To the editor:
Point mutations of the Bcr-Abl kinase domain (KD) are the most frequently identified mechanism of resistance in patients with chronic myeloid leukemia (CML) who fail tyrosine kinase inhibitor (TKI) therapy.1–4 The V299L mutation occurs rarely after imatinib therapy,3 but has been reported to confer resistance to dasatinib and bosutinib.5–6 The objective of this study was to assess the incidence, pattern of V299L development, and response to therapy in patients with TKI-resistant CML at our institution.
Between January 2000 and March 2010, 186 patients were evaluated for Bcr-Abl KD mutations after imatinib failure, 170 patients after dasatinib failure, 72 patients after bosutinib failure, and 125 patients after nilotinib failure. Mutation analysis was interrogated based on evidence of treatment failure per the European LeukemiaNet.7 All patients signed informed consents and were treated on protocols approved by our institutional review board. Informed consent was provided according to the Declaration of Helsinki.
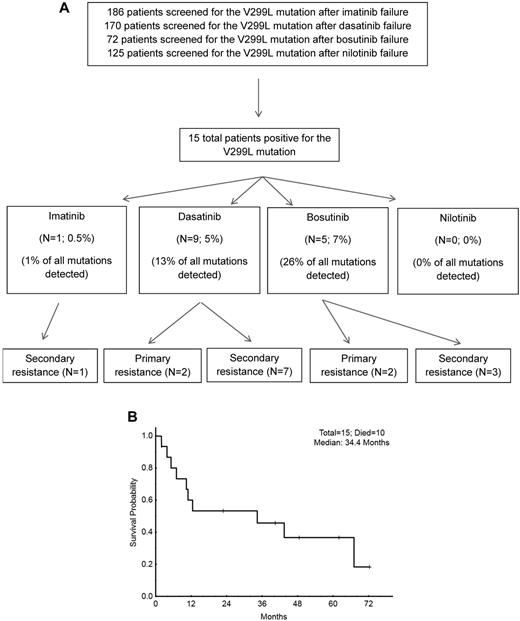
V299L mutations were detected in 15 patients and occurred more frequently on dasatinib and bosutinib than imatinib or nilotinib (P < .001). V299L mutations were identified in 1 of 186 (0.5%) patients after 26 months of imatinib, in 9 of 170 (5%) after a median 14 months of dasatinib, and in 5 of 72 (7%) after a median 5 months of bosutinib. None of the 125 patients receiving nilotinib acquired the V299L mutation (Figure 1A). In 4 patients, another mutation, in the same clone, was discovered at the time of V299L detection: 2 on bosutinib developed an F317L mutation, while 2 on dasatinib developed G250E and F486 mutations, respectively.
Outcome of patients with V299L mutation. (A) Development of resistance after V299L detection. (B) Overall survival after detection of the V299L mutation in patients treated with prior TKI therapy.
Outcome of patients with V299L mutation. (A) Development of resistance after V299L detection. (B) Overall survival after detection of the V299L mutation in patients treated with prior TKI therapy.
V299L was associated with primary resistance in 4 patients and secondary resistance in 11. Among the 15 patients with V299L mutations and TKI resistance, 9 received salvage therapy. One of 4 patients receiving salvage therapy with nilotinib achieved a sustained major molecular response of 40+ months. Another patient not initially responding to omacetaxine achieved a peripheral hematologic response for 6+ months on nilotinib. Both were in chronic phase and responded within 3 months after V299L detection.
After a median follow-up of 23 months from the time V299L was detected, 10 of 15 patients (67%) had died, 4 in chronic phase, 1 in accelerated phase, and all 3 in blast phase. The 2-year overall survival was 55% with a median survival of 34 months (Figure 1B).
The higher incidence of V299L after therapy with Src/Abl kinase inhibitors parallels the findings of in vitro studies and is similar to a prior report noting V299L in 4 of 17 patients (24%) treated with dasatinib after imatinib resistance.8 Another recent study described 12 patients who developed V299L-mediated resistance on bosutinib, all of whom discontinued treatment within 6 months because of treatment failure.6 In contrast, and consistent with the in vitro studies of cells transfected with V299L-containing vectors of Bcr-Abl that showed retention of sensitivity to imatinib and nilotinib,5 2 of the 4 patients treated with nilotinib demonstrated a clinical improvement after detection of V299L. These findings corroborate the potential benefit of the nilotinib use in the treatment of V299L-positive CML.
In conclusion, V299L occurs more frequently during therapy with dual Src/Abl kinase inhibitors dasatinib and bosutinib relative to other TKIs, with no development of V299L here for patients receiving nilotinib. TKIs like nilotinib showing activity against this mutation may be good treatment options for V299L-positive patients.
Authorship
Contribution: E.J. designed the concept, analyzed data, and wrote and approved the manuscript; H.K. designed the concept and wrote and approved the manuscript; V.M. analyzed the data and wrote the manuscript; C.C.Y. analyzed the data and wrote and approved the manuscript; E.B. analyzed the data; and J.C. designed the concept, analyzed the data, and wrote and approved the manuscript.
Conflict-of-interest disclosure: E.J. has received honoraria from BMS, Pfizer, and Novartis. H.K. and J.C. received research grants from Novartis and BMS. The remaining authors declare no competing financial interests.
Correspondence: Elias Jabbour, MD, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: ejabbour@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal