Abstract
Abstract 339
There is little understanding of the maintenance and regeneration of epithelial tissues after allogeneic transplant. Most clinical strategies to limit epithelial damage from graft vs. host disease (GVHD) also limit post-transplant immune function. Damage to the gastrointestinal (GI) tract from GVHD is a major cause of morbidity and mortality, and damage to the thymus from pre-transplant conditioning and GVHD can impair immune reconstitution, predispose patients to infection, and increase the risk of relapse. Therefore, understanding of tissue damage and recovery could lead to strategies selectively protecting epithelial tissues, improving intestinal barrier function, and promoting immune reconstitution without worsening post-transplant immunosuppression.
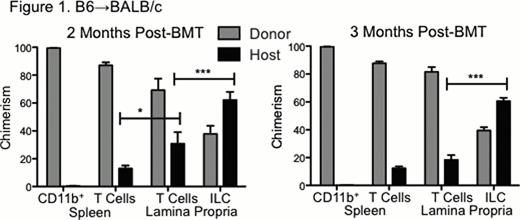
We have recently identified that IL-22 from recipient-derived innate lymphoid cells (ILC) is critical for promoting intestinal recovery from GVHD and for promoting thymic recovery from radiation/pre-transplant conditioning. IL-22 deficient mice demonstrated significantly reduced thymopoiesis after total body irradiation (TBI), and IL-22 deficient murine bone marrow transplant (BMT) recipients demonstrated increased GVHD mortality and intestinal histopathology, deficiency of the antimicrobial molecules Reg3γ and Reg3β, and loss of intestinal stem cells needed for epithelial recovery. The source of thymic and intestinal IL-22 was RORγ+CD3−NKp46−IL-7R+CCR6+ lymphoid-tissue-inducer-like cells. Similar to as had been observed in the thymus, intestinal ILC produced IL-22 in response to IL-23, which was upregulated after TBI (p<.05 small intestine, p<.001 large intestine). IL-22 was also upregulated in response to TBI, but not in p40-deficient mice lacking IL-23 (p<.05 small intestine, p<.01 large intestine). ILC were radioresistant, as lethal TBI led to a three-fold increase in the intestinal ILC:CD4 ratio (p<.05). Furthermore, recipient-derived ILC comprised more than 50% of intestinal lamina propria ILC three months after T cell-depleted BMT, well after donor myeloid reconstitution and after donor reconstitution of the intestinal T cell compartment as well (Figure 1).
Although intestinal ILC could survive lethal TBI, they were significantly depleted by both MHC mismatched (B6BALB/c) and MHC matched (LPB6) GVHD. Similarly, GVHD led to depletion of thymic IL-22+ ILC and reduction in thymic IL-22 levels (p<.001). Thymic IL-22 was critical for maintaining thymopoiesis during GVHD, as IL-22 deficient BMT recipients demonstrated significantly greater loss of double positive (DP) thymocytes after MHC-mismatched BMT. We previously identified that IL-21 receptor (IL-21R) signaling contributes to the migration of alloreactive donor T cells to the GI tract and that IL-21R-deficent donor T cells mediate significantly reduced GI GVHD. Given the similar homing molecules involved in the migration of donor T cells to the GI tract and thymus in GVHD, we evaluated the role of IL-21 in thymic GVHD. Donor T cell IL-21R deficiency led to increased thymopoiesis and DP thymocytes (p<.001), but not in IL-22-deficient recipients. ILC evaluation indicated that this IL-22 dependency was because IL-21R-deficiencient donor T cells had a reduced capacity to eliminate thymic ILC during GVHD (Figure 2). Therefore, donor T cell IL-21 signaling was critical for the elimination of recipient thymic ILC during GVHD, and preservation of the ILC compartment allowed for the IL-22 mediated regeneration of thymopoiesis. Finally, we also found that administration of rIL-22 post-BMT could reverse the thymic damage caused by GVHD and elimination of ILC, restoring the numbers of DP thymocytes to a level similar to what was observed after T cell-depleted BMT.
In summary, IL-22+ ILC are radioresistant and capable of regulating tissue-specific epithelial recovery after allogeneic BMT. However, recipient ILC are extremely sensitive to GVHD, leading to a loss of the IL-22-mediated recovery response. IL-21 blockade can prevent the elimination of recipient thymic ILC by donor T cells in GVHD, and IL-22 administration can restore the thymopoiesis that is lost in GVHD due to ILC elimination. Maintenance of epithelial function post-BMT is thus an active innate immune response requiring cooperation between both recipient stroma and recipient hematopoietic cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal