Key Points
ILC22 and cNK cells can be distinguished on the basis of LFA-1 expression.
ILC22 and cNK cells have differing requirements for their development from hematopoietic stem cells.
Abstract
Human interleukin (IL)-22–producing RORγt+ innate lymphoid cells (ILC22) and conventional natural killer (cNK) cells are present in secondary lymphoid tissues. Both have an immunophenotype corresponding to stage III NK progenitors (CD56+/−CD117highCD94−). Using an in vitro differentiation and primary human tissues, we investigated their developmental relationships. cNK cells showed a CD56+CD117+CD7+/−LFA-1high phenotype and expressed surface receptors, cytokines, and transcription factors found on mature cNK cells. In contrast, ILC22 cells were contained within the CD56+CD117highCD94−CD7−LFA-1− fraction and produced IL-22, IL-8, and granulocyte macrophage colony stimulating factor. Although ILC22 cells expressed NKp44 and CD161, they lacked most other NK receptors and NK-associated transcription factors (T-bet and Eomes) and were incapable of interferon-γ production or cytotoxic responses. Most purified CD56+CD117+CD7+/−LFA-1− remained as ILC22 cells and never became cNK cells. In the absence of IL-15, CD34+ cells showed a complete block in cNK differentiation and instead gave rise to a CD56+ population of ILC22 cells. Conversely, in the absence of IL-7 and stem cell factor, cNK cells were generated but ILC22 cells showed minimal differentiation. Although human ILC22 cells and cNK progenitors have a phenotype that overlaps with stage III NK progenitors, they have unique cytokine requirements and can be distinguished by LFA-1 expression.
Introduction
Recently, it has been proposed that a group of cells with varying functions be classified as innate lymphoid cells (ILC).1,2 These cells are derived from Id2-expressing precursors and are dependent upon common γ-chain cytokine signaling for their development.3 The best-described ILC cells are natural killer (NK) cells (ILC1), though other cell types within the ILC family have been characterized, including type 2 ILCs (ILC2, natural helper cells or nuocytes4 ) and ILCs that express the retinoic acid receptor-related orphan receptor-γt (RORγt) transcription factor (RORγt+ ILCs).1,2 ILC populations are defined in part by transcription factor expression, which dictates function, including cytokine production. For instance, NK cells (ILC1) express T-bet and produce interferon-γ (IFN-γ) and tumor necrosis factor α following interleukin (IL)-12 and IL-18 stimulation. ILC2 cells express the transcription factor ROR-α and secrete the Th2-associated cytokines IL-5 and IL-13 following extracellular parasite infection.4,5 As the name implies, RORγt+ ILCs express the RORγt transcription factor and produce IL-22 (ILC22) and/or IL-17 (ILC17) in response to IL-1β and IL-23 released during bacterial infections and/or gastrointestinal tract injury.6,7 Additionally, RORγt+ ILCs also mediate lymphoid tissue development during fetal life and its regeneration in adult life.1,8
In both humans and mice, RORγt+ ILCs (ILC22 cells) are present in secondary lymphoid tissues (SLTs) such as the tonsils, Peyer patches, and other intestinal lymphoid tissue.6,7,9-13 Research teams have variably named these cells (including NK22, LTi-like, and NCR22), and under the new nomenclature they are now referred to as ILC22 cells. Some investigators have considered ILC22 cells and conventional NK cells (cNK) to be developmentally related to one another given that they both express NK-associated receptors (CD56 and NKp44 for humans, NK1.1 and NKp46 for mice) and are present in the SLTs.10,14,15 In humans, both cell types fall within the stage III NK progenitor cell fraction (CD34-CD56+/−CD117+CD94−),6,7,16 perhaps supporting this concept. Prior studies show that stage III NK progenitors from SLT can further differentiate into stage IV NK cells (CD56+CD94+) but have lost the capacity to give rise to B, T, or dendritic cells.16 Therefore, stage III NK progenitor cells have previously been considered to be committed NK progenitors, leading to the assumption that ILC22 cells are part of the NK lineage. However, recent murine fate-mapping studies refute this concept because cNK progenitors lack evidence for RORγt expression during development, leading to the conclusion that ILC22 and cNK cells are separate lineages in mice.13,17 In further support of separate lineages, Crellin et al18 showed that CD56+CD117+CD127+ cells from human tonsils retain their RORγτ expression and IL-22 production and do not develop into cNK cells after in vitro culture. Thus, in humans the lineage relationship between ILC22 and cNK cells remains unclear. Distinguishing between these two cells types will not only shed light into basic understanding of the developmental relationships between these two cells, but may also lead to novel methods to facilitate posttransplant cNK-cell–mediated graft vs leukemia reactions and ILC22-mediated SLT repair.
We previously reported that umbilical cord blood (UCB) CD34+ progenitors cultured with cytokines and a fetal liver stromal cell line can differentiate into human cNK cells though a series of developmental stages that mirror those in the SLT.19,20 More recently, we also demonstrated that IL-22–producing CD56+ cells (ie, ILC22 cells) are also present in these cultures.7 Using a similar approach Montaldo and colleagues21 showed that some stage III NK progenitors express IL-8 upon CD161 crosslinking. These cells also produced IL-22 and were confined to the stage III fraction that lacked LFA-1, leading to the conclusion that immature stage III cNK cells (lacking LFA-1) produce IL-8 and IL-22 and that acquisition of LFA-1 is a later step in cNK development. However, an alternative explanation is that ILC22 cells and cNK cells are separate lineages distinguished by LFA-1 expression. We set out to understand the phenotype, function, and lineage relationships between these two cell subsets. We show that the stage III NK progenitor fraction is made up of both cNK cells and ILC22 cells with distinct phenotypes, developmental requirements, and functional attributes. These studies also show that ILC22 cells do not give rise to cNK cells or vice versa. Collectively, we conclude that ILC22 cells and cNK cells are separate cell lineages with overlapping phenotypes.
Materials and methods
In vitro generation of human NK cells and ILC22 cells from UCB CD34+ cells
Mouse embryonic liver cell line EL08-D12 cells were maintained in culture medium (40% α minimum essential medium with Glutamax [Invitrogen], 50% myeloculture M5300 medium [Stem Cell Technologies], 10% fetal bovine serum supplemented with 1% penicillin + streptomycin, β-mercaptoethanol [25 μM], and hydrocortisone [1 μM]) on 0.1% gelatin-coated plates at 32°C. Human cord blood CD34+ cells were positively isolated using MACS CD34 microbeads (Miltenyi Biotec) after Ficoll separation. Purified cells (>95% purity) were suspended in B0 medium (Dulbecco’s modified Eagle medium plus Ham’s F-12 medium [2:1] supplemented with 10% of heat-inactivated human AB sera, 1% penicillin + streptomycin, 25 μM β-mercaptoethanol, 20 μg/mL ascorbic acid, and 0.05 μg/mL sodium selenite) seeded on monolayer of 80% to 90% confluent EL08-D12 cells irradiated at 3000 cGy. Human recombinant IL-3 (5 ng/mL), IL-7 (20 ng/mL), IL-15 (10 ng/mL), stem cell factor (SCF; 20 ng/mL), and Flt3 ligand (FLT3L; 10 ng/mL) (all from R&D Systems) were used for supporting cNK cell and ILC generation. Cultures were refed weekly by half-volume change of fresh media with cytokines (except for IL-3, which was used only at day 0).
Flow cytometry
All fluorescence-conjugated antibodies were from BD Biosciences except for IL-1R1, IL-8 (R&D Systems), CD117, IL-22, and granulocyte macrophage colony stimulating factor (GM-CSF; eBioscience). Intracellular staining was performed with cytofix/cytoperm (for cytokines) or Perm III buffer (for phosphorylated proteins; both from BD Biosciences). To detect intracellular cytokines, cultured cells were harvested, resuspended with fresh media, and treated with stimuli (10 ng/mL IL-1β+23 or IL-12+18). The protein transporter inhibitor monensin (BD GolgiStop) was treated 1 hour after stimulation, and cytokines staining was performed after 6 hours. To detect phosphorylated proteins, cultured cells were allowed to rest for 2 hours prior to cytokine stimulation, and cells were harvested and fixed 15 minutes later. Samples were further permeabilized and stained. To measure NK cytotoxicity, CD107a degranulation was used. Briefly, K562 target cells were cocultured with CD56+ cells at 1:1 ratio for 6 hours in the presence of anti-CD107a antibody and GolgiStop. Samples were analyzed with BD Canto II machine and FlowJo software (version 7.6).
Detecting IL-22+ ILCs from primary human tonsillar cells
Deidentified surgically resected human tonsillar tissues were finely cut to small fragments, transferred on a 70-μm strainer, and rendered to a single-cell suspension using a syringe plunger. Mononuclear cells were harvested after ficoll sedimentation, and CD3+, CD14+, and CD19+ cells were depleted by magnetic-bead depletion. Cells were washed, resuspended with B0 media, and rested overnight. To measure IL-22 production, cells were stimulated with IL-1β and IL-23 for 6 hours and then stained with NKp44, CD94, LFA-1, CD3, CD14, CD19, and IL-22 (intracellular) and analyzed by fluorescence-activated cell sorting (FACS). The Lin− fraction (CD3−, CD14−, and CD19−) was electronically gated and analyzed.
Cell sorting
At day 21, cultured cells were harvested and stage III CD56+CD94−CD117high were sorted on the basis of CD7 and LFA-1. ILC22 cells (CD7−LFA-1−) and cNK precursors (CD7+/−LFA-1+) were sorted and cultured with fresh media supplemented with either a combination of cytokines (IL-15, IL-7, SCF, and FLT3L) or IL-15 alone for 1 or 2 weeks further.
Real-time qPCR
Total messenger RNA from specified populations was purified using RNeasy kit (QIAGEN). Complimentary DNA was synthesized using the MMRV RT kit (iScript cDNA Synthesis Kit; Bio-Rad). Real-time qPCR was performed using a StepOnePlus machine according to the manufacturer’s instructions (Applied Biosystems). Primer/probe mix for transcription factors were predesigned TaqMan Gene Expression Assays (AhR: Hs00169233_m1, targeting NM_001621.4, TBX21: Hs00203436_m1, targeting NM_013351.1, and Eomes: Hs00172872_m1, targeting NM_005442.2). RORγt-specific primers (forward: 5′-AGG CGC TGC TGA GAG G-3′; reverse: 5′-CCT TGG CTC CCT GTC CTT-3′ and TaqMan probe 5′-CCT CGC CCC GCC TCT-3′) were designed based on NM_001001523.1. Transcripts were analyzed by the ΔΔCt method and normalized to 18S ribosomal RNA.
All human samples were deidentified and used on protocols approved by the Institutional Review Board.
Statistical analysis
Differences between groups (IL-22–producing cells, LFA-1 cells, and generation of ILC22 and cNK cells using various cytokine combinations) were determined using a Student t test.
Results
Distinguishing human cNK cells from IL-22–producing ILC cells
Human CD34+ cells cocultured with stroma and recombinant cytokines can differentiate into both cNK cells and ILC22 cells,7,19,20,22 but the lineage relationship between these two cell types in humans has been debated.10,15,23,24 To investigate this, CD34+ cells were cultured with IL-3 (for the first week), SCF, FLT3L, IL-7, and IL-15 for 3 weeks. At that time, a CD56-expressing population with a heterogeneous immunophenotype consistent with stage III-V NK cells could be detected (Figure 1A).20 Some CD56+ cells lacked the pan-NK receptor NKp4625 and instead expressed NKp44 (see below, Figure 1E). We have previously shown that these cells exist within the stage III NK progenitor fraction (CD56+/−CD117+CD94−) and express IL-1R1 (CD121a) and CCR6.7 Functionally, they produce IL-22 following IL-1β and IL-23 stimulation and are now termed ILC22 cells.1 Because none of these receptors, either alone or in combination, are specific for the ILC22 subset, we screened IL-1β– and IL-23–stimulated cells with a panel of monoclonal antibodies to identify ILC22 cells. IL-22–expressing cells were completely contained within a stage III population (CD56+CD117highCD94−) that lacked CD94, CD7, and LFA-1 (CD11a) (Figure 1A). Thus, ILC22 cells were defined as expressing CD56 and lacking CD94, CD7, and CD11a. To confirm these findings, we also tested primary tonsil-derived mononuclear cells. In the lineage-negative (CD3−CD14−CD19−) gate, 2 populations could be distinguished using NKp44 and CD94, as previously described.18 The NKp44+CD94− cells lacked LFA-1 and a significant proportion produced IL-22. In contrast, NKp44−CD94+ cells expressed LFA-1 and lacked IL-22 production (Figures 1B-C). Because LFA-1 (CD11a/CD18) is present on the majority of peripheral blood (PB) cNK cells (Figure 1D), and because LFA-1+ in vitro–derived NK cells expressed NK-associated receptors (NKp30, NKp46, NKGA, NKG2D, CD16, 2B4, and KIR [Figure 1E-F] and perforin and granzymes [Figure 1G]), they were considered to be cNK cells. Further proof of this was that CD56+LFA-1+ cells produced IFN-γ in response to IL-12 and IL-18 stimulation and displayed CD107a in after coculture with K562 targets (Figure 1H) whereas CD56+LFA-1− cells did not. Thus, using the combination of LFA-1, CD7, and CD94, we could distinguish between ILC22 cells and cNK cells in both hematopoietic stem cell (HSC)-derived cultures and in primary human tonsils.
IL-22–producing ILCs and cNK cells can be discriminated by LFA-1 and CD7 expression. (A) Cord blood CD34+ progenitor cells were cultured on EL08-D12 feeder cells with IL-3 (for the first week), IL-7, IL-15, SCF, and FLT3L for 21 days. Only CD56+ cells that were negative for CD94, CD7, and LFA-1 expressed IL-22 after IL-1β+23 stimulation. Results are representative of >5 individual donors. (B-C) Differential expression of IL-22 in freshly isolated Lin− (CD3−CD14−CD19−) lymphocytes from human tonsils. Three populations of lymphocytes could be discerned based on NKp44 and CD94 staining. These cells also differed in LFA-1 expression, with all NKp44+CD94− cells lacking LFA-1 and the majority of NKp44−CD94+ cells expressing LFA-1. After IL-1β+23 stimulation, only the NKp44+CD94− cells produced IL-22. Flow cytometry plots from a representative donor are shown in panel B and summary data for 3 donors is shown in panel C. (D) Peripheral blood NK cells mostly express LFA-1 and CD7. (E-F) CD56+LFA-1− ILC cells (solid lines) express NKp44 and CD161 only, but not other NK associated receptors, including KIR (antibody cocktail for 2DL1, 2DL2/3, and 3DL1), CD16, or CD8. cNK cells (dotted lines) were used as the controls. (G) Cytotoxic proteins granzyme B and K and perforin were not expressed in CD56+LFA-1− cells (dotted lines). CD56+LFA-1+ cNK cells were used as the control (dotted lines). (H) ILC22 cells also do not kill the K562 target cells (1:1 ratio for 6 hours) or express IFN-γ in response to IL-12/18 (10 ng/ml each). Gray-filled histograms are mouse immunoglobulin G. Results are representative of >10 donors. IgG, immunoglobulin G.
IL-22–producing ILCs and cNK cells can be discriminated by LFA-1 and CD7 expression. (A) Cord blood CD34+ progenitor cells were cultured on EL08-D12 feeder cells with IL-3 (for the first week), IL-7, IL-15, SCF, and FLT3L for 21 days. Only CD56+ cells that were negative for CD94, CD7, and LFA-1 expressed IL-22 after IL-1β+23 stimulation. Results are representative of >5 individual donors. (B-C) Differential expression of IL-22 in freshly isolated Lin− (CD3−CD14−CD19−) lymphocytes from human tonsils. Three populations of lymphocytes could be discerned based on NKp44 and CD94 staining. These cells also differed in LFA-1 expression, with all NKp44+CD94− cells lacking LFA-1 and the majority of NKp44−CD94+ cells expressing LFA-1. After IL-1β+23 stimulation, only the NKp44+CD94− cells produced IL-22. Flow cytometry plots from a representative donor are shown in panel B and summary data for 3 donors is shown in panel C. (D) Peripheral blood NK cells mostly express LFA-1 and CD7. (E-F) CD56+LFA-1− ILC cells (solid lines) express NKp44 and CD161 only, but not other NK associated receptors, including KIR (antibody cocktail for 2DL1, 2DL2/3, and 3DL1), CD16, or CD8. cNK cells (dotted lines) were used as the controls. (G) Cytotoxic proteins granzyme B and K and perforin were not expressed in CD56+LFA-1− cells (dotted lines). CD56+LFA-1+ cNK cells were used as the control (dotted lines). (H) ILC22 cells also do not kill the K562 target cells (1:1 ratio for 6 hours) or express IFN-γ in response to IL-12/18 (10 ng/ml each). Gray-filled histograms are mouse immunoglobulin G. Results are representative of >10 donors. IgG, immunoglobulin G.
Transcription factors expressed by cNK and ILC22 cells
Given that transcription factors may dictate function, cNK cells (CD56+LFA-1+) and ILC22 cells (CD56+LFA-1−) were purified and assessed for the expression of transcription factors known to be involved in development and function. Compared with cNK cells, ILC22 cells showed significantly higher levels of RORγt and the aryl hydrocarbon receptor (AHR), both of which are essential for ILC22 cell development or function.8,26 In contrast, T-bet (TBX21) and Eomes, which are critical for cNK cell differentiation,27 were significantly higher in cNK cells compared with ILC22 cells. Thus, using LFA-1 to distinguish cNK and ILC22 cells within the stage III NK progenitor fraction (CD56+/−CD117highCD94−), significant differences in key transcription factors for both cell lineages could be detected.
cNK and ILC22 cells: cytokine receptors and response to stimuli
Although both cNK and ILC22 cells expressed the receptor for SCF (c-Kit, CD117), using the combination of CD11a (LFA-1), CD7, and CD94 we consistently found that stage III cNK progenitors expressed CD117 at lower levels than ILC22 cells (Figure 2B). ILC22 cells also displayed receptors for IL-1β (CD121), IL-2 (receptor α, CD25), and IL-23, whereas these were at low levels or absent on cNK cells (Figure 2B). Although CD127 expression is one of the discriminative markers for ILC22,28 we did not detect surface CD127 expression by flow cytometry (not shown) due to its downregulation by rhIL-7, as shown in T cells.29 Previously, we were able to detect CD127 upon withdrawal of IL-7,7 and as shown in supplemental Figure 1, CD127/IL-7 signaling was functional in ILC22 cells. In addition to IL-22 elaboration, ILC22 cells produced GM-CSF and IL-8 in response to IL-1β and IL-23 stimulation, consistent with prior studies.21,30,31 In contrast, these cytokines were not produced by the CD56+LFA-1+ cNK cells (Figure 2C). High levels of OX40 ligand (OX40L) were found in IL-1β+23–stimulated ILC22 cells, but not on resting ILC22 cells or on resting/activated cNK cells. Another ILC22-associated protein, B-cell activating factor (BAFF),6 was present even on resting ILC22 cells, but not on cNK cells (Figure 2C). Importantly, IL-17A messenger RNA or protein was not detected at rest or after stimulation in either cell type (data not shown). Collectively, these data show significant differences in the cytokine elaboration and surface protein expression by ILC22 and cNK cells.
Transcription factors, cytokine receptors, and other molecules expressed in human IL-22–producing ILCs. (A) Expression of RORC2, AHR, TBX21, and Eomes was measured by real-time qPCR from HSC-derived cNK cells (CD56+CD94+CD7+/−CD117lowLFA-1+) and ILC22 cells (CD56+CD94−CD7−CD117highLFA-1+) after sorting. In ILC22 cells AHR and RORγt are highly expressed but are lower-expressed or absent in cNK cells. T-bet and Eomes expression is higher in CD56+LFA-1+ cNK cells. Transcripts in cNK cells were used as reference samples for relative quantification in ILC22 (ΔΔCT method, n = 3). (B) HSC-derived CD56+LFA-1− ILC22 but not cNK cells show expression of IL-1R1 and CD25 and CD117. (C) In addition to IL-22, CD56+LFA-1− ILC22 cells (left) express IL-8, GM-CSF (intracellular), and OX40 ligand (surface) when stimulated with IL-1β+23 (10 ng/ml each for 6 hours). Intracellular BAFF expression is detected in CD56+LFA-1− ILC22 cells without cytokine stimulation. All of the above were not detected on cNK cells (right). Gray-filled histograms are mouse immunoglobulin G, dotted lines are unstimulated, and solid lines are stimulated. Results are representative of 10 donors. IgG, immunoglobulin G.
Transcription factors, cytokine receptors, and other molecules expressed in human IL-22–producing ILCs. (A) Expression of RORC2, AHR, TBX21, and Eomes was measured by real-time qPCR from HSC-derived cNK cells (CD56+CD94+CD7+/−CD117lowLFA-1+) and ILC22 cells (CD56+CD94−CD7−CD117highLFA-1+) after sorting. In ILC22 cells AHR and RORγt are highly expressed but are lower-expressed or absent in cNK cells. T-bet and Eomes expression is higher in CD56+LFA-1+ cNK cells. Transcripts in cNK cells were used as reference samples for relative quantification in ILC22 (ΔΔCT method, n = 3). (B) HSC-derived CD56+LFA-1− ILC22 but not cNK cells show expression of IL-1R1 and CD25 and CD117. (C) In addition to IL-22, CD56+LFA-1− ILC22 cells (left) express IL-8, GM-CSF (intracellular), and OX40 ligand (surface) when stimulated with IL-1β+23 (10 ng/ml each for 6 hours). Intracellular BAFF expression is detected in CD56+LFA-1− ILC22 cells without cytokine stimulation. All of the above were not detected on cNK cells (right). Gray-filled histograms are mouse immunoglobulin G, dotted lines are unstimulated, and solid lines are stimulated. Results are representative of 10 donors. IgG, immunoglobulin G.
Homing and migration receptors on cNK and ILC22 cells
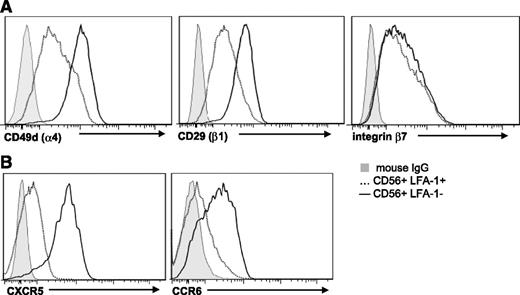
Next, ILC22 cells and cNK cells were investigated for differences in adhesion and chemokine receptors that mediate homing and migration. Given their role in SLT repair after injury,32,33 ILC22 cells were assessed for expression of very late activation Ag 4 (CD49d/CD29), which interacts with vascular cell adhesion molecule 1 on stromal cells in SLT.34 Both CD49d and CD29 were brightly expressed on ILC22 cells relative to cNK cells (Figure 3A). Recent studies also show that murine ILC22 cells display the integrin complex lymphocyte Peyer patch adhesion molecule 1 (CD49d/β7)35,36 that recognizes mucosal addressin cell adhesion molecule 1 on mucosal and inflammatory tissues. Although similar amounts of integrin β7 were present on both cNK cells and ILC22 cells, the higher expression of CD49d on ILC22 cells suggests that lymphocyte Peyer patch adhesion molecule 1 is also higher on human ILC22 cells (Figure 3A). In addition, the chemokine receptors CCR6 and CXCR5 were mainly expressed on ILC22 cells, with only low-level expression on cNK cells (Figure 3B). Because the ligands of these chemokines are involved in mucosal immunity and SLT generation,37-39 ILC22 cell trafficking appears be different from cNK cells.
Adhesion molecule expression on cNK and ILC22 cells. (A) The individual chains of the α4/β1 (very late activation Ag 4) receptor are brightly expressed on HSC-derived CD56+LFA-1− ILC22 cells and to a lesser degree on CD56+LFA-1+ cNK cells. Both CD56+ populations express integrin β7 at similar levels. (B) Expression of CXCR5 and CCR6 is higher on ILC22 cells (solid) relative to cNK cells (dotted line). Gray-filled histograms are mouse immunoglobulin G control. Results are representative of 15 donors. Similar findings were observed in ILC22 cells isolated from tonsils and PB cNK cells. IgG, immunoglobulin G.
Adhesion molecule expression on cNK and ILC22 cells. (A) The individual chains of the α4/β1 (very late activation Ag 4) receptor are brightly expressed on HSC-derived CD56+LFA-1− ILC22 cells and to a lesser degree on CD56+LFA-1+ cNK cells. Both CD56+ populations express integrin β7 at similar levels. (B) Expression of CXCR5 and CCR6 is higher on ILC22 cells (solid) relative to cNK cells (dotted line). Gray-filled histograms are mouse immunoglobulin G control. Results are representative of 15 donors. Similar findings were observed in ILC22 cells isolated from tonsils and PB cNK cells. IgG, immunoglobulin G.
Not all “stage III NK cells” become cNK cells
Prior studies show that stage III NK progenitors (CD56+CD117highCD94−) isolated from these cultures or SLTs can differentiate into stage IV cNK cells (CD56+CD117lowCD94+) upon further culture with cytokines.16,20 However, we have previously observed that not all stage III progenitors differentiate into stage IV cNK cells,20 perhaps suggesting heterogeneity within the stage III fraction. To put these studies in the context of our present findings and to investigate the developmental relationship between ILC22 cells and cNK cells, HSC-derived stage III cells (CD56+CD117highCD94−) were fluorescence-activated cell sorted on the basis of LFA-1 expression (Figure 4A) and further cultured with a combination of cytokines (IL-7, IL-15, SCF, and FLT3L) or IL-15. After 7 days, the vast majority of LFA-1− stage III cells (CD56+CD117highCD7-CD94−LFA-1−) maintained their phenotype in either cytokine combination (Figure 4B) and produced IL-22 (Figure 4C). In contrast, a significant proportion of LFA-1+ stage III progenitors acquired CD94, progressed to stage IV (Figure 4B), and produced IFN-γ (Figure 4C), consistent with their assignment to the cNK lineage. After 14 days, most LFA-1+ stage III cells acquired CD94, whereas in contrast the LFA-1− sorted stage III cells remained negative for LFA-1 and CD94 (Figure 4D), suggesting that LFA-1− cells were not simply less mature than their LFA-1+ counterparts. Thus, human stage III progenitors (CD56+CD117highCD94−) contain overlapping cell subsets including ILC22 cells and cNK progenitors that are discriminated by LFA-1 and CD7 expression. Given that human ILC22 cells did not differentiate into cNK cells under these conditions, the above experiments also support the concept that ILC-22 cells are not cNK cell progenitors and are instead a separate cell lineage.
CD56+LFA-1- ILC22 cells do not become cNK cells. (A) At day 21, stage III progenitors (CD56+CD94-CD117high) were sorted on the basis of LFA-1 expression for ILC22 cells (LFA-1−) and cNK cells (LFA-1+). (B) Cells were cultured with IL-15, IL-7, SCF, and FLT3L or IL-15 alone for additional 7 days. Greater than half of the stage III cells that expressed LFA-1 at the time of sorting acquired CD94 and therefore differentiated into stage IV or V cNK cells. In contrast, stage III cells that were LFA-1− did not acquire CD94 and maintained an ILC22 phenotype in either cytokine condition. (C) LFA-1− stage III cells, but not LFA-1+ cells, produce IL-22 in response to IL-1β+23 after 7 days of culture in IL-15. In contrasts, the LFA-1+ cells express IFN-γ in response to IL-12+18. (D) After cultivation with IL-15 for 14 days, most of LFA-1+ sorted cells acquired CD94, whereas LFA-1− stage III maintained LFA-1−CD94− phenotype. Results are representative of 5 donors. D, day.
CD56+LFA-1- ILC22 cells do not become cNK cells. (A) At day 21, stage III progenitors (CD56+CD94-CD117high) were sorted on the basis of LFA-1 expression for ILC22 cells (LFA-1−) and cNK cells (LFA-1+). (B) Cells were cultured with IL-15, IL-7, SCF, and FLT3L or IL-15 alone for additional 7 days. Greater than half of the stage III cells that expressed LFA-1 at the time of sorting acquired CD94 and therefore differentiated into stage IV or V cNK cells. In contrast, stage III cells that were LFA-1− did not acquire CD94 and maintained an ILC22 phenotype in either cytokine condition. (C) LFA-1− stage III cells, but not LFA-1+ cells, produce IL-22 in response to IL-1β+23 after 7 days of culture in IL-15. In contrasts, the LFA-1+ cells express IFN-γ in response to IL-12+18. (D) After cultivation with IL-15 for 14 days, most of LFA-1+ sorted cells acquired CD94, whereas LFA-1− stage III maintained LFA-1−CD94− phenotype. Results are representative of 5 donors. D, day.
HSC-derived ILC22 differentiation requires IL-7 and SCF, but not IL-15
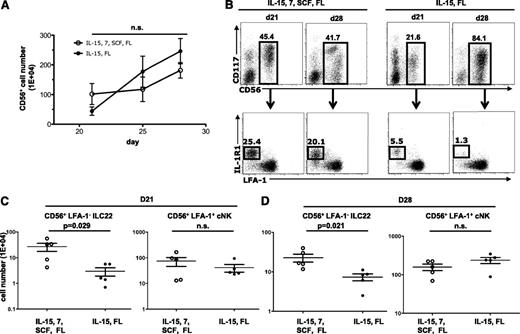
Prior studies show that FLT3L and IL-15 are sufficient for cNK development from CD34+ progenitors.40 To investigate whether these cytokines are necessary for ILC22 development, HSCs were cultured with a combination of cytokines (SCF, FLT3L, IL-7, and IL-15) or with only FLT3L and IL-15. As shown in Figure 5A, the kinetics of total CD56+ cell expansion was similar between the two cultures. In conditions containing all cytokines (SCF, FLT3L, IL-7, and IL-15), both ILC22 and cNK cells could be detected at days 21 to 28 (Figure 5B). In contrast, cultures containing FLT3L and IL-15 showed fewer ILC22 cells. Under these conditions, the majority of CD56+ cells expressed LFA-1, a phenotype consistent with cNK cells (Figure 5B). Data from 5 replicate experiments at days 21 and 28 are shown in Figure 5C-D and demonstrate that FLT3L and IL-15 resulted in significantly fewer ILC22 cells than the combination of cytokines (SCF, FLT3L, IL-7, and IL-15). In contrast, the numbers of cNK cells did not differ between the two conditions for either time point. Thus, FLT3L and IL-15 alone are sufficient for cNK development, but the number of ILC22 cells is markedly reduced in this condition.
cNK cells, but not ILC22 cells, can be generated with IL-15 and FLT3L. (A) CD34+ progenitors were cultured with 4 cytokines (IL-7, IL-15, SCF, and FLT3L) or with only IL-15 and FLT3L. There were no differences in the total numbers of CD56+ cells generated under these two conditions (P > .05) (n = 5). (B) Representative FACS plots showing the percentage of CD56+ cells and the distribution of cells that show an ILC22 phenotype (IL-1R1+ and LFA-1−) at day 21 and day 28 in the above culture conditions. (C-D) In cultures lacking IL-7 and SCF, significantly fewer ILC22 cells (CD56+LFA-1−CD7−) were generated at day 21 (P = .029, n = 5) and day 28 (P = .021, n = 5). In contrast, no differences in cNK cells were detected at either day 21 or day 28. D, day; FL, FLT3L; n.s., not significant.
cNK cells, but not ILC22 cells, can be generated with IL-15 and FLT3L. (A) CD34+ progenitors were cultured with 4 cytokines (IL-7, IL-15, SCF, and FLT3L) or with only IL-15 and FLT3L. There were no differences in the total numbers of CD56+ cells generated under these two conditions (P > .05) (n = 5). (B) Representative FACS plots showing the percentage of CD56+ cells and the distribution of cells that show an ILC22 phenotype (IL-1R1+ and LFA-1−) at day 21 and day 28 in the above culture conditions. (C-D) In cultures lacking IL-7 and SCF, significantly fewer ILC22 cells (CD56+LFA-1−CD7−) were generated at day 21 (P = .029, n = 5) and day 28 (P = .021, n = 5). In contrast, no differences in cNK cells were detected at either day 21 or day 28. D, day; FL, FLT3L; n.s., not significant.
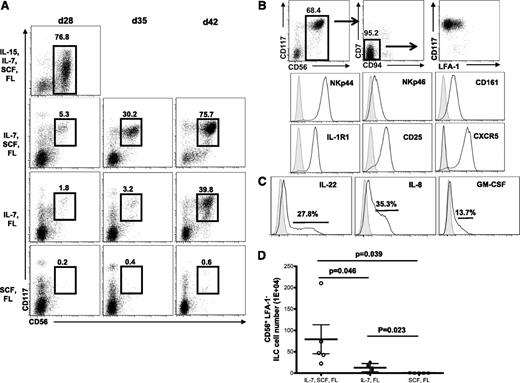
To investigate the role of IL-15 and other cytokines on ILC22 development, CD34+ cells were cultured with a combination of all cytokines used in these experiments (SCF, FLT3L, IL-7, and IL-15), all cytokines except IL-15 (SCF, FLT3L, and IL-7), only IL-7 and FLT-3L, or SCF and FLT-3L. In IL-15–containing conditions, a significant proportion of the cells developed into CD56+ cNK cells by day 28 (Figure 6A). In the absence of IL-15, the majority of cells at day 28 lacked CD56 expression. However, at days 35 and 42, CD56+ cells were detected in cultures lacking IL-15 (IL-7, SCF, and FLT3L or IL-7 and FLT3L) (Figure 6A). The vast majority of these were ILC22 cells by phenotype (CD56+CD117highCD7−CD11a−CD121+CD25+NKp44+) (Figure 6B) and function (IL-22, GM-CSF, and IL-8 producing in response to exogenous IL-1β+23) (Figure 6C). Cultures that contained SCF, IL-7, and FLT3L had significantly more ILC22 cells than those with IL-7 and SCF (P = .046) or SCF and FLT3L, which barely gave rise to ILC22 cells (P = .039, Figure 6D).
ILC22 generation is dependent on IL-7 and SCF, but not IL-15. (A) CD34+ cells were cultured in varying combinations of cytokines as listed. At day 28, very few CD56+ cells were present in cultures lacking IL-15. At later time points, CD56+ cells were generated (day 35 and day 42). Representative data from a single donor is shown. (B) The CD56+ cells that developed in the absence of IL-15 had a phenotype of ILC22 cells (CD56+CD117high CD94/7/LFA-1−) and expressed NKp44, CD161, IL-1R (CD121), CD25, and CXCR5. (C) These cells also showed cytokine expression (IL-22, IL-8, and GM-CSF) in response to IL-1β+23. Data are on CD56+ gated cells and gray-filled histograms are mouse immunoglobulin G controls. (D) The number of ILC22 cells at day 42 in the various cytokine conditions (n = 5). d, day; FL, FLT3L.
ILC22 generation is dependent on IL-7 and SCF, but not IL-15. (A) CD34+ cells were cultured in varying combinations of cytokines as listed. At day 28, very few CD56+ cells were present in cultures lacking IL-15. At later time points, CD56+ cells were generated (day 35 and day 42). Representative data from a single donor is shown. (B) The CD56+ cells that developed in the absence of IL-15 had a phenotype of ILC22 cells (CD56+CD117high CD94/7/LFA-1−) and expressed NKp44, CD161, IL-1R (CD121), CD25, and CXCR5. (C) These cells also showed cytokine expression (IL-22, IL-8, and GM-CSF) in response to IL-1β+23. Data are on CD56+ gated cells and gray-filled histograms are mouse immunoglobulin G controls. (D) The number of ILC22 cells at day 42 in the various cytokine conditions (n = 5). d, day; FL, FLT3L.
The above studies strongly suggested that differential cytokine exposure leads to CD34+ cell differentiation into the cNK or ILC22 lineage. To directly test this, CD34+ cells were isolated from UCB and cultured with either IL-15/FLT3L or IL-7/SCF/FLT3L. As shown in Figure 7A, after 42 days either cNK cells or ILC22 cells were generated, respectively. To further confirm these findings, we compared expression of transcription factors of cells generated in each cytokine condition at day 42. In particular, the expression of cNK-associated transcription factor Eomes was compared with the ILC22-associated transcription factors RORγt and AHR. Cells cultured in IL-7, SCF, and FLT3L showed high RORγt:Eomes and AHR:Eomes ratios, whereas conversely cells cultured in IL-15 and FLT3L had low ratios. Collectively, these studies establish that different cytokines act on HSCs to generate cNK and ILC22 cells, with the latter relying mainly on IL-7, FLT3L, and SCF, but not IL-15.
cNK and ILC22 cells are generated with different cytokine dependency. (A) Day 42 phenotype of CD34+-derived cNK (IL-15 and FLT3L) and ILC22 (IL-7, SCF, and FLT3). (B) Ratio of RORC2:Eomes and Ahr:Eomes (measured by real-time qPCR) in the various culture conditions at days 35 and 42. ILC22 cells (generated in IL-7, SCF, and FLT3L) expressed high ratios of RORγt and AhR to Eomes, whereas in contrast cells cultured in only IL-15 and FLT3L showed inverted RORγt:Eomes and AhR:Eomes ratios (n = 3). d, day; FL, FLT3L.
cNK and ILC22 cells are generated with different cytokine dependency. (A) Day 42 phenotype of CD34+-derived cNK (IL-15 and FLT3L) and ILC22 (IL-7, SCF, and FLT3). (B) Ratio of RORC2:Eomes and Ahr:Eomes (measured by real-time qPCR) in the various culture conditions at days 35 and 42. ILC22 cells (generated in IL-7, SCF, and FLT3L) expressed high ratios of RORγt and AhR to Eomes, whereas in contrast cells cultured in only IL-15 and FLT3L showed inverted RORγt:Eomes and AhR:Eomes ratios (n = 3). d, day; FL, FLT3L.
Discussion
ILC22 cells are resident cells in the SLT that mediate mucosal immunity, immune responses, and/or SLT homeostasis though cytokines (IL-22, IL-8, BAFF, and OX40L) and surface proteins (lymphotoxin).41,42 cNK progenitors are also present in the SLTs, and the relationship between these two cell populations was unclear. Because prior studies show that they have overlapping phenotypes, both were considered to be part of the NK lineage.10 However, recent murine studies questioned this because RORγt reporter mice showed that cNK cells do not proceed through an RORγt+ developmental stage.13,17 Although highly informative, these studies did not provide data that could be used to distinguish these cNK cell progenitors from ILC22 cells. Given that ILC22 cells differ phenotypically in humans and mice and that fate-mapping studies are not possible in humans, species-specific differences have been considered.2,15 To address these issues, we used an established in vitro differentiation system known to give rise to both cNK cells and ILC22 cells7 and employed a functional screen to distinguish between these two cell types. The combination of CD7 and CD11a (LFA-1) discriminated between ILC22 and cNK cells. Using classical NK cell functions (IFN-γ production and cytotoxicity) and the expression of NK-associated transcription factors (Tbet and Eomes) and surface receptors, we show that the cells expressing CD7 and CD11a (LFA-1) were cNK cells. In contrast, cells that lacked CD7 and CD11a expressed high levels of RORγτ and AHR. They also produced IL-22, IL-8, and GM-CSF6,30,31 and expressed BAFF11 and OX40L,43 which are characteristics of ILC22 cells and not cNK cells.
In this work, we also demonstrate that IL-15 is not required for the differentiation of human HSCs into the ILC22 lineage, whereas in contrast this cytokine was necessary for NK development. These results are in line with murine studies of IL-15−/−44 or IL-15R−/−45 mice that lack cNK cells but have seemingly normal lymphoid structures. Conversely, we found that IL-7 and SCF were critically important for ILC22 differentiation, but not for cNK cell development. Again, these findings are mainly consistent with murine data showing that IL-7−/− and IL-7−/−/SCF−/− mice lack ILC cells and show a dramatic reduction in SLT46 but have normal NK cell number and function.47,48 In our studies, a complete lack of ILC22 cells was not observed when HSCs were cultured in the absence of IL-7 and SCF because a small number of ILC22 cells were detected in cultures containing only IL-15 and FLT3L. These findings could represent the production of IL-7 and/or SCF by other cells present in the cultures (such as monocytes or the stromal layer), endogenous cytokines in human sera ,or perhaps differences between the two species.
The stage III NK progenitor fraction was mainly considered to contain cells that were restricted to the NK lineage.16 Consistent with the findings of Crellin et al,18 we demonstrate that the stage III NK progenitor fraction is heterogeneous. In these studies, however, we show that LFA-1 (CD11a) and CD7 can be used to distinguish stage III cNK cells from ILC22 cells. Using FACS and reculture, cNK cells were shown to be derived from CD56+CD117intLFA-1+CD7+/− cells, whereas ILC22 cells were contained within the CD56+CD117highLFA-1−CD7− fraction. Although this latter fraction mainly gave rise to ILC22 cells, a small number of cNK (approximately 2%) could be generated, suggesting the presence of a LFA-1− cNK precursor, consistent with the notion that LFA-1 is acquired during cNK differentiation.21 In addition, prior studies show that IL-7 could be used to differentiate CD56+ cells from human CD34+ bone marrow progenitors. These CD56+ cells also lacked LFA-1, but further cultivation with exogenous IL-2 or IL-15 led to LFA-1 acquisition in some but not all cells.49 Therefore, although expression of LFA-1 is a reliable marker to discriminate cNK cells (and precursors) from ILC22 cells, we do not exclude the possibility of the presence of LFA-1− cNK precursors.
One issue is whether CD7 and CD11a staining can be used to discriminate mature peripheral blood cNK cells from ILC22 cells. CD11a/CD18 (LFA-1) is well known to be involved in NK target-cell recognition and immune synapse formation,50 and accordingly the vast majority (>98%) of PB NK cells express LFA-1 (CD11a). Although a small population of CD7-CD56bright cells have been reported in the PB of healthy individuals (<4%) these have been reported to be myeloid cells that have acquired CD56 expression rather than NK cells that lack CD7.51 Whether or not this small cell fraction expresses ROR-γτ and could belong to the ILC22 lineage is plausible but has not been formally addressed. However, given the expression of SLT adhesion and homing receptors on ILC22 cells, they would not be expected to be abundant in the peripheral circulation.
Here, we discern the lineage relations between human cNK cells and ILC22 cells using CD34+ progenitor cell development assays and primary lymphocytes isolated from tonsil. Human ILC22 and cNK cells have separate phenotypes that can be distinguished based on CD7 and CD11a expression. These cells require differing cytokines for development. In particular, ILC22 cells require IL-7 and SCF, whereas IL-15 is needed for cNK generation. The resulting cells differ in transcription factors, surface proteins, and function. This work also strongly suggests that their developmental pathways are independent and nonintersecting. Understanding the relationship between these two cell types will assist in the understanding of pathophysiology of diseases that ILC22 cells are associated with (such as Crohn disease52,53 ) and the determination of their potential clinical application following chemotherapy and allogeneic transplantation, where disruption of mucosal immunity and SLT injury is common.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Linda Kluge and BioE for providing some of the UCB units used in these studies.
This work was supported by the American Cancer Society (M.R.V.) and the Children’s Cancer Research Fund (M.R.V.) (grant P01 CA65493) (J.S.M., B.R.B.) (grant P01 111412) (M.R.V., J.S.M.) (grant R01 HL55417) (J.S.M.) (grant CA72669) (B.R.B.) (grant PO1067493) (J.S.M.), and (grant ASBMT New Investigators Award) (Y-O.A.).
Authorship
Contribution: Y.-O.A. designed and performed all experiments and wrote the manuscript. B.R.B. and J.S.M. reviewed data and wrote the manuscript. M.R.V. oversaw all aspects of this work including experimental planning and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael R. Verneris, Cancer Center, 425 E River Rd, Ste 660, Minneapolis, MN 55455; e-mail: verneris@umn.edu.