Key Points
Eliminating the binding motif on fibrinogen for ClfA, but not leukocyte integrin Mac-1, improves host survival in Staphylococcus aureus septicemia.
Abstract
Fibrinogen can support host antimicrobial containment/clearance mechanisms, yet selected pathogens appear to benefit from host procoagulants to drive bacterial virulence. Here, we explored the hypothesis that host fibrin(ogen), on balance, supports Staphylococcus aureus infection in the context of septicemia. Survival studies following intravenous infection in control and fibrinogen-deficient mice established the overall utility of host fibrin(ogen) to S. aureus virulence. Complementary studies in mice expressing mutant forms of fibrinogen-retaining clotting function, but lacking either the bacterial ClfA (FibγΔ5) binding motif or the host leukocyte integrin receptor αMβ2 (Fibγ390–396A) binding motif, revealed the preeminent importance of the bacterial ClfA–fibrin(ogen) interaction in determining host survival. Studies of mice lacking platelets or the platelet integrin receptor subunit αIIb established that the survival benefits observed in FibγΔ5 mice were largely independent of platelet αIIbβ3–mediated engagement of fibrinogen. FibγΔ5 mice exhibited reduced bacterial burdens in the hearts and kidneys, a blunted host proinflammatory cytokine response, diminished microscopic tissue damage, and significantly diminished plasma markers of cardiac and other organ damage. These findings indicate that host fibrin(ogen) and bacterial ClfA are dual determinants of virulence and that therapeutic interventions at the level of fibrinogen could be advantageous in S. aureus septicemia.
Introduction
Staphylococcus aureus is a pervasive gram-positive pathogen that underlies a wide spectrum of infections, ranging from minor skin infections to serious life-threatening conditions such as endocarditis, pneumonia, bacteremia, and sepsis.1 S. aureus infections are particularly problematic in immune-compromised individuals and in hospital settings where serious S. aureus infections are often associated with foreign bodies such as catheters, surgical implants, and sutures.2 The emergence of antibiotic-resistant strains of S. aureus (eg, methicillin-resistant S. aureus and vancomycin-resistant S. aureus) and the need for novel treatment strategies has driven renewed interest in better defining mechanisms by which pathogen-encoded virulence factors interact with host proteins to support S. aureus virulence.3
A remarkable number of S. aureus virulence factors that specifically engage host hemostatic system components have been identified, including at least 2 distinct staphylocoagulases that form proteolytically active procoagulant complexes with host prothrombin, a fibrin-selective plasminogen activator, staphylokinase, and multiple fibrin(ogen)-binding proteins.4-9 The bacterial fibrinogen-binding protein, clumping factor A (ClfA), belongs to a family of bacterial proteins collectively referred to as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) that mediate microbial engagement of host extracellular matrix components (eg, fibronectin, collagen, fibrinogen).5,8 ClfA has been recognized as important in pathogen virulence in murine models of bacteremia10,11 and septic arthritis,12,13 as well as in rat and rabbit models of bacterial endocarditis.14,15 The capacity of ClfA to interact with dimeric fibrinogen (Aα2Bβ2γ2) through the carboxy-terminal domain of the fibrinogen γ chain is the key to bacterial aggregation (clumping) observed within host plasma and to bacterial adhesion to immobilized fibrin(ogen)-coated surfaces.8,16 ClfA has been shown to promote bacterial evasion of host defense, in part, by inhibiting neutrophil and macrophage phagocytosis through both fibrinogen-dependent and -independent mechanisms.17 ClfA also appears to stimulate platelet activation via an interaction with the platelet integrin receptor αIIbβ3, an activity enhanced by the presence of fibrinogen.6
One driving force for developing a more detailed understanding of the relationship between host procoagulants and the bacterial products that engage them is the appreciation that key hemostatic factors appear to support microbial virulence in certain contexts while seeming to be central to antimicrobial host defense in others. Working at the level of host containment and/or local immune modulation, host fibrin(ogen) has often been shown to impede bacterial success in settings as divergent as S. aureus, Yersinia enterocolitica, Yersinia pestis, Listeria monocytogenes, and Staphylococcus pyogenes infections.18-21 Multiple innate immune cell components, including neutrophils and macrophages, are responsive to fibrin(ogen). Leukocyte engagement of fibrin(ogen) via the integrin receptor αMβ2 (CD11b/CD18) drives leukocyte activation events that lead to the full implementation of antimicrobial functions, including phagocytosis, generation of reactive oxygen species, nuclear factor κB–mediated alterations in gene expression, and the elaboration of proinflammatory mediators [eg, interleukin-1β(IL-1β), tumor necrosis factor α (TNFα), IL-6, monocyte chemoattractant protein-1 (MCP-1), and interferon-γ (IFN-γ)].22-25 Consistent with the concept that local fibrin(ogen)-αMβ2 interactions support antimicrobial host defense, we previously demonstrated that Fibγ390-396A mice carrying a mutant form of fibrinogen lacking the αMβ2 binding motif but retaining clotting function exhibit a diminished capacity to clear S. aureus in the context of perintonitis.18 Thus, an unresolved microbiological paradox is that host fibrin(ogen) is recognized as both proinflammatory and a potential means of bacterial containment and/or elimination in some settings, but that microbe-driven fibrin deposition also may promote microbial pathogenesis in other contexts.4,9,10,26 Here, we directly explore the hypothesis that fibrin(ogen), on balance, is an asset to S. aureus in the context of septicemia (ie, systemic disease resulting from the dissemination of the microbial pathogen through the circulation) and that virulence is dependent on the functional motif in the distal portion of the fibrinogen γ chain that is recognized by ClfA.
Materials and methods
Mice and bacterial strains
Fibrinogen-deficient, FibγΔ5, and fIIlow mice were described previously.18,27 Mice lacking the integrin subunit αIIb,28 lacking protease-activated receptor-4 (PAR-4),29 or expressing low levels of tissue factor30 (TF30 ) were used for the study. Platelet depletion was achieved by a single intraperitoneal injection of 1 mg/kg anti-mouse αIIbβ3 antibody (1B5) with nonimmune total hamster immunoglobulin G as the control antibody (Innovative Research) 24 hours prior to infection. Wild-type and ClfA/B Newman strains of S. aureus were kindly provided by Timothy Foster (Trinity College, Dublin, Ireland). The USA300 isolate of community-acquired S. aureus (FPR3757) was obtained from the American Type Culture Collection. Bacteria were grown at 37°C overnight in tryptic soy broth (Difco Laboratories), washed in phosphate-buffered solution, and resuspended to an optical density at wavelength 600 nm of 0.5 to 1.0. Colony forming units (CFUs) per milliliter values were directly determined by colony counts of serial dilutions plated on tryptic soy agar (TSA). Mice were infected by tail vein injection. Survival studies were performed at least twice, and similar results were observed in all replicate experiments. All experimental animals used in these studies were gender- and age-matched young adults (both male and female animals between 8 and 12 weeks of age) on a C57Bl/6J genetic background. The Cincinnati Children’s Hospital Institutional Animal Care and Use Committee approved all animal studies.
Bacterial clumping assay and adhesion to immobilized fibrinogen
Fibrinogen was purified from mouse citrate plasma by affinity chromatography as described.18 Fibrinogen-dependent clumping of S. aureus was performed by combining serial dilutions of purified fibrinogen with suspensions of S. aureus on 96 well microtiter plates (NUNC) at room temperature prior to evaluation and photography. The binding of bacteria to immobilized fibrinogen was assessed by coating NUNC enzyme-linked immunosorbent assay (ELISA) plates with purified fibrinogen and incubating with bacterial suspensions prepared from either stationary or exponential phase cultures. Adherent bacteria were fixed and stained with 0.1% crystal violet. The bound crystal violet was solubilized in acetic acid and quantified by spectrophotometry at 595 nm. Each sample was assayed in triplicate.
Bacterial burden and microscopic analysis of tissues
At selected times after infection, mice were anesthetized with ketamine/xylazine/acepromazine; blood and organs were then harvested for analyses. For bacterial load measurements, tissues were disrupted in ice-cold buffered saline using a Tissumizer polytron (Tekmar) and the bacterial CFU within organ homogenates or citrate-anticoagulated whole blood quantitated by plating serial dilutions on TSA media. For microscopic analyses, formalin-fixed tissue sections were stained with hematoxylin and eosin.
Blood cell and coagulation parameters
Blood cell counts in citrate-anticoagulated samples were determined using a HEMAVET 950 (Drew Scientific). Plasma thrombin–antithrombin (TAT) complex levels were determined using the Enzygnost TAT kit (Siemens). Plasma fibrinogen levels were determined using a mouse fibrinogen-specific ELISA assay kit (Immunology Consultants Laboratory, Inc.).
Plasma cytokines and markers of tissue damage
Cytokines were measured in citrate plasma using Milliplex multiplex kits (Millipore) for mouse cytokines/chemokines in conjunction with a luminex technology–based Bio-Plex plate reader (Bio-Rad). In addition, specific cytokines were measured by standard sandwich ELISA using OptEIA kits (BD Biosciences) for IL-6, TNFα, and IL-12p70 and a DuoSet kit (R&D Systems) for MCP-1. Cardiac troponin I levels were determined by ELISA using a high-density mouse cardiac troponin I kit (Life Diagnostics, Inc.). Lactate dehydrogenase and creatine kinase were determined using a colorimetric activity assay (BioAssay Systems). Plasma alanine/aspartate aminotransferase (ALT/AST) levels were determined using an enzyme assay kit (Labs Biotechnology).
Results
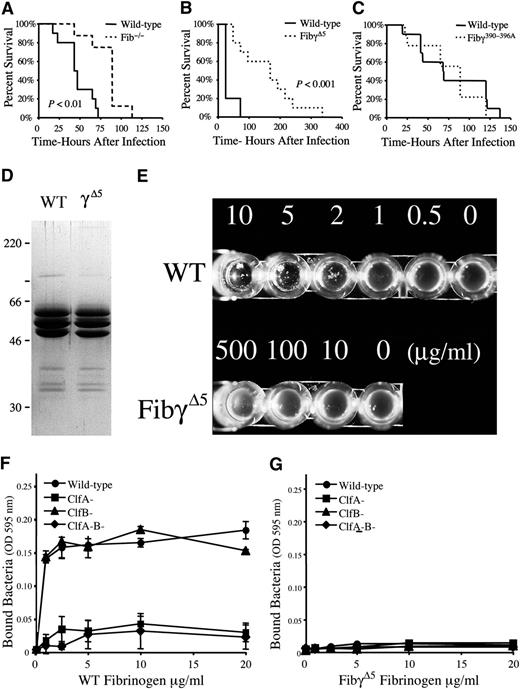
The fibrinogen motif recognized by the S. aureus surface protein ClfA, but not the motif recognized by leukocyte integrin αMβ2, is central to virulence in septicemia
To better understand the fibrinogen-dependent pathways that contribute to bacterial virulence and host defense, S. aureus septicemia studies were performed in mice lacking circulating fibrinogen or expressing mutant forms of fibrinogen lacking selected microbial- or host-receptor binding motifs. Survival analyses following intravenous injection of S. aureus revealed that although both control and fibrinogen-deficient mice succumbed to this challenge, fibrinogen-deficient cohorts exhibited a significant survival advantage, particularly over early times post-infection (Figure 1A); by 3 days post-infection control mice uniformly died or became moribund, whereas only 20% of fibrinogen-null mice succumbed during this period. More detailed studies of mice with more subtle genetic alterations in fibrinogen provided a means to elucidate the functional elements of fibrinogen that drive the high post-infection mortality. FibγΔ5 mice carry a mutant form of fibrinogen that lacks the final 5 amino acids of the γ chain. This imposes no impediment to polymer formation but results in the loss of the binding region proposed to mediate S. aureus ClfA binding. FibγΔ5 displayed a significant survival advantage relative to wild-type animals following intravenous infection (Figure 1B), an advantage that was even more impressive than that gained by the complete elimination of fibrinogen. In contrast, Fibγ390-396A mice expressing a mutant form of fibrinogen that retains normal polymer formation but does not support binding to the leukocyte integrin receptor αMβ2 had a survival profile indistinguishable from wild-type animals (Figure 1C). Thus, the final 5 amino acids of the fibrinogen γ chain, but not the αMβ2 binding moiety, encode a motif crucial to S. aureus virulence/host survival in septicemia.
Host fibrinogen is a determinant of virulence in S. aureus septicemia through a mechanism dependent on the 5 carboxy-terminal residues of the fibrinogen γ chain, a motif critical for ClfA binding. (A) Survival profile of fibrinogen-deficient (N = 8) and wild-type (N = 10) mice challenged with an intravenous injection of 3 × 108 CFUs of S. aureus. Although mice of both genotypes ultimately succumb to infection at these high bacterial doses, note that mice lacking fibrinogen exhibit a significant survival advantage. (B) Comparative survival analysis of cohorts of wild-type and FibγΔ5 mice (N = 10 pairs) challenged with intravenous injection of 3 × 108 CFUs of S. aureus. Note that despite full retention of clotting function, FibγΔ5 mice exhibit a distinct survival advantage over control mice. (C) Comparative survival analysis of wild-type and Fibγ390-396A mice challenged with intravenous injection of 3 × 108 CFUs of S. aureus. Survival statistics were calculated by Kaplan-Meier log rank analysis. (D) Coomassie blue-stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel (reducing conditions) of affinity-purified fibrinogen preparations from wild-type and FibγΔ5 mice. (E) Fibrinogen-dependent clumping of ClfA+S. aureus as a function fibrinogen concentration with wild-type (WT) fibrinogen and fibrinogen-γΔ5. Note that bacterial clumping was readily observed by simple inspection when bacterial suspensions were combined with wild-type fibrinogen at concentrations as low as 1 to 2 μg/mL. In contrast, S. aureus suspensions combined with fibrinogen-γΔ5 failed to support bacterial clumping at any fibrinogen concentration, including concentrations as high as 500 μg/mL. (F, G) Adhesion of wild-type, ClfA−, ClfB−, and ClfA−ClfB−S. aureus to immobilized wild-type fibrinogen (F) and fibrinogen-γΔ5 (G). Data are expressed as the mean ± standard deviation at each fibrinogen concentration.
Host fibrinogen is a determinant of virulence in S. aureus septicemia through a mechanism dependent on the 5 carboxy-terminal residues of the fibrinogen γ chain, a motif critical for ClfA binding. (A) Survival profile of fibrinogen-deficient (N = 8) and wild-type (N = 10) mice challenged with an intravenous injection of 3 × 108 CFUs of S. aureus. Although mice of both genotypes ultimately succumb to infection at these high bacterial doses, note that mice lacking fibrinogen exhibit a significant survival advantage. (B) Comparative survival analysis of cohorts of wild-type and FibγΔ5 mice (N = 10 pairs) challenged with intravenous injection of 3 × 108 CFUs of S. aureus. Note that despite full retention of clotting function, FibγΔ5 mice exhibit a distinct survival advantage over control mice. (C) Comparative survival analysis of wild-type and Fibγ390-396A mice challenged with intravenous injection of 3 × 108 CFUs of S. aureus. Survival statistics were calculated by Kaplan-Meier log rank analysis. (D) Coomassie blue-stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel (reducing conditions) of affinity-purified fibrinogen preparations from wild-type and FibγΔ5 mice. (E) Fibrinogen-dependent clumping of ClfA+S. aureus as a function fibrinogen concentration with wild-type (WT) fibrinogen and fibrinogen-γΔ5. Note that bacterial clumping was readily observed by simple inspection when bacterial suspensions were combined with wild-type fibrinogen at concentrations as low as 1 to 2 μg/mL. In contrast, S. aureus suspensions combined with fibrinogen-γΔ5 failed to support bacterial clumping at any fibrinogen concentration, including concentrations as high as 500 μg/mL. (F, G) Adhesion of wild-type, ClfA−, ClfB−, and ClfA−ClfB−S. aureus to immobilized wild-type fibrinogen (F) and fibrinogen-γΔ5 (G). Data are expressed as the mean ± standard deviation at each fibrinogen concentration.
To confirm that the final 5 amino acids of the fibrinogen γ chain are central to microbial binding, affinity-purified fibrinogen from wild-type and FibγΔ5 mice (Figure 1D) was assayed for the capacity to support ClfA-dependent S. aureus clumping activity and adhesion. Wild-type fibrinogen supported bacterial aggregation at concentrations as low as 1 to 2 μg/mL (Figure 1E), whereas no aggregate formation was observed with any concentration of fibrinogen-γΔ5. Complementary adhesion studies illustrated that immobilized wild-type fibrinogen supported bacterial binding in a fibrinogen concentration–dependent manner. ClfA+, but not ClfA−, S. aureus were adherent to wild-type fibrinogen at coating concentrations as low as 1 μg/mL, whereas essentially no bacterial adhesion to immobilized fibrinogen-γΔ5 could be appreciated even at 20-fold higher concentrations (Figure 1F-G). The ClfA-related S. aureus protein, ClfB, was found to be irrelevant in bacterial adhesion to immobilized murine fibrinogen of any type, regardless of whether assayed in the exponential growth phase (Figure 1F-G) or the stationary phase (data not shown). These studies formally establish that FibγΔ5 mice carry a form of fibrinogen that lacks a key feature supporting S. aureus binding of either soluble or immobilized fibrinogen.
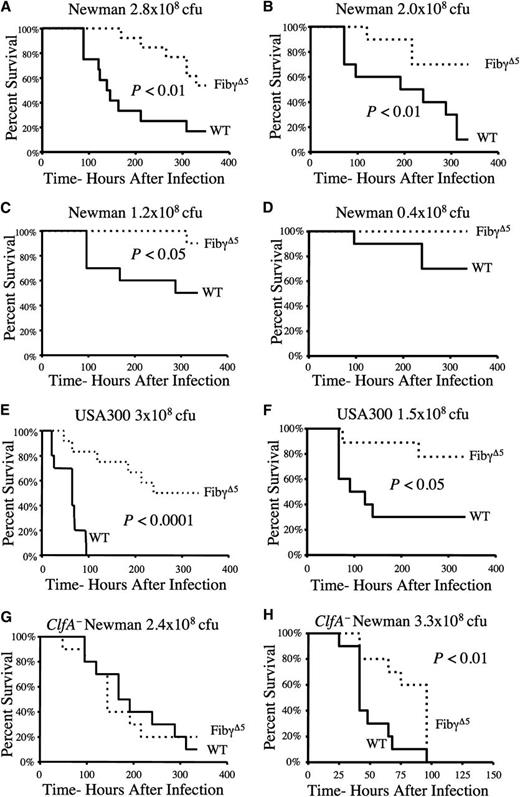
FibγΔ5 mice exhibit a survival advantage over wild-type mice in S. aureus septicemia over a wide range of bacterial doses and through ClfA-dependent and -independent mechanisms
Comprehensive studies using Newman strain S. aureus revealed a significant and consistent survival advantage for FibγΔ5 mice at challenge doses spanning the lethal dose medians 50% to 100% for control animals (Figure 2A–D). At a dose where ∼50% of control mice succumb to infection over a 2-week observation period (1.2 × 108 CFUs), most died within 6 days of initial infection; just 1 in 10 FibγΔ5 mice died after 2 weeks. At the lowest challenge dose examined (0.4 × 108 CFU), approximately 40% of wild-type animals died, whereas no FibγΔ5 mice succumbed to the infection (Figure 2D).
FibγΔ5 exhibit a distinct survival advantage over wild-type mice in S. aureus septicemia over a wide range of bacterial doses and through ClfA-dependent and ClfA-independent mechanisms. (A–D) Survival profile of wild-type and FibγΔ5 mice (N = 10 pairs) following intravenous injection with Newman strain S. aureus at the indicated doses. (E–F) Survival profile of wild-type and FibγΔ5 cohorts (N = 12 pairs) following intravenous administration of the community-associated strain of S. aureus USA300 at the indicated doses. Comparative analysis of survival in control and FibγΔ5 mice challenged in parallel with either (G) 2.4 × 108 CFUs intravenously or (H) 3.3 × 108 CFUs intravenously. ClfA-deficient derivative of S. aureus Newman. Survival was compared using the Kaplan-Meier log rank analysis.
FibγΔ5 exhibit a distinct survival advantage over wild-type mice in S. aureus septicemia over a wide range of bacterial doses and through ClfA-dependent and ClfA-independent mechanisms. (A–D) Survival profile of wild-type and FibγΔ5 mice (N = 10 pairs) following intravenous injection with Newman strain S. aureus at the indicated doses. (E–F) Survival profile of wild-type and FibγΔ5 cohorts (N = 12 pairs) following intravenous administration of the community-associated strain of S. aureus USA300 at the indicated doses. Comparative analysis of survival in control and FibγΔ5 mice challenged in parallel with either (G) 2.4 × 108 CFUs intravenously or (H) 3.3 × 108 CFUs intravenously. ClfA-deficient derivative of S. aureus Newman. Survival was compared using the Kaplan-Meier log rank analysis.
To investigate whether a similar survival advantage could be appreciated in FibγΔ5 mice following infection with other strains of S. aureus, including clinically significant strains, complementary studies were performed with the community-associated methicillin-resistant isolate of S. aureus, USA300. Similar to the pattern observed in cohorts challenged with S. aureus Newman, FibγΔ5 mice displayed a distinct survival advantage over wild-type mice when challenged with S. aureus USA300 (see Figures 2E and 2F). At a challenge dose of 3 × 108 CFUs USA300, there was a complete loss of wild-type mice within 100 hours of infection, whereas <20% of FibγΔ5 mice were lost within this time frame, and just half of FibγΔ5 mice succumbed over the 2-week observation period (Figure 2E). A similar survival pattern was observed at the lower challenge dose of 1.5 × 108 CFUs USA300 (Figure 2F).
In considering the mechanistic basis for the survival advantage observed in FibγΔ5 mice, the following 2 nonmutually exclusive hypotheses were formulated: (1) the loss of bacterial ClfA binding to fibrinogen-γΔ5 results in a survival advantage or (2) the loss of the platelet integrin αIIbβ3–binding element in fibrinogen-γΔ5 underlies the host survival advantage in S. aureus septicemia.31 Under the first theory, the differential survival benefits observed for FibγΔ5 mice would be eliminated if mice were challenged with ClfA-deficient bacteria. Consistent with this concept, the survival profile of FibγΔ5 mice and wild-type mice was indistinguishable following intravenous challenge with 2.4 × 108ClfA−S. aureus (Figure 2G). Comparable survival profiles were observed in multiple independent experiments where the challenge doses were < 3 × 108 CFUs of ClfA−S. aureus (data not shown). Interestingly, at even a marginally higher challenge dose of 3.3 × 108 CFUs ClfA− bacteria, FibγΔ5 mice exhibited a modest but statistically significant prolongation in survival relative to wild-type animals (Figure 2H). These data suggest that at lower challenge doses, the dominant mechanism driving improved survival in FibγΔ5 mice is loss of ClfA–fibrinogen interactions, whereas at challenge doses > ∼3 × 108 CFUs, a second ClfA-independent mechanism (ie, other S. aureus-derived fibrinogen-binding proteins or other host components such as platelets) contributes to improved survival.
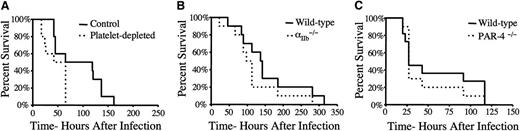
The question of whether the loss of platelet function contributes to the survival advantage observed in S. aureus-challenged FibγΔ5 mice was also investigated. As a test of the overall platelet contribution to infection outcome, host survival was compared in mice immunologically depleted of circulating platelets by intraperitoneal injection of the antiplatelet monoclonal antibody 1B5 24 hours prior to septicemia challenge (>99% of circulating platelets eliminated; data not shown). Platelet depletion did not result in prolonged survival but rather significantly shortened survival times following intravenous injection of wild-type S. aureus Newman (Figure 3A). As a direct complement to our prior studies of FibγΔ5 mice, comparative studies of S. aureus infection were done in cohorts of wild-type and αIIb−/− mice entirely devoid of the platelet integrin receptor αIIbβ3. Unlike FibγΔ5 mice, αIIb−/− mice had a survival profile indistinguishable from wild-type animals following intravenous injection of S. aureus (Figure 3B). Finally, mice deficient in the platelet-associated thrombin-signaling molecule, PAR-4, had a survival profile comparable to that of wild-type control mice following S. aureus infection (Figure 3C). Taken together, these results indicate that deficits in platelet function do not mirror the host survival advantage observed for FibγΔ5 mice following S. aureus infection.
A deficiency in platelet number or loss of platelet function, including a loss of the platelet fibrinogen receptor αIIbβ3, does not present a survival advantage compared to that observed in FibγΔ5mice. (A) Survival analysis of platelet-depleted and control mice (N = 10 pairs) following an intravenous injection of 2.7 × 108 CFUs of Newman strain S. aureus. Mice were treated with either an antiplatelet antibody or a control antibody prior to bacteremia challenge. Note that platelet-depleted mice had a significantly shorter survival profile than control mice. (B) Survival analysis of mice deficient in the platelet integrin receptor subunit αIIb and control mice following intravenous injection of 2 × 108 CFUs Newman strain S. aureus. (C) Survival analysis of control mice (N = 11) and mice deficient in PAR-4 (N = 10) following an intravenous injection of 2.2 × 108 CFUs of Newman strain S. aureus. Survival was compared using the Kaplan-Meier log rank analysis.
A deficiency in platelet number or loss of platelet function, including a loss of the platelet fibrinogen receptor αIIbβ3, does not present a survival advantage compared to that observed in FibγΔ5mice. (A) Survival analysis of platelet-depleted and control mice (N = 10 pairs) following an intravenous injection of 2.7 × 108 CFUs of Newman strain S. aureus. Mice were treated with either an antiplatelet antibody or a control antibody prior to bacteremia challenge. Note that platelet-depleted mice had a significantly shorter survival profile than control mice. (B) Survival analysis of mice deficient in the platelet integrin receptor subunit αIIb and control mice following intravenous injection of 2 × 108 CFUs Newman strain S. aureus. (C) Survival analysis of control mice (N = 11) and mice deficient in PAR-4 (N = 10) following an intravenous injection of 2.2 × 108 CFUs of Newman strain S. aureus. Survival was compared using the Kaplan-Meier log rank analysis.
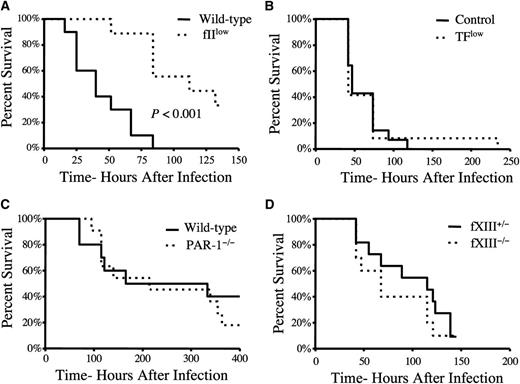
Prothrombin, but not host TF, PAR-1, or fXIII, is a determinant of mortality in S. aureus septicemia
Based on the contribution of fibrinogen to host mortality following intravenous S. aureus infection, we hypothesized that prothrombin was also a determinant of host survival following septicemia. Consistent with this hypothesis, mice carrying ∼10% the normal level of murine prothrombin exhibited a significant survival advantage over wild-type mice following intravenous infection with S. aureus (Figure 4A). Interestingly, TFlow mice expressing ∼1% of TF, the primary host initiator of the thrombin generation, displayed a survival pattern that was indistinguishable from that of control mice (Figure 4B) when given similar doses of S. aureus. This finding is consistent with previous reports highlighting the central importance of pathogen-derived prothrombin activators, staphylocoagulase and von Willebrand factor binding protein (vWbp), in S. aureus sepsis.4,7 Although S. aureus virulence appears to be fibrin(ogen) dependent, the genetic elimination of several other important thrombin targets, including PAR-4 (Figure 3C), PAR-1 (Figure 4C), and the transglutaminase fXIII (Figure 4D), had no appreciable impact on virulence/host survival following acute S. aureus septicemia.
Host prothrombin, but not host TF, PAR-1, or fXIII, drives virulence following intravenous S. aureus infection. (A) Survival analysis of wild-type and fIIlow mice (carrying ∼10% normal level of prothrombin) following intravenous infection with 2 × 108 CFUs of Newman strain S. aureus (N = 10 pairs). (B) Survival analysis of control mice (N = 14) and mice expressing low levels of TF (N = 12) following intravenous infection with 2 × 108 CFUs of Newman strain S. aureus. (C) Survival analysis of wild-type and PAR-1−/− mice (N = 12 pairs) following intravenous infection with 2 × 108 CFUs of Newman strain S. aureus. (D) Survival analysis of fXIII+/− (N = 11) and fXIII −/− mice (N = 10) following intravenous infection with 2 × 108 CFUs of Newman strain S. aureus. Survival was compared using the Kaplan-Meier log rank analysis.
Host prothrombin, but not host TF, PAR-1, or fXIII, drives virulence following intravenous S. aureus infection. (A) Survival analysis of wild-type and fIIlow mice (carrying ∼10% normal level of prothrombin) following intravenous infection with 2 × 108 CFUs of Newman strain S. aureus (N = 10 pairs). (B) Survival analysis of control mice (N = 14) and mice expressing low levels of TF (N = 12) following intravenous infection with 2 × 108 CFUs of Newman strain S. aureus. (C) Survival analysis of wild-type and PAR-1−/− mice (N = 12 pairs) following intravenous infection with 2 × 108 CFUs of Newman strain S. aureus. (D) Survival analysis of fXIII+/− (N = 11) and fXIII −/− mice (N = 10) following intravenous infection with 2 × 108 CFUs of Newman strain S. aureus. Survival was compared using the Kaplan-Meier log rank analysis.
Differential bacterial colonization/outgrowth of tissues in FibγΔ5 mice and wild-type animals following intravenous S. aureus infection
To explore the basis for the prolonged survival in S. aureus-challenged FibγΔ5 mice, qualitative microscopic analyses of bacterial foci and quantitative analyses of bacterial burden were done in major organ systems of control and FibγΔ5 mice as a function of time. Microscopic analyses of tissue sections prepared from heart, kidney, and liver collected 48 hours post-infection revealed numerous bacterial foci/abscesses (generally characterized by a central core of bacteria surrounded by a robust inflammatory cell infiltrate), regardless of mouse genotype (see Figure 5A for gallery of representative views). However, the lesions within tissues of FibγΔ5 mice appeared to be both fewer in number and smaller in size than those observed in wild-type animals 48 hours post-infection, particularly in the heart and kidney. Although the total lesion number at 48 hours (enumerated independently of lesion size) in heart, kidney, and liver sections did not quite reach statistical significance (Figure 5B), consistent with the perceived difference in average lesion size 48 hours post-infection, the total bacterial burden observed in heart and kidney was significantly less in FibγΔ5 mice relative to wild-type control animals (Figure 5C, left panel). A similar pattern of reduced bacterial burden within heart and kidney of FibγΔ5 mice relative to control mice was also observed 24 hours post-infection (Figure 5C, center panel). Interestingly, complementary studies at the very early time point of 30 minutes post-infection (Figure 5C, right panel) or the intermediate time point of 5 hours (data not shown) revealed that bacteria were rapidly marginalized from circulating blood with initial bacterial localization largely to the liver, regardless of genotype. The bacterial counts within whole blood 30 minutes post-infection were slightly <0.1% of the original inoculum and not significantly different in wild-type (6.2 ± 1.6 × 104 CFUs/mL) and FibγΔ5 (7.4 ± 2.2 × 104 CFUs/mL) mice. Circulating CFUs remained at low levels in both wild-type and FibγΔ5 mice when measured at 24 hours post-infection (6.6 ± 4.2 ×103 and 2.6 ± 0.8 ×103 CFUs/mL, respectively) or at 48 hours post-infection (3.5 ± 1.1 × 103 and 1.8 ± 0.7 × 103 CFUs/mL, respectively). We note, parenthetically, that the efficient (>99.9%) margination or loss of viable S. aureus in the circulation was also described by Cheng et al,10 at least in wild-type mice, although this required more than 3 hours. The more precipitous exit of bacteria from the circulation in the present study may be due to slightly different study designs (eg, retro-orbital vs tail vein injection and Balb/C mice vs C57Bl/6 mice). Regardless, the pattern of rapid and comparable elimination of bloodborne CFUs was observed in cohorts of wild-type and FibγΔ5 mice in multiple independent experiments. A significant genotype-dependent difference in initial bacterial localization was detected in the lung 30 minutes post-infection (Figure 5C, right panel), but the initial bacterial localization in heart and kidney was comparable and relatively modest (<0.05% of initial inoculum; <105 CFUs per entire organ) in FibγΔ5 mice and wild-type animals. These data suggest that margination of bloodborne S. aureus and the initial seeding of bacterial emboli into organs are generally similar in control and FibγΔ5 mice, but the selected loss of the fibrinogen ClfA-binding motif results in an impediment in outgrowth in key organ systems.
Comparative analyses of abscess formation and bacterial burden in tissue from FibγΔ5 and wild-type mice following intravenous S. aureus infection. (A) Representative hematoxylin/eosin-stained sections of heart, kidney, and liver from wild-type and FibγΔ5 mice collected 48 hours after intravenous infection with 2.5 × 108 CFUs wild-type S. aureus. Images were captured using an Axioplan 2 microscope with Axiovision Image analysis software (version 4.8.1; Carl Zeiss Microimaging). Scale bar represents 100 μm (upper panels) and 50 μm (lower panels). (B) Bacterial lesion counts in tissue sections prepared from heart, kidney, and liver of wild-type and FibγΔ5 mice 48 hours after intravenous infection. Data are presented as the mean number of lesions per tissue midline section ± standard error of the mean (SEM) with N = 5 per group. (C) Bacterial burdens observed in major organs of FibγΔ5 and control mice at 48 hours (left panel), 24 hours (middle panel), and 30 minutes (right panel) following intravenous injection of 2.5 × 108 CFUs S. aureus (N = 5 per group per time point). Values in solid tissues are the total mean CFUs ± SEM within the entire organ as determined by serial plating aliquots of tissue homogenates. Values in blood are the mean CFUs ± SEM per milliliter in whole blood as determined by plating serial dilutions of whole blood collected from the inferior vena cava. The data were analyzed using the Student t test.
Comparative analyses of abscess formation and bacterial burden in tissue from FibγΔ5 and wild-type mice following intravenous S. aureus infection. (A) Representative hematoxylin/eosin-stained sections of heart, kidney, and liver from wild-type and FibγΔ5 mice collected 48 hours after intravenous infection with 2.5 × 108 CFUs wild-type S. aureus. Images were captured using an Axioplan 2 microscope with Axiovision Image analysis software (version 4.8.1; Carl Zeiss Microimaging). Scale bar represents 100 μm (upper panels) and 50 μm (lower panels). (B) Bacterial lesion counts in tissue sections prepared from heart, kidney, and liver of wild-type and FibγΔ5 mice 48 hours after intravenous infection. Data are presented as the mean number of lesions per tissue midline section ± standard error of the mean (SEM) with N = 5 per group. (C) Bacterial burdens observed in major organs of FibγΔ5 and control mice at 48 hours (left panel), 24 hours (middle panel), and 30 minutes (right panel) following intravenous injection of 2.5 × 108 CFUs S. aureus (N = 5 per group per time point). Values in solid tissues are the total mean CFUs ± SEM within the entire organ as determined by serial plating aliquots of tissue homogenates. Values in blood are the mean CFUs ± SEM per milliliter in whole blood as determined by plating serial dilutions of whole blood collected from the inferior vena cava. The data were analyzed using the Student t test.
Blood cell profile and coagulation parameters in wild-type and FibγΔ5 mice following intravenous S. aureus infection
To determine if the improved survival in FibγΔ5 mice following intravenous infection was linked to early changes in blood cell profile or hematologic parameters, animals were evaluated following S. aureus infection. Here, measurements were taken within the first 48 hours following infection prior to any potentially confounding genotype-dependent loss of animals. Significant reductions were observed in white blood cell and platelet counts (Table 1) in mice administered an intravenous dose of 2.1 × 108 CFUs S. aureus relative to uninfected animals. However, the infection-mediated changes were observed in both control and FibγΔ5 mice. A similar pattern was observed for plasma thrombin-antithrombin (TAT) complexes and fibrinogen levels. Plasma TAT levels were significantly increased 24 hours following infection in mice of both genotypes (Table 2), and although TAT levels remained high in FibγΔ5 mice at 48 hours, the levels returned to baseline in wild-type mice. Plasma fibrinogen concentrations were also similar in mice of each genotype at baseline (Table 2) and, consistent with fibrinogen being a well-known acute phase reactant, fibrinogen levels in both genotypes were increased ∼2 fold following infection. These results suggest that the profound difference in survival observed between wild-type and FibγΔ5 mice was not a reflection of differential infection-driven changes to blood cell profiles, thrombin activation, or fibrinogen levels.
Blood cell parameters in wild-type and FibγΔ5 mice 24 h after intravenous infection with 2.1 × 108 CFUs S. aureus
| Parameter . | Unchallenged . | S. aureus . | ||
|---|---|---|---|---|
| Wild-type N = 5 . | FibγΔ5 N = 5 . | Wild-type N = 7 . | FibγΔ5 N = 5 . | |
| WBC (×109/L) | 3.5 ± 1.1 | 2.1 ± 0.4 | 0.4 ± 0.2* | 0.7 ± 0.2* |
| RBC (×1012/L) | 8.3 ± 0.03 | 7.6 ± 0.4 | 6.8 ± 2.6 | 9.2 ± 0.8 |
| Hemoglobin (g/dL) | 13.1 ± 0.2 | 11.6 ± 0.6 | 11.8 ± 1.4 | 13.1 ± 2.2 |
| Hematocrit (%) | 40.8 ± 0.8 | 37.3 ± 1.5 | 35.8 ± 12.5 | 45.6 ± 2.8 |
| MCV (fL) | 49.1 ± 0.8 | 49.3 ± 0.4 | 52.3 ± 7.3 | 49.8 ± 4.3 |
| RDW (%) | 15.6 ± 0.3 | 16.8 ± 0.6 | 19.1 ± 4.1 | 15.6 ± 0.4 |
| Platelets (×109/L) | 666 ± 42 | 576 ± 113 | 228 ± 77* | 208 ± 66* |
| Parameter . | Unchallenged . | S. aureus . | ||
|---|---|---|---|---|
| Wild-type N = 5 . | FibγΔ5 N = 5 . | Wild-type N = 7 . | FibγΔ5 N = 5 . | |
| WBC (×109/L) | 3.5 ± 1.1 | 2.1 ± 0.4 | 0.4 ± 0.2* | 0.7 ± 0.2* |
| RBC (×1012/L) | 8.3 ± 0.03 | 7.6 ± 0.4 | 6.8 ± 2.6 | 9.2 ± 0.8 |
| Hemoglobin (g/dL) | 13.1 ± 0.2 | 11.6 ± 0.6 | 11.8 ± 1.4 | 13.1 ± 2.2 |
| Hematocrit (%) | 40.8 ± 0.8 | 37.3 ± 1.5 | 35.8 ± 12.5 | 45.6 ± 2.8 |
| MCV (fL) | 49.1 ± 0.8 | 49.3 ± 0.4 | 52.3 ± 7.3 | 49.8 ± 4.3 |
| RDW (%) | 15.6 ± 0.3 | 16.8 ± 0.6 | 19.1 ± 4.1 | 15.6 ± 0.4 |
| Platelets (×109/L) | 666 ± 42 | 576 ± 113 | 228 ± 77* | 208 ± 66* |
MCV, mean corpuscular volume (in femtoliters); RBC, red blood cells; RDW, red cell distrobution width; WBC, white blood cells.
P < 0.05 by Student t test relative to values in unchallenged mice.
Changes in host coagulation parameters following infection with 2.5 × 108 CFUs S. aureus
| Parameter . | Unchallenged . | 24 h post-infection . | 48 h post-infection . | |||
|---|---|---|---|---|---|---|
| Wild-type N = 3 . | FibγΔ5 N = 3 . | Wild-type N = 4 . | FibγΔ5 N = 4 . | Wild-type N = 4 . | FibγΔ5 N = 4 . | |
| TAT (μg/L) | 26 ± 2.1 | 27 ± 3.2 | 54 ± 6.1 | 60 ± 2.4 | 25 ± 3.7 | 40 ± 1.3* |
| Fibrinogen (mg/mL) | 2.8 ± 0.1 | 2.3 ± 0.3 | 4.1 ± 0.7 | 4.3 ± 0.1 | 4.8 ± 0.3 | 5.2 ± 0.1 |
| Parameter . | Unchallenged . | 24 h post-infection . | 48 h post-infection . | |||
|---|---|---|---|---|---|---|
| Wild-type N = 3 . | FibγΔ5 N = 3 . | Wild-type N = 4 . | FibγΔ5 N = 4 . | Wild-type N = 4 . | FibγΔ5 N = 4 . | |
| TAT (μg/L) | 26 ± 2.1 | 27 ± 3.2 | 54 ± 6.1 | 60 ± 2.4 | 25 ± 3.7 | 40 ± 1.3* |
| Fibrinogen (mg/mL) | 2.8 ± 0.1 | 2.3 ± 0.3 | 4.1 ± 0.7 | 4.3 ± 0.1 | 4.8 ± 0.3 | 5.2 ± 0.1 |
P < 0.05 by Student t test relative to value in wild-type mice 48 h after infection.
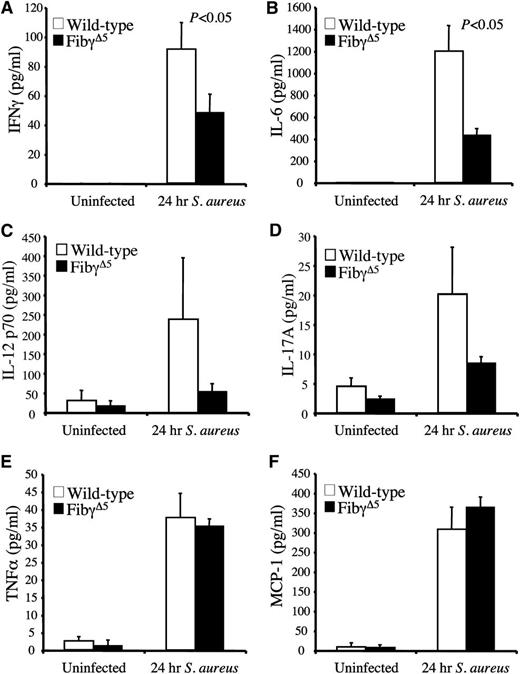
FibγΔ5 mice exhibit diminished circulating proinflammatory cytokines and reduced markers of tissue damage following S. aureus septicemia
We hypothesized that one benefit underlying the superior survival profile of FibγΔ5 mice following S. aureus infection was a blunted inflammatory response and a subsequent diminution in tissue damage. Comparative studies of selected circulating proinflammatory cytokines and chemokines were done in unchallenged control and FibγΔ5 mice and cohorts challenged with S. aureus (∼2.5 × 108 CFUs) 24 hours prior to blood collection. In unchallenged mice, cytokines of all types were predictably low and comparable between genotypes, whereas every cytokine evaluated, including IFNγ, IL-6, TNFα, and MCP-1 (Figure 6A–F), increased dramatically within 24 hours of infection. More significantly, an appreciably lower level of cytokine elaboration into the circulation was observed in FibγΔ5 mice relative to wild-type animals in the case of both IFNγ and IL-6. A similar trend was observed for IL-12p70 and IL-17A, but these differences did not reach statistical significance. However, 2 other cytokines, TNFα and MCP-1, although appreciably elevated following infection, were not differentially controlled based on genotype. Thus, the findings imply some selectivity in the underlying mechanism(s) linking fibrinogen-γΔ5 to inflammatory mediators following septicemia.
Diminished circulating levels of proinflammatory cytokines for FibγΔ5 mice following intravenous S. aureus infection. Circulating levels of IFNγ (A), IL-6 (B), IL-12p70 (C), IL-17A(D), TNFα, (E), and MCP-1 (F) were measured in plasma using a Luminex bead multiplex assay. Cohorts of wild-type and FibγΔ5 mice were either unchallenged (N = 4 per genotype) or administered an intravenous infection of ∼2.5 × 108 CFUs of S. aureus for 24 hours (N = 8 per genotype). The data are represented as the mean ± standard error of the mean and analyzed using the Student t test.
Diminished circulating levels of proinflammatory cytokines for FibγΔ5 mice following intravenous S. aureus infection. Circulating levels of IFNγ (A), IL-6 (B), IL-12p70 (C), IL-17A(D), TNFα, (E), and MCP-1 (F) were measured in plasma using a Luminex bead multiplex assay. Cohorts of wild-type and FibγΔ5 mice were either unchallenged (N = 4 per genotype) or administered an intravenous infection of ∼2.5 × 108 CFUs of S. aureus for 24 hours (N = 8 per genotype). The data are represented as the mean ± standard error of the mean and analyzed using the Student t test.
The concept that FibγΔ5 mice are partially protected from multiorgan tissue damage associated with the infection–host response was explored. Circulating levels of established markers of organ damage were evaluated in unchallenged and challenged wild-type and FibγΔ5 mice at 24 and 48 hours following intravenous administration of ∼2.5 × 108 CFUs S. aureus. Given the profound difference in bacterial outgrowth in the hearts of control and FibγΔ5 mice (see Figure 5C), evidence of differential cardiomyocyte damage was of particular interest. As shown in Figure 7A, the levels of plasma cardiac troponin I (cTnI), a marker exquisitely sensitive to disruption of cardiomyocytes, was virtually undetectable in unchallenged mice of both genotypes but was readily detected in infected animals. However, more significantly, cTnI was significantly higher in the plasma of wild-type mice relative to FibγΔ5 mice at both 24 hours and 48 hours after S. aureus infection (Figure 7A). Additionally, other plasma markers of tissue damage, including creatine kinase (CK) and lactate dehydrogenase (LDH), displayed similar patterns in which levels were extremely low in unchallenged mice but increased in mice of both genotypes following infection. Within 48 hours after infection, both CK (Figure 7B) and LDH (Figure 7C) levels were significantly higher in wild-type mice compared with FibγΔ5 animals challenged in parallel. Similarly, levels of the liver enzymes AST (Figure 7D) and ALT (data not shown) were extremely low in unchallenged mice of each genotype but rose dramatically following infection. Similar to the other markers of tissue damage, AST levels were significantly higher in infected wild-type mice compared with FibγΔ5 mice at both 24 and 48 hours post-infection (Figure 7D). Thus, consistent with the survival advantage, FibγΔ5 mice show a significant degree of protection from multiorgan tissue damage associated with S. aureus septicemia.
Significantly diminished circulating levels of multiple tissue damage markers in FibγΔ5 mice following intravenous S. aureus infection. The levels of cardiac troponin I (A), creatine kinase (B), lactate dehydrogenase (C), and AST (D) were determined in plasma from wild-type and FibγΔ5 mice that were unchallenged (N = 6 per genotype) or administered and intravenous infection of ∼2.5 × 108 CFUs of S. aureus for 24 hours or 48 hours (N = 8 per genotype). The data are represented as the mean ± standard error of the mean and analyzed using the Student t test.
Significantly diminished circulating levels of multiple tissue damage markers in FibγΔ5 mice following intravenous S. aureus infection. The levels of cardiac troponin I (A), creatine kinase (B), lactate dehydrogenase (C), and AST (D) were determined in plasma from wild-type and FibγΔ5 mice that were unchallenged (N = 6 per genotype) or administered and intravenous infection of ∼2.5 × 108 CFUs of S. aureus for 24 hours or 48 hours (N = 8 per genotype). The data are represented as the mean ± standard error of the mean and analyzed using the Student t test.
Discussion
Despite wide differences in virulence, preferred sites of colonization, and mechanisms for evading immune surveillance, many bacterial pathogens have evolved the capacity to engage host hemostatic factors. Fibrin(ogen) is a powerful regulator of local inflammatory processes in numerous inflammatory disease settings (eg, arthritis,32 colitis,33 neuroinflammatory disease34 ) and recognized as a potential modifier of host responses to microbial infection.19,32,35 Comparative studies of Y. pestis, L. monocytogenes, Y. enterocolitica, and group A streptococci infection in mice with and without fibrin(ogen) have established that genetic elimination of host fibrin(ogen) resulted in markedly increased microbial virulence.19,20,35 Similarly, previous studies of S. aureus peritonitis indicated that either the loss of fibrinogen or genetics-based alterations in fibrin(ogen) αMβ2–binding function resulted in a failure of S. aureus clearance within the peritoneal cavity and dramatically increased microbial virulence in that specific experimental setting.18 Thus, in multiple contexts focusing on widely divergent bacterial pathogens, fibrin(ogen) has been repeatedly shown to favor the host in successfully coping with infection, including certain forms of S. aureus infection. By contrast, the present study underscores that fibrin(ogen) also can be of use to microbial pathogens. Taken together with earlier studies of S. aureus infection, these studies establish the following: (1) host fibrin(ogen) can either be a benefit (eg, septicemia) or a serious liability (eg, peritonitis) to the very same microbe, depending on the precise context, and (2) specific functional properties of fibrin(ogen) can benefit the host (eg, αMβ2 binding) in some contexts (eg, peritonitis), whereas other functional properties can benefit the microbe (eg, ClfA binding) in other contexts (eg, septicemia). These context-dependent benefits and liabilities of host fibrin(ogen) probably underlie the curious capacity of S. aureus to express fibrinogen-binding proteins (eg, ClfA) and procoagulants (eg, staphylocoagulase and vWbp) as well as potent fibrinolytic products (eg, staphylokinase).
Microbe-induced platelet adhesion, aggregation, and/or activation events are thought to be important in intravascular infections by multiple pathogens and are viewed to be particularly important in S. aureus colonization of tissues and infective endocarditis.9,14,36 Studies of S. aureus mutants with single and combined deficits in ClfA, staphylocoagulase, and vWbp suggest that all 3 of these bacterial proteins cooperate in supporting platelet aggregation, trapping, and activation events, at least in vitro.7,37 Although the 2 noncatalytic bacterial prothrombin activators, staphylocoagulase and vWbp, do not directly activate platelets through the cleavage of PARs, both have been shown to indirectly drive platelet aggregation and activation events secondary to the conversion of fibrinogen to fibrin.38-40 Based on the dual functionality of the distal region of the fibrinogen γ chain, the survival advantage observed in FibγΔ5 mice following S. aureus septicemia could be primarily a consequence of the loss of fibrin(ogen) interactions with bacterial ClfA or primarily due to the loss of fibrin(ogen) engagement by platelet αIIbβ3, or a combination of both.
Two findings suggest that the loss of ClfA binding in FibγΔ5 mice is dominant in determining host survival. First, the survival profiles of FibγΔ5 and control cohorts were significantly different following infection with multiple ClfA+S. aureus strains, including the most common community-acquired methicillin-resistant strain USA300. However, survival profiles were indistinguishable in cohorts challenged with ClfA− bacteria, at least at bacterial doses < 3 × 108 CFUs. Thus, the known elimination of the platelet αIIbβ3–fibrin(ogen) interaction in FibγΔ5 mice31 presented no appreciable survival benefit relative to control mice in a setting where bacterial ClfA was not available. Second, mice lacking platelets or genetically devoid of platelet αIIbβ3 exhibited no appreciable survival advantage over control mice when challenged with ClfA+ bacteria. If anything, survival was found to be worse in cohorts depleted of platelets. However, the experimental inference that the loss of platelet receptor−fibrinogen interactions in FibγΔ5 mice may be of less significance to overall virulence than the loss of bacterial ClfA−fibrinogen interaction requires some caution; this may hold only in selected experimental contexts.
Our complementary finding that FibγΔ5 mice exhibit a modest survival advantage over control mice when challenged with very high doses of ClfA− bacteria (eg, > 3 × 108 CFUs intravenously; Figure 2H) leaves open the possibility that a ClfA-independent function of the last 5 residues of the fibrin(ogen) γ chain (eg, the capacity to bind platelet αIIbβ3) may contribute to S. aureus virulence in contexts where the circulating bacterial load is exceptional. Although the precise benefits and liabilities of platelets in infection remain to be fully understood, any advantages to S. aureus of platelet association/activation in evasion of immune surveillance and/or colonization may be tempered by some disadvantages, including exposure to platelet-derived antimicrobial peptides.41-43 The present study does not exclude a contribution of platelet receptor–fibrinogen interactions to infection sequelae, such as endocarditis,15 but suggests that ClfA-mediated interactions with fibrinogen dominate in determining early host survival in the setting of acute septicemia.
Recent findings suggest staphylocoagulases and ClfA are dual determinants supporting abscess formation and virulence following intravenous infection. The elimination of either bacterial ClfA or staphylocoagulase-directed prothrombin protease activity reduces abscess size, and the combined elimination of ClfA, coagulase and vWbp renders S. aureus incapable of abscess formation and avirulent.4,26,44 Our data provide direct evidence, experimentally unavailable through studies of ClfA− bacteria, that ClfA–fibrin(ogen) interaction, as opposed to ClfA interactions with other putative host ligands (eg, fibronectin37 and complement factor 145 ), is the driver of abscess formation, bacterial burden, organ damage, and increased inflammatory responses in the host. The present study illustrates that a genetically-based diminution in circulating prothrombin improved host survival following infection. The study also extends prior findings showing that S. aureus virulence strongly depends on bacterial coagulase activity and that virulence can be suppressed by inhibiting staphylocoagulase-prothrombin complexes.40,44 The complementary finding that a genetics-based diminution of the host initiator of coagulation, TF, results in no apparent host survival advantage following S. aureus septicemia provides compelling new evidence that microbe staphylocoagulase-driven procoagulant activity is far more important to overall virulence than host TF-driven thrombin generation. This general notion is also compatible with the fact that loss of PAR-1 and PAR-4, 2 well-known nonfibrinogen targets of α-thrombin, but not substrates for the coagulase–prothrombin proteolytic complex, had little impact on S. aureus virulence in septicemia.
The precise mechanism(s) by which abscess outgrowth and virulence are impeded in FibγΔ5 mice remains to be fully understood, but our data support the notion that ClfA–fibrin(ogen) interactions, together with coagulase-based fibrin deposition, promote the development of life-threatening vegetations such as those observed in endocarditis.9,40,44 One general hypothesis is that fibrin-stabilized bacterial complexes allow S. aureus to evade immune surveillance mechanisms and that the loss of capacity to engage fibrin(ogen) may make the microbe more susceptible to immune clearance. This view is consistent with studies suggesting that ClfA may offer some means of limiting phagocytosis by macrophages and polymorphonuclear leukocytes13,17 and that staphylocoagulases may also provide a mechanism for immune protection in vivo.44 Other nonmutually exclusive mechanisms may underlie the survival benefits in FibγΔ5 mice, including that an impediment in the formation of bacterial thromboemboli reduces microvascular occlusive events and host inflammatory shock responses. Another possible view is that the loss of the interaction of ClfA with host fibrin(ogen) has an impact on bacterial gene expression and microbial properties, possibly through an agr operon/RNAIII–dependent quorum-sensing mechanism whereby bacteria undergo a density-dependent conversion in phenotype from tissue-adhering to tissue-damaging.11 Rothfork et al demonstrated that pharmacological depletion of fibrinogen limited S. aureus–RNAIII promoter activity and RNAIII target gene expression of known virulence factors (ie, α-hemolysin and hla).11 A linkage between ClfA and tissue damage is consistent with the finding of elevated circulating tissue damage markers (ie, cTnI, CK, LDH) in wild-type mice relative to FibγΔ5 mice following intravenous challenge. However, to our knowledge, no study has specifically evaluated whether ClfA binding to fibrin(ogen) per se influences quorum sensing. Studies in FibγΔ5 mice would be ideal for such analyses because ClfA–fibrinogen interactions would be eliminated in a setting where both ClfA binding to other putative ligands and host clotting function remains intact.
The data presented here indicate that limiting fibrinogen–ClfA engagement could be, in itself, extremely effective in reducing the morbidity and mortality associated with S. aureus septicemia. The present study also provides the proof of principle that therapeutic gains can be made at the level of fibrinogen without necessarily compromising clotting function. This concept of disrupting ClfA–fibrin(ogen) interactions with specific antibodies,46,47 vaccines,48,49 or fibrinogen-directed agents (this report) as a means of impeding S. aureus infections has become even more attractive in view of recent studies suggesting that the therapeutic gains of this intervention may be more effective if combined with staphylocoagulase blocking agents (eg, small molecule inhibitor of coagulase- or vWbp-prothrombin proteolytic activity). Of course, inferences regarding human subjects based on findings in laboratory animals must be drawn with extreme caution. However, the carboxy-terminal final 5 amino acids of the fibrinogen γ chain are 100% identical in mouse and human molecules, and prior biochemical studies have established similar binding affinities between human and mouse fibrinogen for ClfA. In addition, previously reported crystal structures indicate that the 9 terminal amino acids in the fibrinogen γ chain that are primarily involved in the antiparallel β strands binding to ClfA are also conserved in the mouse and human γ chains.50 Nevertheless, corroborative translational findings will be necessary to firmly define the biological importance of ClfA–fibrinogen interactions in infection outcomes in humans. Because multiple microbes appear to express products designed to engage hemostatic factors, a better understanding of the mechanisms by which microbes interact and capitalize on these host factors may reveal novel adjunct therapies across multiple human pathogens.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kathryn McElhinney, Keith Kombrinck, Elizabeth Blevins, Alice Jone, and Whitney Miller for excellent technical assistance. We also thank Eric Mullins for his helpful suggestions in the preparation of this manuscript. We thank the following for their contributions to this study: Dr. Barry Coller, Rockefeller University, New York, who provided mice lacking the integrin subunit αIIb and anti-mouse αIIbβ3 antibody (1B5); Dr. Shaun Coughlin, University of California, San Francisco, who provided mice lacking protease-activated receptor-4 (PAR-4); Dr. Nigel Mackman, University of North Carolina, Chapel Hill, who provided mice expressing low levels of TF.
This research was supported by grants from the National Institutes of Health (HL085357, HL096126, AI020624, and AR056990).
National Institutes of Health
Authorship
Contribution: M.J.F. designed and performed the experiments, interpreted the data, and wrote the manuscript. X.D., J.M.P., H.R., and J.S.P. performed experiments and helped in interpreting the data. E.S. and M.H. aided in data interpretation and manuscript preparation. J.L.D. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: E.S. and M.H. are coinventors on a granted patent and a pending patent regarding MSCRAMM:fibrinogen interactions and have an interest in ECM Technology LLC, Houston, TX, which is developing medical products related to MSCRAMMs. M.H. has a research grant from Pfizer to study the structures of ClfA and ClfB.
Correspondence: Jay L. Degen, Children’s Hospital Research Foundation, Division of Experimental Hematology–ML7015, 3333 Burnet Ave, Cincinnati, Ohio 45229-3039; e-mail: jay.degen@cchmc.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal