Key Points
Long-term lenalidomide/dexamethasone/biaxin in newly diagnosed myeloma is safe and effective.
No increased incidence of second primary malignancies seen in lenalidomide without alkylators.
Abstract
The combination of clarithromycin, lenalidomide, and dexamethasone (BiRd) was evaluated as therapy for treatment-naive symptomatic multiple myeloma (MM), with overall response at 2 years of 90%. We reviewed the long-term follow-up of initial BiRd therapy. Seventy-two patients were given dexamethasone 40 mg weekly, clarithromycin 500 mg twice daily, and lenalidomide 25 mg daily on days 1 to 21 of a 28-day cycle. After a median follow-up of 6.6 years, overall response rates were 93%, with a very good partial response or better of 68%. Median progression-free survival was 49 months. Evaluation for the development of second primary malignancies (SPMs) was conducted, and no increase in incidence was noted in our cohort of patients who received frontline immunomodulatory therapy. BiRd remains a highly potent and safe regimen for frontline therapy in patients with MM without apparent increase in risk of SPMs. This trial was registered at www.clinicaltrials.gov as #NCT00151203.
Introduction
Novel agents, including immunomodulatory drugs, have revolutionized the treatment of multiple myeloma (MM). Lenalidomide has proven efficacy both in the relapse/refractory1,2 and frontline3 settings, and thus has become a valuable regimen for patients with MM. The addition of clarithromycin to the combination of lenalidomide/dexamethasone yielded improved response rates and survival benefit.4,5
In 2008, we first reported the BiRd regimen (clarithromycin [Biaxin], lenalidomide [Revlimid], and dexamethasone) to be effective for symptomatic patients with newly diagnosed MM.4 The original study recruited 72 treatment-naive patients. BiRd had an overall response rate of 90.3%, as compared with 79.1% seen in a lenalidomide/dexamethasone cohort.5 Toxicities observed were manageable and mostly hematologic.4 As clarithromycin potentiates the effects of corticosteroids,6 dexamethasone dose reductions were performed, resulting in decreased toxicity. In addition, the regimen did not interfere with CD34+ cell mobilization or engraftment in patients eligible for autologous stem cell transplantation (ASCT).7 These results established BiRd as an effective induction regimen, as well as a valuable option for treatment of transplant-ineligible MM patients. This experience has been compounded by the observation of deepening responses to prolongation of lenalidomide-based therapy beyond induction, even achieving molecular complete response (CR) in a number of patients.7,8
All enrolled study patients continue to be monitored, now with nearly 7 years of follow-up9 , providing valuable data on newly diagnosed patients receiving continuous lenalidomide. As data accrues in the long-term use of lenalidomide, concern has arisen for development of second primary malignancies (SPMs), including myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML) and lymphoma.10-12 This signal was noted in studies of lenalidomide maintenance following therapy with melphalan, both in the context of ASCT or lower doses for up to 1 year in transplant-ineligible patients. Given this emergent data, we examined the alkylator-naive BiRd patient cohort for the incidence of SPMs.
Study design
The phase 2 BiRd trial enrolled patients from December 2004 to November 2006, all of whom continue to be monitored until death, with none lost to follow-up. The BiRd regimen consists of dexamethasone 40 mg orally weekly, clarithromycin 500 mg orally twice daily, and lenalidomide 25 mg orally daily on days 1 to 21 of a 28-day cycle. Patients received prophylaxis with aspirin 81 mg daily, omeprazole 20 mg daily, and double-strength trimethoprim/sulfamethoxazole twice daily 3 times per week. Patients could choose, with physician discretion, to undergo ASCT at maximum response, or remain on continuous BiRd. Posttransplant maintenance chemotherapy was not given. The end points of the study were response to treatment, time to first and maximum response, and adverse events. Response criteria were adopted from the International Myeloma Working Group criteria.13 Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Patients were maintained on BiRd until progression of disease (12 patients), ASCT (33 patients), or development of intolerable side effects (6 patients).
Assessment for SPMs was performed via chart review. Evaluation included time of diagnosis, transplant status, number of BiRd cycles received, and whether or not patients were still on treatment. Investigative bone marrow aspirate and biopsy were performed on all patients remaining on study.
Primary efficacy analyses were performed according to the intent-to-treat principle. Survival curves began at time of enrollment, and were measured to date of progression (progression-free survival [PFS]), date of death (overall survival [OS]), or date of event (event-free survival [EFS]), including MM progression, SPM diagnosis, abandonment due to toxicity, or death (Figure 1). PFS, OS, and EFS were analyzed using Kaplan-Meier survival analysis, and 95% confidence intervals (CIs) were constructed using the Greenwood formula. The log-rank test was used to compare PFS/OS by cytogenetics and ASCT. All P values are 2-sided with statistical significance evaluated at the .05 α level. All analyses were performed in SAS version 9.3 (SAS Institute, Inc, Cary, NC) and STATA version 12.0 (StataCorp, College Station, TX).
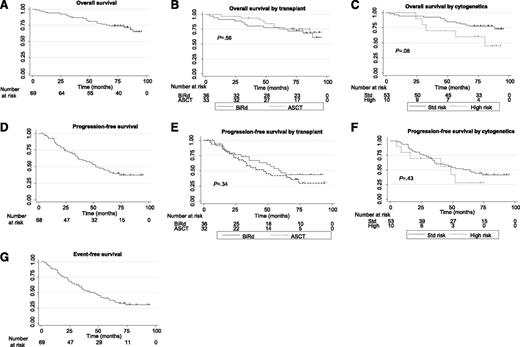
Survival curves. (A) OS. N = 69 patients, 21 deaths. Median OS not reached; 5-year OS = 75.2% (95% CI = 63.1%, 83.8%). (B) OS by transplant status. No transplant/continuous BiRD: 36 patients, 12 deaths, median OS = not reached; 5-year OS = 75.0% (95% CI = 57.5%, 86.1%). Transplant: 33 patients, 9 deaths, median OS = not reached; 5-year OS = 75.2% (95% CI = 56.4%, 86.7%). (C) OS by cytogenetics. Standard risk: 53 patients, 13 deaths, median OS = not reached; 5-year OS = 79.3% (95% CI = 65.7%, 87.9%). High risk: 10 patients, 5 deaths, median OS = 80 months; 5-year OS = 60.0% (95% CI = 25.3%, 82.7%). (D) PFS. N = 68 patients, 39 progressions. Median PFS = 52 months; 5-year PFS = 41.2% (95% CI = 28.9%, 53.1%). (E) PFS by transplant status: no transplant/continuous BiRD: 36 patients, 18 progressions, median PFS = 60 months; 5-year PFS = 43.4% (95% CI = 25.9%, 59.7%). Transplant: 32 patients, 21 progressions, median PFS = 47 months; 5-year PFS = 38.8% (95% CI = 22.1%, 55.2%). (F) PFS by cytogenetics. Standard risk: 53 patients, 29 progressions, median PFS = 64 months; 5-year PFS = 46.4% (95% CI = 32.2%, 59.4%). High risk: 10 patients, 6 progressions, median PFS = 49 months; 5-year PFS = 28.0% (95% CI = 4.4%, 59.7%). (G) EFS. N = 69 patients, 46 progressions, second malignancies, or deaths. Median EFS = 47 months; 5-year EFS = 34.2% (95% CI = 23.1%, 45.7%).
Survival curves. (A) OS. N = 69 patients, 21 deaths. Median OS not reached; 5-year OS = 75.2% (95% CI = 63.1%, 83.8%). (B) OS by transplant status. No transplant/continuous BiRD: 36 patients, 12 deaths, median OS = not reached; 5-year OS = 75.0% (95% CI = 57.5%, 86.1%). Transplant: 33 patients, 9 deaths, median OS = not reached; 5-year OS = 75.2% (95% CI = 56.4%, 86.7%). (C) OS by cytogenetics. Standard risk: 53 patients, 13 deaths, median OS = not reached; 5-year OS = 79.3% (95% CI = 65.7%, 87.9%). High risk: 10 patients, 5 deaths, median OS = 80 months; 5-year OS = 60.0% (95% CI = 25.3%, 82.7%). (D) PFS. N = 68 patients, 39 progressions. Median PFS = 52 months; 5-year PFS = 41.2% (95% CI = 28.9%, 53.1%). (E) PFS by transplant status: no transplant/continuous BiRD: 36 patients, 18 progressions, median PFS = 60 months; 5-year PFS = 43.4% (95% CI = 25.9%, 59.7%). Transplant: 32 patients, 21 progressions, median PFS = 47 months; 5-year PFS = 38.8% (95% CI = 22.1%, 55.2%). (F) PFS by cytogenetics. Standard risk: 53 patients, 29 progressions, median PFS = 64 months; 5-year PFS = 46.4% (95% CI = 32.2%, 59.4%). High risk: 10 patients, 6 progressions, median PFS = 49 months; 5-year PFS = 28.0% (95% CI = 4.4%, 59.7%). (G) EFS. N = 69 patients, 46 progressions, second malignancies, or deaths. Median EFS = 47 months; 5-year EFS = 34.2% (95% CI = 23.1%, 45.7%).
The research was approved by the institutional review board of the Weill Medical College of Cornell University, New York Presbyterian Hospital, in accordance with federal regulations and the Declaration of Helsinki. All participants gave written informed consent prior to enrollment. All authors had access to the primary clinical trial data.
Results and discussion
Seventy-two patients enrolled on the BiRd study. With a median follow-up of 6.6 years, 10 patients remain on lenalidomide (3 with dexamethasone), and 47 have received second-line therapy. Thirty-three patients have now undergone ASCT, thereafter followed by active observation alone. Median PFS was 49 months, and median EFS was 47 months. While the median OS has not been reached, 5-year OS was 75.2%. Post hoc analysis by transplant status showed no effect on PFS or OS, indicating that the benefit of lenalidomide maintenance exists even in the absence of transplantation; however, formal conclusions regarding ASCT should be addressed by prospective randomized studies. Patients who underwent ASCT received a median of 10 cycles (range, 2-36) of BiRd, compared with 26 cycles (range, 3-93) for those on continuous therapy. Eleven patients (5 BiRd, 6 ASCT) had high-risk cytogenetics, which did not significantly affect PFS or OS, although a trend is seen in OS.
Systematic review for SPMs in our patient cohort revealed 6 new diagnoses of invasive cancer, and 6 of skin noninvasively (4 basel cell carcinoma, 2 squamous cell carcinoma). The invasive cancers included colon (2), prostate, pancreas, metastatic melanoma, and lung carcinoid. Notably, there were no cases of MDS/AML, even after careful bone marrow evaluation of all patients remaining on study. The median time to SPM diagnosis was 35 months (range, 5-71), with an average of 31 cycles (range, 3-68) of lenalidomide (BiRd). Seven subjects were receiving lenalidomide, and 2 had undergone ASCT.
Response rates at time of original publication and with long-term follow-up show deepening responses with prolongation of treatment. For BiRd 2008 (2-year follow-up), overall response rate was 90%, complete response was 39%, very good partial response was 21%, and partial response was 17%. For BiRd 2012 (6.6-year follow-up), overall response rate was 93%, complete response was 43%, very good partial response was 25%, and partial response was 25%. SPM data are outlined in Table 1.
SPMs
| SPM . | N . | Median time to SPM diagnosis, months (range) . | ASCT . | Gender . | On study . |
|---|---|---|---|---|---|
| Solid tumors (stage) | 6 | 35 (8-71) | |||
| Metastatic melanoma | 1 | 8 mo | No | M | On |
| Colon (IV) | 1 | 25 mo | No | M | On |
| Colon (III) | 1 | 31 mo | No | F | Off |
| Pancreas (II) | 1 | 39 mo | Yes | M | Off |
| Prostate (II) | 1 | 53 mo | No | M | On |
| Lung carcinoid (I) | 1 | 71 mo | Yes | F | Off |
| Hematologic tumors | 0 | ||||
| Basal cell carcinoma | 4 | 42 (5-64) | No | 2F, 2M | On |
| Squamous cell carcinoma | 2 | 29.5 (28-33) | No | 2M | On/Off |
| SPM . | N . | Median time to SPM diagnosis, months (range) . | ASCT . | Gender . | On study . |
|---|---|---|---|---|---|
| Solid tumors (stage) | 6 | 35 (8-71) | |||
| Metastatic melanoma | 1 | 8 mo | No | M | On |
| Colon (IV) | 1 | 25 mo | No | M | On |
| Colon (III) | 1 | 31 mo | No | F | Off |
| Pancreas (II) | 1 | 39 mo | Yes | M | Off |
| Prostate (II) | 1 | 53 mo | No | M | On |
| Lung carcinoid (I) | 1 | 71 mo | Yes | F | Off |
| Hematologic tumors | 0 | ||||
| Basal cell carcinoma | 4 | 42 (5-64) | No | 2F, 2M | On |
| Squamous cell carcinoma | 2 | 29.5 (28-33) | No | 2M | On/Off |
Invasive tumors were seen at a rate expected by SEER database. No hematologic malignancies were seen. No association noted with multiple variables analyzed.
F, female; M, male; SEER, Surveillance Epidemiology and End Results.
Development of SPM showed no correlation with age, gender, stage, prior malignancy, chromosomal abnormality, ASCT, cycles of lenalidomide, or whether patients remained on study by Cox regression analysis. Eligibility criteria for the BiRd trial required no malignancy for 5 years prior to enrollment. Review of this data identified 11 patients with a history of malignancy prior to MM diagnosis, none of whom relapsed. When compared with the Surveillance Epidemiology and End Results (SEER) database incidence of invasive cancers for patients in the same age group, the incidence of SPMs observed in our cohort (2.85 per 100 person-years [1.04-6.31]) was not statistically different from expected (2.1 per 100 person-years).
Three studies recently reported results of double-blind phase 3 multicenter randomized trials on long-term use of lenalidomide following alkylating therapy,10-12 where post hoc analyses noted an increase in SPMs. The BiRd study provides a unique cohort of patients who received long-term continuous lenalidomide as induction therapy with no post-ASCT maintenance, and suggests that timing lenalidomide prior to and without alkylating chemotherapy is not associated with an increase in SPMs. We identified no biases; however, our phase 2 study was not comparative by design. Further evaluation of the temporal relationship of lenalidomide with alkylating therapy may help clarify its possible role in the development of SPMs.
BiRd is a highly effective regimen in patients with newly diagnosed MM, with remarkable response rates and survival outcomes, and may provide a less toxic alternative to transplantation. It compares favorably with other 3-drug regimens, with little long-term toxicity. In our cohort of treatment-naive patients, no cases of secondary MDS/AML were seen, in contrast to reports in relapse/refractory patients who received lenalidomide as third- or fourth-line therapy14 or as posttransplant maintenance.10,11 As outcomes in patients with MM continue to improve, so will our understanding of the long-term effects of novel agents.
Presented as an oral abstract at the 2011 annual meeting of the American Society of Clinical Oncology, Chicago, IL, June 5, 2011.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The original study was supported by Celgene Corp, which provided free lenalidomide and data support. This investigation was supported in part by the Leukemia & Lymphoma Society SCOR grant, and a National Institutes of Health National Cancer Institute K23 award (CA109260-01). P.C. and A.R. were partially supported by grant UL1-RR024996 of the Clinical and Translational Science Center at Weill Cornell Medical College.
National Institutes of Health
Authorship
Contribution: A.R. updated the database, performed SPM evaluation, wrote the manuscript, and coordinated input from all the authors; D.J. generated the database, coordinated translational efforts, and generated figures; P.C. performed biostatistical analysis; R.P. provided significant input on trial design and expert patient care; T.M., F.Z., and K.P. provided significant contribution in patient care; S.C.-K. coordinated translational research and gave direction in science; M.C. contributed in original idea generation and treatment program concept, reviewed the manuscript, and contributed with expert patient care; R.N. conceived the treatment program, wrote and implemented the protocol, and coordinated data and database.
Conflict-of-interest disclosure: R.N., M.C., and T.M. have served as consultants and participate as lecturers in the speaker’s bureau for Celgene. R.N. received research support and drug for the implementation of this trial. The remaining authors declare no competing financial interests.
Correspondence: Ruben Niesvizky, Multiple Myeloma Center, Division of Hematology and Medical Oncology, Weill-Cornell Medical College, New York Presbyterian Hospital-Cornell Medical Center, 428 East 72nd St, Suite 300, New York, NY 10021; e-mail: run9001@med.cornell.edu.