Key Points
Fancc−/− mice experience previously unrecognized late gestational lethality.
Fancc−/− fetal mouse hematopoiesis is quantitatively and qualitatively deficient.
Abstract
Hematopoietic failure is the predominant clinical manifestation of Fanconi anemia (FA), a rare, recessively inherited disorder. Mutations in 1 of 15 genes that coordinately function in a complex pathway to maintain DNA integrity also predispose patients to constitutional defects in growth and development. The hematologic manifestations have been considered to reflect the progressive loss of stem cells from the postnatal bone marrow microenvironment. Ethical concerns preclude the study of human hematopoiesis in utero. We report significant late gestational lethality and profound quantitative and qualitative deficiencies in the murine Fancc−/− fetal liver hematopoietic stem and progenitor cell pool. Fancc−/− fetal liver hematopoietic stem and progenitor cells revealed a significant loss of quiescence and decline in serial repopulating capacity, but no substantial difference in apoptosis or levels of reactive oxygen species. Our studies suggest that compromised hematopoiesis in Fancc−/− animals is developmentally programmed and does not arise de novo in bone marrow.
Introduction
Bone marrow (BM) failure is the most common cause of morbidity and mortality from Fanconi anemia (FA), a recessively inherited disorder resulting from biallelic mutations in 1 of 15 known genes that cooperate in a DNA repair pathway.1,2 The presumptive postnatal loss of hematopoietic stem and progenitor cells (HSPC) from the marrow is thought to account for patient symptom onset during early school age and is generally consistent with experimental evidence.3 However, cytopenias often precede clinical symptoms in FA patients or affected siblings.4 Although systematic studies of in utero development of FA patients have not been conducted, reports of reduced cord blood progenitor frequency, evidence in embryonic stem cell lines, and studies of rare fetal tissues all support the notion of early compromise in HSPC function.5-10 Here, we studied fetal liver (FL) hematopoiesis in a murine model of FA complementation group C (Fancc−/−) to determine if hematopoietic deficits were limited to HSPC losses from the BM compartment, or whether they may have origins earlier in development.11 Our study for the first time reveals broad defects of hematopoietic development in Fancc−/− animals, which include a compromised fetal liver HSPC pool as well as late gestational lethality.
Study design
Mice
Mice were handled in accordance with the Oregon Health and Science University Institutional Animal Care and Use Committee. FL from 14.5 days post coitum timed pregnancies (vaginal plug method) from C57BL/6 Fancc+/− (CD45.2) dams were dissected to prepare single-cell suspensions. Fetal tails were genotyped using the Phire PCR Kit (Thermo Scientific, Waltham, MA). BM cells from adult animals were harvested as previously described.12
Cellular assays
Cytogenetic analyses were conducted on FL cells by the Oregon Health and Science University Cytogenetics Core Laboratory. Methylcellulose progenitor assays (M3434; StemCell Technologies, Vancouver, BC, Canada) were performed according to manufacturer’s instructions.
Cytokine arrays
Unfractionated FL cells were cultured in StemSpan SFEM (StemCell Technologies) at 1.6 × 107 cells/mL. Conditioned media was applied to Mouse Cytokine Antibody Array, Panel A (R&D Systems, Minneapolis, MN) following manufacturer’s instructions and imaged on a Fujifilm LAS2000. Pixel density was analyzed using Multi Gauge software (Fujifilm, Tokyo, Japan).
Transplantation
Unfractionated FL cells (2 × 106) were injected via tail vein into irradiated (530 cGy) congenic CD45.1 recipients. Serial chimerism was analyzed as described.12 For 2° transplant, 2 × 106 whole BM cells from 1° recipients (n = 2/genotype) were transplanted (each) into 5 irradiated (775 cGy) CD45.1 recipients.
Flow cytometry
For c-Kit+ Sca-1+ Lin− (KSL) and AA4.1+ Sca-1+ Linlow/− (ASL) HSPC analyses, cells were stained using antibodies; lineage antibodies were against B220, Gr-1, CD3, CD4, CD5, and Ter119 (excluding Mac-113 ). For cell-cycle analysis, we used anti-Ki-67 antibody and Hoechst 33342.14 Apoptosis assays used Annexin V (BD) and reactive oxygen species (ROS) levels were assayed by carboxy-H2DCFDA staining (Invitrogen, Carlsbad, CA). Data were collected on FACSCalibur and BD Influx (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (TreeStar, Ashland, OR).
Quantitative real-time PCR
KSL or ASL FL cells were sorted by FACS on a BD Influx. RNA was purified using RNeasy Mini Kit (Qiagen, Germantown, MD), and then converted to complementary DNA using SuperScript III First Strand (Invitrogen). The complementary DNA was analyzed by real-time polymerase chain reaction (PCR) (StepOne Plus) using Power SYBR Green Master Mix (both Applied Biosystems, Carlsbad, CA). Fancc−/− and wild-type (WT) samples were normalized to Gapdh, and Fancc−/− gene expression was normalized to WT. Relevant transcripts were previously described.9,15
Statistical analysis
Numerical results are expressed as mean (± standard error of the mean as indicated) and compared using an independent Student t test. Genotype distributions were compared by χ-square test.
Results and discussion
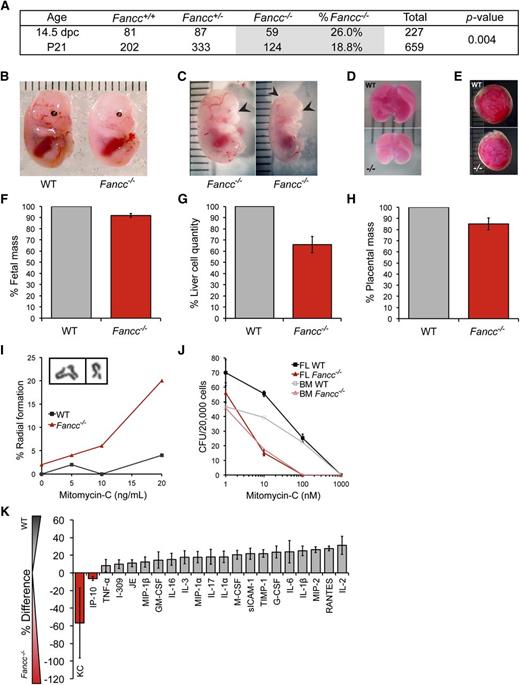
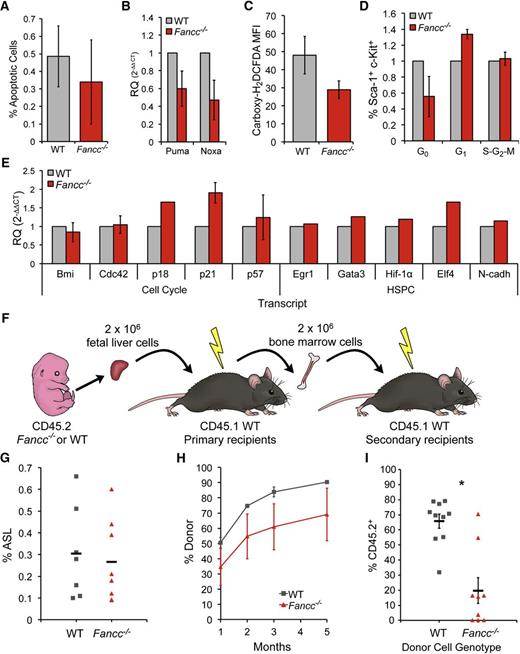
A wide range of FA-associated constitutional defects arising in utero are diagnosed at birth. Hematopoietic manifestations of FA in infants, by comparison, are rare.4 Fancc−/− mice provide a validated, if imperfect, model of FA hematopoiesis. Here, we focused on FL hematopoiesis (14.5 days post coitum) as a key stage during hematopoietic development.16 Using heterozygous breeder animals, the genotype distribution across 28 litters yielded 26% Fancc−/− fetuses (n = 59−/−, 81+/+, 87+/−), at near-Mendelian frequency. This exceeds the frequency at weaning of 18.9% (n = 659 total; P = .004), also reported by others, and for the first time reveals late gestational lethality in FA mice (Figure 1A).17,18 Beyond occasional gross morphological abnormalities (microphthalmia, anophthalmia, anencephaly), Fancc−/− fetuses weighed on average 8% less than WT littermates, with a concomitant 34% reduction in whole FL cellularity and a 15% decrease in placental mass (Figure 1B-H). Fancc−/− FL cells exhibited characteristic mitomycin-c–induced radial formation (Figure 1I) and chromosomal breaks (not shown).11,19 Clonogenic hematopoietic progenitor growth in methylcellulose was reduced by 20% in Fancc−/− FL compared with WT (Figure 1J), implying an aggregate 47% decline in progenitor frequency per fetus. Although interleukin-1, tumor necrosis factor-α, and interferon-γ can suppress postnatal hematopoiesis in Fancc−/− animals, we found no differences in cytokine secretion by unfractionated FL cells to account for the reduction in fetal Fancc−/− progenitor cells (Figure 1K).20 Our data reveal other phenotypic differences between fetal FA HSPC and those present in the postnatal bone marrow compartment. Unlike adult FA HSPC, characterized by exaggerated apoptosis and elevated levels of ROS, we found no difference in the frequency of apoptotic events (Annexin V and expression of pro-apoptotic genes Puma and Noxa) or ROS levels (by carboxy-H2DCDFA stain) between Fancc−/− and WT Sca-1+ AA4.1+ (fetal HSPC marker21 ) FL cells (Figure 2A-C).22,23 Cell-cycle analysis revealed fewer Fancc−/− than WT c-Kit+ Sca-1+ (KS) Ki-67neg (G0) HSPC (Figure 2D), echoing the reported loss in quiescence in murine FA BM KSL cells, and suggested potential G1 arrest and senescence.14,19,24 This is further consistent with quantitative reverse-transcription-PCR studies of sorted FL HSPCs demonstrating Fancc−/− upregulation of p21 and p18, cell-cycle regulators involved in hematopoietic failure in FA (Figure 2E).6,9
Prenatal manifestation of several FA-associated defects. (A) Fancc+/− crosses yielded an approximately Mendelian frequency of Fancc−/− fetal mice, whereas frequencies of Fancc−/− weanlings were sub-Mendelian. χ2 test comparing fetuses to weanlings yielded a P value of .004. (B) Representative photo of WT and Fancc−/− day 14.5 fetal mice. Units are in millimeters. (C) Malformations observed in some Fancc−/− fetuses: microphthalmia (left); anophthalmia and anencephaly (right). (D) Representative photos of 14.5 days post coitum WT and Fancc−/− fetal livers, and (E) placentas. (F) Fetal mass normalized to WT mean within each litter (nlitters = 5, nWT = 19, nFancc−/− = 14). (G) Cell counts from single cell suspensions of fetal livers normalized to WT mean (nlitters = 6, nWT = 16, nFancc−/− = 13). (H) Placenta weights (nWT = 25, nFancc−/− = 14), P = .009. (I) Chromosome radial formation (%) in WT and Fancc−/− fetal liver cells cultured in mitomycin-c (MM-C; n = 50/condition). Inset image of representative radial (left) and break (right). (J) FL and adult BM hematopoietic progenitor colony formation in methylcellulose (CFU-C) (nFL = 3/genotype, nBM = 1/genotype). Unfractionated cells were plated in the presence or absence of MM-C at 2 × 104 per 35 mm dish and colonies of >50 cells were counted on day 12. (K) Mean percent difference in the secretion of select cytokines from Fancc−/− vs WT fetal liver-conditioned media assayed by cytokine arrays in 3 independent experiments; the cytokines shown are those that yielded consistent results across all 3, whereas those that did not show differential expression are not shown. Error bars represent standard error of the mean. BM, bone marrow; FL, fetal liver; MM-C, mitomycin-c; WT, wild type.
Prenatal manifestation of several FA-associated defects. (A) Fancc+/− crosses yielded an approximately Mendelian frequency of Fancc−/− fetal mice, whereas frequencies of Fancc−/− weanlings were sub-Mendelian. χ2 test comparing fetuses to weanlings yielded a P value of .004. (B) Representative photo of WT and Fancc−/− day 14.5 fetal mice. Units are in millimeters. (C) Malformations observed in some Fancc−/− fetuses: microphthalmia (left); anophthalmia and anencephaly (right). (D) Representative photos of 14.5 days post coitum WT and Fancc−/− fetal livers, and (E) placentas. (F) Fetal mass normalized to WT mean within each litter (nlitters = 5, nWT = 19, nFancc−/− = 14). (G) Cell counts from single cell suspensions of fetal livers normalized to WT mean (nlitters = 6, nWT = 16, nFancc−/− = 13). (H) Placenta weights (nWT = 25, nFancc−/− = 14), P = .009. (I) Chromosome radial formation (%) in WT and Fancc−/− fetal liver cells cultured in mitomycin-c (MM-C; n = 50/condition). Inset image of representative radial (left) and break (right). (J) FL and adult BM hematopoietic progenitor colony formation in methylcellulose (CFU-C) (nFL = 3/genotype, nBM = 1/genotype). Unfractionated cells were plated in the presence or absence of MM-C at 2 × 104 per 35 mm dish and colonies of >50 cells were counted on day 12. (K) Mean percent difference in the secretion of select cytokines from Fancc−/− vs WT fetal liver-conditioned media assayed by cytokine arrays in 3 independent experiments; the cytokines shown are those that yielded consistent results across all 3, whereas those that did not show differential expression are not shown. Error bars represent standard error of the mean. BM, bone marrow; FL, fetal liver; MM-C, mitomycin-c; WT, wild type.
Serial repopulating defects and cell cycle abnormalities in Fancc−/− fetal liver HSPCs. (A) Annexin V flow cytometry analysis for AA4.1+ Sca-1+ cells, P = .65. (B) Quantitative reverse-transcription-PCR gene expression analysis of Puma (p53-upregulated modulator of apoptosis) and Noxa (Phorbol-12-myristate-13-acetate-induced protein-1), pro-apoptotic, p53 target genes in Fancc−/− and WT FL sorted ASL cells (n = 3/genotype), PPuma = 0.17, PNoxa= 0.13. (C) ROS flow cytometry analysis for AA4.1+ Sca-1+ cells, shown as median fluorescence intensity, P = .17. (D) Cell-cycle analysis for c-Kit+ Sca-1+ cells. Fancc−/− average 45% decrease in Ki-67− (G0) cells, 34% increase in G1, and 3% increase in S-G2-M cells. Results normalized to WT littermates. (E) Quantitative reverse-transcription-PCR gene expression analysis of transcripts from sorted ASL or LSK cells from Fancc−/− and WT FL. Bmi1 (BMI1 polycomb ring finger oncogene), Cdc42 (small GTPase cell division control protein42), p57 (cyclin-dependent kinase inhibitor 1C), EGR1 (early growth response 1), Gata3 (GATA binding protein 3), Hif-1α (hypoxia inducible factor-1α), and Elf4 (E74-like factor 4), N-cadh (N-cadherin). (F) Serial transplantation scheme: 2 × 106 unfractionated CD45.2 FL cells were transplanted into conditioned CD45.1 recipients. Five months later, 2 × 106 whole BM cells from 1° recipients were transplanted into CD45.1 hosts. (G) Percent ASL cells in WT and Fancc−/− FL, P = .36. (H) Donor chimerism (% CD45.2+) of peripheral blood in 1° transplant (nWT FL donors = 4, nFancc−/−FL donors = 3; nWT recipients = 7, nFancc−/−recipients = 5), P = .13 for 1 month, .12 for 2 months, .10 for 3 months, and .14 for 5 months. (I) CD45.2+ chimerism (% FL donor-derived) for 2° transplantation. Two 1° recipients served as donors from each cohort (nWT recipients = 10, nFancc−/−recipients = 10), P = .0002. Error bars represent standard error of the mean. BM, bone marrow; FL, fetal liver; WT, wild type.
Serial repopulating defects and cell cycle abnormalities in Fancc−/− fetal liver HSPCs. (A) Annexin V flow cytometry analysis for AA4.1+ Sca-1+ cells, P = .65. (B) Quantitative reverse-transcription-PCR gene expression analysis of Puma (p53-upregulated modulator of apoptosis) and Noxa (Phorbol-12-myristate-13-acetate-induced protein-1), pro-apoptotic, p53 target genes in Fancc−/− and WT FL sorted ASL cells (n = 3/genotype), PPuma = 0.17, PNoxa= 0.13. (C) ROS flow cytometry analysis for AA4.1+ Sca-1+ cells, shown as median fluorescence intensity, P = .17. (D) Cell-cycle analysis for c-Kit+ Sca-1+ cells. Fancc−/− average 45% decrease in Ki-67− (G0) cells, 34% increase in G1, and 3% increase in S-G2-M cells. Results normalized to WT littermates. (E) Quantitative reverse-transcription-PCR gene expression analysis of transcripts from sorted ASL or LSK cells from Fancc−/− and WT FL. Bmi1 (BMI1 polycomb ring finger oncogene), Cdc42 (small GTPase cell division control protein42), p57 (cyclin-dependent kinase inhibitor 1C), EGR1 (early growth response 1), Gata3 (GATA binding protein 3), Hif-1α (hypoxia inducible factor-1α), and Elf4 (E74-like factor 4), N-cadh (N-cadherin). (F) Serial transplantation scheme: 2 × 106 unfractionated CD45.2 FL cells were transplanted into conditioned CD45.1 recipients. Five months later, 2 × 106 whole BM cells from 1° recipients were transplanted into CD45.1 hosts. (G) Percent ASL cells in WT and Fancc−/− FL, P = .36. (H) Donor chimerism (% CD45.2+) of peripheral blood in 1° transplant (nWT FL donors = 4, nFancc−/−FL donors = 3; nWT recipients = 7, nFancc−/−recipients = 5), P = .13 for 1 month, .12 for 2 months, .10 for 3 months, and .14 for 5 months. (I) CD45.2+ chimerism (% FL donor-derived) for 2° transplantation. Two 1° recipients served as donors from each cohort (nWT recipients = 10, nFancc−/−recipients = 10), P = .0002. Error bars represent standard error of the mean. BM, bone marrow; FL, fetal liver; WT, wild type.
We previously showed that the frequency of immunophenotypically defined HSPC (c-Kit+ Sca-1+ Lin−; KSL) in Fancc−/− BM is not substantially different from WT animals.12 Similarly, we found a minimal decrease in Fancc−/− FL AA4.1+ Sca-1+ Linlow/− (ASL) HSPCs (Figure 2G). Deficient repopulation is widely considered a hallmark of compromised FA HSPC function.8,24 We therefore tested serial repopulating ability by transplanting 2 × 106 CD45.2 Fancc−/− or WT unfractionated FL cells into sublethally irradiated, congenic CD45.1 mice. Peripheral blood donor chimerism 5 months after transplantation showed an average genotype difference of 24% (P = .14) (Figure 2F-H). However, during serial repopulation, the average donor chimerism between 1° and 2° recipients decreased from 87% to 66% in WT, compared with 66% to 20% for Fancc−/− donor cells (21% vs 46% decline, respectively) (Figure 2I). Thus, the average difference in chimerism between genotypes grew from 24% in 1° recipients to 70% in 2° recipients (P = .0002 for 2° transplant).
Fancc−/− mice do not demonstrate the slowly progressive cytopenias seen in patients, but they have provided valuable insight into FA HSPC biology. Our data reveal that the hematopoietic defect in Fancc−/− mice has origins in development without compartmental or temporal restriction to the BM. These observations provide the cohesive developmental context for prior reports that suggested prenatal HSPC loss and have potential implications for ongoing efforts to harness induced pluripotency for FA.5-7 It will be important to delineate how the FA phenotype impacts HSPC pool expansion and seeding of the fetal BM niche during development. Finally, compromised prenatal hematopoiesis may determine the timing of diagnostic procedures, a reassessment of its mechanistic origin, and prompt strategies to preempt hematopoietic failure in FA patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr. Amy Skinner, Dr. Jianya Huan, Tae Hoon Ha, Kyle Lenz, Andrea McBeth, Pamela Canaday, Dorian La Tocha, and the Oregon Health and Science University Cytogenetics Core.
A.N.K.-L. was supported by T32 Molecular Hematology Training Grant HL778116 and N.L. Tartar Trust Grant. Presented in part at the 23rd Annual Fanconi Anemia Research Fund Scientific Symposium, Barcelona, Spain, 2011, and the 53rd Annual Meeting of the American Society of Hematology, San Diego, CA, 2011.
Authorship
Contribution: A.N.K.-L. designed and performed experiments and wrote the manuscript; N.A.G. performed experiments and interpreted data; P.K. designed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Kurre, Oregon Health & Science University, Papé Family Pediatric Research Institute, L499, 701 SW Gaines Rd, Portland, OR 97239-3098; e-mail: kurrepe@ohsu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal