To the editor:
The familial aggregation of thrombocytopenia and hematopoietic malignancies is seen in several autosomal-dominant familial myelodysplastic syndrome/acute leukemia predisposition syndromes (MDS/AL PS).1 We hypothesized that some thrombocytopenic HLA-matched related subjects who donate hematopoietic stem cells (HSCs) for a first-degree relative with an hematopoietic malignancy represent unrecognized cases of MDS/AL PS.
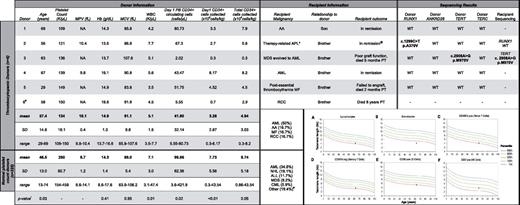
To test this, we studied the characteristics of 331 HLA-matched related HSC donors who underwent peripheral blood stem cell (PBSC) mobilization at The University of Chicago from 2001 to 2011 (Figure 1; supplemental Methods, supplemental Table 1, and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Approval was obtained from The University of Chicago institutional review board for these studies, and informed consent was provided according to the Declaration of Helsinki. Donor complete blood counts drawn before mobilization identified 6 (1.8%) subjects with thrombocytopenia, defined as a platelet count ≤ 150 000/μL. Thrombocytopenic donors were older, had lower total WBC counts, fewer circulating PBSCs after mobilization, and fewer PBSCs collected than those with normal platelet counts.
Tabular data. Thrombocytopenic and normal platelet count donor and recipient pair clinical characteristics and mutation screening results. (A-F) Median telomere length (in kb) in donor 3 plotted versus age. Lines on the graph represent telomere length measurement ranges in normal controls: blue line indicates the 99th percentile; top green line, the 90th percentile; middle green line, the 50th percentile; bottom green line, the 10th percentile; and red line, the 1st percentile. The telomere length reference ranges according to population age are: very short (< 1st percentile), short (≥ 1st and < 10th percentile), and normal (≥ 10th and < 90th percentile). All donors had ANKRD26 SNV rs7897309 (c.59A>G) and donor 5 had SNV rs41299222 (c.-140C>G). *Also had a history of follicular lymphoma; @Follicular lymphoma recurred 4 years post-allogeneic hematopoietic stem cell transplant #Donor 6 was excluded from mutation analysis due to lack of a family member with hematopoietic malignancy. #Other includes: multiple myeloma, pure red cell aplasia, Hodgkin lymphoma, paroxysmal nocturnal hemoglobinuria, chronic lymphocytic leukemia, myelofibrosis, aplastic anemia, and renal cell carcinoma. !All P values determined by Wilcoxon rank-sum test. AA indicates aplastic anemia; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; CML, chronic myeloid leukemia; dash (-), test not done; Hb, hemaglobin; MDS, myelodysplastic syndrome; MCV, mean corpuscular volume; MF, myelofibrosis; MPV, mean platelet volume; NA, data not available; NHL, non-Hodgkin lymphomia; PB, peripheral blood; PT, posttransplant; RCC, renal cell carinoma; SD, standard deviation; WBC, white blood cell count; and WT, wild-type.
Tabular data. Thrombocytopenic and normal platelet count donor and recipient pair clinical characteristics and mutation screening results. (A-F) Median telomere length (in kb) in donor 3 plotted versus age. Lines on the graph represent telomere length measurement ranges in normal controls: blue line indicates the 99th percentile; top green line, the 90th percentile; middle green line, the 50th percentile; bottom green line, the 10th percentile; and red line, the 1st percentile. The telomere length reference ranges according to population age are: very short (< 1st percentile), short (≥ 1st and < 10th percentile), and normal (≥ 10th and < 90th percentile). All donors had ANKRD26 SNV rs7897309 (c.59A>G) and donor 5 had SNV rs41299222 (c.-140C>G). *Also had a history of follicular lymphoma; @Follicular lymphoma recurred 4 years post-allogeneic hematopoietic stem cell transplant #Donor 6 was excluded from mutation analysis due to lack of a family member with hematopoietic malignancy. #Other includes: multiple myeloma, pure red cell aplasia, Hodgkin lymphoma, paroxysmal nocturnal hemoglobinuria, chronic lymphocytic leukemia, myelofibrosis, aplastic anemia, and renal cell carcinoma. !All P values determined by Wilcoxon rank-sum test. AA indicates aplastic anemia; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; CML, chronic myeloid leukemia; dash (-), test not done; Hb, hemaglobin; MDS, myelodysplastic syndrome; MCV, mean corpuscular volume; MF, myelofibrosis; MPV, mean platelet volume; NA, data not available; NHL, non-Hodgkin lymphomia; PB, peripheral blood; PT, posttransplant; RCC, renal cell carinoma; SD, standard deviation; WBC, white blood cell count; and WT, wild-type.
Mutation screening of RUNX1, TERT, TERC, and ANKRD26 in the 5 thrombocytopenic donors with a first-degree relative with a hematopoietic malignancy identified a RUNX1 variant of unknown significance in donor 2, which did not segregate with the disease. In donor 3, we identified a novel missense TERT variant, p.M970V, which was also present in the recipient's leukemic cells. Flow FISH of donor-derived cells showed short or very short telomeres in all lymphocyte subsets (Figure 1A-F), which is suggestive of deleterious functional consequences in vivo, as seen in inherited telomere biology disorders (TBDs).
Detailed review of the medical histories of this donor/recipient pair supported the diagnosis of a TBD. First, the donor's predonation laboratory studies revealed an unexplained macrocytosis. Second, this donor collected a combined stem cell yield of 1 × 106 CD34+ cells/kg after 2 PBSC collections and a BM harvest. After transplantation using the combined stem cell yield, the recipient never achieved a normal platelet count, required intermittent transfusions for anemia, and died 5 months later due to complications from graft failure. A similar combination of macrocytosis, cytopenias, failure to mobilize PBSCs, and a family member with a hematopoietic malignancy has been reported in subjects with deleterious TERC mutations,2 which, like mutations in TERT, cause an autosomal-dominant heritable TBD often lacking the mucocutaneous triad seen in classic dyskeratosis congenita.3,4
Our ability to identify a novel TERT variant in 1 of 5 (20%) thrombocytopenic donors supports our hypothesis that careful analysis of healthy HSC donors can lead to the identification of cases of MDS/AL PS. The peritransplantation evaluation of first-degree relatives of patients with hematopoietic malignancies presents a unique opportunity to delineate the detailed history that allows identification of the often subtle presentations of MDS/AL PS. Macrocytosis or mild cytopenias alone warrant work-up for MDS/AL PS, which should include telomere length testing to rule out TBDs. Failure to recognize MDS/AL PS in the transplantation setting can have significant consequences, including poor graft function or donor-derived leukemias, if affected relatives are used as donors1 ; excessive transplantation-related morbidity from some preparative regimens in those with TBDs5 ; lack of monitoring for related health issues (eg, pulmonary fibrosis and other cancers in TBD); and a missed opportunity to identify and counsel all at-risk family members.
Authorship
The online version of this letter contains a data supplement.
Acknowledgments: The authors gratefully acknowledge the patients, donors, and their family members who graciously participated in this study; Dr Alison Bertuch for many helpful discussions; Dr Michael Chicka (Prevention Genetics, Marshfield, WI) for performing custom RUNX1 array comparative genomic hybridization; and Repeat Diagnostics Inc (North Vancouver, BC) for performing telomere length testing.
Contribution: J.E.C. and L.A.G. designed the research; J.E.C., E.N., R.M., K.R., and B.L. performed the experiments; J.E.C., E.N., R.M., and K.R. analyzed the data; J.E.C., R.L., A.S.A., K.v.B., and L.A.G. provided clinical care; A.S.A. and K.v.B. provided the donor mobilization data; A.W. provided cryopreserved patient samples; and all authors wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for K.v.B. is Division of Hematology and Medical Oncology, Weill Cornell Medical College, New York, NY.
Correspondence: Lucy A. Godley, MD, PhD, 5841 S Maryland Ave MC2115; Chicago, IL 60637; e-mail: lgodley@medicine.bsd.uchicago.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal