Abstract
Abstract  3557
3557
Secondary Acute Myeloid Leukemia (AML) accounts for approximately 10% of AML's and are often associated with adverse outcomes compared to de novo AML. In addition to cytogenetics, multiple gene mutations have been incorporated in the de novo AML risk stratification as independent prognostic and predictive factors. Little is known about these molecular markers in secondary AML. We analyzed the characteristics and outcomes of AML patients arising from myelodysplastic syndrome (MDS), myeloproliferative neoplasm (MPN), treatment-related AML (t-AML), or with prior history of cancer not treated with chemotherapy or radiation compared to de novo AML, and investigated the frequency and prognostic relevance of molecular markers among those groups.
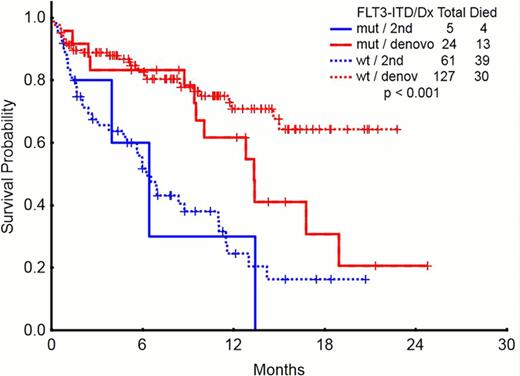
We analyzed the outcomes of adult patients with AML receiving induction chemotherapy at MDACC (n= 248) from 2010 to 2012. Median age was 67 years (range 17–82) and 142 (57.2 %) patients were >60 yrs. 108 (44%) were female. 146 (59 %) had de novo AML, 43 (17%) had t-AML, 23 (9%) had post-MDS, 16 (7%) post-MPN, and 20 (8%) AML with antecedent cancer not treated with chemotherapy and/or radiation therapy. Median white blood cell count (WBC) at diagnosis was 4.45 × 109/L (r: 0.4–186.5), and 82 (33%) pts had WBC >10 x109/L. Cytogenetics were diploid in 91 (37%), inv 16 in 16 (7%), t(8;21) in 12 (5%), trisomy 8 in 10 (4%), chromosome −5 and/or −7 in 60 (24%), 11q in 11 (4%), miscellaneous in 30 (12%), and insufficient metaphases on 18 (7%). 43(17%) had FLT3 mutations including 26 (10%) with FLT3-ITD, 14 (6%) FLT3-D835, and 3 (1%) double mutant. 32 (13 %) had NPM 1, 15 (6%) CBFb-MYH1, 10 (4%) ABL1/ETO, 6 (2%) JAK2, 34 (14 %) RAS, 1 (0.4%) cKIT, 18 (7%) CEBPA, 10 (4%) IDH1, and 8 (3.2%) IDH2. The pts were treated with several different induction chemotherapies. Idarubicin and cytarabine (IA)-based were more frequent in de novo and in 2nd cancer groups, while hypomethylating agents were more common in post-MDS and post-MPN groups. CR rates were higher in de novo AML than the rest of the groups, and early deaths were more common in the post-MPN group. The frequency of mutations was similar among groups with the exception of JAK 2 mutation, which was more frequent in post-MPN (Table 1). In pts with secondary AML, FLT3 mutations do not seem to further worsen their outcome (median survival 6.2 months for FLT3 wt and 6.4 for FLT3 ITD in 2nd AML; corresponding values for de novo AML are not reached and 13.3 months, respectively). (Figure 1) EFS and OS was worse in the post-MDS and post-MPN groups compared to de novo AML and second cancer group (p>0.001).
In patients with secondary AML, antecedent of MDS or MPN are associated with unique molecular signatures (eg, rare FLT3-ITD in post MDS, frequent JAK2 in post-MPN) and have an inferior outcome. In contrast AML in pts with history of previous cancers not previously exposed to chemotherapy or radiation therapy survival outcomes are similar to de novo AML. Mutational status may not be as predictive of outcome among patients with secondary AML as it is for de novo AML.
Characteristics and molecular abnormalities in AML arising from MDS, MPN and second cancers
| . | De novo (n=143) . | t-AML (n-=43) . | Antecedent MDS (n=23) . | Antecedent MPN (n=16) . | second cancer (n=20) . | p . |
|---|---|---|---|---|---|---|
| AGE | 59.5 | 60 | 69 | 70.5 | 67 | |
| Cytogenetics (%) | ||||||
| Diploid | 41 | 14 | 44 | 25 | 55 | |
| t(8;21) | 5 | 5 | 0 | 0 | 10 | |
| inv 16 | 8 | 2 | 0 | 4 | 10 | |
| Trisomy 8 | 5 | 0 | 4 | 4 | 0 | |
| -5 and/or -7 | 19 | 44 | 22 | 31 | 15 | |
| 11q abnormality | 4 | 14 | 0 | 0 | 0 | |
| Miscelaneous, other | 11 | 19 | 13 | 13 | 5 | |
| ND | 6 | 2 | 17 | 19 | 5 | |
| Outcomes (%) | ||||||
| CR | 69 | 42 | 26 | 19 | 80 | |
| CR p/i | 3 | 2 | 13 | 25 | 10 | |
| Median DFS (months) | 11.7 | 3.5 | 1.6 | 1.4 | 10.9 | <0.001 |
| Median OS (months) | 18.9 | 11.7 | 6.9 | 4.9 | 16.8 | <0.001 |
| AML1/ETO | 5 | 5 | 0 | 0 | 5 | 0.832 |
| CBFb-MYH11 | 8 | 2 | 0 | 6 | 10 | 0.547 |
| FLT3-ITD | 13 | 7 | 0 | 13 | 25 | 0.208 |
| JAK2 | 1 | 0 | 0 | 31 | 0 | 0.001 |
| NPM1 | 18 | 12 | 0 | 19 | 30 | 0.086 |
| RAS | 16 | 5 | 4 | 0 | 25 | 0.486 |
| KIT | 1 | 0 | 0 | 0 | 0 | 0.952 |
| CEBPA | 9 | 2 | 9 | 0 | 10 | 0.445 |
| IDH1 | 3 | 5 | 9 | 0 | 10 | 0.279 |
| IDH2 | 3 | 2 | 4 | 6 | 5 | 0.879 |
| . | De novo (n=143) . | t-AML (n-=43) . | Antecedent MDS (n=23) . | Antecedent MPN (n=16) . | second cancer (n=20) . | p . |
|---|---|---|---|---|---|---|
| AGE | 59.5 | 60 | 69 | 70.5 | 67 | |
| Cytogenetics (%) | ||||||
| Diploid | 41 | 14 | 44 | 25 | 55 | |
| t(8;21) | 5 | 5 | 0 | 0 | 10 | |
| inv 16 | 8 | 2 | 0 | 4 | 10 | |
| Trisomy 8 | 5 | 0 | 4 | 4 | 0 | |
| -5 and/or -7 | 19 | 44 | 22 | 31 | 15 | |
| 11q abnormality | 4 | 14 | 0 | 0 | 0 | |
| Miscelaneous, other | 11 | 19 | 13 | 13 | 5 | |
| ND | 6 | 2 | 17 | 19 | 5 | |
| Outcomes (%) | ||||||
| CR | 69 | 42 | 26 | 19 | 80 | |
| CR p/i | 3 | 2 | 13 | 25 | 10 | |
| Median DFS (months) | 11.7 | 3.5 | 1.6 | 1.4 | 10.9 | <0.001 |
| Median OS (months) | 18.9 | 11.7 | 6.9 | 4.9 | 16.8 | <0.001 |
| AML1/ETO | 5 | 5 | 0 | 0 | 5 | 0.832 |
| CBFb-MYH11 | 8 | 2 | 0 | 6 | 10 | 0.547 |
| FLT3-ITD | 13 | 7 | 0 | 13 | 25 | 0.208 |
| JAK2 | 1 | 0 | 0 | 31 | 0 | 0.001 |
| NPM1 | 18 | 12 | 0 | 19 | 30 | 0.086 |
| RAS | 16 | 5 | 4 | 0 | 25 | 0.486 |
| KIT | 1 | 0 | 0 | 0 | 0 | 0.952 |
| CEBPA | 9 | 2 | 9 | 0 | 10 | 0.445 |
| IDH1 | 3 | 5 | 9 | 0 | 10 | 0.279 |
| IDH2 | 3 | 2 | 4 | 6 | 5 | 0.879 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal