Abstract
Chemotaxis promotes neutrophil participation in cellular defense by enabling neutrophil migration to infected tissue and is controlled by persistent cell polarization. One long-standing question of neutrophil polarity has been how the pseudopod and the uropod are coordinated. In our previous report, we suggested that Rho GTPase Cdc42 controls neutrophil polarity through CD11b signaling at the uropod, albeit through an unknown mechanism. Here, we show that Cdc42 controls polarity, unexpectedly, via its effector WASp. Cdc42 controls WASp activation and its distant localization to the uropod. At the uropod, WASp regulates the reorganization of CD11b integrin into detergent resistant membrane domains; in turn, CD11b recruits the microtubule end binding protein EB1 to capture and stabilize microtubules at the uropod. This organization is necessary to maintain neutrophil polarity during migration and is critical for neutrophil emigration into inflamed lungs. These results suggest unrecognized mechanism of neutrophil polarity in which WASp mediates long-distance control of the uropod by Cdc42 to maintain a proper balance between the pseudopod and the uropod. Our study reveals a new function for WASp in the control of neutrophil polarity via crosstalk between CD11b and microtubules.
Introduction
Neutrophils are the most abundant leukocyte and fastest moving cell in the body. Neutrophils play a central role in innate immunity as cellular defense against infecting microorganisms and inflammatory processes and can contribute to hyperinflammatory reactions causing tissue injury. Neutrophils move rapidly toward sites of infection through a multiple-step process that involves tethering, rolling, adhesion, and transmigration to reach the site of infection in the tissue. Moreover, a new step in the neutrophil extravasation cascade, so-called locomotion, has been recently identified where neutrophils crawl onto endothelium toward the nearest endothelial junctions before transmigrating into tissues.1-3 Failure to regulate any of these events of neutrophil extravasation may lead to abnormal innate immune responses, including immunodeficiency or aberrant inflammatory reactions. Therefore, understanding the molecular mechanisms that control neutrophil migration is of significant therapeutic importance.
Neutrophils are one of the fastest migrating cells in response to a shallow chemoattractant gradient. Migrating neutrophils are highly polarized cells, which enables them to persistently migrate along the chemotactic gradient. During this process, filamentous actin (F-actin) polymerizes asymmetrically at the cell leading edge, and provides the protrusive forces to propel the cell membrane forward. At the same time, lateral membrane protrusions (so-called secondary/abnormal protrusions) are inhibited by actomyosin contractile complexes forming along the cell sides and the trailing edge, or uropod.4-6 Members of the Rho GTPase family, including Rho, Rac, and Cdc42, are key regulators of chemotaxis. They cycle between an inactive, GDP-bound and active, GTP-bound forms via guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). In many migrating cells, Rac activity is highly polarized at the leading edge and promotes actin assembly into lamellipodia by activating the WASp/WAVE family of proteins.1-3 In primary neutrophils, which express 2 related Rac proteins, Rac1 and Rac2, we and others showed that Rac2 is the main regulator of actin assembly.7-10 In contrast, RhoA and its effector Rho-associated protein kinase (ROCK) stimulate the formation of myosin filaments at the uropod via phosphorylation of myosin light-chain (p-MLC).11,12 It remains unclear how the pseudopod and the uropod are coordinated for efficient chemotaxis.
Cdc42 is a key regulator of cell polarity13 by determining where lamellipodia forms, probably by confining Rac activity at the leading edge.14 In addition, Cdc42 can control polarity by orienting the microtubule organization center in front of the nucleus and the capture of microtubules at the leading edge via Par6-Par3-atypical PKC complex, in fibroblasts.15 Although Cdc42 has been widely studied in various cell types, including fibroblasts, lymphocytes, macrophages, and dendritic cells, its physiologic role in neutrophils remains largely unexplored. The neutrophil chemotaxis mechanism is very unique and the knowledge gained from other cell types may not apply to these cells. We and others have previously reported that Cdc42 appears to play unique and distinctive roles in maintaining polarity, acting from a distance using so-called “long-range” signaling pathways. Whereas it is located at the front of the cells, Cdc42 amplifies RhoA signaling at the uropod, in HL-60 neutrophilic cells.16,17 In primary mouse neutrophils, we showed that Cdc42 controls polarized migration by regulating p-MLC at the uropod in a β2-integrin–dependent fashion.18 However, key components of this long-range signaling pathway that are important for regulating neutrophil migration in the innate immune response are unknown.
We have now identified unexpected key elements mediating Cdc42 functions in neutrophil migration. Cdc42 controls neutrophil polarity via its effector WASp. In this model, WASp is dispensable for F-actin polymerization at the leading edge. Instead, on activation by Cdc42, WASp acts at the uropod and facilitates microtubule capture and stability at the uropod via CD11b clustering. Our study thus reveals novel functions for WASp in microtubule organization in neutrophil chemotaxis. Importantly, this pathway is critically required for neutrophil transmigration into lungs and for lung inflammation. Therefore, the Cdc42/WASp axis emerges as a critical and physiologic regulator of neutrophil migration by coordinating the pseudopod and the uropod.
Methods
Mouse strains
The conditional MxCreTg/+; Cdc42flox/flox and control MxCreTg/+; wild-type (WT) mice were previously described.18 All mice were treated with polyI:polyC (300 μg per mouse; GE Healthcare). CD11b-deficient animals (Itgamtm1Myd) were purchased from The Jackson Laboratory. Wiskott-Aldrich syndrome protein-deficient mice (WASp−/−) were previously described.19 All animals were bred in the Cincinnati Children's Research Foundation pathogen-free animal facility. All experimental procedures were approved by the institutional animal committee at the Cincinnati Children's Research Foundation.
Neutrophil migration in vitro
Neutrophil isolation was previously described.18 Time-lapse video microscopy was performed in a Zigmond chamber (Neuro Probe) on surface coated with Fibrinogen (Fg) in gradient of 10μM fMLP.18 Migration was recorded with a Zeiss Axiovert 200 microscope at 10×/0.3 NA objective, equipped with ORCA-ER camera (Hamamatsu) and driven by ImageJ 1.43J software. Analysis of cell migration (speed [Sp] and straightness [St; distance from origin to total distance covered]) was performed in the motile population that had moved more than 20 μm using ImageJ Version 1.43J software. Quantifications were performed on at least 30 cells from individual experiment/video and from 3 independent experiments.
Retroviral transduction of mutant vectors
The Cdc42 mutant Cdc42S71P was generated with a PCR-based technique and subcloned in the bicistronic retrovirus vector Mieg3.20 To determine WASp and PAK binding, National Institutes of Health (NIH) 3T3 cells overexpressing HA3-tagged–Cdc42S71P were subjected to the pull-down assay using the Cdc42 effector probe, GST-WASp or GST-PAK, as previously described.20 MxCreTg/+;WT and MxCreTg/+; Cdc42flox/flox bone marrow cells were transduced with retroviral supernatant.10,21 EGFP+ cells were isolated and transplanted into lethally irradiated C57Bl/6 animals. Five weeks after bone marrow reconstitution, all animals were treated with polyI:polyC. Neutrophils were used for experiments.21
Immunofluorescence
Neutrophils were stimulated with fMLP in HBSS containing 0.1% BSA, 1mM Ca2+, 1mM Mg2+, and on Fg-coated slides or anti-CD11b–coated slides, for 10 minutes at 37°C. The anti-CD11b–coated slides are for antibody-mediated crosslinking experiments to enforce CD11b activation.18,22 The cells were fixed and stained with antibodies as previously described.18 To examine the role of EB1, neutrophils were transfected with EB1-CΔAC (kindly provided by Dr Akhmanova23 [Department of Cell and Molecular Biology, Northwestern University Medical School, Chicago]) by nucleofection and analyzed by IF. For details see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Preparation of whole cell lysates, cytosolic and DRM fractions
To examine protein expression in various cellular domains, neutrophils were either maintained in suspension without stimulation or stimulated with fMLP and plated on Fg-coated or anti-CD11b–coated plates. After removal of nonadherent cells, adherent cells were lysed with buffer for whole cell lysate, cytosolic or detergent-resistant membrane (DRM) faction. For details see supplemental Methods.
Western blotting
Cell lysates containing equal amounts of protein were separated by SDS-PAGE and probed for Glu-tubulin (kindly provided by Dr Gundersen24 [Department of Pathology and Cell Biology, Columbia University, New York]), α-tubulin (Sigma-Aldrich) or β-tubulin (Sigma-Aldrich), p38 (Cell Signaling Technology), phospho-Y291 WASp (Abcam), WASp (Santa Cruz), CD11b (Abcam), EB-1 (Santa Cruz), actin (Sigma-Aldrich), Arp2 (Santa Cruz), Cdc42 (BD Bioscience), phospho-T423 PAK1 (Cell Signaling Technology), HA-probe (Santa Cruz). For detection of ganglioside (GM1)–enriched domain using dot-blots, samples were spotted onto nitrocellulose, blocked, and then probed with peroxidase conjugated cholera toxin (Sigma-Aldrich).
F-actin quantitation by flow cytometry
Flow cytometry was used to quantitate the total amount of F-actin per neutrophil.9 Neutrophils were stimulated with fMLP in HBSS containing 0.1% BSA, 1mM Ca2+, 1mM Mg2+ at 37°C. Cells were fixed with formaldehyde, permeabilized with 0.1% Triton X-100, and stained with rhodamine-phalloidin. The results are reported as arbitrary unit of mean cellular fluorescence (AU). To normalize data between experiments, the mean cellular fluorescence of untreated WT neutrophils was arbitrarily assigned a value of 100%.
LPS-induced lung inflammation model
The mice were challenged with 33 μg LPS by intratracheal instillation after ketamine and xylazine anesthesia. Bronchoalveolar lavages (BALs) and lung histology were performed as previously described.21 Images are representative of 2 experiments with 3 mice each group. Lung function was determined as in Perkins et al.25 For details see supplemental Methods.
Statistics
All the experiments were performed at least 3 times, data are mean ± SD, Prism 5 software was used for statistical analysis t test. P < .05 was consider as significant.
Results
Cdc42 controls neutrophil emigration to inflamed lungs.
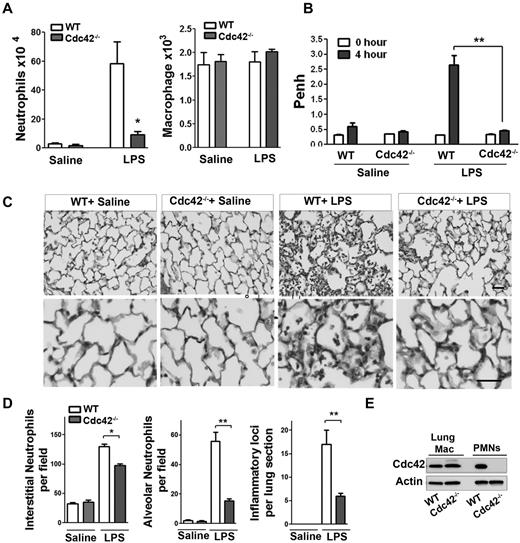
To examine the physiologic relevance of Cdc42 in neutrophil migration in vivo, we used a model of LPS-induced lung inflammation. Because Cdc42 is expressed in all tissues, we used adoptive transfer of marrow cells from MxCreTg+;Cdc42flox/flox and control CreTg+;Cdc42wt/wt into lethally irradiated C57BL/6 WT mice to allow selective Cdc42 deletion in blood cells.21 Five weeks after hematopoietic reconstitution, mice were treated with polyI:C to induce Cdc42 loss in neutrophils (supplemental Figure 1A). The total white blood count, including neutrophils, was normal in WT and Cdc42−/− reconstituted animals (not shown). To examine neutrophil emigration into lung alveoli, broncho-alveolar lavage fluid (BALF) was performed 4 hours after LPS exposure. BALF of Cdc42−/− reconstituted mice that received LPS contained significantly fewer neutrophils than similarly challenged WT reconstituted mice, whereas the numbers of macrophages remained unchanged (Figure 1A). Lung functions of these animals were then determined by barometric plethysmography and Penh values were calculated.25 Penh values of LPS challenged Cdc42−/− reconstituted animals were significantly less than WT, indicating a more normal respiratory function in Cdc42−/− reconstituted mice (Figure 1B). Finally, lung histology showed decreased neutrophil infiltration into interstitial tissues, reduced inflammatory foci and limited neutrophil numbers in the alveoli of Cdc42−/− reconstituted mice, compared with WT (Figure 1C-D). The lung resident macrophages were still WT in mice reconstituted with Cdc42−/− cells, consistent with a slow reconstitution of these cells after transplantation (Figure 1E). Hence, Cdc42 loss ameliorates lung function because of reduced neutrophil inflammation in a short-term acute lung inflammation model.
Cdc42 is critical for neutrophil recruitment into inflamed lungs. (A) Mice reconstituted with WT or Cdc42−/− hematopoietic cells were treated with polyI:C for Cdc42 deletion in hematopoietic system including neutrophils. Broncho-alveolar lavage (BALF) was performed 4 hours after LPS challenge in the lung. Numbers of neutrophils (*P = .0067) and macrophages (ns) recovered in brancho-alveolar lavage fluid were counted (mean ± SD, 3 independent experiments). (B) Lung function (respiratory rate) in WT or Cdc42−/− mice challenged with saline and LPS were measured as Penh frequency by barometric plethysmograph (Buxco Research Systems; **P = .0003, t test. (C) Lung histology; lungs were harvested 24 hours after challenge and fixed, and lung histology was performed. Sections were stained with H&E, scale bar, 50 μm. Images were captured at room temperature using a Leica DMI6000 microscope at 10× objective N/A0.3 with Leica camera driven by Openlab Version 5.5.0 software. (D) Quantification of lung histology parameters as indicated (*P < .001, **P < .003). (E) Genotype of lung resident macrophages extracted from total lung assessed by WB.
Cdc42 is critical for neutrophil recruitment into inflamed lungs. (A) Mice reconstituted with WT or Cdc42−/− hematopoietic cells were treated with polyI:C for Cdc42 deletion in hematopoietic system including neutrophils. Broncho-alveolar lavage (BALF) was performed 4 hours after LPS challenge in the lung. Numbers of neutrophils (*P = .0067) and macrophages (ns) recovered in brancho-alveolar lavage fluid were counted (mean ± SD, 3 independent experiments). (B) Lung function (respiratory rate) in WT or Cdc42−/− mice challenged with saline and LPS were measured as Penh frequency by barometric plethysmograph (Buxco Research Systems; **P = .0003, t test. (C) Lung histology; lungs were harvested 24 hours after challenge and fixed, and lung histology was performed. Sections were stained with H&E, scale bar, 50 μm. Images were captured at room temperature using a Leica DMI6000 microscope at 10× objective N/A0.3 with Leica camera driven by Openlab Version 5.5.0 software. (D) Quantification of lung histology parameters as indicated (*P < .001, **P < .003). (E) Genotype of lung resident macrophages extracted from total lung assessed by WB.
Cdc42 controls neutrophil chemotaxis via WASp.
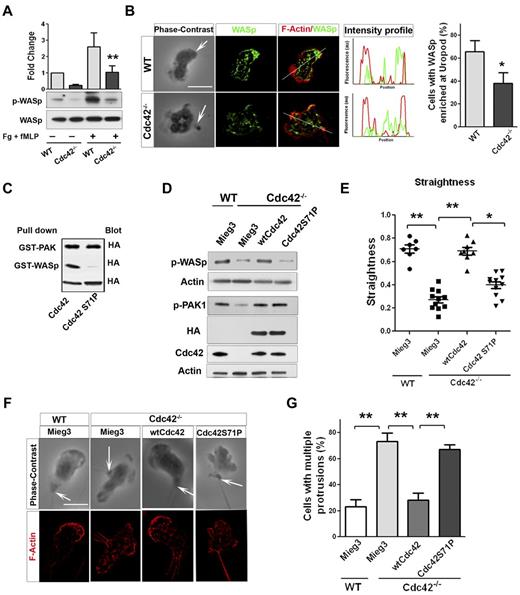
Neutrophil migration is a critical step of neutrophil emigration into tissues and can be dependent on CD11b/ β2-integrin signaling.26 Interestingly, we previously showed that Cdc42 controls neutrophil migration and polarity via CD11b.18 We thus analyzed the underlying mechanism of these events in greater details. Neutrophil chemotaxis results from a fine balance between the pseudopod and the uropod.1-3 Interestingly, although Cdc42 is distributed in the front (supplemental Figure 1B), it regulates uropod functions to maintain polarity during migration.18 We also showed that Cdc42 is doing so by controlling CD11b clustering at the uropod. To further dissect how Cdc42 controls uropod functions, we looked for effectors. We examined the potential role of the actin remodeling Wiskott Aldrich syndrome protein (WASp),27 because it has been implicated in β2-integrin clustering and function in neutrophils.28 We first examined WASp phosphorylation on Tyr291 in response to the chemokine fMLP and on surface containing fibrinogen.18 Stimulation-induced WASp phosphorylation was significantly decreased in Cdc42−/− neutrophils compared with WT cells (Figure 2A). We next examined the subcellular localization of WASp, using IF staining. Surprisingly, WASp accumulated at the uropod in majority of WT neutrophils, whereas WASp was homogenously distributed in Cdc42−/− neutrophils (Figure 2B). Therefore, Cdc42 controls WASp activity and its distant distribution to the uropod, suggesting that WASp may mediate Cdc42 control of the uropod.
Cdc42 controls neutrophil polarity via WASp. (A) WT and Cdc42−/− neutrophils were un-stimulated or stimulated with fMLP and plated on Fg-coated slides for 10 minutes. Western blots from whole-cell lysate (WCL), probed with p-WASp (Y-291) and total WASp. Representative blot of 3 independent experiments (mean ± SD; **P = .0059). (B) Immunofluorescence of WASp and F-actin in fMLP and Fg-stimulated neutrophils. Arrow indicates the tail of neutrophils identified on phase contrast images based on the classic morphology of the tail at the uropod (in all the subsequent images). Histogram is percent of cells exhibiting WASp enriched at uropod (mean ± SD; *P = .0097; 3 independent experiments). (C) WTCdc42 and Cdc42S71P proteins were subjected to the pull down assay to assess ability of the protein to bind WASp or PAK. The amount of immunoprecipitated GTP-Cdc42 is revealed by immunoblot with anti-HA. WCL blotting was used for equal input (lowest blot). (D) Immunoblot of WCL for expression of wtCdc42 and Cdc42S71P in Cdc42−/− neutrophils and for phosphorylation of PAK (p-PAK1) and WASp (pWASp). P-PAK and pWASp blots were performed independently and actin loading control is shown for each. (E) Neutrophil migration was examined by time lapse video microscopy in gradient of fMLP and on surface coated with fibrinogen, in Zigmond chamber. Measurement of straightness of migration was performed in ImageJ Version 1.43J software. Scatter plot of individual cells (*P < .05, **P < .01; 3 independent experiments). (F) Immunofluorescence analysis of F-actin distribution on stimulation with fMLP and Fg, F-actin was labeled with Rhodamine Phalloidin and slides were mounted in SlowFade Gold Antifade. (G) Histogram is percent of cells exhibiting multiple F-actin protrusions as seen in panel F (mean ± SD; **P < .001; 3 independent experiments). Fluorescence images were captured at room temperature using a Leica DMI6000 fluorescence microscope at 63× objective N/A1.3 with ORCA-ER C4742-95 camera driven by Openlab Version 5.5.0 software.
Cdc42 controls neutrophil polarity via WASp. (A) WT and Cdc42−/− neutrophils were un-stimulated or stimulated with fMLP and plated on Fg-coated slides for 10 minutes. Western blots from whole-cell lysate (WCL), probed with p-WASp (Y-291) and total WASp. Representative blot of 3 independent experiments (mean ± SD; **P = .0059). (B) Immunofluorescence of WASp and F-actin in fMLP and Fg-stimulated neutrophils. Arrow indicates the tail of neutrophils identified on phase contrast images based on the classic morphology of the tail at the uropod (in all the subsequent images). Histogram is percent of cells exhibiting WASp enriched at uropod (mean ± SD; *P = .0097; 3 independent experiments). (C) WTCdc42 and Cdc42S71P proteins were subjected to the pull down assay to assess ability of the protein to bind WASp or PAK. The amount of immunoprecipitated GTP-Cdc42 is revealed by immunoblot with anti-HA. WCL blotting was used for equal input (lowest blot). (D) Immunoblot of WCL for expression of wtCdc42 and Cdc42S71P in Cdc42−/− neutrophils and for phosphorylation of PAK (p-PAK1) and WASp (pWASp). P-PAK and pWASp blots were performed independently and actin loading control is shown for each. (E) Neutrophil migration was examined by time lapse video microscopy in gradient of fMLP and on surface coated with fibrinogen, in Zigmond chamber. Measurement of straightness of migration was performed in ImageJ Version 1.43J software. Scatter plot of individual cells (*P < .05, **P < .01; 3 independent experiments). (F) Immunofluorescence analysis of F-actin distribution on stimulation with fMLP and Fg, F-actin was labeled with Rhodamine Phalloidin and slides were mounted in SlowFade Gold Antifade. (G) Histogram is percent of cells exhibiting multiple F-actin protrusions as seen in panel F (mean ± SD; **P < .001; 3 independent experiments). Fluorescence images were captured at room temperature using a Leica DMI6000 fluorescence microscope at 63× objective N/A1.3 with ORCA-ER C4742-95 camera driven by Openlab Version 5.5.0 software.
To further examine this possibility, a Cdc42 mutant that is unable to bind WASp (Cdc42S71P) but binds other Cdc42 effector, such as PAK (Figure 2c), was expressed in Cdc42−/− cells via retroviral vector, Mieg3.10 Immunoblot indicated that the amount of ectopic Cdc42S71P protein expressed in Cdc42−/− cells was similar to that of ectopic WT Cdc42 (Figure 2D). Importantly, Cdc42S71P expression did not restore p-WASP, but did restore the abnormal level of p-PAK in Cdc42−/− neutrophils, similarly to WT Cdc42 (Figure 2D), indicating that this protein is functional. Migration was examined in fMLP gradient using time-lapse video-microscopy.18 WT cells transduced with empty vector (WT-mieg3) were highly polarized with a single pseudopodium facing the fMLP gradient. They produced few lateral protrusions and moved persistently toward fMLP (supplemental Video 1). Conversely, Cdc42−/−-mieg3 neutrophils developed lateral protrusions that caused deviation of the cell trajectory (supplemental Video 2). As such, they exhibited lower straightness of migration,18 that is, ratio of distance from origin to total distance, than WT-mieg3 cells (Figure 2E), suggesting loss of polarity. Remarkably, expression of wtCdc42 but not Cdc42S71P restored persistent migration to WT levels (Figure 2E, supplemental Videos 3-4).
We next analyzed F-actin distribution using IF. Consistent with polarity loss,18 Cdc42−/−-mieg3 neutrophils exhibited multiple F-actin protrusions compared with WT-mieg3 neutrophils, and Cdc42S71P failed to rescue this abnormal morphology (Figure 2F-G). Finally, because Cdc42 controls polarity via CD11b clustering at the uropod,18 we examined CD11b distribution in polarized neutrophils. The enrichment of CD11b at the uropod seen in WT-mieg3 neutrophils was not restored in Cdc42−/− neutrophils by Cdc42S71P but was by wtCdc42 (supplemental Figure 2). These data strongly suggest a direct role for WASp in neutrophil polarity.
Loss of WASp leads to defective neutrophil chemotaxis and polarity
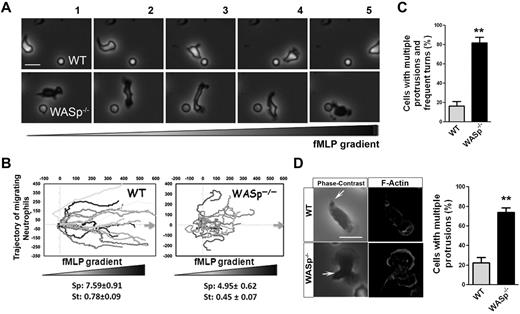
To examine the role of WASp in neutrophil chemotaxis, we used WASp−/− neutrophils.19,29 WASp−/− neutrophils, similar to Cdc42−/− neutrophils, extended lateral protrusions during migration (Figure 3A, supplemental Videos 5-6), as consequence they were unable to efficiently migrate up the chemotactic gradient (Figure 3B). Consistently, WASp−/− cells exhibited multiple F-actin fronts (Figure 3C-D). Finally, as previously reported,28 WASp−/− neutrophils failed to redistribute CD11b into clusters at the uropod (supplemental Figure 3A-C). Therefore, WASp appears to be important for suppressing inappropriate lateral protrusions at the uropod and maintaining the polarity axis during migration. This seemed counter-intuitive because Cdc42 and WASp are known to stimulate F-actin polymerization.27 F-actin polymerization was thus quantified in response to fMLP, using flow cytometry. fMLP stimulation resulted in F-actin polymerization responses in WASp−/− and Cdc42−/− cells similar to WT cells (supplemental Figure 3D-E). In fact, this is consistent with a study showing a normal F-actin response to fMLP stimulation of neutrophils extracted from WAS patients30 and Cdc42-deficient dendritic cells.31 Thus, the neutrophil F-actin polymerization process per se is not dependent on Cdc42/WASp, rather Cdc42/WASp controls its spatial distribution in the cell. Together, the Cdc42/WASp axis coordinates the pseudopod and the uropod for efficient neutrophil chemotaxis.
WASp−/− neutrophils have defective chemotaxis and exhibit loss of polarity. (A) WT and WASp−/− neutrophil migration by time-lapse video microscopy in a gradient of fMLP and on surface coated with Fg, in a Zigmond chamber. Representative images (1 minute between each frame) of migrating cells, fMLP concentration increases from left to right. Images were captured at 37°C using a Zeiss Axiovert 200 microscope at 10× objective N/A0.3 with ORCA-ER C4742-95 camera driven by Openlab Version 5.5.0 software. (B) Cell trajectory analysis; the schema represents the migration trajectory of cells moving up fMLP gradient for 20 minutes. Trajectories were tracked with ImageJ Version 1.43J software. Speed (sp; μm/min) and straightness (st) of migration are indicated at the bottom (mean ± SD; n = 60; *P < .01; 3 independent experiments). (C) Histogram represents the percentage of cells with changes in direction arising from inappropriate lateral protrusions as seen during time-lapse video microscopy. Data are from 80 cells (mean ± SD; 3 independent videos; *P = .00013). (D) Immunofluorescence analysis of F-actin on stimulation with fMLP and Fg, F-actin was labeled with Rhodamine Phalloidin and slides were mounted in SlowFade Gold Antifade. Scale bar, 10 μm. Histogram is percent of cells with multiple protrusions. (mean ± SD; 3 independent experiments; **P < .01). Fluorescence images were captured at room temperature using a Leica DMI6000 fluorescence microscope at 63× objective N/A1.3 with ORCA-ER C4742-95 camera driven by Openlab Version 5.5.0 software.
WASp−/− neutrophils have defective chemotaxis and exhibit loss of polarity. (A) WT and WASp−/− neutrophil migration by time-lapse video microscopy in a gradient of fMLP and on surface coated with Fg, in a Zigmond chamber. Representative images (1 minute between each frame) of migrating cells, fMLP concentration increases from left to right. Images were captured at 37°C using a Zeiss Axiovert 200 microscope at 10× objective N/A0.3 with ORCA-ER C4742-95 camera driven by Openlab Version 5.5.0 software. (B) Cell trajectory analysis; the schema represents the migration trajectory of cells moving up fMLP gradient for 20 minutes. Trajectories were tracked with ImageJ Version 1.43J software. Speed (sp; μm/min) and straightness (st) of migration are indicated at the bottom (mean ± SD; n = 60; *P < .01; 3 independent experiments). (C) Histogram represents the percentage of cells with changes in direction arising from inappropriate lateral protrusions as seen during time-lapse video microscopy. Data are from 80 cells (mean ± SD; 3 independent videos; *P = .00013). (D) Immunofluorescence analysis of F-actin on stimulation with fMLP and Fg, F-actin was labeled with Rhodamine Phalloidin and slides were mounted in SlowFade Gold Antifade. Scale bar, 10 μm. Histogram is percent of cells with multiple protrusions. (mean ± SD; 3 independent experiments; **P < .01). Fluorescence images were captured at room temperature using a Leica DMI6000 fluorescence microscope at 63× objective N/A1.3 with ORCA-ER C4742-95 camera driven by Openlab Version 5.5.0 software.
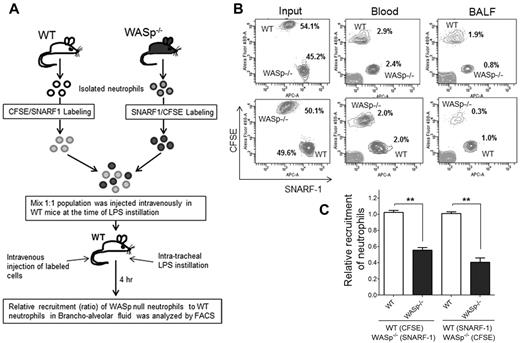
Finally, to assess WASp−/− neutrophil migration in vivo, an adoptive transfer experiment of WT and WASp−/− neutrophils was performed.32 WASp−/− mice or bone marrow transfer methods could not be used because WASp−/− mice exhibited higher numbers of neutrophils in the peripheral blood than WT control mice, which is cell autonomous because WASp in expressed only in hematopoietic cells. Thus, WT and WASp−/− neutrophils were isolated and labeled with 2 different dyes, carboxyfluorescein diacetate succinimidyl esters (CFSE; green) or chloromethyl SNARF-1 acetate (red), respectively.32 A mixed (1:1, WT-CFSE:WASp−/−–SNARF1 or WT-SNARF1:WASp−/−–CFSE) population was then transferred into WT recipients (Figure 4A). The animals were then challenged with LPS and the relative numbers of adoptively transferred neutrophils that emigrated into lung alveoli was assessed by flow cytometry. Reduced proportion of WASp−/− neutrophils were detected in BALF 4 hours after LPS challenge relative to WT neutrophils, indicating defective migratory ability of WASp−/− neutrophils in vivo relative to WT (Figure 4B-C).
WASp is necessary for neutrophil emigration into lungs. (A) Experimental design of recruitment of adoptively transferred neutrophils in the lung. WT and WASp−/− neutrophils were labeled either with CSFE (in green) or with SNARF1 (in red). Labeled cells were mixed (1:1; eg, WT-CFSE:WASp−/−-SNARF1 or WT-SNARF1:WASp−/−-CFSE) and transferred to WT recipients at the time of LPS lung challenged. The amount of labeled neutrophils recovered in BALF 4 hours after challenge was evaluated by flow cytometry. (B) Representative flow cytometry charts of labeled neutrophils in the mixed population (left panel), in the blood (middle panel), in the BALF (right panel). (C) Relative recruitment of neutrophils calculated as ratio of WASP relative to WT (mean ± SD; n = 5, each experiment; **P < .01). See supplemental Methods for detail of methodology.
WASp is necessary for neutrophil emigration into lungs. (A) Experimental design of recruitment of adoptively transferred neutrophils in the lung. WT and WASp−/− neutrophils were labeled either with CSFE (in green) or with SNARF1 (in red). Labeled cells were mixed (1:1; eg, WT-CFSE:WASp−/−-SNARF1 or WT-SNARF1:WASp−/−-CFSE) and transferred to WT recipients at the time of LPS lung challenged. The amount of labeled neutrophils recovered in BALF 4 hours after challenge was evaluated by flow cytometry. (B) Representative flow cytometry charts of labeled neutrophils in the mixed population (left panel), in the blood (middle panel), in the BALF (right panel). (C) Relative recruitment of neutrophils calculated as ratio of WASP relative to WT (mean ± SD; n = 5, each experiment; **P < .01). See supplemental Methods for detail of methodology.
Cdc42/ WASp controls neutrophil polarity via microtubule stabilization
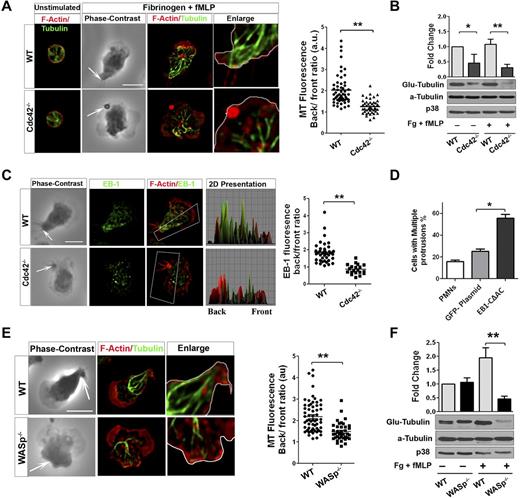
During neutrophil migration, microtubules reorient toward the uropod and are critical for maintaining polarity.33 Because Cdc42 is a known regulator of microtubules,15 we explored a possible link between Cdc42, WASP, and microtubules in controlling polarity. We analyzed microtubule organization in WT and Cdc42−/− neutrophils on stimulation. On stimulation of WT neutrophils, MTs were straight and polarized toward the back (Figure 5A, supplemental Figure 4A). Furthermore, they appeared to make contact with the plasma membrane at the uropod. In contrast, Cdc42−/− neutrophils exhibited crimped MTs that were oriented toward the front and the back without connecting with the uropod (Figure 5A). During cell migration, MTs are locally captured and stabilized at the cell cortex from capping of MT plus-end proteins.34 Consequently, the α-tubulin subunit of MTs becomes posttranslationally modified by detyrosination (so-called Glu-tubulin).34 We examined MT stability using Glu-tubulin detection.24 On stimulation, Cdc42−/− neutrophils exhibited a marked reduction in Glu-tubulin relative to WT cells (Figure 5B). This was associated with an abnormal distribution of the MT plus-end protein EB1. EB1 was enriched at the uropod in WT but not in Cdc42−/− neutrophils (Figure 5C). We then used nocodazole, which disrupts MTs, as well as the expression of a dominant-negative EB1, GFP-EB1-CΔAC.23 Both nocodazole treatment (supplemental Figure 4B) or expression of GFP-EB1-CΔAC (Figure 5D, supplemental Figure 4C) induced a loss of polarity, with multiple protrusions developing in WT cells.
Cdc42/WASp axis controls neutrophil polarity via microtubule orientation and stability. Neutrophils were un-stimulated or stimulated with fMLP on Fg. (A) Immunofluorescence of F-actin and microtubules. Scatter plot for MT polarity as ratio of MT intensity at the back versus the front. Scattered plot representation of at least 90 cells analyzed from 3 independent experiments **P < .0001. Arrow indicates the tail of neutrophils identified on phase contrast images based on the classic morphology of the tail at the uropod (B) Western blots of WCL probed with Glu-tubulin for stabilized microtubules. α-tubulin and p38 were used for total tubulin and loading control (mean ± SD; 3 independent experiments; *P = .0059). (C) Immunofluorescence analysis of EB1 and F-actin. 2D representation of intensity profile of EB1 and F-actin in the region of interest indicated by the box were analyzed in ImageJ Version 1.43J software. Scatter graph for ratio of intensity of EB-1 at back versus front (identified using F-actin). Thirty cells were analyzed from individual experiment and 3 independent experiments were performed (*P = .003). (D) WT neutrophils were transfected with GFP-EB1CΔAC or GFP plasmid vector control using nucleofactor and stimulated with fMLP and Fg and stained for F-actin. Histogram is cells with more than 1 protrusion. Fifty cells were analyzed from individual experiment and 3 independent experiments were performed (mean ± SD; *P = .0027). (E) Immunofluorescence analysis of F-actin and microtubules after stimulation. Scatter plot for MT polarity quantification (mean ± SD; **P = .000017). (F) Western blots of WCL probed with Glu-tubulin (**P = .0024). In fluorescence images tubulin and EB-1 were stained with secondary antibody conjugated with Alexa Fluor 488, while F-actin was labeled with Rhodamine Phalloidin and slides were mounted in SlowFade Gold Antifade. Fluorescence images were captured at room temperature using a Leica DMI6000 fluorescence microscope at 63× objective N/A1.3 with ORCA-ER C4742-95 camera driven by Openlab Version 5.5.0 software.
Cdc42/WASp axis controls neutrophil polarity via microtubule orientation and stability. Neutrophils were un-stimulated or stimulated with fMLP on Fg. (A) Immunofluorescence of F-actin and microtubules. Scatter plot for MT polarity as ratio of MT intensity at the back versus the front. Scattered plot representation of at least 90 cells analyzed from 3 independent experiments **P < .0001. Arrow indicates the tail of neutrophils identified on phase contrast images based on the classic morphology of the tail at the uropod (B) Western blots of WCL probed with Glu-tubulin for stabilized microtubules. α-tubulin and p38 were used for total tubulin and loading control (mean ± SD; 3 independent experiments; *P = .0059). (C) Immunofluorescence analysis of EB1 and F-actin. 2D representation of intensity profile of EB1 and F-actin in the region of interest indicated by the box were analyzed in ImageJ Version 1.43J software. Scatter graph for ratio of intensity of EB-1 at back versus front (identified using F-actin). Thirty cells were analyzed from individual experiment and 3 independent experiments were performed (*P = .003). (D) WT neutrophils were transfected with GFP-EB1CΔAC or GFP plasmid vector control using nucleofactor and stimulated with fMLP and Fg and stained for F-actin. Histogram is cells with more than 1 protrusion. Fifty cells were analyzed from individual experiment and 3 independent experiments were performed (mean ± SD; *P = .0027). (E) Immunofluorescence analysis of F-actin and microtubules after stimulation. Scatter plot for MT polarity quantification (mean ± SD; **P = .000017). (F) Western blots of WCL probed with Glu-tubulin (**P = .0024). In fluorescence images tubulin and EB-1 were stained with secondary antibody conjugated with Alexa Fluor 488, while F-actin was labeled with Rhodamine Phalloidin and slides were mounted in SlowFade Gold Antifade. Fluorescence images were captured at room temperature using a Leica DMI6000 fluorescence microscope at 63× objective N/A1.3 with ORCA-ER C4742-95 camera driven by Openlab Version 5.5.0 software.
Interestingly, WASp−/− neutrophils failed to orient and capture MT at the uropod (Figure 5E). Furthermore, WASp−/− neutrophils exhibited less EB1 at the uropod (supplemental Figure 4D) and a marked decreased Glu-tubulin on stimulation (Figure 5F). Consistent with these findings, expression of Cdc42S71P in Cdc42−/− cells did not restore abnormal MT organization (not shown). Thus, these results suggested that Cdc42 controls MT orientation and stability at the uropod to balance the front and the back via WASp.
Cdc42/WASp regulates MT stability via CD11b rearrangement in the uropod membrane domains
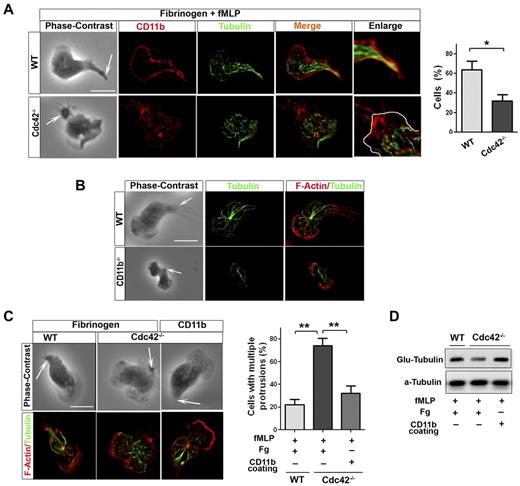
Because integrins have been involved in MT stabilization,35 CD11b might capture MTs at the uropod in neutrophils. In polarized WT neutrophils, MTs appear to contact with the plasma membrane at the uropod where CD11b is enriched (Figure 6A). Interestingly, this contact was lost in Cdc42−/− (Figure 6A) as well as in WASp−/− neutrophils (not shown). We thus investigated CD11b functions in MT polarity. CD11b appears to be necessary for MT polarity, as MTs failed to contact with the uropod in CD11b−/− neutrophils, although CD11b−/− neutrophils have limited spreading area18 (Figure 6B). We then used surface coated with anti-CD11b Ab, which cross-links CD11b,18,22 to enforce CD11b activation in Cdc42−/− or WASp−/− neutrophils and examine effect on MT polarity. As control for this, we show that anti-CD11b coating but not Fg alone induces cell adhesion, thus indicating that anti-CD11b coating but not Fg induces CD11b activation (supplemental Figure 5). MT polarization and stabilization, as assessed by Glu-tubulin measurement, were restored in Cdc42−/− neutrophils plated on used surface coated with anti-CD11b Ab (Figure 6C-D). Anti-CD11b also restored polarity of WASp−/− neutrophils (supplemental Figure 6). Thus, Cdc42 controls MT capture and stabilization at the uropod via CD11b clustering, something that is critical for neutrophil polarity.
Cdc42 controls microtubule stabilization and polarity via CD11b clustering. (A) Immunofluorescence of CD11b and microtubules of neutrophils stimulated with fMLP and plated on Fg-coated slides. Cells showing microtubules-CD11b contacts were enumerated, *P = .0062. (B) Immunofluorescence of F-actin and microtubules of WT and CD11b−/− neutrophils stimulated with fMLP on glass. Data are representative of 3 independent experiments (scale bar, 10 μm). (C) Immunofluorescence of F-actin and microtubules of neutrophils stimulated with fMLP and plated on Fg-coated slides or on slides coated with anti-CD11b to induced CD11b clustering. Cells with more than 1 protrusion were enumerated (**P = .00067 and .0045). (D) Western blots of WCL from cells that were stimulated on Fg or CD11b coated plates, were probed with Glu-tubulin for stabilized microtubules. In fluorescence images tubulin was stained with secondary antibody conjugated with Alexa Fluor 488, while F-actin and CD11b were labeled with Rhodamine Phalloidin and secondary antibody conjugated with Alexa Fluor 594 respectively, slides were mounted in SlowFade Gold Antifade. Fluorescence images were captured at room temperature using a Leica DMI6000 fluorescence microscope at 63× objective N/A1.3 with ORCA-ER C4742-95 camera driven by Openlab Version 5.5.0 software.
Cdc42 controls microtubule stabilization and polarity via CD11b clustering. (A) Immunofluorescence of CD11b and microtubules of neutrophils stimulated with fMLP and plated on Fg-coated slides. Cells showing microtubules-CD11b contacts were enumerated, *P = .0062. (B) Immunofluorescence of F-actin and microtubules of WT and CD11b−/− neutrophils stimulated with fMLP on glass. Data are representative of 3 independent experiments (scale bar, 10 μm). (C) Immunofluorescence of F-actin and microtubules of neutrophils stimulated with fMLP and plated on Fg-coated slides or on slides coated with anti-CD11b to induced CD11b clustering. Cells with more than 1 protrusion were enumerated (**P = .00067 and .0045). (D) Western blots of WCL from cells that were stimulated on Fg or CD11b coated plates, were probed with Glu-tubulin for stabilized microtubules. In fluorescence images tubulin was stained with secondary antibody conjugated with Alexa Fluor 488, while F-actin and CD11b were labeled with Rhodamine Phalloidin and secondary antibody conjugated with Alexa Fluor 594 respectively, slides were mounted in SlowFade Gold Antifade. Fluorescence images were captured at room temperature using a Leica DMI6000 fluorescence microscope at 63× objective N/A1.3 with ORCA-ER C4742-95 camera driven by Openlab Version 5.5.0 software.
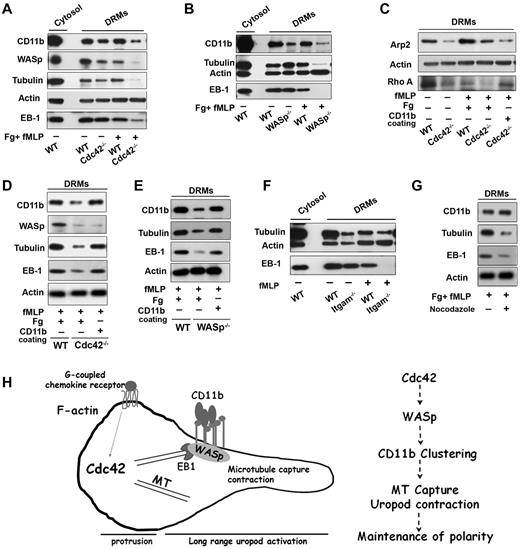
Plasma membrane reorganization is essential for polarity and migration.36-38 During neutrophil polarization, the uropod plasma membrane reorganizes into DRM domains containing CD44 and ICAM, whereas the front becomes enriched in CD45 within detergent-sensitive membranes.36-38 In this process, CD11b and CD44 codistribute at the uropod in a Cdc42-dependent manner, suggesting that the rearrangement of the uropod membrane during neutrophil polarization depends on Cdc42.18 We observed that CD44 distribution at the uropod was also decreased in WASp−/− cells (supplemental Figure 7A). To further investigate crosstalk between WASp, CD11b and microtubules at the plasma membrane, we biochemically extracted the DRM fraction of neutrophils using cold Triton detergent (supplemental Figure 7B).39 Interestingly, WASp along with CD11b and tubulin coassociated in DRMs of WT cells, both in resting and activated (ie, polarized) neutrophils (Figure 7A). However, DRMs isolated from Cdc42−/− cells contained significantly less CD11b, WASp, and tubulin, most dramatically on stimulation (Figure 7A). Importantly, no differences in expression of these proteins in total cell lysate were detected (supplemental Figure 7C). Similar results were observed in WASp−/− neutrophils (Figure 7B). Furthermore, EB1 was present in WT DRMs but was almost absent in stimulated Cdc42−/− and WASp−/− DRMs, respectively (Figure 7A-B). Thus, Cdc42 controls the reorganization of the uropod membrane on stimulation, containing WASp, CD11b, EB1, and microtubules.
Cdc42/WASp axis regulates CD11b, microtubule association to rafts microdomains. Neutrophils were unstimulated or stimulated with fMLP and plated on Fg-coated slides or on CD11b-coated plates, as indicated in each panels. Western blots of cytosolic and DRM fractions were probed for WASp, CD11b, tubulin, EB-1, actin, Arp-2, or Rho A proteins as indicated in panels A, C, and D Cdc42−/−, (B-E) WASp−/−, (F) ITGAM−/− (CD11b−/−) cells with their respective WT controls, (G) DRM fraction of WT cells treated with nocodazole. Blots are representative of 3 to 5 independent experiments. (H) Model of long range control of uropod function by Cdc42 in neutrophil polarity: Cdc42 controls WASp activation at the uropod, which induces CD11b clustering and activation; in turn, CD11b recruits EB1 to capture microtubules and activate uropod function.
Cdc42/WASp axis regulates CD11b, microtubule association to rafts microdomains. Neutrophils were unstimulated or stimulated with fMLP and plated on Fg-coated slides or on CD11b-coated plates, as indicated in each panels. Western blots of cytosolic and DRM fractions were probed for WASp, CD11b, tubulin, EB-1, actin, Arp-2, or Rho A proteins as indicated in panels A, C, and D Cdc42−/−, (B-E) WASp−/−, (F) ITGAM−/− (CD11b−/−) cells with their respective WT controls, (G) DRM fraction of WT cells treated with nocodazole. Blots are representative of 3 to 5 independent experiments. (H) Model of long range control of uropod function by Cdc42 in neutrophil polarity: Cdc42 controls WASp activation at the uropod, which induces CD11b clustering and activation; in turn, CD11b recruits EB1 to capture microtubules and activate uropod function.
Interestingly, Arp2, which nucleates actin on activation by WASp,27 was also increased in WT DRMs on stimulation (Figure 7C). The amount of Apr2 in DRMs from Cdc42−/− (Figure 7C) and WASp−/− (not shown) neutrophils after stimulation, was significantly less that in WT cells. Hence, Cdc42/WASp axis may recruit Arp2/3 complex to control CD11b clustering at the uropod. To sort out these events, we used CD11b cross-linking experiments.18 CD11b cross-linking did not restore the DRM redistribution of WASp, and Arp2 in Cdc42−/− cells, confirming that they acted upstream of CD11b (Figure 7C-D). In contrast, EB1 and tubulin were reassociated with DRMs of Cdc42−/− cells and WASp−/− cells, respectively (Figure 7C-E), after CD11b crosslinking. Thus, on activation by WASp, CD11b recruits EB1 to capture and stabilize microtubules. In support of this, DRMs extracted from CD11b−/− neutrophils contained less tubulin and EB-1 (Figure 7F). Conversely, treatment of WT cells with nocodazole led to a drastic decrease in tubulin and EB1 in DRMs, whereas CD11b appeared unaltered (Figure 7G). Finally, we previously showed that CD11b can activate RhoA and MLC-driven contraction.18 Interestingly, RhoA was almost missing in DRMs of Cdc42−/− cells but its presence was restored in the same cells by anti-CD11b crosslinking (Figure 7C).
Together, these findings suggest that Cdc42 controls the reorganization of DRM domains containing CD11b via WASp, and that, in turn, CD11b capture and stabilize microtubules via EB1 to maintain neutrophil polarity during migration.
Discussion
Our study reveals an unexpected mechanism of neutrophil chemotaxis. In neutrophils, Cdc42 uses its effector WASp to control polarity for efficient chemotaxis and transmigration. In this model, WASp appears to act at the uropod at a distance from Cdc42, and facilitate microtubule capture and stabilization at the membrane through CD11b signaling. Therefore, WASp seems to mediate “long distance” crosstalk between Cdc42 and the uropod to maintain polarity during migration. Importantly, the Cdc42/WASp polarity pathway controls neutrophil extravasation to lung alveoli during acute lung inflammation.
Neutrophil polarity is a self-organizing process that leads to the segregation of F-actin filaments at the front and bundles of acto-myosin at the uropod.1-3 One long-standing question of neutrophil polarity has been how the pseudopod and the uropod are coordinated. One concept is that the pseudopod restricts itself by generating “long distance signaling” that would amplify the uropod, thereby limiting protrusions to the leading edge. We and other have shown that Cdc42 controls the neutrophil uropod from a distance.16,18 In this study, we provide evidence that WASp mediates Cdc42 functions in the “long distance” control of neutrophil polarity during directed migration leading to microtubule capture at the uropod. This was first suggested by expression of Cdc42S71P, a mutant form of Cdc42 that no longer binds WASp, which was unable to restore abnormal polarity of Cdc42−/− neutrophils. Although we cannot exclude the involvement of other effectors in addition to WASp, the fact that Cdc42S71P rescued PAK but not WASp phosphorylation in Cdc42−/− neutrophils without restoring their polarity, strongly supports a key role for WASp in neutrophil polarity. This was clearly confirmed by studies showing that WASp−/− neutrophils exhibit all characteristic of loss of polarity because of loss of uropod functions, that is, multiple membrane protrusions and frequent change in direction. Interestingly, WASp appears to act at the uropod at a distance from Cdc42. WASp was enriched at the uropod of polarized neutrophils whereas Cdc42 was not. However, this distribution was Cdc42-dependent, suggesting that WASp relocalizes to the uropod secondary to Cdc42 activation. WASp activation is complex. WASp initially exists in a closed inactive state in which the Cdc42-binding domain contacts the verprolin, cofilin, acidic (VCA) domain.27 Binding to GTP-Cdc42 releases the VCA domain leading to unfolding WASp. Unfolding WASp activates WASp and exposes Y291 for phosphorylation, which enhances its function. In addition, phosphorylation of WASp primes the molecule to further activation by other stimuli, such as Src kinases, when Cdc42 is no longer binding. In this model, WASp secondary activation requires its release from Cdc42. Phosphorylation of WASp is thought to be important for its subcellular localization.40,41 Hence, pWASp may relocate through vesicle transport in domains where Cdc42 is not enriched. Because Cdc42 is known to control polarity via its effector aPKC leading to MTOC reorientation and microtubule capture at the leading edge in fibroblasts,14,15 our work unveils a novel mechanism in which Cdc42 controls neutrophil polarity via WASp, and suggests that WASp is a key factor of the “long-distance” control of the uropod by the pseudopod.
Our model is consistent with the concept of long-distance control of the uropod through transport of signaling molecules. Recently, Houk et al elegantly demonstrated that membrane tension is a key alternative mechanism.42 Using morphologic perturbations and cell severing to prevent diffusion-based exchanges between the front and the cell body, they show that diffusion-based mechanisms are insufficient for long-range inhibition of the pseudopod. Instead, membrane tension appears to be one mode of long-range inhibition mechanisms. Membrane tension nearly doubles during leading-edge protrusions, and tension increase is sufficient for long-range inhibition of Rac activation, whereas reducing membrane tension activates actin assembly throughout the cell.42 Membrane tension is known to coordinate the front and the back, communicating through the membrane, by mechanically hindering actin polymerization.43 Although through an unknown mechanism, tension appears to do so by inhibiting signaling molecules necessary for F-actin polymerization, in neutrophils.42 For a global regulatory role of membrane tension, tension must remain high and constant to prevent the emergence of secondary protrusions. Yet, neutrophil shape remains highly dynamic during polarized migration. Tension alone may not be sufficient to explain persistent polarity during migration and other elements probably contribute to it, such as microtubules33 and the plasma membrane composition.36-38 Furthermore, long-range control of the uropod involves other mechanism than inhibiting Rac, for example, activation of RhoA and downstream effector p-MLC.17,18 In our model, Cdc42 stimulates p-MLC.18 Interestingly, membrane tension is tightly dependent on the biochemical composition of the plasma membrane and membrane-cytoskeleton adhesion.43 Because these parameters are highly heterogeneous and dynamic during neutrophil migration, membrane tension will probably intertwine with other mechanisms, and/or differentially contribute various mode of migration, that is, neutrophil crawling on endothelium or transendothelial extravasation through tight space. It will be interesting to investigate possible crosstalk between tension-based and diffusion-based polarity mechanisms.
WASp is a member of the WASp/WAVE/Scar family protein that acts as a cytoskeleton adapter to promote actin remodeling via Arp2/3.20 As such, WASp is classically involved in actin rearrangement processes at the cell leading edge leading to membrane protrusions. WASp also controls podosome assembly and integrin-dependent adhesion, and is implicated in cell migration.27,28,44 Previous studies have shown impaired neutrophil migration, altered F-actin polymerization, and impaired adhesion under flow and β2-integrin clustering in WASp−/− neutrophils.28,29,45 However, the precise role of WASP in neutrophil migration was not established. Although WASp is known to induce actin remodeling, F-actin content was unchanged in WASp−/− neutrophils stimulated with fMLP in cell suspension, compared with WT neutrophils, and measured by flow cytometry. Similarly, neutrophils extracted from WAS patients exhibited normal actin polymerization responses.30 Conversely, WASp−/− neutrophils have abnormally multiple F-actin protrusions when plated on Fg. Because F-actin quantification by flow cytometry measures total cellular content of F-actin polymerization, whereas F-actin analysis of cells stimulated in Fg-coated plates provides information on the spatial distribution of F-actin, our data strongly suggest that WASp is dispensable for F-actin polymerization per se; instead, it controls the spatial distribution of polymerized F-actin into a single leading edge during neutrophil polarization and migration. Mechanistically, WASp limits protrusions by facilitating microtubule capture at the uropod via CD11b signaling. We show that WASp controls integrin clustering, which is associated with lipid-detergent resistant membrane at the uropod. CD11b then recruits the MT-binding protein EB1 allowing for MT stabilization at the uropod membrane. The rearrangement of the plasma membrane into domains containing different membrane receptors and lipids between the front and the back, with DRM domains at the uropod, is important for neutrophil polarization.36-38 Our study is consistent with this view and further indicates that WASp is important for the reorganization of DRM domains containing CD44 and CD11b, and that the presence of CD11b within DRMs is necessary for MT orientation and stabilization. Microtubules exhibit dynamics instability with alternate phase of elongation and shortening. Capping from plus-end binding proteins at the plasma membrane is necessary for their capture and stabilization.34 In fibroblasts, integrin regulates lipid raft localization to capture microtubule locally.35 We show that CD11b recruits the MT-binding proteins EB1 to capture and stabilize MTs. Microtubules provide tracks for vesicle and protein transport and play regulatory role. Therefore, microtubule capture at the uropod may help delivering regulators of RhoA activity facilitating local activation of actomyosin contraction. Our previous study showed that Cdc42 controls polarity via CD11b–mediated RhoA signaling pathway at the uropod,18 providing first evidence of the direct involvement of CD11b in neutrophil polarity. The PIP5K1C kinase appears to be important for CD11b activation of RhoA at the uropod.46 How these elements are precisely integrated during neutrophil migration will be interesting to analyze further. Nevertheless, our study now reveals a novel interplay between CD11b integrin, lipid raft and microtubules that is necessary to maintain polarity and is regulated by WASp in neutrophils.
Our study also proposes that the Cdc42/WASp axis regulates inside/out CD11b integrin signaling and further reinforces the growing notion that integrins are critical to the regulation of polarity.47 We show that CD11b clustering is abrogated in Cdc42 and WASp-deficient neutrophils, and CD11b cross-linking restored their polarity without restoring the recruitment of WASp to the uropod membrane, hence placing the Cdc42/WASp axis upstream of integrin functions. The exact mechanism of β2 integrin clustering in neutrophils is poorly understood. A mechanism of diffusion of integrin in the plasma membrane may be controlled by inside-out signaling.48 In resting neutrophils, β2 integrins are linked to intracellular actin via talin, thus preventing their mobility onto the plasma membrane. On activation, integrins are released from this constraint, which contributes to their diffusion onto the membrane and their clustering. New bound with actin are then formed via α-actinin to consolidate integrin clusters.48,49 Because Arp2 became enriched in DRMs at the uropod on activation, and CD11b cross-linking did not restore the Apr2 defect in the uropod membrane of Cdc42 and WASp−/− neutrophils, WASp may locally promote de novo actin remodeling at integrin site that may be cross-linked via α-actinin to reconnect the integrin to the cytoskeleton and facilitate their clustering.
Our study reports a novel signaling pathway of neutrophil polarity that is critical for neutrophil extravasation into lung alveoli during inflammation. Mutations within WASp are responsible for a severe immunodeficiency disorder known as WASp.50 The primary manifestation of the disease is an increased susceptibility to various pathogens, which are compatible with neutrophil function defects. Therefore, our study provides important regulation of neutrophil polarity through Cdc42-WASp-CD11b-Microtubule cascade in neutrophil-related innate immunity and inflammation conditions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr J. Cancelas (Division of Experimental Hematology and Cancer Biology, Cincinnati Children's Hospital Medical Center [CCHMC]) for important comments on the paper. The authors thank the mouse core, Jeff Bailey and Victoria Summey, for bone marrow transplantation; and the flow cytometry core for assistance with cell sorting at CCHMC.
The work was supported by NIH (HL090676-MDF; 5P01 HL059561-11-SS).
National Institutes of Health
Authorship
Contribution: S.K. designed and performed experiments, analyzed the data, and wrote the paper; J.X. performed experiments; C.P. performed experiments and analyzed the data; F.G. performed experiments; S.S. contributed vital reagents by providing the WASp knock out mouse; F.D.F. provided key advice in research design, data analysis, and editing the paper; Y.Z. contributed vital reagents by providing the Cdc42 knock out mouse, provided key advice in research design, data analysis and editing the paper; and M.-D.F. designed and directed the program research, analyzed data, and wrote and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marie-Dominique Filippi, Division of Experimental Hematology and Cancer Biology, S7.605, Cincinnati Children's Research Foundation, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: marie-dominique.filippi@cchmc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal