In this issue of Blood, Malinge et al1 describe new reciprocal interactions between Ikaros, Notch, and GATA transcription factors during megakaryocyte development. Ikaros represses megakaryocytic genes and selected Notch targets, while being turned off by GATA1 upon terminal differentiation.
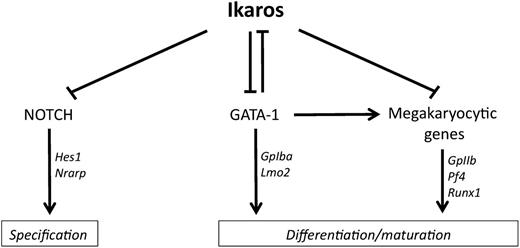
Regulation of megakaryopoiesis by Ikaros, Notch, and GATA-1. Findings from Malinge et al1 indicate that Ikaros controls megakaryocyte development both by inhibiting Notch signaling and by repressing multiple megakaryocyte differentiation genes, including GATA-1. Conversely, GATA-1 downregulates Ikaros expression. See Figure 7 in the article by Malinge et al that begins on page 2440.
Regulation of megakaryopoiesis by Ikaros, Notch, and GATA-1. Findings from Malinge et al1 indicate that Ikaros controls megakaryocyte development both by inhibiting Notch signaling and by repressing multiple megakaryocyte differentiation genes, including GATA-1. Conversely, GATA-1 downregulates Ikaros expression. See Figure 7 in the article by Malinge et al that begins on page 2440.
Both Ikaros and Notch proteins were first identified for their essential functions in lymphocyte development as well as for their dysregulation in lymphoid malignancies. The Krüppel-type zinc finger transcription factor Ikaros (encoded by IKZF1) regulates expression of multiple genes critical for the development of lymphoid lineages. In mice, Ikaros loss-of-function is associated with the emergence of aggressive T-cell lymphoblastic leukemias. In humans, high-risk B-cell acute lymphoblastic leukemias harbor recurrent genomic deletions at the IKZF1 locus.2 In addition, emerging work suggests that Ikaros also functions in the myeloid, erythroid, and megakaryocytic lineages. For example, an Ikzf1-gfp reporter allele revealed a strong correlation between high Ikaros expression in hematopoietic progenitors and myeloid cell fate, while low Ikaros expression identified progenitors committed to megakaryocytic and erythroid differentiation.3 Moreover, Ikaros-deficient mice have thrombocytosis, suggesting that Ikaros can negatively regulate megakaryopoiesis.
Notch signaling was first studied for its essential role in T-cell development. In addition, activating NOTCH1 mutations are present in a high proportion of T-cell acute lymphoblastic leukemias in mice and humans.4 In this context, Notch and Ikaros appear to have antagonistic functions. Expression of dominant-negative Ikaros isoforms lacking DNA binding can cooperate with Notch activation in T-cell leukemia.5 Ikaros may directly antagonize the effects of the Notch transcriptional activation complex at target gene loci. At least in mice, Ikaros can also repress transcription from internal Notch1 promoter elements that drive expression of truncated constitutively active Notch receptors.6,7 Interestingly, recent work indicates that Notch signaling also plays important functions in the myeloid and megakaryocytic lineages.8,9 These observations have set the stage to investigate whether Notch and Ikaros interact and exert new functions outside of lymphoid progenitors.
In this issue, Malinge et al1 focus their attention on the role of Ikaros in megakaryopoiesis. As a rationale to initiate this work, the authors built on their past observations that Notch signaling supports enhanced megakaryopoiesis in vitro and in vivo.9 In addition, acute megakaryocytic leukemias (AMKL) carrying the recurrent OTT-MAL translocation were shown to activate an aberrant Notch signature.10 Based on the hypothesis that Ikaros may antagonize Notch in these cells by analogy to lymphoid cells, Malinge et al1 report that Ikaros overexpression inhibits both Notch-driven megakaryocyte specification and expansion of an AMKL cell line expressing the OTT-MAL fusion protein. When analyzing the mechanisms of this effect, however, the authors discovered that Ikaros had a broad impact on the megakaryocyte transcriptional network, including many Notch-independent effects. Together with other regulators of megakaryocytic differentiation, GATA1 expression was downregulated by Ikaros. Reciprocally, the abundance of Ikaros transcripts was reduced by GATA1 expression, while direct binding of GATA1 to the Ikzf1 locus was identified by chromatin immunoprecipitation. These findings suggest that Ikaros expression is downregulated by the GATA2/GATA1 switch that occurs during megakaryopoiesis, perhaps to allow full expression of multiple genes repressed by Ikaros that are necessary for terminal differentiation. Interestingly, this phenomenon was specific to the megakaryocyte lineage and not observed in erythroid progenitors, in which Ikaros appeared to cooperate with GATA1 to promote erythroid differentiation. These observations suggest the existence of multiple lineage-specific effects of Ikaros. In support of this idea, Malinge et al1 described a panel of putative direct Ikaros target genes in megakaryocyte progenitors that showed only limited overlap with genes previously identified as putative targets in thymocytes.
Altogether, findings reported in this paper expand the scope of Ikaros functions and unravel a critical transcriptional network at the heart of megakaryocytic differentiation (see figure). More work is needed to fully dissect the reciprocal interactions between nodes of this network, especially because some of these interactions could be indirect. Another remarkable finding is the high degree of lineage specificity with which Ikaros operates and regulates target genes. It is likely that differential usage of transcriptional cofactors and epigenetic regulators accounts for target gene specificity in individual lineages. Finally, although mostly focused on normal hematopoiesis, work presented by Malinge and colleagues could have implications for the understanding of malignant disease, in particular AMKL. Ikaros overexpression profoundly inhibited the expansion of an AMKL cell line. In contrast, Ikaros loss cooperated with Gata1 deficiency in mice to block erythroid development and increase the proliferation of megakaryocyte progenitors.1 This constellation of findings could have oncogenic effects, for example, in AMKL carrying an enhanced Notch signature or in AMKL associated with Down syndrome, in which loss-of-function GATA1 mutations are typically observed. Thus, it could be interesting to study the pattern of Ikaros isoforms expressed in AMKL (as dominant-negative isoforms could block Ikaros activity) and the status of the IKZF1 locus in AMKL subtypes (because genomic deletions could be present, as previously reported in B-cell acute lymphoblastic leukemia and more recently in myeloid malignancies). In hematopoiesis, it appears that the wings of Ikaros have not fallen off, and we should continue the journey toward new discoveries.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal