Key Points
In newly diagnosed ITP, addition of rituximab to dexamethasone yields higher sustained response rates than dexamethasone alone.
Abstract
In this study, we report the results from the largest cohort to date of newly diagnosed adult immune thrombocytopenia patients randomized to treatment with dexamethasone alone or in combination with rituximab. Eligible were patients with platelet counts ≤25×109/L or ≤50×109/L with bleeding symptoms. A total of 133 patients were randomly assigned to either dexamethasone 40 mg/day for 4 days (n = 71) or in combination with rituximab 375 mg/m2 weekly for 4 weeks (n = 62). Patients were allowed supplemental dexamethasone every 1 to 4 weeks for up to 6 cycles. Our primary end point, sustained response (ie, platelets ≥50×109/L) at 6 months follow-up, was reached in 58% of patients in the rituximab + dexamethasone group vs 37% in the dexamethasone group (P = .02). The median follow-up time was 922 days. We found longer time to relapse (P = .03) and longer time to rescue treatment (P = .007) in the rituximab + dexamethasone group. There was an increased incidence of grade 3 to 4 adverse events in the rituximab + dexamethasone group (P = .04). In conclusion, rituximab + dexamethasone induced higher response rates and longer time to relapse than dexamethasone alone. This study is registered at http://clinicaltrials.gov as NCT00909077.
Introduction
Primary immune thrombocytopenia (ITP) is characterized by immune-mediated peripheral platelet destruction and impaired platelet production in the bone marrow1,2 with consequent increased risk of bleeding.3 Recommended first-line treatment of symptomatic ITP is glucocorticoids, but the majority of adult patients relapse within the first year of treatment and require additional therapy including splenectomy.4
Rituximab (RTX) is recommended as a second-line treatment of ITP,4 and several studies have shown that RTX induces an initial response in up to 60% of patients with refractory ITP.5 However, the few studies evaluating long-term effects of RTX in refractory ITP have shown that sustained response rates are in the range of 20% to 35%, especially in patients with a longer duration of ITP.6,7
Only 2 previous studies have investigated the role of RTX in first-line treatment of newly diagnosed ITP. Zaja et al reported higher sustained response rates after 6 months of treatment with dexamethasone (DXM) and RTX vs DXM in patients with newly diagnosed ITP.8 Arnold et al recently reported the results of a pilot study of RTX vs placebo as adjuvant to standard-of-care treatment in a mixed cohort of newly diagnosed and relapsed ITP patients with no difference in platelet counts after 6 months in the 2 groups.9 In this study, we present the results of the largest cohort to date of newly diagnosed ITP patients treated with RTX+DXM vs DXM alone with up to 4 years of follow-up.
Methods
Study design
We conducted a randomized open-label phase 3 trial in 9 Danish departments of hematology. The study was initiated in 2006 and results were analyzed in November 2011. The study is closed for inclusion, and a final report on follow-up data is expected in 2015.
Patients
Eligible patients were newly diagnosed adult patients ≥18 years of age with confirmed ITP and platelet counts ≤25 × 109/L or ITP with platelet counts ≤50 × 109/L and concomitant bleeding symptoms. The clinical criteria for the diagnosis of ITP were: (1) isolated thrombocytopenia with platelet count <100 × 109/L, (2) normal bone marrow biopsy with a normal or increased number of megakaryocytes, (3) normal spleen size assessed by ultrasonography, (4) exclusion of secondary immune- or drug-induced thrombocytopenias, (5) exclusion of virally induced thrombocytopenia, and (6) normal levels of thyroid-stimulating hormone. Fertile women were included only if adequate contraceptives had been used for a minimum of 3 months before enrollment. Patients were allowed to have received oral prednisolone for up to 1 week before enrollment. Exclusion criteria were low performance status (≥2 using the World Health Organization score system), previous therapy with RTX or therapy with other immunomodulating agents within 1 month of enrollment, serious comorbidities, pregnancy or lactation, contraindications for RTX (eg, anti-murine antibodies), or seropositive tests for human immune deficiency virus, hepatitis B, hepatitis C, cytomegalovirus, Epstein-Barr virus, anticardiolipid antibodies, or antinuclear antibodies. A bone marrow biopsy and an abdominal ultrasonography were performed in all patients to exclude hematological malignancy. The Committees on Biomedical Research Ethics of the Capital Region of Denmark approved the protocol and all patients received oral and written information, according to international guidelines, before giving written consent in accordance with the Declaration of Helsinki.
Study procedures.
Eligible patients were randomized 1:1 by use of precoded envelopes to receive either a combination of RTX+DXM or DXM as monotherapy. RTX was administered in a standardized dosage of 375 mg/m2,10 on day 2 of enrollment and thereafter once weekly for a total of 4 weeks. Premedication included oral paracetamol (acetaminophen) and intravenous antihistamine to reduce infusion-related discomfort. DXM was administered orally in a dosage of 40 mg once daily for 4 consecutive days. Patients were recruited between 2006 and 2011, and in 2009 an amendment to the protocol allowed nonresponders (see “Enrollment and follow-up”) in both arms to repeat the DXM treatment every 1 to 4 weeks for a total of up to 6 treatment cycles.11 The choice of DXM vs prednisolone, both recommended as first-line treatments of ITP, was based on reports from a study of 125 patients with newly diagnosed ITP treated with 1 cycle of DXM in which 50% showed sustained response after 6 months.12 Safety interim analyses after 2 years found that we could not reproduce the response rates reported in the previous study (data not shown). Because subsequent reports had shown a benefit of repeated cycles of DXM,11 we made an amendment to the protocol in 2009 allowing patients to receive up to 6 cycles of DXM. Platelet counts were measured on all study visits and immunoglobulin (Ig) levels were measured at baseline and after 3, 6, 12, and 24 months.
Study outcomes.
The primary end point was a sustained partial or complete response at 6 months’ follow-up. Secondary end points included time to relapse, time to rescue treatment, and rates of splenectomy. Complete response was defined as platelet count ≥100 × 109/L, partial response as platelet count ≥ 50 × 109/L, and no response as the absence of a rise in platelet counts or a continued count of ≤ 50 × 109/L. Patients withdrawn from the study because of no response and patients receiving rescue treatment before 6 months were defined as nonresponders at 6 months. Initial response was defined as an increase in platelet counts of at least 30 × 109/L, platelet counts >50 × 109/L on day 10, and no clinical bleeding. Relapse was defined as a drop in platelet count to ≤30 × 109/L following an initial partial or complete response. Adverse events were recorded at all clinical visits and graded according to the Common Toxicity Criteria version 2.0.
Statistical analysis
Differences in achieving complete or partial response between the 2 groups and differences in adverse events were analyzed by the Fisher’s exact test. Fraction of relapse-free patients, time to relapse, time to rescue treatment, and rates of splenectomy were compared using the Kaplan-Meier method and log-rank test. For descriptive statistics, we used the Mann-Whitney test, Wilcoxon signed-rank test, and Fisher’s exact test. All P values were 2-sided, and P values below .05 were considered significant. IBM SPSS Statistics version 19.0.0 (2010, IBM, Somers, NY) or R Statistical Software version 2.14.1 (2011, R Foundation for Statistical Computing, Vienna, Austria) were used for all analyses.
Funding
The investigators were responsible for the design of the trial and writing of the protocol, analysis and interpretation of data and preparation of the manuscript. The funder provided the study drug, and had no role in design or conduct of the trial, analysis or interpretation of data, or preparation or approval of the manuscript.
Results
Enrollment and follow-up
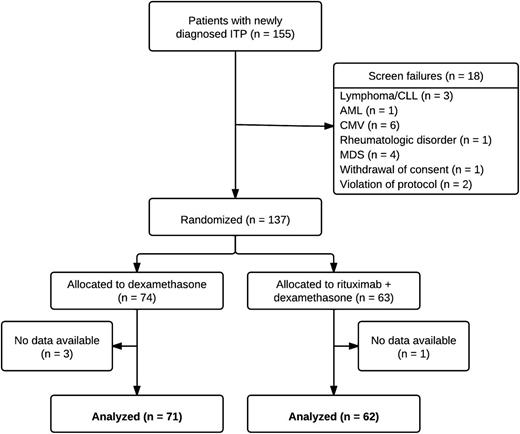
Patients were enrolled in the study from its initiation between March 2006 and August 2011. In this period, 155 patients were included, 137 being subsequently randomized to receive RTX+DXM (n = 63, no data available for 1 patient) or DXM alone (n = 74, no data available for 3 patients). The median time from screening to randomization was 1 day (interquartile range [IQR] 0–3 days) in both groups. The excluded 18 patients were identified as secondary ITP (n = 15), withdrew consent (n = 1), or did not meet inclusion criteria (n = 2; 1 patient had a third relapse of ITP; 1 patient had received intravenous immunoglobulin [IVIG] 1 week before enrollment) (Figure 1). Baseline characteristics were balanced between the 2 groups, and no patients were splenectomized at time of inclusion (Table 1). Non-ITP medications administered in >5% of patients included antihypertensives, anti-diabetics, proton pump inhibitors, cholesterol-lowering drugs, and pain medication. In the RTX+DXM group, 19 patients (31%) were administered ≥2 cycles of DXM vs 31 patients (44%) in the DXM group, P = .09 (Table 1).
Patient flowchart. AML, acute myeloid leukemia; CLL, chronic lymphatic lymphoma; MDS, myelodysplastic syndrome.
Patient flowchart. AML, acute myeloid leukemia; CLL, chronic lymphatic lymphoma; MDS, myelodysplastic syndrome.
Baseline characteristics
| Parameter . | RTX+DXM (n = 62) . | DXM (n = 71) . | P value for difference between groups . |
|---|---|---|---|
| Age, median (IQR) | 51 y (36-63) | 58 y (41-70) | .2 |
| Females, n (%) | 36 (58%) | 34 (48%) | .3 |
| Baseline platelet count ×109/L, median (IQR) | 13 (6-20) | 14 (8-23) | .4 |
| Follow-up period in days, median (IQR) | 921 (526-1152) | 922 (190-1126) | .3 |
| Dexamethasone cycles during treatment | |||
| 1 cycle, no. of patients (%) | 43 (69%) | 40 (56%) | .09 |
| 2-3 cycles, no. of patients (%) | 16 (26%) | 22 (31%) | |
| >4 cycles, no. of patients (%) | 3 (5%) | 9 (13%) | |
| Parameter . | RTX+DXM (n = 62) . | DXM (n = 71) . | P value for difference between groups . |
|---|---|---|---|
| Age, median (IQR) | 51 y (36-63) | 58 y (41-70) | .2 |
| Females, n (%) | 36 (58%) | 34 (48%) | .3 |
| Baseline platelet count ×109/L, median (IQR) | 13 (6-20) | 14 (8-23) | .4 |
| Follow-up period in days, median (IQR) | 921 (526-1152) | 922 (190-1126) | .3 |
| Dexamethasone cycles during treatment | |||
| 1 cycle, no. of patients (%) | 43 (69%) | 40 (56%) | .09 |
| 2-3 cycles, no. of patients (%) | 16 (26%) | 22 (31%) | |
| >4 cycles, no. of patients (%) | 3 (5%) | 9 (13%) | |
IQR, interquartile range.
This is the first report from a prospective cohort study; thus, follow-up time varied within the groups with no differences between the 2 groups (Table 1). Patients withdrawn from the study because of no response and patients receiving rescue treatment before 6 months (not including study-permitted additional cycles of dexamethasone) were defined as nonresponders for the duration of the study. Thus, 60 patients (97%) in the RTX+DXM group and 63 patients (89%) in the DXM group had completed 6 months follow-up at the time of data analyses. Three-years of follow-up were attained by 26 patients (42%) in the RTX+DXM group and 25 patients (35%) in the DXM group.
In the RTX+DXM group, 8 patients were lost to follow-up during the first 3 years vs 6 patients in the DXM group. Patients were lost to follow-up according to their own request (n = 9) or of unknown causes (n = 5). Five patients in the RTX+ DXM group were withdrawn early from the study vs 9 patients in the DXM group. Reasons for early withdrawal were: complete remission (n = 2); pregnancy (n = 2); unacceptable adverse events (n = 3); newly diagnosed comorbidities (n = 3); patient deceased (n = 4). Patients with relapse before the time of withdrawal were counted as nonresponders and included as events in the time-to-relapse and, if relevant, time-to-rescue-treatment analyses. The patients withdrawn because of adverse events or comorbidities and those deceased were censored at their withdrawal but were considered nonresponders in the intention-to-treat analyses. The 2 pregnant patients were censored at time of withdrawal. The 2 patients withdrawn because of complete remission were censored at their withdrawal; both had been included in the study for more than 3 years.
Response rates
Sustained partial or complete response at 6 months’ follow-up was achieved in 58% of patients in the RTX+DXM group and 37% of patients in the DXM monotherapy group (P = .02). Study-permitted supplemental cycles of DXM were received by 5 responding patients in the RTX+DXM group vs 10 responding patients in the DXM group. In an intention-to-treat analysis, including patients either dying or withdrawn because of adverse events, partial or complete response was achieved in 57% of the patients in the RTX+DXM group and 35% of the patients in the DXM monotherapy group (P = .01). In the RTX+DXM group, 11 of 26 nonresponding patients (42%) had an initial response to therapy but relapsed after a median (range) of 25 days (15 to 55 days). Accordingly, 18 of 45 nonresponding patients in the DXM group (40%) had an initial response but relapsed after a median (range) of 32 days (13 to 139 days). Study-permitted supplemental cycles of DXM were administered in 14 of the 26 nonresponding patients in the RTX+DXM group and in 21 of the 45 nonresponders in the DXM group.
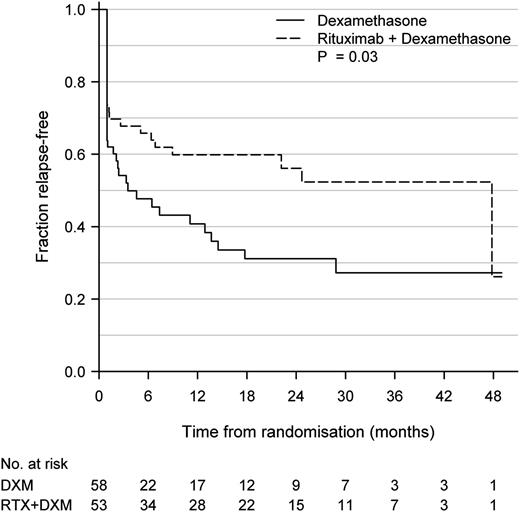
At 12 months’ follow-up, sustained partial or complete response was achieved in 53% of patients in the RTX+DXM group and 33% patients in the DXM monotherapy group (P < .05). During a 3-year observational period (RTX+DXM median 921 days [IQR 526-1152]; DXM median 922 days [IQR 190-1126]), there was significantly longer time to relapse defined as a drop in platelet counts to <50 × 109/L following an initial, partial, or complete response in the RTX+DXM group as compared with the DXM group (P = .03, Figure 2). We also assessed the time to rescue treatment, defined as the time point when patients were administered rescue medicine for ITP at the discretion of their hematologist. In patients who had initially achieved a partial or complete response, median time to rescue treatment in the DXM group was 7.4 months and median time to rescue treatment was not reached in the RTX+DXM group after 48 months. There was significantly longer time to rescue treatment in the RTX+DXM than in the DXM group (P = .007). Median platelet count at time of rescue treatment was 15 × 109/L (IQR 7-24 × 109/L). We found no differences in rates of splenectomy in the 2 groups: 6 patients (10%) were splenectomized in the RTX+DXM group vs 5 patients (7%) in the DXM group (P = .8).
Kaplan-Meier plot of time to relapse in patients achieving initial, partial, or complete response. Relapse was defined as a drop in platelet count to ≤50 × 109/L following a complete response (platelet count ≥100 × 109/L), partial response (platelet count ≥50 × 109/L), or initial response (increase in platelet counts of at least 30 × 109/L, platelet counts >50 × 109/L on day 10 and no clinical bleeding). No. at risk indicates number of patients with sustained complete or partial response. P = .03.
Kaplan-Meier plot of time to relapse in patients achieving initial, partial, or complete response. Relapse was defined as a drop in platelet count to ≤50 × 109/L following a complete response (platelet count ≥100 × 109/L), partial response (platelet count ≥50 × 109/L), or initial response (increase in platelet counts of at least 30 × 109/L, platelet counts >50 × 109/L on day 10 and no clinical bleeding). No. at risk indicates number of patients with sustained complete or partial response. P = .03.
Adverse events
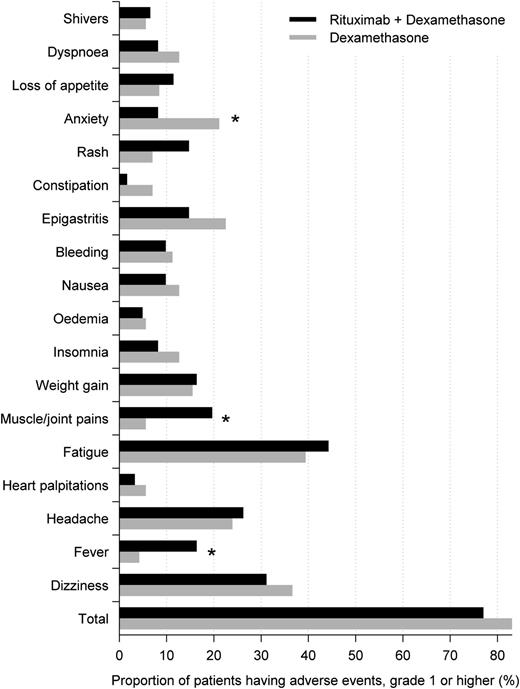
Adverse events were, in general, mild and balanced between the 2 groups. Figure 3 shows adverse events reported by >5% of patients, the most frequent being fatigue, dizziness, headache, epigastritis, and anxiety. There was an overrepresentation of fever and muscle/joint pains in the RTX+DXM group (17.6 vs 6.4%, P = .12) and of anxiety in the DXM group (21.3 vs 3.9%, P = .01). All adverse events reported in this study have previously been described in the product summaries of RTX and DXM. There were 16 reported serious adverse events in the RTX+DXM group, including 1 death, as compared with 8 serious adverse events in the DXM group, including 3 deaths (P = .04) (Table 2). None of the deaths were considered related to treatment. In the RTX+DXM group, 5 patients were hospitalized because of thrombocytopenia vs no patients in the DXM group. In 4 of the 5 patients, thrombocytopenia occurred within 2 to 4 weeks after inclusion in the study. One of these patients did not receive rescue treatment and platelet counts increased to >100 × 109 after completion of rituximab therapy; the other 3 patients required long-term prednisolone treatment and were thus considered nonresponders in the protocol. The last patient was an 83-year-old nonresponder who had received multiple rescue treatments including DXM, prednisolone, and IVIG. She was hospitalized with thrombocytopenia 2 years after rituximab and treated with DXM and prednisolone. The thrombocytopenias may be related to ITP or may be related to treatment with RTX as this has previously been reported.13 One patient in the RTX+DXM group and 2 patients in the DXM group were withdrawn from the study because of adverse events.
Adverse events. Adverse events reported by >5% of patients in either the rituximab + dexamethasone group or dexamethasone group. *P < .05.
Adverse events. Adverse events reported by >5% of patients in either the rituximab + dexamethasone group or dexamethasone group. *P < .05.
Serious adverse events and infections
| Parameter . | RTX+DXM . | DXM . |
|---|---|---|
| Total serious adverse events | 16 | 9 |
| Thrombocytopenia (without bleeding) requiring hospitalization | 5 | 0 |
| Bleeding grade 3-4 | 2 | 1 |
| Deaths | 1* | 3† |
| Fever | 2 | 1 |
| Pain | 1 | 1 |
| Dizziness | 1 | 1 |
| Infusion-related anaphylaxis | 1 | 0 |
| Delayed neutropenia | 1 | 0 |
| Vasculitis | 1 | 0 |
| Cataract | 1 | 0 |
| Hyperglycemia | 0 | 1 |
| Chest pain | 0 | 1 |
| Total reported infections | 11 | 9 |
| Upper respiratory tract | 4 | 1 |
| Topical | 4‡ | 1§ |
| Oral candida | 0 | 3 |
| Pneumonia | 2 | 2 |
| Urinary tract | 1 | 2 |
| Herpes genitalis | 1 | 1 |
| Parameter . | RTX+DXM . | DXM . |
|---|---|---|
| Total serious adverse events | 16 | 9 |
| Thrombocytopenia (without bleeding) requiring hospitalization | 5 | 0 |
| Bleeding grade 3-4 | 2 | 1 |
| Deaths | 1* | 3† |
| Fever | 2 | 1 |
| Pain | 1 | 1 |
| Dizziness | 1 | 1 |
| Infusion-related anaphylaxis | 1 | 0 |
| Delayed neutropenia | 1 | 0 |
| Vasculitis | 1 | 0 |
| Cataract | 1 | 0 |
| Hyperglycemia | 0 | 1 |
| Chest pain | 0 | 1 |
| Total reported infections | 11 | 9 |
| Upper respiratory tract | 4 | 1 |
| Topical | 4‡ | 1§ |
| Oral candida | 0 | 3 |
| Pneumonia | 2 | 2 |
| Urinary tract | 1 | 2 |
| Herpes genitalis | 1 | 1 |
84-y-old female deceased from pneumonia and heart failure 33 mo after inclusion.
83-y-old female deceased from atrial fibrillation and sepsis 3 wk after inclusion; 80-y-old male deceased in own home 4 d after inclusion, cause unknown; 80-y-old woman deceased 33 mo after inclusion, cause unknown.
Bursitis, otitis, bartholinitis, and eye infection.
Furunculi.
In both groups, age at the time of inclusion was in the range of 18 to 84 years.
Ig serum levels
Pre- and posttreatment serum levels of IgG, IgM, and IgA were available for 42 patients in the RTX+DXM group and 36 patients in the DXM group. At 3 months’ follow-up, there was a significant decrease in IgG concentrations in the RTX+DXM group when compared with baseline values (median [range] 8.7 g/L [4.1-14.5 g/L] vs 11.2 g/L [0.6-28 g/L], P < .0001), these decreased levels persisted through the first year. At 2 years’ follow-up, there was no difference in IgG levels from baseline values. A significant decrease in IgM levels following RTX treatment persisted even 2 years after treatment, with baseline median [range] 0.9 g/L [0.3-8.4 g/L] vs at 2 years 0.7 g/L [0.1-4.9 g/L], P = .0014. Levels of IgA also decreased slightly but significantly with median [range] values after 2 years 2 g/L [0.3-4.5 g/L] vs baseline 2.4 g/L [0.1-8.4 g/L], P = .034. In the DXM group, IgG values decreased from baseline median [range] 11 g/L [2.4-17.4 g/L vs after 2 years 9.5 g/L [7.3-13.4 g/L], P = .0048, as did levels of IgA (baseline median [range] 2.0 g/L [0.3-5.7 g/L] vs after 1 year 1.7 g/L [0.2-5 g/L], P = .0015). No changes in IgM were found in the DXM group.
Discussion
We present the first results from a prospective study on the largest cohort to date of newly diagnosed ITP patients treated with RTX+DXM vs DXM alone. Our findings suggest that up-front addition of RTX to DXM in treatment of newly diagnosed ITP improves outcomes and yields longer sustained response rates than DXM alone.
Our primary end point was sustained complete or partial response after 6 months of follow-up, which was reached in 57% in the RTX+DXM group vs 37% in the DXM group (P = .02). Our findings are largely in line with those reported by Zaja et al, who found sustained response rates after 6 months in 63% of 49 patients receiving RTX+DXM vs 36% of 52 patients treated with DXM alone.8 When regarding time to relapse over a 4-year follow-up period, we also found a significant difference between the 2 groups favoring RTX+DXM (Figure 2), although numbers at risk were substantially lower toward the end of the follow-up period.
Patients with chronic ITP are often observed without treatment, even when platelet counts are low, because the potential adverse effects of treatments generally do not match the estimated risk of bleeding in the individual patients. Thus, 1 of our secondary end points was time to rescue treatment including prednisolone, IVIG, splenectomy, or any other second-line treatments. Regarding this end point, we also found a significant difference between the 2 groups in favor of the group receiving RTX+DXM.
Splenectomy is a recommended second-line therapy for ITP, but the choice of splenectomy is highly dependent on the preferences of the hematologist and the patient. In this study, the choice of splenectomy reflected current clinical practice at the individual treatment facility because no common guidelines were outlined in the protocol. No differences in splenectomy between the 2 groups were observed.
Persistent hypogammaglobulinemia following rituximab as monotherapy or in combination with methotrexate has been reported in up to 23% of patients with rheumatoid arthritis.14 There have also been reports of decreasing serum IgG and IgM levels in children with ITP treated with rituximab.15 Even though the rituximab-induced hypogammaglobulinemia has not been associated with an increased risk of infections,14,15 there are still concerns about the long-term side effects of rituximab. In concordance with these previous reports, we found that levels of IgM decreased significantly following RTX treatment and remained decreased even 2 years after treatment. No changes in serum IgM were found in patients treated with DXM alone. IgG and IgA levels decreased after RTX+DXM as well as after DXM monotherapy, but remained within normal range in both groups. IgG values were available in 7 of 10 patients reporting infections during or after RTX+DXM treatment; in 3 of the 7, we found a decrease in IgG levels following RTX, although none of them had IgG levels below normal range. The infections reported in these 3 patients were upper respiratory tract infection, eye infection, and urinary tract infection.
There were no previously undescribed adverse events reported, and no significant differences in the total number of adverse events between the 2 groups. There were more serious adverse events in both groups than reported in the 2 previous studies.8,9 This may to some extent be ascribed to variations in the grading of adverse events. The 4 deaths in the cohort all occurred in patients older than 80 years of age, and the deaths were not considered to be treatment-related by the investigator.
All patients in this study were evaluated with a bone marrow smear and biopsy, chest radiograph, and abdominal ultrasonography before inclusion to exclude secondary ITP from the cohort. Patient outcomes were evaluated by both platelet counts and time to rescue treatment. Taken together, our findings show that RTX+DXM is superior to DXM monotherapy in the treatment of newly diagnosed ITP. On the basis of these results, we propose that RTX should be considered early in the treatment course of newly diagnosed ITP patients, even before splenectomy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.ll
There is an Inside Blood commentary on this article in this issue.
Acknowledgments
This work was supported (in part) by research funding from Roche A/S Denmark.
Authorship
Contribution: S.G. performed data analyses and wrote and edited the manuscript; H.B. designed the study, served as investigator, and edited the manuscript; H.F. served as investigator and wrote and edited the manuscript; B.A.J. served as investigator and edited the manuscript; M.K.J. served as investigator and edited the manuscript; L.K. served as investigator and edited the manuscript; T.W.K. performed statistical data analyses and wrote and edited the manuscript; H.L. served as investigator and edited the manuscript; H.T.M.-A. served as investigator and edited the manuscript; C.H.N. wrote and edited manuscript; O.J.N. served as investigator and edited the manuscript; T.P. served as investigator and edited the manuscript; S.P. served as investigator and edited the manuscript; I.H.R. served as investigator and edited the manuscript; D.R.-J. served as investigator and edited the manuscript; and H.C.H. designed the study, served as investigator, and wrote and edited the manuscript.
Conflict-of-interest disclosure: S.F. has received research support from Amgen and GlaxoSmithKline; H.F. has been a member of an advisory board for GlaxoSmithKline; C.H.N. has received consultant fees from Roche A/S; T.P. is the recipient of research grants from Roche; and I.H.R. is an advisory board member of Bristol-Myers Squibb.
Correspondence: Sif Gudbrandsdottir, Department of Hematology, Copenhagen University Hospital Roskilde, Koegevej 7-13, 4000 Roskilde, Denmark; e-mail: sif.gudbrandsdottir@gmail.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal