Abstract
Plasmacytoid dendritic cells (PDCs) produce type I interferons (IFNs) in response to viral nucleic acids to exert antiviral immunity. However, PDCs are related to the progress and severity of autoimmune diseases, such as systemic lupus erythematosus, because they respond to host DNA. Therefore, the regulation of PDC activation is critical for maintaining adequate immune responses. Here we show that an inhibitory major histocompatibility complex class I receptor, paired immunoglobulin-like receptor B (PIR-B), suppressed Fms-like tyrosine kinase 3 ligand-induced PDC differentiation in BM cells, as well as Toll-like receptor 9-mediated IFN-α production by PDCs, through the dephosphorylation of STAT1/STAT2. In particular, PIR-B inhibited IFN-α–mediated STAT phosphorylation, suggesting that PIR-B negatively regulates the positive feedback mechanism of IFN-α secretion triggered by Toll-like receptor 9. These results demonstrate a novel regulatory role for PIR-B in PDCs.
Introduction
Plasmacytoid dendritic cells (PDCs) are DC subsets specialized for the production of type I interferons (IFN-α/β) in response to viral infection.1,2 PDCs exclusively express Toll-like receptor 7 (TLR7) and TLR9, which recognize single-strand RNA and double-strand DNA, respectively.1,2 Stimulation of TLR7/TLR9 leads to the production of pro-inflammatory cytokines and type I IFNs. However, IFN-α/β receptor-mediated signaling also requires the production of type I IFNs because PDCs lacking IFN-α/β receptor barely secrete type I IFNs, demonstrating the positive feedback mechanism of type I IFN secretion initiated by TLR7/TLR9.1–3
Several receptors regulate IFN secretion by PDCs. Siglec H and NKp44 inhibit TLR9-induced type I IFN secretion.2,4,5 Moreover, immunoglobulin (Ig)–like transcript 7 represses the production of type I IFNs by interacting with its ligand, BM stromal cell antigen 2 (BST2).6 BST2 is induced on surrounding cells; hence, the interaction of Ig-like transcript 7 with BST2 provides a negative feedback system for regulating the excessive production of IFNs by PDCs. However, the involvement of other inhibitory receptors and whether there is an autoregulatory system on PDCs remain unclear.
Paired Ig-like receptor B (PIR-B) is also an inhibitory receptor that recruits SH2 domain-containing tyrosine phosphatase-1 (SHP-1) to its phosphotyrosylated cytoplasmic domain.7 PIR-B is a pair receptor, which consists of activating-type PIR-A. Although previous studies showed that PIR-B regulated various immune cells,8 it remains unclear whether PIR-B is expressed on PDCs and whether PIR-B affects the function of PDCs.
In the present study, we show that PIR-B can regulate not only Fms-like tyrosine kinase 3 ligand (Flt3-L)–induced PDC differentiation but also TLR9-mediated IFN-α production. In particular, PIR-B dampens IFN-α–mediated signaling, suggesting that PIR-B attenuates the positive feedback mechanism of IFNs secretion triggered by TLR9.
Methods
Mice
Pirb−/− mice were generated9 and backcrossed with C57BL/6 (B6) mice. Experimental protocols were approved by the Animal Studies Committee at the Institute of Development, Aging, and Cancer, Tohoku University, or Kanazawa Medical University.
Flow cytometry and immunoblot assay
Flow cytometry and immunoblot assays were done according to standard methods. Antibodies for flow cytometry were obtained from BioLegend. Antibodies for immunoblot assay were obtained from Cell Signaling Technology and Sigma-Aldrich.
Results and discussion
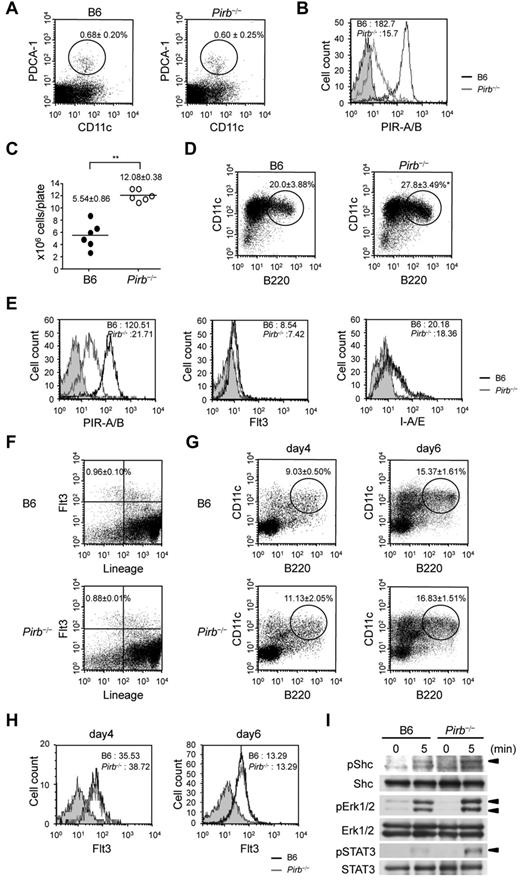
We first investigated the population of splenic PDCs (PDCA-1+ B220+ cells) in B6 and Pirb−/− mice. The population of splenic PDCs was comparable between B6 and Pirb−/− mice (Figure 1A), suggesting that PIR-B is not implicated in the development of PDCs in vivo.
PIR-B represses the development of Flt3-L–induced PDCs. (A) Splenocytes isolated from 8-week-old mice are stained with anti–PDCA-1 and CD11c. The percentages of PDCA-1+CD11c+ PDCs (circled). Data are mean ± SEM (n = 4 mice per group). (B) Flow cytometric analysis of PIR-A/B expression on B6 and Pirb−/− splenic PDCs. Black line indicates B6 PDCs; and gray line, Pirb−/− PDCs. Occupied gray area represents isotype control. Mean fluorescent intensities of PIR-A/B expression. (C) BM cells from B6 and Pirb−/− mice are incubated with Flt3-L (150 ng/mL) for 8 days. The total cell numbers of BM cells. ● represents B6 BM cells; and ○, Pirb−/− BM cells. Data are mean ± SEM (n = 6 mice per group). (D) The percentages of Flt3-L–induced PDCs (circled). Data are mean ± SEM (n = 3 mice per group). (E) Flow cytometric analysis of PIR-A/B, Flt3, and I-A/E expression on B6 and Pirb−/− Flt3-L–induced PDCs. Mean fluorescent intensities. (F) The progenitor fraction of PDCs (Flt3+lineage−) in B6 and Pirb−/− BM cells. Data are mean ± SEM (n = 3 mice per group). (G) The percentages of B220+CD11c+ BM cells at 4 and 6 days after Flt3-L administration (circled). Data are mean ± SEM (n = 3 mice per group). (H) Flow cytometric analysis of Flt3 expression on day 4 and 6 B220+CD11c+ BM cells. Mean fluorescent intensities. (I) B220+CD11c+ BM cells at 6 days after Flt3-L administration were cultured in Flt3-L–free conditions for 3 hours to reduce endogenous signaling activity and were then stimulated with Flt3-L (300 ng/mL). Immunoblot analysis of phospho-STAT3 (pSTAT3), STAT3, pErk1/2, Erk1/2, pShc, and Shc. All results are representative of 3 separate experiments. All statistical analyses were performed using Student t test: *P < .05, **P < .01.
PIR-B represses the development of Flt3-L–induced PDCs. (A) Splenocytes isolated from 8-week-old mice are stained with anti–PDCA-1 and CD11c. The percentages of PDCA-1+CD11c+ PDCs (circled). Data are mean ± SEM (n = 4 mice per group). (B) Flow cytometric analysis of PIR-A/B expression on B6 and Pirb−/− splenic PDCs. Black line indicates B6 PDCs; and gray line, Pirb−/− PDCs. Occupied gray area represents isotype control. Mean fluorescent intensities of PIR-A/B expression. (C) BM cells from B6 and Pirb−/− mice are incubated with Flt3-L (150 ng/mL) for 8 days. The total cell numbers of BM cells. ● represents B6 BM cells; and ○, Pirb−/− BM cells. Data are mean ± SEM (n = 6 mice per group). (D) The percentages of Flt3-L–induced PDCs (circled). Data are mean ± SEM (n = 3 mice per group). (E) Flow cytometric analysis of PIR-A/B, Flt3, and I-A/E expression on B6 and Pirb−/− Flt3-L–induced PDCs. Mean fluorescent intensities. (F) The progenitor fraction of PDCs (Flt3+lineage−) in B6 and Pirb−/− BM cells. Data are mean ± SEM (n = 3 mice per group). (G) The percentages of B220+CD11c+ BM cells at 4 and 6 days after Flt3-L administration (circled). Data are mean ± SEM (n = 3 mice per group). (H) Flow cytometric analysis of Flt3 expression on day 4 and 6 B220+CD11c+ BM cells. Mean fluorescent intensities. (I) B220+CD11c+ BM cells at 6 days after Flt3-L administration were cultured in Flt3-L–free conditions for 3 hours to reduce endogenous signaling activity and were then stimulated with Flt3-L (300 ng/mL). Immunoblot analysis of phospho-STAT3 (pSTAT3), STAT3, pErk1/2, Erk1/2, pShc, and Shc. All results are representative of 3 separate experiments. All statistical analyses were performed using Student t test: *P < .05, **P < .01.
We next examined PIR-B expression on splenic PDCs. Pirb−/− PDCs showed a low level of PIR-A expression, demonstrating a dominant expression of PIR-B on PDCs (Figure 1B). PDCs are also derived from Flt3 ligand (Flt3-L)–induced BMDCs.1 Thus, we induced BMDCs from BM cells for 8 days under Flt3-L containing medium, and PDCs were purified from BMDCs. In contrast to in vivo results, the total cell numbers of BMDCs in Pirb−/− mice were increased (Figure 1C). The ratio of PDCs in BMDCs was also augmented in Pirb−/− mice, suggesting that PIR-B repressed FLt3-L–mediated signaling (Figure 1D). Conversely, PIR-B was also dominantly expressed on FLt3-L–induced PDCs (Figure 1E). The expression level of Flt3 and MHC class II was comparable between B6 and Pirb−/− PDCs (Figure 1E).
To elucidate how PIR-B regulates PDC differentiation induced by Flt3-L, we checked PDC progenitor fractions.10 The population of lineage−Flt3+ BM cells between B6 and Pirb−/− mice was comparable (Figure 1F). Therefore, we investigated the PDC subset at day 4 and day 6 after administration with Flt3-L. The population and numbers of Pirb−/− B220+CD11c+ cells were almost the same as that of B6 B220+CD11c+ cells (Figure 1G; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). B6 and Pirb−/− B220+CD11c+ cells also expressed the same level of Flt3 (Figure 1H). Conversely, the expression of PIR-B on B6 B220+CD11c+ cells was remarkably up-regulated at day 6 (supplemental Figure 1B), suggesting that PIR-B regulates PDC differentiation from day 6 to day 8. Therefore, we tested Flt3 signaling in B220+CD11c+ cells at day 6. The phosphorylation of Shc, Erk1/2, and STAT3 was enhanced in Pirb−/− B220+CD11c+ cells (Figure 1I), demonstrating that PIR-B suppressed Flt3-L–mediated signaling. However, the depletion of PIR-B did not affect PDC development in vivo (Figure 1A). GM-CSF suppresses Flt3-L–dependent PDC development.11 Furthermore, the population of immediate PDC precursors, which have plasticity to conventional DCs under GM-CSF administration,12 was augmented in Pirb−/− mice (supplemental Figure 2). The in vivo development of PDCs in Pirb−/− mice might be blocked by GM-CSF.
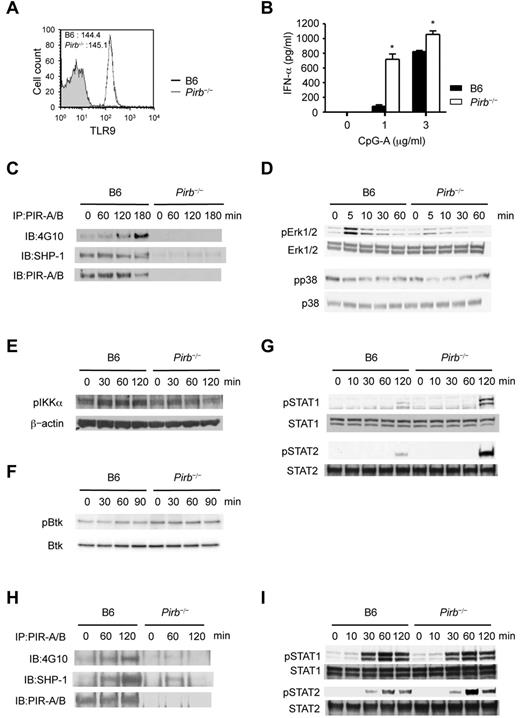
To examine the role of PIR-B in TLR9-mediated activation, we confirmed the same level of cytoplasmic TLR9 expression in B6 and Pirb−/− PDCs (Figure 2A). Thus, we measured the amount of IFN-α produced by B6 and Pirb−/− PDCs after CpG-A stimulation. IFN-α produced by Pirb−/− PDCs was higher than that by B6 PDCs (Figure 2B), indicating that PIR-B suppressed TLR9-mediated activation. There are 2 signaling pathways activated by TLR9 stimulation in PDCs: one leads to the production of pro-inflammatory cytokines and the other to the production of type I IFNs.13 After CpG-A stimulation, PIR-B phosphorylation and the association with SHP-1 were increased in B6 PDCs (Figure 2C). We investigated pro-inflammatory cytokine signaling pathways, such as ERK and p38. However, the phosphorylation of ERK and p38 was slightly reduced in Pirb−/− PDCs (Figure 2D). Therefore, we examined the alternate pathway that leads to IFN production. However, Pirb−/− PDCs also showed reduced IκB kinase-α phosphorylation (Figure 2E). In B-1 cells, PIR-B represses TLR9-mediated activation through Bruton tyrosine kinase (Btk) dephosphorylation.14 However, the Btk phosphorylation barely increased in B6 and Pirb−/− PDCs (Figure 2F). Furthermore, the IL-12 production was reduced in Pirb−/− PDCs (supplemental Figure 3). These results indicated that PIR-B did not repress TLR9-mediated signaling in PDCs.
PIR-B negatively regulates the positive feedback mechanism of IFN-α secretion triggered by TLR9. (A) Flow cytometric analysis of cytoplasmic TLR9 expression on B6 and Pirb−/− Flt3-L–induced PDCs. Mean fluorescent intensities. (B) B6 and Pirb−/− PDCs are incubated with CpG-A (OD2216) for 24 hours at the indicated concentration. The amounts of IFN-α are measured by ELISA assay. Data are mean ± SEM (n = 4). The results are representative of 3 separate experiments. Statistical analyses were performed using Student t test: *P < .05. (C-G) Immunoblot analysis of B6 and Pirb−/− PDCs stimulated with CpG-A (3 μg/mL). (C) Immunoblots of PIR-B phosphotyrosine (4G10), SHP-1, and PIR-B after the precipitation with anti–PIR-A/B. (D) Immunoblots of pErk1/2, Erk, pp38, and p38. (E) Immunoblots of pIκB kinase α- and β-actin. (F) Immunoblots of pBtk and Btk. (G) Immunoblots of pSTAT1, STAT1, pSTAT2, and STAT2. (H-I) Immunoblot analysis of B6 and Pirb−/− PDCs stimulated with IFN-α (100 U/mL). (H) Immunoblots of PIR-B phosphotyrosine (4G10), SHP-1, and PIR-B after the precipitation with anti–PIR-A/B. (I) Immunoblots of pSTAT1, STAT1, pSTAT2, and STAT2. The immunoblot results are representative of 3 separate experiments.
PIR-B negatively regulates the positive feedback mechanism of IFN-α secretion triggered by TLR9. (A) Flow cytometric analysis of cytoplasmic TLR9 expression on B6 and Pirb−/− Flt3-L–induced PDCs. Mean fluorescent intensities. (B) B6 and Pirb−/− PDCs are incubated with CpG-A (OD2216) for 24 hours at the indicated concentration. The amounts of IFN-α are measured by ELISA assay. Data are mean ± SEM (n = 4). The results are representative of 3 separate experiments. Statistical analyses were performed using Student t test: *P < .05. (C-G) Immunoblot analysis of B6 and Pirb−/− PDCs stimulated with CpG-A (3 μg/mL). (C) Immunoblots of PIR-B phosphotyrosine (4G10), SHP-1, and PIR-B after the precipitation with anti–PIR-A/B. (D) Immunoblots of pErk1/2, Erk, pp38, and p38. (E) Immunoblots of pIκB kinase α- and β-actin. (F) Immunoblots of pBtk and Btk. (G) Immunoblots of pSTAT1, STAT1, pSTAT2, and STAT2. (H-I) Immunoblot analysis of B6 and Pirb−/− PDCs stimulated with IFN-α (100 U/mL). (H) Immunoblots of PIR-B phosphotyrosine (4G10), SHP-1, and PIR-B after the precipitation with anti–PIR-A/B. (I) Immunoblots of pSTAT1, STAT1, pSTAT2, and STAT2. The immunoblot results are representative of 3 separate experiments.
Conversely, the robust production of type I IFN by PDCs requires a positive feedback mechanism mediated by autocrine type I IFNs.1–3 Thus, to elucidate whether PIR-B affects type I IFN-mediated signaling, we examined the IFN-α/β receptor downstream signaling molecules, STAT1/STAT2, which activate the transcription of IFN production. We found that phosphorylation of STAT1/STAT2 increased 2 hours after CpG-A stimulation (Figure 2G), suggesting that autocrine type I IFNs activate STAT phosphorylation. Moreover, Pirb−/− PDCs showed enhanced phosphorylation of STAT1/STAT2 compared with B6 PDCs. Therefore, we stimulated PDCs with IFN-α. PIR-B phosphorylation and the association with SHP-1 were increased in B6 PDCs (Figure 2H). The phosphorylation status of STAT2 (but not STAT1) was increased in Pirb−/− PDCs (Figure 2I). These findings suggested that PIR-B repressed the type I IFNs secreted by the positive feedback mechanism. PIR-B functions as an autoregulatory system for preventing excessive secretion of type I IFNs in PDCs, different from the regulatory system of the Ig-like transcript 7-BST2 interaction. Collectively, PIR-B regulates cytokines-mediated signaling in PDCs, suggesting that PIR-B could be a therapeutic target for autoimmune disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Core Research for Evolutional Science and Technology Program of the Japan Science and Technology Agency (T.T.), the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant-in-aid; A.N. and T.T.), and the Global Century Center of Excellence Program “Innovative Therapeutic Development Toward the Conquest of Signal Transduction Diseases with Network Medicine” (T.T.).
Authorship
Contribution: Y.M., A.N., and T.T. designed research and wrote the manuscript; Y.M., A.N., S.E., K.T., and T.Y.-W. performed the experiments; and T.N. supervised the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Toshiyuki Takai, Department of Experimental Immunology, Institute of Development, Aging and Cancer, Tohoku University, Seiryo 4-1, Aoba-ku, Sendai 980-8575, Japan; e-mail: tostakai@idac.tohoku.ac.jp; and Akira Nakamura, Department of Immunology, Kanazawa Medical University, Daigaku 1-1, Uchinada, Ishikawa 920-0293, Japan; e-mail: aki-n@kanazawa-med.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal