmIL-1Ra immediately before 3 Gy TBI separate experiments. n = 5 mice per group. *P < .03, TBI alone group vs TBI followed by heat treatment. †P < .04, TBI + heat group vs TBI + heat + rmIL-1Ra group. n.d. indicates not detected.
IL-1 is important for the thermally mediated increase in neutrophil numbers after TBI. Cell lysates from the intestine of C57BL/6 mice that received either 3 Gy (A,C) or 6 Gy (B,D) TBI with or without heat treatment were collected, and ELISAs were performed using 50 μg lysate per well to determine concentrations of IL-1β (A-B) or IL-1α (C-D). (E) Blocking IL-1 activity with rmIL-1Ra (given immediately before 3 Gy TBI and again every 12 hours for 3 days) reduces the effect of heating. Ly6G+ cell counts were performed on mice that received saline (solid bars) or rmIL-1Ra (hatched bars) in no treatment, TBI alone and TBI + heat groups. Each graph is a representative of at least 2 Ly6G+ cell counts were performed on mice that received rmIL-1Ra immediately before 3 Gy TBI separate experiments. n = 5 mice per group. *P < .03, TBI alone group vs TBI followed by heat treatment. †P < .04, TBI + heat group vs TBI + heat + rmIL-1Ra group. n.d. indicates not detected.
Heat treatment does not increase intestinal damage after TBI exposure
Because granulopoiesis and neutrophil release from the bone marrow can also be increased after injury, we evaluated the possibility that heat exposure might be inducing an increase in circulating neutrophil numbers via aggravation of TBI-induced intestinal injury. To evaluate this possibility, intestinal tissue from mice treated with TBI alone, heat alone, and TBI followed by heat treatment was collected 12 and 24 hours after radiation, and the number of apoptotic cells in the intestine was evaluated by immunohistochemistry. Heating alone had no effect on the number of apoptotic cells detected in the intestine (Figure 6). As expected, TBI increased the number of apoptotic cells in the intestine 12 hours after radiation exposure (∼ 4.3-fold increase over untreated); however, heat treatment after TBI did not further increase the number of cells undergoing apoptosis. By 24 hours after TBI, the number of apoptotic cells in the TBI and TBI plus heat groups were similar to that observed in mice that received no treatment. There was no noticeable increase in leukocyte infiltration within the intestinal tissues; further, we noted a significant decrease in intestinal IL-6 and KC (a potent neutrophil chemoattractant; also known as CXCL1) concentrations in mice given heat treatment after TBI versus TBI alone (supplemental Figure 5).
Mild heating does not further increase the number of apoptotic cells in the intestine of mice after TBI. C57BL/6 mice were left untreated (NT) or treated with heat alone, 3 Gy TBI alone, or TBI followed 2 hours later with a 6-hour heat treatment. Intestine samples were collected 12 and 24 hours after treatment, and formalin-fixed, paraffin-embedded sections were stained for apoptosis. The number of apoptotic cells per field (when counted at 40×) was quantified at both 12 and 24 hours after treatment. Ten fields were counted per slide. Arrows indicate positive staining. n = 3 mice per group. Images are intestine samples from 12 hours after treatment (original magnification ×40).
Mild heating does not further increase the number of apoptotic cells in the intestine of mice after TBI. C57BL/6 mice were left untreated (NT) or treated with heat alone, 3 Gy TBI alone, or TBI followed 2 hours later with a 6-hour heat treatment. Intestine samples were collected 12 and 24 hours after treatment, and formalin-fixed, paraffin-embedded sections were stained for apoptosis. The number of apoptotic cells per field (when counted at 40×) was quantified at both 12 and 24 hours after treatment. Ten fields were counted per slide. Arrows indicate positive staining. n = 3 mice per group. Images are intestine samples from 12 hours after treatment (original magnification ×40).
Discussion
We show here that raising body temperature after TBI can accelerate recovery of circulating neutrophils in a G-CSF–, IL-17–, and IL-1–dependent manner. Because this mild heating protocol does not appear to exacerbate the level of radiation damage to the intestinal microenvironment, mild warming strategies may be a viable therapeutic approach that could potentially reduce the risk of infection after treatment-induced neutropenia through the physiologically stimulated amplification of known cytokine-dependent neutrophil homeostatic pathways. Moreover, it appears that even a single heat treatment can lead to accelerated neutrophil recovery after TBI. Because G-CSF is typically administered daily for 10-14 days to lessen the severity of neutropenia, it will be important to explore what differences these 2 therapies have on the kinetics of hematopoiesis, the survival of the hematopoietic stem and progenitor cells after TBI, and any change in neutrophil migration patterns that may influence neutrophil numbers in the periphery.
Normally, endogenous G-CSF levels are tightly regulated because of its critical role in maintaining neutrophil numbers in the periphery. IL-17 signaling regulates G-CSF expression, as well as the expression of other cytokines that increase circulating neutrophil numbers.25,27,34-36 The effect of IL-17 on neutrophil numbers stems from its ability to increase G-CSF mRNA stability that ultimately leads to a systemic increase in G-CSF concentrations.23 Mice lacking IL-17 have previously been shown to be more sensitive to TBI than normal mice in that they have a reduced LD50 from 8.5 Gy in female C57BL/6 mice to 7.25 Gy in female IL17ra−/− mice and also experience increased myelotoxicity after radiation confirming an important role of IL-17 in protection from radiation-induced hematologic damage.37 Notably, we found significantly enhanced IL-17 production in the intestine in our animal model when TBI was followed by heat treatment within the first 24 hours after radiation, whereas, by themselves, TBI or heat induces low levels of IL-17 production at the radiation doses used. It is unclear whether TBI-induced intestinal damage or TBI-induced neutropenia, or both, are required to drive this IL-17 production. However, it is intriguing that an early thermally induced change in IL-17 concentrations resulting from a temporary elevation in body temperature may influence a long-lasting increase in neutrophil numbers in the peripheral blood after TBI. This observation warrants further experimentation to measure cytokine concentrations after longer time points to determine whether there are later secondary effects of heat treatment that alter the cytokine milieu after TBI.
The intestine appears to be a primary site for IL-17 production in mice that were exposed to TBI/heat treatment, although the precise cellular source remains to be identified. Several cell types normally found in the intestine can produce IL-17, including the Th17 cells, γδ T cells, NK cells, NK-T cells, and Paneth cells, a specialized epithelial cell of the small intestinal crypts that plays a role in the innate immune response.23,38 These, or other as yet unidentified cell types, could be the thermally sensitive target. IL-17 production is known to be up-regulated in many of these cells by TNF-α, IL-1, IL-6, TGF-β, and/or IL-23 signaling as well as by binding of bacterial products to Toll-like receptors.23,26,28-32 We observed a significant increase in IL-1β and IL-1α concentrations after TBI/heat treatment in the intestinal tissue; importantly, blocking IL-1RI led to a significant loss of the thermally mediated enhancement of neutrophil recovery (Figure 5). Further experiments are needed to determine whether IL-1 signaling leads to the thermally mediated increase in IL-17 concentrations, although IL-1β neutralization studies suggest a partial role for this cytokine in regulating IL-17 in our experimental model (supplemental Figure 6). Additional study is also needed to identify specific heat-sensitive steps in this process, including a better understanding of why IL-17 production in the intestinal microenvironment may be more thermally sensitive than in other tissue sites.
It is well known that IL-1 therapy can accelerate hematopoietic recovery after radiation.39-41 Shen et al reported that heat treatment (40°C for 1 hour) could elevate IL-1 concentrations and concurrently saw a significant increase in white blood cell counts in the thymus, spleen, and bone marrow of mice when heat was given 20 hours before 9 Gy TBI.42 However, which leukocyte population was being thermally regulated and whether IL-1 was directly responsible for this effect were not examined. More recent studies that used a similar heating protocol given 20 hours before TBI demonstrated that IL-1α, IL-6, and TNF-α levels were elevated in mice that received heat treatment and were associated with an increase in white blood cells in the spleen, thymus, bone marrow, and peripheral blood.43 In our experimental model, we observed no change in TNF-α concentrations, a decrease in IL-6 concentrations, and an increase in both G-CSF and IL-17. Therefore, we hypothesize that the thermal alteration of cytokine concentrations differs based on the timing of heat administration in relation to TBI. However, both heating protocols appear to be associated with a thermal-mediated increase in IL-1 concentrations when in combination with TBI, suggesting that the production of this particular cytokine is highly heat sensitive.
IL-1, like IL-17, can also directly enhance G-CSF production.44 Whether IL-1α and/or IL-1β are leading to a direct increase in G-CSF concentrations in our model remains unclear, but we think this is unlikely because IL17ra−/− mice exhibit no change in serum G-CSF concentrations after TBI/heat treatment. IL-1 signaling is also associated with an increase in IL-6 concentrations.45 However, in our experimental model, we saw a significant decrease in IL-6 levels after TBI/heat treatment. Further experimentation is needed to determine why IL-6 concentrations are decreased and whether this change plays a role in the thermal enhancement of neutrophil recovery after TBI.
Although a role for granulopoiesis in neutrophil reconstitution is supported by the increase in G-CSF concentrations seen with TBI is followed by heat treatment (Figure 3), the rapid increase in progenitor cell numbers observed as early as 12 hours (Figure 2) suggests that granulopoiesis alone may not be solely responsible for the thermal effects in the bone marrow. This suggests that there may be at least 2 mechanisms by which heat treatment increases neutrophil progenitor numbers: (1) increased survival of existing progenitor cells because of protection from radiation damage and/or (2) increased production of new neutrophil progenitors. To clearly define which, if not both, events are occurring in our experimental model, further experimentation is required.
Finally, we have wondered why neutrophil homeostasis may be sensitive to body temperature elevation. One possibility is that, because a sustained elevation in body temperature, or fever, occurs naturally after infection and inflammation (events that are associated with changes in neutrophil homeostasis), our mild heating protocol may be activating evolutionarily conserved, thermally sensitive points in this endogenous physiologic program. Body temperature elevation may act in synchrony with stimuli from infection or injury (in this case TBI) to alert the marrow of the potential need for accelerated neutrophil production. Several proinflammatory mediators are up-regulated after infection, such as IL-1, TNF-α, and IL-17, all of which are known to induce G-CSF expression, which in turn alters granulopoiesis,46 ultimately increasing neutrophil numbers in the periphery. Intriguingly, some of these same proinflammatory mediators are themselves potent pyrogens,45,47 known to drive the naturally occurring increase in body temperature after infection. Therefore, these data may reveal the existence of novel, dynamically regulated, and thermally sensitive interactions among cytokines involved with body temperature regulation and hematopoiesis, which comes into play after stress.
Although further study is needed to determine how humans will respond to heat treatment after cytotoxic therapies and to identify optimal heating schedules/protocols, our findings suggest overall that mild thermal therapy should be tested as a feasible strategy to raise endogenous G-CSF levels in a controlled physiologic manner, which may reduce the need for additional recombinant cytokine therapy.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Bonnie Hylander, Sandra Gollnick, and Andrei Gudkov for helpful discussions; Jason Eng and Katie Kokolus for suggestions on the manuscript; Dr Melissa Grimm for providing the IL17ra−/− mice; and Kelly Dunbar and Jeanne Prendergast for laboratory assistance.
This work was supported by the National Institutes of Health (RO1CA135368, T32 CA085183-10, NAID RO1A1079253) and the Roswell Park Alliance Foundation.
National Institutes of Health
Authorship
Contribution: M.L.C. designed and performed experiments and assisted in writing the manuscript; M.J.N., T.A.M., and C.S.-R. performed experiments and provided discussion; B.H.S. provided discussion and technical advice; P.L.M. provided significant discussion and technical advice; and E.A.R. assisted in the design of experiments, provided discussion, and assisted in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elizabeth A. Repasky, Department of Immunology, Roswell Park Cancer Institute, Buffalo, NY 14263; e-mail: elizabeth.repasky@roswellpark.org.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal