Abstract
Abstract 3820
Progressive cytopenias are the cause of death for the majority of patients with myelodysplastic syndromes (MDS). In order to investigate if the proliferative activity of CD34+ cells in MDS bone marrow correlates with prognosis, we measured expression levels of the proliferation inhibitor CDKN1C (P57KIP2) in two independent study cohorts.
Gene expression profiling data on bone marrow CD34+ cells were obtained from 183 MDS patients (55 with RA, 48 with RARS, 37 with RAEB1 and 43 with RAEB2). mRNA expression levels of CDKN1C (P57KIP2) were correlated with overall survival (OS) and proliferation markers such as PCNA, cyclins and cyclin dependent kinases.
In a second independent patient cohort comprising 93 patients (17 had RA, 13 REAB1, 27 RAEB2, 29 AML arising from MDS and 7 CMML), protein expression levels of CDKN1C (P57KIP2) were evaluated by immune histochemistry (IHC) in trephine biopsies. Protein expression levels of CDKN1C (P57KIP2) were correlated with clinical outcome, OS and the proliferation marker KI67 in CD34+ cells (double staining).
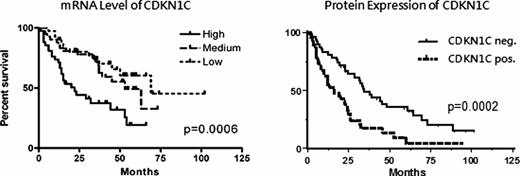
In purified CD34+ cells, mRNA expression of CDKN1C (P57KIP2) correlated with higher risk and poorer overall survival of patients with MDS (p=0.0006, n=183, Figure1). Furthermore, increased CDKN1C (P57KIP2) expression was significantly associated with loss of CD38 (p=0.002) as well as loss of proliferating cell nuclear antigen (PCNA, p<0.001), cyclins and cyclin-dependent kinases (p<0.001) underlining the role of CDKN1C (P57KIP2) as a proliferation inhibitor.
Similarly, protein expression of CDKN1C (P57KIP2) determined in trephine biopsies predicted a poor prognosis of patients with MDS (p=0.0003, HR=2.2, n=93, Figure 1). No expression of CDKN1C (P57KIP2) could be demonstrated in 10 control patients with normal bone marrow. Separate evaluation of WHO risk categories revealed that in patients with less than 10% blasts (RA, RAEB1 and CMML1), CDKN1C (P57KIP2) was not predictive for OS. In contrast, patients with high CDKN1C (P57KIP2) expression and RAEB2 (p=.0.02, HR 2.4) or AML arising from MDS (p=0.002, HR 2.9) had a significantly worse OS than patients with low CDKN1C (P57KIP2) levels. CDKN1C (P57KIP2) expression analysis within the group of allogeneic stem cell recipients showed no impact on survival after transplant. Multivariate cox regression analysis with the confounding co-variates: age, IPSS score factors (cytopenias, cytogenetic risk profile, blast count) and primary versus secondary MDS confirmed the independent impact of CDKN1C (P57KIP2) expression on OS (p=0.0002, HR 2.9). CDKN1C (P57KIP2) protein expression could also be demonstrated in sorted CD34+ cells of MDS patients by western blot analysis and was significantly higher than in CD34- cells (p=0.03, n=7).
KI67 expression was evaluated in CD34+ cells by IHC double staining in 34 trephine biopsies and 10 control patients with normal bone marrow. Percentages of CD34+ and KI67 positive cells were higher in control patients and patients with low risk MDS (RA, RAEB1) than in patients with high risk MDS (RAEB2) (p<0.01). Patients with AML (>20% blasts) had significantly higher levels of KI67 (p<0.05). In patients with MDS (<20% blasts) KI67 percentages correlated inversely with CDKN1C (P57KIP2) expression (p=0.04).
High CDKN1C (P57KIP2) expression in CD34+ cells of patients with MDS is associated with a reduced fraction of proliferative CD34+ cells and determines a worse prognosis independently of factors used to calculate the IPSS score. Allogeneic stem cell transplantation could overcome the worse prognosis of high CDKN1C (P57KIP2) expression. Further studies are warranted to determine the impact of CDKN1C (P57KIP2) expression to guide clinical decisions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal