Abstract
We used a novel NF-08-TM transplant protocol based on intravenous busulfan, cyclophosphamide, fludarabine, and thiotepa in 82 consecutive patients with β-thalassemia major (TM), including 52 with allogeneic peripheral blood stem cell transplantation (PBSCT) from unrelated donors (UDs) with well-matched human leukocyte antigens and 30 with hematopoietic stem cell transplantation (HSCT) from matched sibling donors (MSDs). The median age at transplantation was 6.0 years (range, 0.6-15.0 years), and the ratio of male-to-female patients was 56:26. The median follow-up time was 24 months (range, 12-39 months). The estimated 3-year overall survival and TM-free survival were 92.3% and 90.4% in the UD-PBSCT group and 90.0% and 83.3% in the MSD-HSCT group. The cumulative incidences of graft rejection and grades III-IV acute graft-versus-host disease were 1.9% and 9.6%, respectively, in the UD-PBSCT group and 6.9% and 3.6%, respectively, in the MSD-HSCT group. The cumulative incidence of transplant-related mortality was 7.7% in the UD-PBSCT group and 10.0% in the MSD-HSCT group. In conclusion, UD-PBSCTs using the well-tolerated NF-08-TM protocol show similar results to MSD-HSCTs and can be used to treat β-thalassemia patients in the absence of MSDs.
Introduction
β-thalassemia major (TM), an inherited anemia that is associated with reduced or absent β-globin synthesis, results in an imbalanced accumulation of α-globin chains and ineffective erythropoiesis with hemolysis. Hemoglobin disorders constitute the most common class of monogenic disorders in the world, with > 330 000 affected infants born annually, 17% of which suffer from thalassemias.1 The optimization of both erythrocyte transfusion and iron chelating therapy has resulted in a remarkable improvement in the life expectancy of patients with TM.2,3 However, complications related to iron overload cannot be completely managed through chelating therapy, and compliance with a chronic transfusion regimen is difficult to maintain throughout a lifetime. The only curative therapy remains the replacement of the defective erythropoiesis by allogeneic hematopoietic stem cell transplantation (HSCT).4,5 However, this option is unavailable to many patients as a result of a lack of compatible donors, and the risks of developing life-threatening complications and of graft failure are high.
Since the first allograft was performed 30 years ago6 , hundreds of patients with TM have been cured by HSCT. Bone marrow transplantation (BMT) from a sibling donor with well-matched human leukocyte antigens (HLAs) has been used in most applications of HSCT.7-9 However, suitable unrelated donors (UDs) can be identified using stringent criteria for immunogenetic compatibility by high-resolution molecular typing of class I and class II HLA loci, and UDs have been proven to be a valuable alternative for leukemia patients lacking a well-matched sibling donor (MSD).9-11 Despite this success in leukemia patients, UD-peripheral blood stem cell transplantation (UD-PBSCT) is not widely practiced in thalassemia patients because of higher graft-versus-host disease (GVHD) risk and increased transplant-related mortality (TRM).12,13 Here, we use the NF-08-TM HSCT protocol based on intravenous busulfan (Bu), cyclophosphamide (Cy), fludarabine (Flu), and thiotepa (TT) to treat children with TM and compare the outcomes obtained using UD-PBSCT with the outcomes obtained from MSD-HSCT.
Methods
Patients
The NF-08-TM HSCT protocol was approved by the local institutional review board, and all of the parents of the patients signed a written, informed consent form in accordance with the Declaration of Helsinki. All of the patients included in the study were willing to receive transplants after they participated in an exhaustive discussion of the risk/benefit ratios of the transplantation procedures with their physicians. The inclusion criteria for patients who underwent either MSD-BMT or UD-PBSCT were (1) a diagnosis of TM with hemoglobin electrophoresis, a genetic diagnosis of β-thalassemia and blood transfusion dependence, (2) a cardiac ejection fraction of > 50%, and (3) normal pulmonary function tests and pulmonary examination results. The exclusion criteria for patients included aspartate aminotransferase levels that were more than quadruple the upper limit of the normal range and positive serology for HIV. No patient tested positive for either the hepatitis C virus or the hepatitis B virus before undergoing the transplantation procedure.
Donors and HLA typing
HLA-A, HLA-B, HLA-C, and HLA-DRB1 were typed at the allele level by polymerase chain reactions with sequence-specific primers and by sequence-based typing. Only 1 allelic mismatch within HLA-A, HLA-B, HLA-C, or HLA-DRB1 was accepted in UD-PBSCT, and no more than 1 antigenic mismatch in MSD-HSCT was permitted. One exception to these standards was granted, however, because 1 donor-recipient pair in the UD-PBSCT group had allelic mismatches for both HLA-C and HLA-DRB1.
Transplantation characteristics
Between December 1, 2008 and June 31, 2011, 100 consecutive patients with TM were enrolled in the NF-08-TM protocol, of which the doses of chemotherapy were adjusted based on the patient's age, ferritin level, and liver size (Table 1). Because the numbers of patients in group I (n = 9) and in group III (n = 9) were small, this report focused on the 82 patients in group II who received a uniform conditioning with 55 mg/kg/day Cy (Baxter Oncology; day −10 to day −9); 40 mg/m2/day Flu (Intendis Manufacturing; day −8 to day −4); 10 mg/kg/day TT (Shanghai Xudong; day −5); and intravenous Bu (Busulfex, PLD BioPharma; day −8 to day −6) at a dose that was dependent on the age of the patient (Table 2). The average plasma steady-state concentration (Css) of Bu was tested on day −8 and targeted to range between 300 and 600 μg/L. The dose of Bu was increased or decreased by 1.2 mg/kg when Css was < 300 μg/L or > 600 μg/L and was given in divided doses on day −6. All patients received 3 mg/kg azathioprine and 30 mg/kg hydroxyurea daily beginning at day −45 before transplantation, as described previously.14 On day 0, patients in the UD-PBSCT group received granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cells (PBSCs), whereas patients in the MSD-HSCT group received bone marrow (BM, n: 14), BM with precryopreserved cord blood (BM + CB, n: 12), or PBSCs (n: 4). The mean mononuclear cell (MNC) dose that was infused in MSD-HSCT was 3.38 × 108/kg body weight of the recipient (BWR; range, 2.10-9.00 × 108/kg BWR). In the MSD-HSCT group, the mean MNC dose was 2.63, 2.54, and 8.30 × 108/kg BWR for the BMT, BM + CB transplant (BM + CBT), and PBSCT subgroups, respectively. For the recipients in the UD-PBSCT group, the MNC dose was 8 × 108/kg BWR in 27 cases, 9 × 108/kg BWR in 24 cases, and 7 × 108/kg BWR in 1 case. The mean dose of CD34+ cells that were infused was 6.81 × 106/kg BWR (range, 2.70-13.00 × 106/kg BWR) in the UD-PBSCT group and 14.21 × 106/kg BWR in the MSD-HSCT group (range, 5.49-42.60 × 106/kg BWR). Within the MSD-HSCT group, the mean CD34+ cell doses were 15.68, 13.87, and 9.22 × 106/kg BWR in the BMT, BM + CBT, and PBSCT subgroups, respectively. The CD3+ cells were not measured in either group.
NF-index of categorization of NF-08-TM
| Group . | Ferritin . | Hepatomegaly . | Age, y . |
|---|---|---|---|
| I | < 3000 μg/L | < 2.5 cm under the costal margin | < 4 |
| II | Neither group I nor group III | ||
| III | > 5000 μg/L | > 4 cm | > 8 |
| Group . | Ferritin . | Hepatomegaly . | Age, y . |
|---|---|---|---|
| I | < 3000 μg/L | < 2.5 cm under the costal margin | < 4 |
| II | Neither group I nor group III | ||
| III | > 5000 μg/L | > 4 cm | > 8 |
Conditioning regimens of NF-08-TM
| Conditioning . | Cy (mg/kg/d), d −10 to −9 . | IV Busulfex (mg/kg/d),* d −8 to −6 . | Thiotepa (mg/kg/d bid), d −5 . | Fludarabine (mg/m2/d), d −8 to −4 . |
|---|---|---|---|---|
| Regimen for group I | 60 | 5 | 40 | |
| 1-2 y | 4.4 | |||
| > 2 y | 4.0 | |||
| Regimen for group II | 55 | 5 | 40 | |
| < 6 y | 3.6 | |||
| > 6 y | 3.2 | |||
| Regimen for group III | 50 | 5 | 40 | |
| < 8 y | 3.0 | |||
| > 8 y | 2.8 |
| Conditioning . | Cy (mg/kg/d), d −10 to −9 . | IV Busulfex (mg/kg/d),* d −8 to −6 . | Thiotepa (mg/kg/d bid), d −5 . | Fludarabine (mg/m2/d), d −8 to −4 . |
|---|---|---|---|---|
| Regimen for group I | 60 | 5 | 40 | |
| 1-2 y | 4.4 | |||
| > 2 y | 4.0 | |||
| Regimen for group II | 55 | 5 | 40 | |
| < 6 y | 3.6 | |||
| > 6 y | 3.2 | |||
| Regimen for group III | 50 | 5 | 40 | |
| < 8 y | 3.0 | |||
| > 8 y | 2.8 |
Average plasma Css of busulfan was tested on day −8 and targeted to range between 300 and 600 μg/L. The dose of busulfan was increased or decreased by 1.2 mg/kg when Css was < 300 μg/L or > 600 μg/L and was given in divided doses on day −6.
With respect to the prophylaxis of GVHD, cyclosporine A (Cs-A) was started at 1.5 mg/kg/day i.v. from day −10 to day −2, before being increased to 3 mg/kg/day at day −1 up to day 25, and subsequently administered orally at targeted concentrations of 200 ± 50 ng/mL. The dose of Cs-A was tapered from day 60 until its discontinuation at the end of 1 year. Mycophenolate mofetil (CellCept) was administered on day 1 at 15 mg/kg bid and was discontinued on day 30 if there were no signs of ≥ grade II acute GVHD (aGVHD). Thymoglobulin (Genzyme Europe; day −3 to day −1) was used at 2.5 mg/kg/day from day −3 to day −1. In the UD-PBSCT group, instead of thymoglobulin, ATG-Fresenius ([ATG-F] Fresenius Biotech; day −3 to day −1) was used (15 or 30 mg/kg/day for the first 43 patients in a randomized study; 30 mg/kg/day for the 9 remaining patients). In addition, short-term methotrexate (MTX) was administered on days 1, 3, and 6 at 15, 10, and 10 mg/m2, respectively. Heparin and ursodiol were used for the prophylaxis of hepatic veno-occlusive disease (VOD). The dose of heparin was gradually increased from 100 to 200 U/kg to maintain the levels of activated partial thromboplastin time that were slightly higher than normal. After VOD onset, a diuretic agent was used. Ursodiol was used until the end of the third month after the transplant.
Myeloid engraftment was defined to occur on the first of 3 consecutive days in which the absolute neutrophil count (ANC) was > 0.5 × 109/L. Platelet engraftment was defined to occur during the first of 7 consecutive days with an unsupported platelet count > 20 × 109/L. The engraftment was documented by the in situ Y-chromosome hybridization of bone marrow or blood samples in sex-mismatched donor-recipient pairs and by the analysis of variable number tandem repeat polymorphisms as well as the microsatellite analysis of bone marrow, blood samples, or both in sex-matched donor-recipient pairs. The measurement for donor chimerism after transplantation was performed at the first, second, third, fourth, fifth, sixth, and 12th months after transplantation. Once the donor-derived cells constituted < 98% of the cells sampled from the patient, the dose of Cs-A was reduced. Donor lymphocyte infusion was performed if no response after 4 weeks of observation and the donor-derived cells constituted < 95% of the cells sampled from the patient.
The overall survival (OS) represents those patients who did not die from any cause from the beginning of the conditioning. Thalassemia-free survival (TFS) was calculated from the beginning of the conditioning until either thalassemia recurrence with transfusion dependence or death from any cause. Primary graft failure (PGF) was defined as the absence of donor-originated hematopoietic reconstitution on day 28 after the allograft infusion. Graft rejection (GR) was defined to encompass thalassemia recurrence at any time after engraftment. TRM was defined as deaths that were related to the transplant instead of the recurrence of thalassemia. The aGVHD and chronic GVHD (cGVHD) were defined in accordance with the classification system of Glucksberg and Przepiorka. VOD was diagnosed in accordance with the Baltimore criteria.
Supportive care
All patients were treated in positive-pressure isolation rooms and received a low-bacteria diet and nonabsorbable oral antibiotics for 3 days. No standard prophylactic broad-spectrum bacterial antibiotic was administered to patients when neutropenia occurred. IVIg was administered at 500 mg/kg weekly until myeloid engraftment occurred after the transplant. G-CSF was not administered to any of the patients. Patients with febrile neutropenia were treated according to the guidelines of the Infectious Diseases Society of America.15,16 The diagnosis and treatment of invasive fungal infections were performed as described previously.17 The prophylaxis of fungal infection with intravenously administered itraconazole was started from day 5 and continued until the engraftment. All of the blood products except the allograft were filtered, rather than irradiated, before transfusion. The thresholds of RBC and platelet transfusions were hemoglobin < 8 g/dL and platelets < 20 × 103/mm3, respectively. All of the patients were given preemptive prophylactic treatment for cytomegalovirus (CMV) infection. The CMV status was monitored weekly by quantitative PCR for 6 months, and patients with CMV reactivation were treated with either ganciclovir or foscarnet. None of the patients were monitored for infection by the Epstein-Barr virus after the transplant. Chelation therapy, phlebotomy, or both for iron overload were never administered until either 1 year after the transplant or the termination of the immunosuppressant drugs.
Statistical methods
The reference date of the analysis was July 1, 2012. The median duration of the follow-up treatment was 24 months (range, 12-39 months). No patient was lost during the follow-up treatment. For continuous variables, results were expressed in terms of either medians or means, and ranges were provided. For the calculation of transfusion-independent survival, data on patients were recorded at the time of death, graft failure, or the last follow-up treatment. The probabilities of survival and transfusion-independent survival were estimated by the product-limit method of Kaplan and Meier and expressed as percentages. The SPSS 13.0 software package (SPSS) was used for the analyses. A P value < .05 was considered to be statistically significant.
Results
Engraftment
The characteristics of the 82 patients are summarized in Table 3. Eighty of the patients had successful engraftment, with > 95% donor-derived cells by day 28 after transplantation and became transfusion-independent. Two patients who received MSD-HSCT died before engraftment. One patient who received UD-PBSCT rejected his graft during the second month after transplant. No donor lymphocyte infusion was needed in the 2 study groups. We found no significant difference in ANC engraftment time between the MSD-HSCT and UD-PBSCT groups (17.5 days vs 19.0 days, P = .230; Table 4). The platelet engraftment time was also similar for the 2 groups (17.0 days vs 15.5 days, P = .344). The MSD-HSCT group had a shorter duration of ANC < 500/mm3 than the UD-PBSCT group (18.0 days vs 22.0 days, P = .010). After engraftment, 10 patients in the UD-PBSCT group developed leukopenia (white blood cells < 3.0 × 109/L lasting for 4 weeks or longer) with or without decrease of hemoglobin and/or platelet; 7 of them responded well to steroid treatment. The 3 patients who did not respond to steroids remained clinically healthy, and their white blood cell counts increased subsequently over time spontaneously. All 3 of these patients received UD-PBSCT with 1 allelic mismatch; 2 of them received a transplant with an HLA-A allelic mismatch, and the other patient received a transplant with an HLA-C allelic mismatch.
Characteristics of patients, donors, and transplants
| Characteristic . | MSD-HSCT (n = 30) . | UD-PBSCT (n = 52) . | Total (n = 82) . | P . |
|---|---|---|---|---|
| Categorization of thalassemia | ||||
| Patient age | 0.897 | |||
| Median, range | 6.0 (0.6-13) | 6.0 (2-15) | 6.0 (0.6-15) | |
| Patient sex | ||||
| Male (%) | 20 (66.7) | 36 (69.2) | 56 (68.3) | .810 |
| Female (%) | 10 (33.3) | 16 (30.8) | 26 (31.7) | |
| Donor sex | ||||
| Male (%) | 16 (53.3) | 29 (55.8) | 45 (54.9) | .831 |
| Female (%) | 14 (46.7) | 23 (44.2) | 37 (45.1) | |
| Donor age | ||||
| Median, range | 3 (1-19) | 28 (20-51) | 24 (1-51) | .000 |
| Serum ferritin | ||||
| Median, range | 3252 (233-7198) | 3271.6 (515-8250) | 3252 (233-8250) | .437 |
| Donor/patient ABO-match | ||||
| Match (%) | 20 (66.7) | 18 (34.6) | 38 (46.3) | .005 |
| Mismatch (%) | 10 (33.3) | 34 (65.4) | 44 (53.7) | |
| Stem cell source | ||||
| BM (%) | 14 (46.7) | 0 (0) | 14 (17.1) | .000 |
| PBSC (%) | 4 (13.3) | 52 (100) | 56 (68.3) | |
| BM + UCB (%) | 12 (40.0) | 0 (0) | 12 (14.6) | |
| HLA matching status | .007 | |||
| 8/8 of the HLA-A, HLA-B, HLA-C, and HLA-DRB1 loci are matched (%) | 28 (93.3) | 32 (61.5) | 60 (73.2) | |
| 7/8 matched (%) | 2 (6.7)* | 19 (36.5)† | 21 (25.6) | |
| 6/8 matched (%) | 0 (0) | 1 (2.0)‡ | 1 (1.2) |
| Characteristic . | MSD-HSCT (n = 30) . | UD-PBSCT (n = 52) . | Total (n = 82) . | P . |
|---|---|---|---|---|
| Categorization of thalassemia | ||||
| Patient age | 0.897 | |||
| Median, range | 6.0 (0.6-13) | 6.0 (2-15) | 6.0 (0.6-15) | |
| Patient sex | ||||
| Male (%) | 20 (66.7) | 36 (69.2) | 56 (68.3) | .810 |
| Female (%) | 10 (33.3) | 16 (30.8) | 26 (31.7) | |
| Donor sex | ||||
| Male (%) | 16 (53.3) | 29 (55.8) | 45 (54.9) | .831 |
| Female (%) | 14 (46.7) | 23 (44.2) | 37 (45.1) | |
| Donor age | ||||
| Median, range | 3 (1-19) | 28 (20-51) | 24 (1-51) | .000 |
| Serum ferritin | ||||
| Median, range | 3252 (233-7198) | 3271.6 (515-8250) | 3252 (233-8250) | .437 |
| Donor/patient ABO-match | ||||
| Match (%) | 20 (66.7) | 18 (34.6) | 38 (46.3) | .005 |
| Mismatch (%) | 10 (33.3) | 34 (65.4) | 44 (53.7) | |
| Stem cell source | ||||
| BM (%) | 14 (46.7) | 0 (0) | 14 (17.1) | .000 |
| PBSC (%) | 4 (13.3) | 52 (100) | 56 (68.3) | |
| BM + UCB (%) | 12 (40.0) | 0 (0) | 12 (14.6) | |
| HLA matching status | .007 | |||
| 8/8 of the HLA-A, HLA-B, HLA-C, and HLA-DRB1 loci are matched (%) | 28 (93.3) | 32 (61.5) | 60 (73.2) | |
| 7/8 matched (%) | 2 (6.7)* | 19 (36.5)† | 21 (25.6) | |
| 6/8 matched (%) | 0 (0) | 1 (2.0)‡ | 1 (1.2) |
One antigenic mismatch at the HLA-A locus and 1 antigenic mismatch at the HLA-DRB1 locus.
Allelic mismatches occurred at the HLA-A locus in 7 donor-recipient pairs, at the HLA-DRB1 locus in 9 donor-recipient pairs, at the HLA-C locus in 2 donor-recipient pair, and at the HLA-B locus in 1 donor-recipient pair.
Allelic mismatches at both the HLA-C and the HLA-DRB1 loci occurred in 1 donor-recipient pair.
Clinical outcome according to transplant groups
| . | MSD-HSCT . | UD-PBSCT . | Total . | P . |
|---|---|---|---|---|
| Engraftment, median day (range)* | ||||
| ANC > 500/mm3 | 17.5 (12-30) | 19 (11-26) | 19 (11-30) | .230 |
| PLT > 20 × 103/mm3 | 17 (9-56) | 15.5 (8-42) | 16 (8-56) | .344 |
| Hgb > 8.0 g/dL | 13 (2-42) | 13 (6-28) | 13 (2-42) | .073 |
| Duration of ANC < 500/mm3 | 18 (1-33) | 22 (13-32) | 21 (1-33) | .010 |
| Acute GVHD | ||||
| Grade III-IV (%) | 1 (3.6) | 5 (9.6) | 6 (7.5) | .328 |
| Transplantation-related complications | ||||
| HC (%) | 3 (10.7) | 9 (17.3) | 12 (15.0) | .431 |
| CMV reactivation (%) | 9 (32.1) | 22 (42.3) | 31 (38.8) | .373 |
| VOD (%) | 3 (10.7) | 2 (3.8) | 5 (6.3) | .226 |
| Mucositis (%) | 10 (35.7) | 33 (63.5) | 43 (53.8) | .018 |
| IFD (%) | 1 (3.6) | 5 (9.6) | 6 (7.5) | .328 |
| Results of transplant | ||||
| OS (at 3 y) | .900 | .923 | .915 | .678 |
| TFS (at 3 y) | .833 | .904 | .878 | .309 |
| TRM (at 3 y) | .100 | .077 | .085 | .678 |
| GR (at 3 y) | .069 | .019 | .037 | .259 |
| . | MSD-HSCT . | UD-PBSCT . | Total . | P . |
|---|---|---|---|---|
| Engraftment, median day (range)* | ||||
| ANC > 500/mm3 | 17.5 (12-30) | 19 (11-26) | 19 (11-30) | .230 |
| PLT > 20 × 103/mm3 | 17 (9-56) | 15.5 (8-42) | 16 (8-56) | .344 |
| Hgb > 8.0 g/dL | 13 (2-42) | 13 (6-28) | 13 (2-42) | .073 |
| Duration of ANC < 500/mm3 | 18 (1-33) | 22 (13-32) | 21 (1-33) | .010 |
| Acute GVHD | ||||
| Grade III-IV (%) | 1 (3.6) | 5 (9.6) | 6 (7.5) | .328 |
| Transplantation-related complications | ||||
| HC (%) | 3 (10.7) | 9 (17.3) | 12 (15.0) | .431 |
| CMV reactivation (%) | 9 (32.1) | 22 (42.3) | 31 (38.8) | .373 |
| VOD (%) | 3 (10.7) | 2 (3.8) | 5 (6.3) | .226 |
| Mucositis (%) | 10 (35.7) | 33 (63.5) | 43 (53.8) | .018 |
| IFD (%) | 1 (3.6) | 5 (9.6) | 6 (7.5) | .328 |
| Results of transplant | ||||
| OS (at 3 y) | .900 | .923 | .915 | .678 |
| TFS (at 3 y) | .833 | .904 | .878 | .309 |
| TRM (at 3 y) | .100 | .077 | .085 | .678 |
| GR (at 3 y) | .069 | .019 | .037 | .259 |
All of the patients were included except for the 2 patients who died before engraftment with MSD-HSCT.
OS and TFS
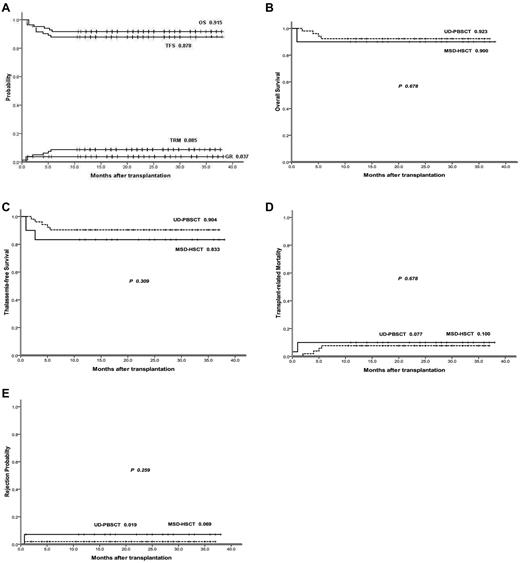
Figure 1A illustrates the Kaplan-Meier probabilities of OS (91.5%), TFS (87.8%), GR (3.7%), and TRM (8.5%). One patient in the MSD-HSCT group died during the conditioning treatment. An additional 6 patients died after receiving transplants, with a median time to death of 3 months after transplantation (range, 1-6 months). With respect to causes of death, in the MSD-HSCT group, 1 patients died from infection and 2 patients died from VOD. In the UD-PBSCT group, 1 patient died from infection and 2 patients died from severe aGVHD. The patient with allelic mismatches for both HLA-C and HLA-DRB1 in the UD-PBSCT group was alive without TM and blood transfusion independence.
Group II patient summary. (A) OS, TFS, TRM, and GR for all of the group II patients for the NF-08-TM HSCT protocol. (B) OS of the MSD-HSCT group versus OS of the UD-PBSCT group. (C) TFS of the MSD-HSCT group versus TFS of the UD-PBSCT group. (D) TRM of the MSD-HSCT group versus TRM of the UD-PBSCT group. (E) GR of the MSD-HSCT group versus GR of the UD-PBSCT group.
Group II patient summary. (A) OS, TFS, TRM, and GR for all of the group II patients for the NF-08-TM HSCT protocol. (B) OS of the MSD-HSCT group versus OS of the UD-PBSCT group. (C) TFS of the MSD-HSCT group versus TFS of the UD-PBSCT group. (D) TRM of the MSD-HSCT group versus TRM of the UD-PBSCT group. (E) GR of the MSD-HSCT group versus GR of the UD-PBSCT group.
The estimated 3-year OS was 90.0% in the MSD-HSCT group and 92.3% in the UD-PBSCT group (P = .678; Figure 1B). The estimated 3-year TFS was 83.3% for the MSD-HSCT group and 90.4% in the UD-PBSCT group (P = .309; Figure 1C). TRM also was found to be similar between the 2 groups (Figure 1D). In total, 2 patients rejected the graft in the MSD-HSCT group and 1 patient rejected the graft in the UD-PBSCT group (Figure 1E).
GVHD
Of the 80 patients who had successful engraftment, 6 developed grades III-IV aGVHD. The incidences of grades III-IV aGVHD were 3.6% in the MSD-HSCT group versus 9.6% in the UD-PBSCT group (P = .328). Among the 6 children with grades III-IV aGVHD, 5 had received UD-PBSCT and had gut and hepatic organ involvement; only 1 patient who received MSD-HSCT developed grade III aGVHD in a gut organ, and this patient had a complete response to the administration of high-dose steroids. In total, 2 patients died from GVHD, all from the UD-PBSCT group. No extensive cGVHD was diagnosed among any patients in the 2 study groups.
Transplantation-related complications
CMV reactivation (CMV antigenemia or DNAemia) occurred in 31 (38.8%) of the 82 patients. These 31 patients included 9 (32.1%) of the 30 patients who received MSD-HSCT and 22 (42.3%) of the 52 patients who received UD-PBSCT (P = .373). No patients died from CMV infection. In the MSD-HSCT group, 1 patient was diagnosed with a probable, invasive fungal disease. Probable invasive fungal disease was diagnosed in 5 of the patients in the UD-PBSCT group. Thus, the incidence of fungal infection was lower for the MSD-HSCT group (3.6%) than for the UD-PBSCT group (9.6%), but the difference was not significant statistically (P = .328). The conditioning regimen was well tolerated with limited organ toxicity; only 1 patient died before transplantation and 1 patient died before engraftment. The most frequently observed toxic effect was mucositis, occurring in 43 (53.8%) of the 82 enrolled patients. VOD accompanied by elevated bilirubin (> 2 mg/dL) was diagnosed in 5 cases, and 2 patients died from the comorbidity of VOD and infection. VOD seemed to be unrelated to the serum ferritin levels. In the 2 patients who died from VOD, the serum levels of ferritin were 233.0 and 3672.0 ng/mL.
Discussion
Although blood transfusions and chelation therapy have greatly improved the quality of life and life expectancy of patients with TM, medical costs and the demand for red blood cells have increased as a result of longer life spans and increasing comobility that are generated by transfusions and chelation therapy.18 Therefore, there is a great need to provide a reliable, cost-effective transplantation procedure that leads to transfusion independence. MSD-HSCT is well established as the first treatment option for younger patients with TM.19 However, in China, the majority of patients with TM do not have an HLA-matched donor within the family because of the nation's 1-child policy. For this reason, we have been exploring the possibility of TM treatments that use allografts from alternative donors.
Until recently, most TM patients in China received irregular transfusions without leukodepletion, resulting in high sensitization to HLAs. In addition, the patients had rarely been in compliance with regular iron chelation therapy owing to health care costs. Almost all parents were unwilling to accept liver biopsy simply for classification and, as a result, the Pesaro's risk groupings are not applicable in the Chinese population.19 Our study demonstrates that the outcomes of UD-PBSCT were similar to the outcomes of MSD-HSCT for children with TM with no significant difference in OS or TFS between the 2 groups. The results for the UD-PBSCT group were comparable to the findings of La Nasa et al20 with respect to the experiences of the Italian Bone Marrow Transplant Group; this study reported that in 68 TM cases with a median follow-up length of 43 months, the probabilities of OS, TFS, GR, and TRM were 79.3%, 65.8%, 14.4%, and 20.7%, respectively. Our findings are also in accordance with data reported by Locatelli et al,21 who found that out of 122 UD-BMT cases that included 26 adults, the 5-year probabilities of OS and TFS were 84% and 75%, respectively. Compared with these aforementioned studies, our follow-up time was shorter and the age of our patients was younger. Our results were also comparable to the outcomes of Jaing et al22 for unrelated cord blood transplants for patients with TM: of the 40 unrelated cord blood transplants that were studied, the 5-year OS and TFS was 88.3% ± 6.7% and 73.9% ± 7.4%, respectively, and the cumulative incidence of TRM at 2 years was 11.7% ± 6.7%.
Thalassemia patients typically display relatively high GR rates owing to the higher probability of being sensitized to allo-HLA through long-term, repeated transfusions. Although Fleischhauer et al demonstrated that GR after UD-HSCT for thalassemia was associated with nonpermissive HLA-DPB1 disparities in the host-versus-graft direction,23 the importance of HLA-DPB1 donor-recipient matching remains debatable. In the present study, GR occurred in only 1 of the 52 (66 if group I and group III cases included) patients in the UD-PBSCT group by the second month after transplantation. It is possible that the PBSCT was easier to stably engraft owing to the high number of hematopoietic stem cells and lymphocytes, which overcame the high rejection tendency of thalassemia transplants. Mathews et al demonstrated that bone marrow was associated with delayed engraftment (median time to ANC > 1000/mm3 was 19 days; range, 15-21 days), greater morbidity, and a higher incidence of mixed chimerism on day 28 after transplantation (50%) than PBSCT (n = 18; median time to ANC > 1000/mm3 was 15 days; range, 12-28 days; day 28 mixed chimerism in 12% of patients).24 The use of PBSCT was not associated with a significantly increased risk of either acute or chronic GVHD.
With the use of treosulphan in the conditioning regimen, the clinical outcomes of high-risk TM patients who underwent an HLA-matched related PBSCT have improved recently.24 In the present study, we modified the classic preparative regimen based on Bu-Cy through the addition of TT and Flu, and we reduced the dose of Bu by one third to decrease lung and liver toxicity. TT has been shown to be highly myeloablative and effective at immunosuppression, leading to enhanced engraftment with lower overall rates of not only VOD and other metrics of liver toxicity but also pulmonary toxicity in a murine model.25 Bu-TT-Cy is an effective preparative regimen for T cell–depleted allo-BMT in genetic diseases, providing sustained engraftment.26 We gave Cy first during the conditioning process to reduce the heart toxicity of the conditioning by avoiding the simultaneous administration of ATG-F and Cy. This initial treatment with Cy also may have induced myeloid and lymphoid cells to undergo cell cycling through the in vivo depletion of lymphocytes and the mobilization of myeloid cells, thus resulting in more effective myeloablation and immunosuppression by the subsequent conditioning agents. The lower death incidence from fungal infections that were observed in our study relative to previously published work might be attributed to a lack of G-CSF use after transplantation in our protocol.27
Similar to the 82 group II patients reported here, the other 18 patients (9 in group I and 9 in group III) treated concurrently on the NF-08-TM protocol with group-adjusted conditioning also had good outcome, with 16 of them (88.9%) alive without TM after receiving MSD-HSCTs (n = 4) or UD-PBSCTs (n = 14). No GR was observed, and only 2 patients died, 1 in group I after receiving MSD-HSCT and 1 in group III after an UD-PBSCT. Future studies including more patients in groups I and III, however, are essential because the number of participants is too small.
Although no significant difference in aGVHD incidence was noted in comparisons of the UD-PBSCT group and the MSD-HSCT group, there was a tendency toward more severe GVHD in the UD-PBSCT group (grades III-IV, 9.6% vs 3.6%). Because 2 of the 3 patients with an allelic mismatch and grades III-IV aGVHD received ATG-F at 15 mg/kg during conditioning and died, in December 2010, we had to discontinue the randomized study in which the effects of 2 different doses of ATG-F (15 mg/kg BWR vs 30 mg/kg BWR) administered in UD-PBSCT were compared. Since that time, the dose of ATG-F has been administered in accordance with donor-recipient HLA match status; ATG-F was given at 15 mg/kg/day in the matched portion of the UD-PBSCT group and at 30 mg/kg/day in the portion of the UD-PBSCT group with 1 allelic mismatch.
We observed a lower incidence of aGVHD than in a previous study in which 40% of the 59 evaluable transplanted patients developed grades II-IV aGVHD, 17% had grades III-IV aGVHD, and 18% developed cGVHD.20 Similarly, in a study of 9 patients with TM who underwent HSCT, nearly half of the patients developed aGVHD (grade II, 30%; grade IV, 15%) and 3 patients developed cGVHD (limited, n = 2; extensive, n = 1).28 The following factors most likely contribute to our results: (1) the combination of multiple drugs, including ATG-F, Cs-A, mycophenolate mofetil, and MTX for the prophylaxis of GVHD reduced the incidence of GVHD in our study29,30 ; (2) the patients enrolled in our study had a median age of 6.0 years, which was much younger than the age of the patients examined by La Nasa et al20 ; (3) none of the patients in the current study was exposed to G-CSF, and G-CSF can increase the incidence and severity of GVHD31,32 ; and (4) our patient-donor pairs had relatively homogenous genetic backgrounds with extended haplotype sharing; most of them are from southern Han Chinese origin and extensively share certain common HLA haplotypes.33 La Nasa et al has reported that BMT from well-selected unrelated donors sharing 1 or 2 extended haplotypes offered results comparable to those obtained in transplantations using HLA-identical family donors.34 Recent studies from the Japan Marrow Donor Registry have demonstrated that highly conserved common HLA haplotypes, ranging from the centromeric HLA-DPB1 to the telomeric HLA-A, display a strong correlation with reduced incidences of aGVHD.35 It is possible that our patient groups share certain low-GVHD haplotypes that are characteristic of the southern Han Chinese people, although obtaining confirmation of this conjecture would require further analysis.
In conclusion, our study demonstrates that there were no significant differences in OS, TFS, GF, aGVHD, and TRM between the MSD-HSCT group and the UD-PBSCT group when the NF-08-TM protocol was used. UD-PBSCT in thalassemia patients yields excellent engraftment rate and TFS; thus, this approach could be a good alternative if MSD is not available for patients with TM. Given the limitations of our single-center study, multicenter studies of UD-PBSCT using the NF-08-TM HSCT protocol are warranted.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Wing Leung (Department of Bone Marrow Transplantation and Cellular Therapy, St Jude Children's Research Hospital, Memphis, TN) and Dr Chi-Kong Li (Department of Paediatrics, Prince of Wales Hospital, Hong Kong) for the contribution of designing the NF-08-TM HSCT protocol and reviewing this manuscript. Andrew R. Dyer and Dr Xiangjun Liu (BFR Diagnostics, Beijing, China) provided drafts and editorial assistance during preparation of this manuscript.
Authorship
Contribution: C.L. participated in conception and design of the study, with other authors performing transplant and follow-up; H.L. and Y.W. assembled the data and performed the statistical analysis; C.L. had primary responsibility for manuscript preparation; and all authors participated in interpretation of data, manuscript preparation, and approval of the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chunfu Li, Department of Pediatrics, Nanfang Hospital, No 1838, Northern Guangzhou Avenue, Guangzhou, China 510515; e-mail: chunfugzcn@126.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal