Key Points
MYC-driven lymphomas demonstrate activation of mTORC1 and an endogenous DNA damage response.
BEZ235 inhibits PI3K-related DNA damage response kinases and mTORC1, inducing p53-independent upregulation of proapoptotic BMF.
Abstract
Pharmacological strategies capable of directly targeting MYC are elusive. Previous studies have shown that MYC-driven lymphomagenesis is associated with mammalian target of rapamycin (mTOR) activation and a MYC-evoked DNA damage response (DDR) transduced by phosphatidylinositol-3-kinase (PI3K)-related kinases (DNA-PK, ATM, and ATR). Here we report that BEZ235, a multitargeted pan-PI3K/dual-mTOR inhibitor, potently killed primary Myc-driven B-cell lymphomas and human cell lines bearing IG-cMYC translocations. Using pharmacologic and genetic dissection of PI3K/mTOR signaling, dual DDR/mTORC1 inhibition was identified as a key mediator of apoptosis. Moreover, apoptosis was initiated at drug concentrations insufficient to antagonize PI3K/mTORC2-regulated AKT phosphorylation. p53-independent induction of the proapoptotic BH3-only protein BMF was identified as a mechanism by which dual DDR/mTORC1 inhibition caused lymphoma cell death. BEZ235 treatment induced apoptotic tumor regressions in vivo that correlated with suppression of mTORC1-regulated substrates and reduced H2AX phosphorylation and also with feedback phosphorylation of AKT. These mechanistic studies hold important implications for the use of multitargeted PI3K inhibitors in the treatment of hematologic malignancies. In particular, the newly elucidated role of PI3K-related DDR kinases in response to PI3K inhibitors offers a novel therapeutic opportunity for the treatment of hematologic malignancies with an MYC-driven DDR.
Introduction
Oncogenic MYC dysregulation is one of the most frequent aberrancies in human cancer.1 In addition to the immunoglobulin (IG) promoter-cMYC translocations that are a hallmark of Burkitt lymphoma, diverse mechanisms upregulate MYC expression across the spectrum of lymphoid malignancies, from lymphoblastic lymphoma to diffuse large B-cell lymphoma and multiple myeloma.2-4 Unlike oncogenic kinases, transcription factors such as MYC are difficult to pharmacologically antagonize.5 Burkitt lymphoma is curable with aggressive multiagent chemotherapy.6 However, treatment morbidity is high, and delivery of adequate dose-intensity may be precluded by a patient’s advanced age or poor performance status. In the relapsed setting, acquired chemoresistance reduces the possibility of effective salvage and cure.7 Novel therapeutic approaches capable of circumventing MYC overexpression to allow de-escalation of conventional therapy and overcome chemoresistant relapses are required.
Prosurvival signaling pathways such as the phosphatidylinositol-3-kinase/mammalian target of rapamycin (PI3K/mTOR) cascade represent an emerging therapeutic target in lymphoma.8,9 Importantly, the MYC-regulated transcriptional network and PI3K/mTOR pathway interact at multiple levels.10-12 The PI3K family includes ataxia telangiectasia–mutated (ATM), ataxia telangiectasia and Rad3–related (ATR) and DNA-dependent protein kinase (DNA-PK), all of which are activated by DNA damage.13 MYC induces DNA damage response (DDR) through the generation of reactive oxygen species and formation of aberrant DNA-replication intermediates.14 Thus, MYC resides upstream of PI3K-related DDR kinases, and MYC activation is associated with upregulation of a DDR.15 Because experimental Burkitt models also show mTOR activation,16,17 we hypothesized that antagonism of PI3K/mTOR signaling would provide a rational strategy for treating MYC-driven lymphomas.
Rapamycin analogs selectively target mTORC1 and show single-agent activity in a range of B-cell malignancies.8,9 Rapamycin was reported to have minimal proapoptotic effects in an Myc-driven lymphoma model and only synergized with chemotherapy in lymphomas expressing constitutively active AKT.18 It is now reported that the mTORC1 specificity of rapamycin analogs may limit efficacy due to feedback activation of upstream PI3K signaling.19-22 Accordingly, ATP-competitive inhibitors have been developed that inhibit PI3K itself and both rapamycin-sensitive (mTORC1) and -insensitive (mTORC2) mTOR complexes (Figure 1A).23 These dual PI3K/mTOR inhibitors are purported to have increased antilymphoma effects because of their ability to suppress prosurvival feedback, in particular, the activity of AKT.24
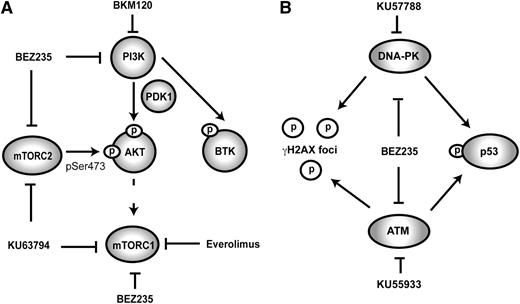
Schematic representation of PI3K-related signaling and associated inhibitors. (A) ATP-competitive dual PI3K/mTOR inhibitors such as BEZ235 antagonize PI3K and both mTOR complexes. Rapamycin analogs such as Everolimus cause allosteric hindrance of mTORC1 without direct inhibitory effects on other PI3K-related kinases. KU63794 is an ATP-competitive mTORC1/2 inhibitor; BKM120 is an ATP-competitive PI3K inhibitor. (B) Because of close structural homology between PI3K/mTOR and the PI3K-related DNA damage response kinases (ATM/ATR and DNA-PK), BEZ235 can antagonize the DNA damage response. KU57788 and KU55933 are selective ATP-competitive antagonists of DNA-PK and ATM, respectively. Phosphorylation of p53 (at Ser15) and γH2AX is the composite end point of the activity of ATM/ATR and DNA-PK.
Schematic representation of PI3K-related signaling and associated inhibitors. (A) ATP-competitive dual PI3K/mTOR inhibitors such as BEZ235 antagonize PI3K and both mTOR complexes. Rapamycin analogs such as Everolimus cause allosteric hindrance of mTORC1 without direct inhibitory effects on other PI3K-related kinases. KU63794 is an ATP-competitive mTORC1/2 inhibitor; BKM120 is an ATP-competitive PI3K inhibitor. (B) Because of close structural homology between PI3K/mTOR and the PI3K-related DNA damage response kinases (ATM/ATR and DNA-PK), BEZ235 can antagonize the DNA damage response. KU57788 and KU55933 are selective ATP-competitive antagonists of DNA-PK and ATM, respectively. Phosphorylation of p53 (at Ser15) and γH2AX is the composite end point of the activity of ATM/ATR and DNA-PK.
BEZ235 (Novartis) is a PI3K/mTOR inhibitor that shows preclinical activity against a range of lymphoid malignancies.23-26 It shows greater than 10 000-fold selectivity for class I PI3K over other clinically relevant protein kinases, has a favorable toxicity profile, and is currently undergoing phase II evaluation in solid organ cancers. We evaluated the efficacy of BEZ235 in the murine Eμ-Myc model of aggressive lymphoma27 and against IG-cMYC-translocated human cell lines. Subsequently, we defined the processes underpinning the robust therapeutic effects of this compound. BEZ235 induced apoptosis in both IG-cMYC–positive human cell lines and in a range of independently derived primary Eμ-Myc lymphomas. By comparing BEZ235 to more selective kinase inhibitors (Table 1; Figure 1A-B) and using a range of genetically manipulated lymphomas, we identified key targets and downstream apoptotic effectors necessary for BEZ235-induced apoptosis. Unexpectedly, BEZ235 responses occurred at concentrations that blocked mTORC1 but were insufficient to inhibit the phosphorylation of AKT. However, apoptosis induction correlated with potent inhibition of PI3K-related DDR kinases. The induction of cell death was p53-independent and correlated with induction of BMF, a BH3-only protein reportedly upregulated in response to suppression of 5′cap-dependent translation following inhibition of mTOR and DDR kinases.28,29
PI3K-related kinase isoform selectivity of compounds used in in vitro studies
| Drug . | Specified targets . | IC50 . | References . | |||
|---|---|---|---|---|---|---|
| PI3K . | mTOR . | DNA-PK . | ATM . | |||
| BEZ235 | PI3K/mTOR | 4-75 nM | 8 nM | 1.7 nM | <100 nM | 23, 38, 39, 40 |
| BKM120 | PI3K | 2-262 nM | 2.9 μM | >10 μM | N/R | 46 |
| Everolimus | mTORC1 | N/A | <10 nM | N/A | N/A | 23 |
| KU63794 | mTORC1/2 | >10 μM | 10 nM | N/R | N/R | 47 |
| KU57788 | DNA-PK | 5 μM | 1.7 μM | 14 nM | >100 nM | 48 |
| KU55933 | ATM | 17 μM | 9 μM | 2.5 μM | 13 nM | 45 |
| Drug . | Specified targets . | IC50 . | References . | |||
|---|---|---|---|---|---|---|
| PI3K . | mTOR . | DNA-PK . | ATM . | |||
| BEZ235 | PI3K/mTOR | 4-75 nM | 8 nM | 1.7 nM | <100 nM | 23, 38, 39, 40 |
| BKM120 | PI3K | 2-262 nM | 2.9 μM | >10 μM | N/R | 46 |
| Everolimus | mTORC1 | N/A | <10 nM | N/A | N/A | 23 |
| KU63794 | mTORC1/2 | >10 μM | 10 nM | N/R | N/R | 47 |
| KU57788 | DNA-PK | 5 μM | 1.7 μM | 14 nM | >100 nM | 48 |
| KU55933 | ATM | 17 μM | 9 μM | 2.5 μM | 13 nM | 45 |
Kinase inhibition 50% inhibitory concentration (IC50) for each compound as reported in selected references. Targets inhibited in the nanomolar concentration range are in italics.
N/A, not applicable, N/R, not reported.
These data demonstrate that in aggressive MYC-driven lymphomas, combined DDR/mTOR kinase inhibition bypasses AKT activation to induce p53-independent BMF-mediated apoptosis. We conclude that combined DDR and mTORC1 inhibition represents a novel therapeutic strategy for the management of MYC-driven lymphoma.
Materials and methods
Eμ-Myc lymphomas, cell lines, and reagents
Eμ-Myc lymphomas were harvested from lymph nodes into a cell suspension for tissue culture, transplantation, or cryopreservation. Eμ-Myc cultures were performed in tissue-culture grade 6-well plates (Greiner Bio-One, Monroe, NC) in high-glucose Dulbecco’s modified Eagle medium with 10% fetal calf serum, penicillin (100 u/mL), streptomycin (100 mg/mL), 0.1 mM L-asparagine, and 50 μM 2-mercaptoethanol. Eμ-Myc mice were crossed onto a LoxP-flanked Rictor background30 to generate Eμ-Myc/LoxP-Rictor-LoxP lymphomas. Development of Eμ-Myc/p53−/−, Eμ-Myc/Atm−/−, Eμ-Myc/Bad−/−, Eμ-Myc/Bim−/−, Eμ-Myc/Puma−/−, Eμ-Myc/Noxa−/−, and Eμ-Myc/Bmf−/− lymphomas was achieved as previously described.15,31-33 All lymphomas were derived on a pure C57BL/6 background, except Eμ-Myc/LoxP-Rictor-LoxP lymphomas, which were mixed C57BL/6 × B129.
Retroviral transduction was performed on freshly isolated lymphoma; retrovirus-containing supernatant was produced by transfecting packaging cells with murine stem-cell virus-internal ribosomal entry site-green fluorescence protein (MSCV-I-GFP) using standard calcium phosphate transfection methods. MSCV-I-GFP/Bcl2 was cloned as previously described.34 MSCV-I-GFP/Akt constructs were a gift from Hans-Guido Wendel11 ; MSCV-I-GFP/Cre was a gift from Kay Macleod.35 Viral supernatant was used to transduce primary lymphoma cells in RetroNectin (TaKaRa Bio, Shiga, Japan) -precoated 6-well plates (Becton Dickinson, Franklin Lakes, NJ). After 48 hours, GFP-positive cells were isolated by and re-injected into C57BL/6 recipients for expansion.
Ramos, Namalwa, and RPMI-8226 cell lines were purchased from the American Type Culture Collection (Manassas, VA). The BL-41 cell line was purchased from Leibniz-Institut DSMZ (Braunschweig, Germany). All human cell lines were cultured in Gibco RPMI-1640 media supplemented with 10% fetal calf serum, penicillin (100 u/mL), and streptomycin (100 mg/mL).
BEZ235, BKM120, and Everolimus were provided by Novartis. KU63794, KU57788, and KU55933 were purchased from SYNthesis. For in vitro use, drugs were stored as 10 mM stock solution in dimethylsulfoxide, except for Everolimus, which was maintained in ethanol. Etoposide and dexamethasone were diluted from clinical pharmacy stock (Peter MacCallum Cancer Centre). For in vivo experiments, crystalline BEZ235 powder was freshly prepared by sonication into suspension in 0.5% methylcellulose solution.
Western blotting
Whole-cell lysates were prepared by using ice-cold Rac lysis buffer with complete EDTA-free protease and phosSTOP inhibitor cocktails (Roche Diagnostics, Sydney, NSW, Australia). Western blots were performed according to standard techniques by separation of whole-cell lysates by sodium dodecyl sulfate polyacrylamide gel electrophoresis and electroblotting onto polvinylidene fluoride membranes. Primary antibodies against phospho-Ser240/244 ribosomal protein (RP) S6, p-Thr37/46 4E-binding protein 1 (4EBP-1), p-Ser473 AKT, p-Tyr223 BTK, p-Ser241 PDK-1, p-Ser139 γH2AX, p-Ser15 p53, Rictor, Raptor, cMYC, total RPS6, and total 4EBP-1 were all from Cell Signaling Technology (Danvers, MA). Other primary antibodies were total p53 (Novocastra, Newcastle, UK), β-actin and α-tubulin (Sigma-Aldrich, St. Louis, MO), p19ARF (Santa Cruz Biotechnology, Santa Cruz, CA), BIM (Stressgen, Ann Arbor, MI), BAD (Assay Designs, Ann Arbor, MI), CrmA (BD Pharmingen, San Jose, CA), murine BMF (Axxora, Farmingdale, NY), and human BMF (Abcam, Cambridge, UK). Quantitation of scanned blots was performed by using ImageJ software, Version 1.45S (National Institutes of Health, Bethesda, MD).
In vitro cell death assays
Eμ-Myc lymphoma cells (4 × 104) or human cell lines (10 × 104) were cultured with indicated compounds or vehicle for 24 to 48 hours in 400 μL of media in 24-well plates (Greiner Bio-One). Cell viability was measured by propidium iodide (PI) uptake, cell cycle analysis (nuclear DNA content by PI staining), annexin-V staining, and tetramethylrhodamine ethyl ester uptake as previously described.34
In vivo assays
Animal experiments were performed according to Australian National Health and Medical Research Council guidelines with prior institutional ethics approval. Syngeneic C57BL/6 mice (Walter and Eliza Hall Institute, Melbourne, VIC, Australia) were intravenously injected with 50 to 100 × 105 cells and monitored by blood sampling and lymph node palpation. Treatment (BEZ235 or 0.5% methylcellulose vehicle) was administered by daily oral gavage. Peripheral blood counts were measured by automated hematology analyzer (Advia 120 Hematology Systems, Deerfield, IL). Positron emission tomography (PET) scanning was performed as previously described.36
Statistical analysis
Statistical analyses were performed by using GraphPad Prism Software, Version 5.0d (GraphPad, La Jolla, CA). Synergy quotients (SQs) for drug combinations were calculated as previously described (in which SQ > 1 indicates a greater-than-additive effect for a drug combination).37
Results
BEZ235 induces apoptosis of Eμ-Myc lymphomas and IG-cMYC-translocated human lymphoid cell lines
BEZ235 has a 50% inhibitory concentration between 4 and 75 nM for PI3K isoforms using in vitro kinase assays (Table 1).23 In cellular systems, mTOR inhibition was reported in the low nanomolar range,23 and at submicromolar concentrations, potent inhibition of DNA PK38,39 and ATM40 has been described. To determine whether BEZ235 killed Eμ-Myc lymphomas at on-target concentrations, a panel of primary lymphomas was treated and assessed for apoptosis by annexin-V/PI staining at 24 hours (Figure 2A; supplemental Figure 1A). The majority of lymphomas showed dose-dependent increases in apoptosis at concentrations corresponding to the expected range of activity for BEZ235. This apoptotic response could not be sufficiently explained by the mTORC1 inhibitory effects of BEZ235, because isolated mTORC1 antagonism by the potent rapamycin analog Everolimus (Afinitor [RAD001], Novartis) showed reduced proapoptotic activity by comparison (Figure 2B). To confirm apoptosis, loss of mitochondrial outer membrane potential and nuclear fragmentation were assessed by tetramethylrhodamine ethyl ester staining and nuclear DNA content, respectively (supplemental Figure 1B). Intrinsic apoptotic pathway engagement was necessary for BEZ235-induced cell death, because BCL2 overexpression in a representative lymphoma prevented the apoptotic response (Figure 2C). In the presence of this apoptotic block, BEZ235 had cytostatic effects (supplemental Figure 1C). BEZ235 bypassed the extrinsic apoptotic pathway, as demonstrated by apoptosis induction despite overexpression of the caspase-8 inhibitor cytokine response modifier A (CrmA) (Figure 2D).
BEZ235 induces apoptosis of Eμ-Myc lymphomas and IG-cMYC-translocated cell lines at on-target concentrations. (A) Six independently derived Eμ-Myc lymphomas were cultured in vitro for 24 hours with increasing concentrations of BEZ235 (or dimethylsulfoxide [DMSO] vehicle alone at maximal concentration) and subjected to flow cytometric analysis for annexin-V/PI positivity. The lymphomas have been stratified from most to least BEZ235 sensitive (at 125 nM) from left to right. A unique identifier for each lymphoma is provided above the bar graph. (B) A representative lymphoma (#4242) was treated with BEZ235 or Everolimus for 24 hours prior to flow cytometric analysis for annexin-V/PI positivity. *P < .05 comparing treatments (BEZ235 vs Everolimus) at each concentration (2-way analysis of variance [ANOVA]). (C) The same lymphoma (#4242) was transduced with murine stem-cell virus expressing Bcl2 or empty vector control (MSCV) and treated for 24 hours with BEZ235 prior to flow cytometric analysis for annexin-V/PI positivity. *P < .05 comparing genotypes (Bcl2 vs empty vector) at each concentration (2-way ANOVA). (D) Lymphoma (#4242) was transduced with murine stem-cell virus expressing CrmA or empty vector control (MSCV) and treated for 24 hours with BEZ235 prior to flow cytometric analysis for annexin-V/PI positivity. There was no significant difference in response between genotypes (CrmA vs empty vector) at any drug concentration (P > .05; 2-way ANOVA). Inset: western blot of CrmA expression in the same lymphoma cells with β-actin provided as a loading control. (E) Human IG-cMYC-translocated cell lines were treated for 48 hours with BEZ235 (or DMSO vehicle) and subjected to flow cytometric analysis for annexin-V/PI positivity. (F) The same human cell lines were treated for 48 hours with Everolimus prior to flow cytometric analysis for annexin-V/PI positivity. All graphs represent the mean (± standard error of the mean [SEM]) for at least 3 independent experiments.
BEZ235 induces apoptosis of Eμ-Myc lymphomas and IG-cMYC-translocated cell lines at on-target concentrations. (A) Six independently derived Eμ-Myc lymphomas were cultured in vitro for 24 hours with increasing concentrations of BEZ235 (or dimethylsulfoxide [DMSO] vehicle alone at maximal concentration) and subjected to flow cytometric analysis for annexin-V/PI positivity. The lymphomas have been stratified from most to least BEZ235 sensitive (at 125 nM) from left to right. A unique identifier for each lymphoma is provided above the bar graph. (B) A representative lymphoma (#4242) was treated with BEZ235 or Everolimus for 24 hours prior to flow cytometric analysis for annexin-V/PI positivity. *P < .05 comparing treatments (BEZ235 vs Everolimus) at each concentration (2-way analysis of variance [ANOVA]). (C) The same lymphoma (#4242) was transduced with murine stem-cell virus expressing Bcl2 or empty vector control (MSCV) and treated for 24 hours with BEZ235 prior to flow cytometric analysis for annexin-V/PI positivity. *P < .05 comparing genotypes (Bcl2 vs empty vector) at each concentration (2-way ANOVA). (D) Lymphoma (#4242) was transduced with murine stem-cell virus expressing CrmA or empty vector control (MSCV) and treated for 24 hours with BEZ235 prior to flow cytometric analysis for annexin-V/PI positivity. There was no significant difference in response between genotypes (CrmA vs empty vector) at any drug concentration (P > .05; 2-way ANOVA). Inset: western blot of CrmA expression in the same lymphoma cells with β-actin provided as a loading control. (E) Human IG-cMYC-translocated cell lines were treated for 48 hours with BEZ235 (or DMSO vehicle) and subjected to flow cytometric analysis for annexin-V/PI positivity. (F) The same human cell lines were treated for 48 hours with Everolimus prior to flow cytometric analysis for annexin-V/PI positivity. All graphs represent the mean (± standard error of the mean [SEM]) for at least 3 independent experiments.
BEZ235-induced apoptosis occurs independently of p53
MYC-driven lymphomagenesis is associated with lesions that bypass the effects of MYC-induced p53 activation,41 with one third of Eμ-Myc lymphomas41 and 10% of Burkitt lymphomas42 (including the Ramos cell line) showing p53 loss or mutation at diagnosis. p53 loss results in chemoresistance and is associated with a poorer prognosis.7 To assess the role of p53 in BEZ235-induced apoptosis, we subjected the same panel of Eμ-Myc lymphomas to etoposide treatment in vitro (Figure 3A-B). Consistent with a prior publication,41 20% of the lymphoma panel showed primary etoposide resistance. These etoposide-resistant lymphomas showed p53 loss and feedback upregulation of p19ARF (supplemental Figure 2A). There was no correlation between etoposide and BEZ235 sensitivity, suggesting that the latter induced apoptosis via p53-independent mechanisms (Figure 3B). To confirm this, etoposide-resistant Eμ-Myc lymphomas derived on a p53-null background and lymphomas with previously characterized p53 mutations33 were tested for BEZ235 sensitivity (Figure 3C). All of the p53-incompetent and chemoresistant (Figure 3D) lymphomas were susceptible to BEZ235 at concentrations as low as 125 nM. Consistent with p53-independent activity, lymphomas deficient in the p53-regulated BH3-only proteins, PUMA, and NOXA31 were also BEZ235 sensitive (Figure 3E). Thus BEZ235 induces p53-independent apoptosis in chemoresistant lymphomas.
BEZ235-induced apoptosis correlates poorly with chemosensitivity and occurs independently of p53 and the p53-regulated BH3-only proteins PUMA and NOXA. (A) Five independently derived Eμ-Myc lymphomas were cultured for 24 hours with 20 nM etoposide and analyzed for annexin-V/PI positivity. (To facilitate comparison with Figure 2, the lymphomas have been stratified from most to least BEZ235 sensitive [at 125 nM] from left to right). The unique identifier for each lymphoma is provided above the bar graph. “R” denotes primary etoposide resistance (defined as AC50 [50% apoptosis concentration] < 20 nM at 24 hours). (B) Correlation of the percentage of cells undergoing apoptosis at 24 hours for an extended panel of lymphomas (n = 11) treated with 250 nM BEZ235 or 20 nM of etoposide (r, Pearson’s correlation coefficient). (C) Eμ-Myc lymphomas derived on a p53 null background (p53 null) or with previously characterized inactivating p53 mutations (p53 mutated [mut.]) were treated for 24 hours with BEZ235 prior to analysis for annexin-V/PI positivity. (D) The same p53-incompetent lymphomas were treated with etoposide (20 nM) for 24 hours prior to analysis for annexin-V/PI positivity. (E) Lymphomas derived on a Puma- (Puma−/−) or Noxa- (Noxa−/−) deficient background and a Puma/Noxa-competent (#4242) control were treated with BEZ235 for 24 hours prior to analysis for annexin-V/PI positivity. All graphs represent the mean (± SEM) for at least 3 independent experiments. WT, wild-type.
BEZ235-induced apoptosis correlates poorly with chemosensitivity and occurs independently of p53 and the p53-regulated BH3-only proteins PUMA and NOXA. (A) Five independently derived Eμ-Myc lymphomas were cultured for 24 hours with 20 nM etoposide and analyzed for annexin-V/PI positivity. (To facilitate comparison with Figure 2, the lymphomas have been stratified from most to least BEZ235 sensitive [at 125 nM] from left to right). The unique identifier for each lymphoma is provided above the bar graph. “R” denotes primary etoposide resistance (defined as AC50 [50% apoptosis concentration] < 20 nM at 24 hours). (B) Correlation of the percentage of cells undergoing apoptosis at 24 hours for an extended panel of lymphomas (n = 11) treated with 250 nM BEZ235 or 20 nM of etoposide (r, Pearson’s correlation coefficient). (C) Eμ-Myc lymphomas derived on a p53 null background (p53 null) or with previously characterized inactivating p53 mutations (p53 mutated [mut.]) were treated for 24 hours with BEZ235 prior to analysis for annexin-V/PI positivity. (D) The same p53-incompetent lymphomas were treated with etoposide (20 nM) for 24 hours prior to analysis for annexin-V/PI positivity. (E) Lymphomas derived on a Puma- (Puma−/−) or Noxa- (Noxa−/−) deficient background and a Puma/Noxa-competent (#4242) control were treated with BEZ235 for 24 hours prior to analysis for annexin-V/PI positivity. All graphs represent the mean (± SEM) for at least 3 independent experiments. WT, wild-type.
BEZ235-induced apoptosis correlates with suppression of mTORC1 and DDR kinases, but not PI3K/AKT
BEZ235 is a PI3K superfamily inhibitor that antagonizes all PI3K isoforms, rapamycin-sensitive (mTORC1) and -insensitive (mTORC2) complexes,23 and the PI3K-related DDR kinases, including DNA-PK38,39 and ATM/ATR40 (Figure 1; Table 1). To determine which PI3K family kinases are functionally regulated by BEZ235 in Eμ-Myc lymphomas, we next profiled the inhibitory effects of BEZ235 on a range of PI3K-related phosphorylation substrates, using Everolimus as a comparator. BEZ235 and Everolimus suppressed mTORC1-regulated RPS6 phosphorylation at low nanomolar concentrations (Figure 4A; supplemental Figure 3A-C). Compared with Everolimus, BEZ235 more potently suppressed 4EBP-1 phosphorylation (Figure 4A; supplemental Figure 3A-B), which is consistent with previously reported effects of ATP-competitive blockade on rapamycin-insensitive mTORC1 substrates.43 Consistent with a direct effect of kinase inhibition (rather than protein degradation), total protein levels remained unchanged (Figure 4A).
BEZ235-induced apoptosis is associated with suppression of mTORC1 and DDR-regulated substrates, but not inhibition of PI3K/mTORC2. (A) Eμ-Myc (#4242) lymphoma cells were treated with Everolimus, BEZ235 or vehicle (DMSO) at the concentrations indicated for 1 hour prior to harvesting and preparation of protein lysates. Protein was then separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) prior to immunoblotting with phospho-specific antibodies for mTORC1-regulated substrates as indicated. Total protein and actin or tubulin loading controls are provided for each blot. (B) Lysates from the same treatment conditions were blotted for mTORC2- (phospho-Ser473 AKT) and PI3K- (phospho-Ser241 PDK-1 and Tyr223 BTK) regulated targets as indicated. (C) Eμ-Myc (#4242) lymphoma cells were treated with Everolimus, BEZ235, or vehicle (DMSO) at the concentrations indicated for 1 hour prior to harvesting and preparation of protein lysates, separation by SDS-PAGE, and immunoblotting for phospho-RPS6, γH2AX, and β-actin as indicated. (D) Eμ-Myc #4242 MSCV/Bcl2 lymphoma, either nonirradiated or irradiated (6 Gy), prior to 30-minute treatment with BEZ235 (500 nM), KU55933 (4 μM), or vehicle (DMSO) was harvested, separated by SDS-PAGE, and immunoblotted for DDR-kinase and mTOR-regulated phosphorylation substrates as indicated. Control samples were obtained from nonirradiated p53 mutant (#106) and p53 null (#3239) lymphomas. Each western blot is representative of at least 3 independent experiments.
BEZ235-induced apoptosis is associated with suppression of mTORC1 and DDR-regulated substrates, but not inhibition of PI3K/mTORC2. (A) Eμ-Myc (#4242) lymphoma cells were treated with Everolimus, BEZ235 or vehicle (DMSO) at the concentrations indicated for 1 hour prior to harvesting and preparation of protein lysates. Protein was then separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) prior to immunoblotting with phospho-specific antibodies for mTORC1-regulated substrates as indicated. Total protein and actin or tubulin loading controls are provided for each blot. (B) Lysates from the same treatment conditions were blotted for mTORC2- (phospho-Ser473 AKT) and PI3K- (phospho-Ser241 PDK-1 and Tyr223 BTK) regulated targets as indicated. (C) Eμ-Myc (#4242) lymphoma cells were treated with Everolimus, BEZ235, or vehicle (DMSO) at the concentrations indicated for 1 hour prior to harvesting and preparation of protein lysates, separation by SDS-PAGE, and immunoblotting for phospho-RPS6, γH2AX, and β-actin as indicated. (D) Eμ-Myc #4242 MSCV/Bcl2 lymphoma, either nonirradiated or irradiated (6 Gy), prior to 30-minute treatment with BEZ235 (500 nM), KU55933 (4 μM), or vehicle (DMSO) was harvested, separated by SDS-PAGE, and immunoblotted for DDR-kinase and mTOR-regulated phosphorylation substrates as indicated. Control samples were obtained from nonirradiated p53 mutant (#106) and p53 null (#3239) lymphomas. Each western blot is representative of at least 3 independent experiments.
Everolimus treatment upregulated the phosphorylation of PI3K-regulated substrates, including Bruton’s tyrosine kinase (BTK), phospholipid-dependent kinase (PDK)-1 and AKT (Figure 4B; supplemental Figure 3A). This feedback phenomenon is well described in other cellular systems and is postulated to abrogate the proapoptotic effects of isolated mTORC1 inhibition.19,22,44 Unexpectedly, BEZ235 (at proapoptotic concentrations sufficient to inhibit mTORC1) also resulted in PI3K/AKT activation (Figure 4B; supplemental Figure 3A), despite its purported PI3K and mTORC2 inhibitory activity.23 This pattern of response was consistent among several lymphomas (supplemental Figure 3B). Thus, the greater proapoptotic effects of BEZ235 over Everolimus could not be adequately explained by antagonism of PI3K or mTORC2.
With no evidence of upstream PI3K/AKT suppression by BEZ235, we assessed its activity against PI3K-related DDR kinases, using γH2AX as a biomarker. H2AX phosphorylation is a composite end point of DNA-PK and ATM/ATR kinases at sites of DNA double-stranded breaks13 (Figure 1B). Constitutive DDR activity was noted in Eμ-Myc lymphomas, evidenced by readily detectable basal H2AX phosphorylation (Figure 4C-D; supplemental Figure 3C). Consistent with PI3K-related DDR kinase inhibition, BEZ235 (but not Everolimus) reduced γH2AX at concentrations similar to those required to inhibit mTORC1 (Figure 4C). To further clarify this observation, the effects of BEZ235 on basal and radiation-induced phosphorylation of p53 on Ser15 (an ATM- and DNA-PK–regulated phosphorylation site) were also examined by using an ATM inhibitor (KU5593345 ) as a comparator (Figure 4D). An Eμ-Myc MSCV-Bcl2 lymphoma was used specifically to avoid protein degradation that might occur with radiation-induced apoptosis. Short-term exposure to BEZ235 abrogated both basal- and radiation-induced p53 phosphorylation on Ser15. Furthermore, BEZ235 (but not KU55933) reduced radiation-induced p53 phosphorylation in a lymphoma derived on an Atm null background (supplemental Figure 3C),15 indicating ATM-independent suppression of the DDR. Thus, consistent with prior reports,38-40 BEZ235 is a DDR-kinase inhibitor.
Selective inhibition of individual PI3K-related kinases is less effective than BEZ235
The proapoptotic effects of BEZ235 occurred at concentrations sufficient to inhibit mTORC1 and H2AX/p53 phosphorylation, but not PI3K and mTORC2 targets. To further define the relative importance of these kinases in apoptotic responses, we used a panel of inhibitors that selectively inhibited different BEZ235 targets (Figure 1; Table 1). Specifically, BEZ235 was compared with BKM120 (PI3K-selective inhibitor46 ), KU63794 (ATP-competitive mTORC1/2 inhibitor47 ), KU55933 (ATM inhibitor), and KU57788 (DNA-PK inhibitor48 ). Although these inhibitors have an affinity for their respective targets in the nanomolar concentration range (Table 1), none recapitulated the apoptotic response observed with BEZ235 (Figure 5A).
Narrow-spectrum kinase inhibition has less proapoptotic activity than BEZ235. (A) A representative Eμ-Myc lymphoma (#4242) was treated in vitro for 48 hours with increasing concentrations of BEZ235 or a panel of more selective kinase inhibitors (BKM120, KU63794, KU59933, or KU57788) prior to flow cytometric analysis for annexin-V/PI positivity. (B) Eμ-Myc lymphoma derived on a LoxP-Rictor-LoxP background was transduced with MSCV-Cre recombinase (Cre) or empty vector (MSCV). Protein lysates were then separated by SDS-PAGE prior to immunoblotting for Rictor or Raptor expression. (C) Matched Rictor expressing (MSCV) and Rictor knockout (Cre) lymphomas were treated for 24 hours with BEZ235 or Everolimus prior to flow cytometric analysis for annexin-V/PI positivity. (D) Eμ-Myc lymphoma cells were treated with increasing concentrations of ATM inhibitor (KU55933), DNA-PK inhibitor (KU57788), or combined KU55933 and KU57788 alone or in combination with a fixed dose of Everolimus (100 nM) for 24 hours prior to flow cytometric analysis for annexin-V/PI staining. # indicates greater-than-additive apoptotic effect (SQ > 1) for the two-drug combination. All results are represented as the mean (± SEM) of at least 3 independent experiments.
Narrow-spectrum kinase inhibition has less proapoptotic activity than BEZ235. (A) A representative Eμ-Myc lymphoma (#4242) was treated in vitro for 48 hours with increasing concentrations of BEZ235 or a panel of more selective kinase inhibitors (BKM120, KU63794, KU59933, or KU57788) prior to flow cytometric analysis for annexin-V/PI positivity. (B) Eμ-Myc lymphoma derived on a LoxP-Rictor-LoxP background was transduced with MSCV-Cre recombinase (Cre) or empty vector (MSCV). Protein lysates were then separated by SDS-PAGE prior to immunoblotting for Rictor or Raptor expression. (C) Matched Rictor expressing (MSCV) and Rictor knockout (Cre) lymphomas were treated for 24 hours with BEZ235 or Everolimus prior to flow cytometric analysis for annexin-V/PI positivity. (D) Eμ-Myc lymphoma cells were treated with increasing concentrations of ATM inhibitor (KU55933), DNA-PK inhibitor (KU57788), or combined KU55933 and KU57788 alone or in combination with a fixed dose of Everolimus (100 nM) for 24 hours prior to flow cytometric analysis for annexin-V/PI staining. # indicates greater-than-additive apoptotic effect (SQ > 1) for the two-drug combination. All results are represented as the mean (± SEM) of at least 3 independent experiments.
Combined mTORC1/2 inhibition with KU63794 had weak effects on Eμ-Myc lymphoma viability (Figure 5A), suggesting BEZ235 responses were not due to mTORC2 blockade. Although mTORC1 is amenable to specific inhibition by rapamycin analogs, no mTORC2-selective inhibitor exists. To exclude mTORC2 as an important target in BEZ235-induced apoptosis, we generated an Eμ-Myc lymphoma on a LoxP-Rictor-LoxP background.30 Expression of Cre-recombinase in this lymphoma genetically ablated Rictor, a critical component of the mTORC2 complex (Figure 5B). Consistent with the minor response to pharmacologic mTORC1/2 inhibition, Rictor knockout had little effect on lymphoma cell viability. Furthermore, matched Rictor-deficient and Rictor-expressing lymphomas showed equivalent sensitivity to BEZ235 and Everolimus (Figure 5C). Thus, mTORC2 inhibition is not a critical determinant of the response to BEZ235.
Combined DNA-PK/mTOR inhibition induces apoptosis of Eμ-Myc lymphomas and human IG-cMYC-positive cell lines
Selective inhibitors of PI3K-related kinases were less potent than BEZ235 in the induction of apoptosis (Figures 2B,E-F, and 5A), suggesting that the multitargeted nature of BEZ235 might be responsible for its apoptotic effects. Because BEZ235-induced apoptosis correlated with dephosphorylation of mTORC1 and DDR kinase targets, we next investigated the effects of combined DDR kinase/mTORC1 inhibition. Titration of the ATM inhibitor (KU55933) onto an mTORC1-inhibitory concentration of Everolimus (100 nM) failed to induce additional apoptosis (Figure 5D). In contrast, supplementation of Everolimus with increasing concentrations of DNA-PK inhibitor (KU57788) resulted in enhanced apoptosis that more closely resembled the effects of single-agent BEZ235 (Figure 5D). Similarly, Everolimus combined with KU57788 to induce additive apoptosis in human IG-cMYC-positive cell lines (supplemental Figure 4A-B). In the absence of concurrent mTOR inhibition, combined KU55933 and KU57788 resulted in some additional apoptotic activity over single-drug treatment (Figure 5D). However, the addition of KU55933 to combined KU57788 and Everolimus did not further augment apoptosis (Figure 5D). Thus, while it is likely that BEZ235-induced apoptosis is attributable to the antagonism of multiple DDR kinases and mTOR, selective DNA-PK and mTORC1 inhibition is sufficient to induce cell death.
BEZ235 bypasses AKT to induce BMF expression through combined antagonism of DNA-PK and mTORC1
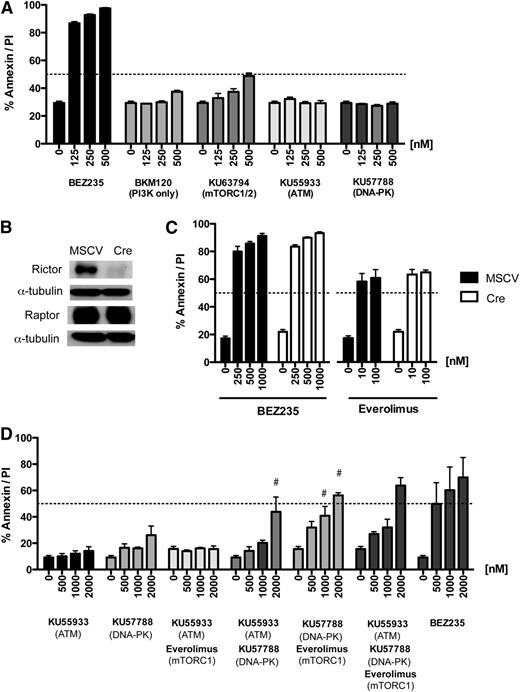
Having identified DDR kinases and mTORC1 as important BEZ235 targets, we next determined the effects DDR/mTORC1 kinase inhibition on candidate apoptosis regulators. BEZ235-induced cell death occurred despite increased AKT phosphorylation (Figure 4B), suggesting that the antiapoptotic effects of AKT activation11 were bypassed by combined DDR/mTOR blockade. This was further supported by robust proapoptotic activity of BEZ235 against lymphoma transduced with a constitutively active (myristoylated) AKT construct11 (Figure 6A; supplemental Figure 5A). Moreover, the AKT-regulated BH3 proteins BIM and BAD49 were dispensable for BEZ235-induced apoptosis (Figure 6B), and BEZ235 treatment failed to induce BIM or alter cellular levels or the electrophoretic mobility of BAD (supplemental Figure 5B). Together, these data indicate that BEZ235 induces apoptosis through targets acting independently of AKT.
Combined DDR kinase and mTORC1 inhibition bypasses AKT to induce BMF-mediated apoptosis. (A) A representative Eμ-Myc lymphoma (#4242) was transduced with a constitutively active AKT expression vector (Akt) or empty vector control (MSCV) and treated with increasing concentrations of BEZ235 for 24 hours prior to flow cytometric analysis for annexin-V/PI staining. (B) Lymphomas derived on a Bad- or Bim-deficient background and a Bad/Bim wild-type control (#4242) were treated for 24 hours with BEZ235 prior to analysis for annexin-V/PI positivity. (C) Schematic representation of the proposed kinase activities mediating BMF upregulation. Dashed lines indicate putative indirect interactions. (D) Lymphomas derived on a Bmf-deficient background (#14 and #31) and a Bmf wild-type control (#4242) were treated for 24 hours with BEZ235 prior to analysis for annexin-V/PI positivity. *P < .05 comparing genotypes (Bmf+/+ vs Bmf−/−; 2-way ANOVA) (E) Eμ-Myc lymphoma (#4242 MSCV/Bcl2) was treated for 24 hours with vehicle (DMSO), Everolimus (100 nM), DNA-PK inhibitor (KU57788; 1 μM), or combined Everolimus/KU57788 or BEZ235 (250 nM) prior to harvesting and preparation of protein lysates and immunoblotting for BMF. The fold-induction of BMF expression (densitometry relative to DMSO control and normalized to tubulin) is indicated above. (F) Lymphomas derived on a Bmf-deficient background (#14 and #31) and a Bmf wild-type control (#4242) were treated for 24 hours with Everolimus, KU57788, or combined Everolimus/KU57788 prior to analysis for annexin-V/PI positivity. # Indicates greater-than-additive effect for drug combination (SQ > 1). All results are representative of at least 3 independent experiments; bar graphs indicate mean (± SEM).
Combined DDR kinase and mTORC1 inhibition bypasses AKT to induce BMF-mediated apoptosis. (A) A representative Eμ-Myc lymphoma (#4242) was transduced with a constitutively active AKT expression vector (Akt) or empty vector control (MSCV) and treated with increasing concentrations of BEZ235 for 24 hours prior to flow cytometric analysis for annexin-V/PI staining. (B) Lymphomas derived on a Bad- or Bim-deficient background and a Bad/Bim wild-type control (#4242) were treated for 24 hours with BEZ235 prior to analysis for annexin-V/PI positivity. (C) Schematic representation of the proposed kinase activities mediating BMF upregulation. Dashed lines indicate putative indirect interactions. (D) Lymphomas derived on a Bmf-deficient background (#14 and #31) and a Bmf wild-type control (#4242) were treated for 24 hours with BEZ235 prior to analysis for annexin-V/PI positivity. *P < .05 comparing genotypes (Bmf+/+ vs Bmf−/−; 2-way ANOVA) (E) Eμ-Myc lymphoma (#4242 MSCV/Bcl2) was treated for 24 hours with vehicle (DMSO), Everolimus (100 nM), DNA-PK inhibitor (KU57788; 1 μM), or combined Everolimus/KU57788 or BEZ235 (250 nM) prior to harvesting and preparation of protein lysates and immunoblotting for BMF. The fold-induction of BMF expression (densitometry relative to DMSO control and normalized to tubulin) is indicated above. (F) Lymphomas derived on a Bmf-deficient background (#14 and #31) and a Bmf wild-type control (#4242) were treated for 24 hours with Everolimus, KU57788, or combined Everolimus/KU57788 prior to analysis for annexin-V/PI positivity. # Indicates greater-than-additive effect for drug combination (SQ > 1). All results are representative of at least 3 independent experiments; bar graphs indicate mean (± SEM).
Downstream of AKT, mTORC1 phosphorylates 4EBP-1, releasing eIF4E to augment 5′cap-dependent protein translation. When compared with Everolimus, we consistently observed increased 4EBP-1 dephosphorylation by BEZ235 (Figure 4A; supplemental Figure 3A-B). The dephosphorylation of rapamycin-insensitive 4EBP-1 phosphorylation sites has been attributed to the nonallosteric mechanism of action of ATP-competitive mTOR inhibitors.43 Others have reported that PI3K-related DDR kinases can phosphorylate 4EBP-1.28 The BH3-only protein BMF is upregulated in response to the inhibition of 5′cap-dependent protein translation.29,50 We therefore hypothesized that the apoptotic activity of BEZ235 was due to the repression of rapamycin-insensitive mTORC1, DDR kinase outputs to 4EBP-1, and upregulation of BMF (Figure 6C). Consistent with this hypothesis, and in contrast to the robust apoptotic responses observed in lymphomas derived on Bim-, Bad-, Puma-, and Noxa deficient backgrounds (Figures 3E and 6B), Bmf-deficient lymphomas showed primary resistance to BEZ235 (Figure 6D). Moreover, BEZ235 treatment resulted in BMF induction that was consistently greater than that seen with Everolimus (Figure 6E; supplemental Figure 5B). BEZ235 also induced BMF expression in IG-cMYC-positive human cell lines (supplemental Figure 5C). Importantly, the DNA-PK inhibitor (KU57788) also upregulated BMF levels, with the combination of Everolimus and KU57788 showing additive effects on BMF expression (Figure 6E). Finally, Bmf-deficient lymphomas showed resistance to the combination of KU57788 and Everolimus treatment when compared with a Bmf-expressing control (Figure 6F). This resistance phenotype showed specificity for combined DNA-PK/mTORC1 inhibition, because these Bmf-null lymphomas were sensitive to conventional proapoptotic agents as otherwise determined by their p53 status (supplemental Figure 5D).29,50 Together, these results suggest that the combined DDR kinase and mTORC1 inhibition by BEZ235 converges to upregulate BMF and activate the intrinsic apoptotic pathway.
BEZ235 induces apoptotic tumor regressions and prolongs survival of mice bearing Eμ-Myc lymphoma
To determine the in vivo efficacy of BEZ235, Eμ-Myc lymphomas were transplanted into cohorts of syngeneic recipient mice. These mice were allowed to develop overt lymphadenopathy, and then daily oral dosing of BEZ235 (50 mg/kg) was commenced. Therapeutic responses were assessed by estimation of leukocytosis (Figure 7A), 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) scanning (Figure 7B; supplemental Figure 6A), apoptosis induction (Figure 7C) and spleen weight (supplemental Figure 6B). Within two days of treatment, marked reductions in leukocytosis, decreased spleen weights and a near complete metabolic response by 18F-FDG-PET were observed. Lymphoma harvested from BEZ235-treated mice showed increased apoptosis relative to vehicle-treated controls (Figure 7C). Early relapse was noted by day 7. Lymph node lysates obtained at the time of maximum response (day 4) were probed for the effects of BEZ235 on PI3K- and mTOR-regulated phosphorylation targets (Figure 7D). At this point, the in vivo apoptotic response correlated with suppression of mTORC1 substrates and the DDR (Figure 7D). Again, BEZ235 induced AKT phosphorylation (Figure 7D) and strongly upregulated BMF expression compared with vehicle controls (Figure 7E). Thus, BEZ235 induced apoptotic tumor regressions associated with suppression of mTORC1 and γH2AX, but with increased AKT phosphorylation and BMF induction.
BEZ235 induces apoptotic regressions and prolongs survival in Eμ-Myc lymphoma. Cohorts of mice (n = 9 per treatment condition) were injected intravenously with Eμ-Myc cells and allowed to develop disseminated lymphoma prior to initiation of daily treatment with BEZ235 (50 mg/kg by mouth) or vehicle (0.5% methylcellulose [MC]). (A) Peripheral white blood cell (WBC) counts for BEZ235 and vehicle-treated mice. (B) Subgroups of mice (n = 3 per treatment group) were monitored by 18F-FDG-PET scanning, with PET avidity quantified as a tumor:background ratio for axillary and inguinal lymph nodes. (C) Lymph node apoptosis was assessed by using fluorescent-activated cell sorting for sub G1 fraction on nuclear PI staining for cell suspensions prepared from 3 individual mice per treatment group at each time point. (D) Lymph nodes collected from mice on day 4 of treatment were harvested for protein lysates, separated by SDS-PAGE, and probed for phosphorylated proteins as indicated in figure legends. Lysate obtained from an untreated lymphoma transduced with a constitutively activated (myristoylated [MYR]) AKT is provided as a control. Each lane represents a lymph node lysate from an individual mouse. (E) Lymph nodes obtained from mice on day 4 of treatment with BEZ235 or vehicle were immunoblotted for BMF expression. Each lane represents a lymph node lysate from an individual mouse. (F) Kaplan-Meier survival curves for cohorts of mice (n = 10 per treatment condition) bearing p53-null (#3239) lymphoma and treated daily with BEZ235 or vehicle from day 3 after receiving transplanted cells (period of dosing indicated in gray). (G) Survival curves for cohorts of mice (n = 10 per treatment condition) bearing p53-competent (#102) lymphoma treated daily with BEZ235 from day 3 after receiving transplanted cells. In each case, a statistically significant prolongation of survival was observed (log-rank P < .05,). *P < .05 (Student t test).
BEZ235 induces apoptotic regressions and prolongs survival in Eμ-Myc lymphoma. Cohorts of mice (n = 9 per treatment condition) were injected intravenously with Eμ-Myc cells and allowed to develop disseminated lymphoma prior to initiation of daily treatment with BEZ235 (50 mg/kg by mouth) or vehicle (0.5% methylcellulose [MC]). (A) Peripheral white blood cell (WBC) counts for BEZ235 and vehicle-treated mice. (B) Subgroups of mice (n = 3 per treatment group) were monitored by 18F-FDG-PET scanning, with PET avidity quantified as a tumor:background ratio for axillary and inguinal lymph nodes. (C) Lymph node apoptosis was assessed by using fluorescent-activated cell sorting for sub G1 fraction on nuclear PI staining for cell suspensions prepared from 3 individual mice per treatment group at each time point. (D) Lymph nodes collected from mice on day 4 of treatment were harvested for protein lysates, separated by SDS-PAGE, and probed for phosphorylated proteins as indicated in figure legends. Lysate obtained from an untreated lymphoma transduced with a constitutively activated (myristoylated [MYR]) AKT is provided as a control. Each lane represents a lymph node lysate from an individual mouse. (E) Lymph nodes obtained from mice on day 4 of treatment with BEZ235 or vehicle were immunoblotted for BMF expression. Each lane represents a lymph node lysate from an individual mouse. (F) Kaplan-Meier survival curves for cohorts of mice (n = 10 per treatment condition) bearing p53-null (#3239) lymphoma and treated daily with BEZ235 or vehicle from day 3 after receiving transplanted cells (period of dosing indicated in gray). (G) Survival curves for cohorts of mice (n = 10 per treatment condition) bearing p53-competent (#102) lymphoma treated daily with BEZ235 from day 3 after receiving transplanted cells. In each case, a statistically significant prolongation of survival was observed (log-rank P < .05,). *P < .05 (Student t test).
Continued daily BEZ235 dosing significantly prolonged survival in both p53-null (#3239) (Figure 7F) and p53-competent (#102) (Figure 7G) lymphomas. The survival advantage conveyed by BEZ235 treatment in BCL2-overexpressing lymphoma (MSCV/Bcl2) was grossly attenuated when compared with isogenic (MSCV/empty vector) control (3.5 days vs 9 days prolongation in median survival, respectively, for #4242 lymphoma; data not shown). In all experiments, the initial suppression of leukemic burden was ultimately lost despite continued dosing (supplemental Figure 6C). Therefore, consistent with in vitro data (Figures 2 and 3), BEZ235 has in vivo activity that is independent of p53 and evident in chemorefractory lymphoma but attenuated by overexpression of BCL2.
Discussion
Therapeutic targeting of PI3K/mTOR signaling has an emerging role in the treatment of lymphoma.9 However, the efficacy of rapamycin analogs may be limited by their propensity to induce feedback upregulation of prosurvival PI3K/AKT signaling. Indeed, in some experimental models, this has a permissive effect on tumor survival.44 To counter prosurvival feedback, dual PI3K/mTOR inhibitors such as BEZ235 have been developed.23 We hypothesized that BEZ235 would show increased antilymphoma activity in the context of MYC dysregulation, in which MYC is known to activate mTORC116,17 and an endogenous DDR that is transduced by PI3K-related kinases (including ATM/ATR and DNA-PK).15 By using a combined genetic and pharmacological approach, we demonstrated that BEZ235 potently induced p53-independent apoptosis that correlated with mTORC1 and DDR suppression and BMF induction. Unexpectedly, apoptotic responses occurred despite feedback PI3K/AKT activation and resulted in impressive in vivo responses.
These observations hold important implications if dual inhibitors such as BEZ235 are to be used in the clinic. First, p53-independent apoptosis induction is desirable in poor-risk lymphomas characterized by 17p deletions. Moreover, BEZ235 may have activity in chemorefractory relapses associated with secondary perturbations of p53. However, responses were markedly attenuated by BCL2 overexpression, indicating that BEZ235 will have reduced activity against “double-hit” lymphomas harboring cMYC and BCL2 translocations. The nonsustained therapeutic response to BEZ235 suggests that single-agent treatment will result in the development of secondary resistance, particularly in aggressive lymphomas exhibiting genomic instability. Therefore, rational combinations with existing therapies should be investigated. The potential for on-target interactions between DDR inhibition and coadministered conventional DNA-damaging cytotoxic modalities requires investigation. Antagonism of the DDR may sensitize it to sequentially scheduled chemotherapy48 or radiotherapy.39,40 Additionally, p53-independent BMF induction by BEZ235 could be beneficial in combination with corticosteroids (which also induce BMF29 ), potentially abrogating glucocorticoid resistance.
Despite the dual inhibitory nature of BEZ235, we demonstrated a propensity to activate feedback prosurvival AKT signaling. This suggests that at submicromolar proapoptotic concentrations, the PI3K-inhibitory effects of BEZ235 are insufficient to compete with feedback loops induced by mTORC1 inhibition. It is also possible that BEZ235 is more potent at inhibiting mTORC1 than PI3K. Alternatively, other mechanisms may activate AKT by PI3K-independent mechanisms. This seems unlikely, because BEZ235 treatment also increased the phosphorylation of PDK-1 and BTK, suggesting widespread upstream kinase activation. Because the propensity for BEZ235-induced feedback loops might be antagonized by dose escalation, there is a precedent for dosing ATP-competitive inhibitors at the maximum-tolerated dose.
Although there are limitations to extrapolating pharmacodynamic end points from mouse to human, mice receiving BEZ235 at the maximum-tolerated dose showed progressive induction of AKT phosphorylation. AKT activation occurred at maximum depth of response and at anticipated steady-state drug levels prior to clinical relapse. The observation of increasing AKT activation prior to subsequent relapse suggests that this phenomenon might rescue lymphoma from the apoptotic effects of BEZ235 in vivo. Alternative combination strategies that could circumvent feedback PI3K activation (for example by the addition of a BTK or AKT inhibitor) are suggested. Finally, if BEZ235 can activate prosurvival signaling in vivo, the potential to antagonize the effects of coadministered proapoptotic agents should be recognized.
In summary, our mechanistic data provide a rationale for the clinical translation of BEZ235 in selected hematologic tumors that show reliance on mTORC1 and an endogenous DDR in the context of MYC deregulation. We provide evidence that broad-spectrum PI3K-family inhibition provides benefit over more selective compounds, through molecular mechanisms that bypass AKT and p53. The efficacy of this approach in other MYC-dysregulated hematologic malignancies merits further investigation. Future studies that fully define the role of PI3K-related kinases in the transduction of prosurvival signaling associated with an MYC-driven DDR will help determine effective combination strategies for clinical evaluation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank Dr Hans-Guido Wendel for the provision of Myr-Akt constructs. The authors also thank Prof A. Strasser, Dr A. Villunger, Dr E. Hawkins, Dr P. Bouillet, Prof S. Cory, Prof J. Adams and Dr C. Scott for provision of reagents and their critical evaluation of this manuscript.
J.S. was supported by funding from the Leukaemia Foundation of Australia and the Co-operative Research Centre for Biomedical Imaging Development. R.W.J. is a Principal Research Fellow of the National Health and Medical Research Council of Australia (NHMRC) and is supported by NHMRC Program and Project Grants, the Susan G. Komen Breast Cancer Foundation, Cancer Council Victoria, The Leukaemia Foundation of Australia, Victorian Breast Cancer Research Consortium, and the Victorian Cancer Agency. R.D.H. and R.B.P. are Senior Research Fellows of the NHMRC and are supported by NHMRC Project Grants and the Leukaemia Foundation of Australia.
Authorship
Contribution: J.S. designed and performed research, collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript. B.P.M., A.N., K.M.H., J.R.D., A.J.B., R.R., and C.C. performed research and collected, analyzed, and interpreted data. C.A.S., M.R., M.N.H., and M.W. contributed vital new reagents. R.D.H., R.B.P., G.A.M., and R.W.J. designed research and wrote the manuscript.
Conflict-of-interest disclosure: Novartis Pharmaceuticals provides grant support to the laboratory of R.W.J. The remaining authors declare no competing financial interests.
Correspondence: Ricky W. Johnstone, Gene Regulation Laboratory. Peter MacCallum Cancer Centre, Locked Bag 1, A’Beckett St, Melbourne, VIC 8006, Australia; e-mail: ricky.johnstone@petermac.org.

![Figure 2. BEZ235 induces apoptosis of Eμ-Myc lymphomas and IG-cMYC-translocated cell lines at on-target concentrations. (A) Six independently derived Eμ-Myc lymphomas were cultured in vitro for 24 hours with increasing concentrations of BEZ235 (or dimethylsulfoxide [DMSO] vehicle alone at maximal concentration) and subjected to flow cytometric analysis for annexin-V/PI positivity. The lymphomas have been stratified from most to least BEZ235 sensitive (at 125 nM) from left to right. A unique identifier for each lymphoma is provided above the bar graph. (B) A representative lymphoma (#4242) was treated with BEZ235 or Everolimus for 24 hours prior to flow cytometric analysis for annexin-V/PI positivity. *P < .05 comparing treatments (BEZ235 vs Everolimus) at each concentration (2-way analysis of variance [ANOVA]). (C) The same lymphoma (#4242) was transduced with murine stem-cell virus expressing Bcl2 or empty vector control (MSCV) and treated for 24 hours with BEZ235 prior to flow cytometric analysis for annexin-V/PI positivity. *P < .05 comparing genotypes (Bcl2 vs empty vector) at each concentration (2-way ANOVA). (D) Lymphoma (#4242) was transduced with murine stem-cell virus expressing CrmA or empty vector control (MSCV) and treated for 24 hours with BEZ235 prior to flow cytometric analysis for annexin-V/PI positivity. There was no significant difference in response between genotypes (CrmA vs empty vector) at any drug concentration (P > .05; 2-way ANOVA). Inset: western blot of CrmA expression in the same lymphoma cells with β-actin provided as a loading control. (E) Human IG-cMYC-translocated cell lines were treated for 48 hours with BEZ235 (or DMSO vehicle) and subjected to flow cytometric analysis for annexin-V/PI positivity. (F) The same human cell lines were treated for 48 hours with Everolimus prior to flow cytometric analysis for annexin-V/PI positivity. All graphs represent the mean (± standard error of the mean [SEM]) for at least 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/15/10.1182_blood-2012-08-446096/4/m_2964f2.jpeg?Expires=1769155704&Signature=hcH~vYYWpeKUe9CvTOVkgckbYXZBrFncuylh0jRkw4is0pQybI3rTbQ0K0r5BTR2k1wwbIn~nf~~8nblCsOlMJx1aPcICEMVduC0-1OEJK-rAbPmyNK4B~cXRq3O9q7IGK1aTHBTux2Uv0sUMYIaQnURxO5WnhT7gvo-7tj7CgK5~mhIUtiO8heJWioXZH6I9cvWLfQoZwoMeHIXPXR4fbm9ATfR0xVYX~jm2-~0soRBB6Wa2ox7Is8sQRlljSQ6iNmT35V2twAj8SZCNBTzKUCb1F8h5fHvFtyOSEi6b-Eeq-H2EuspYrbvb9TGJlMNjuZz0-RRiHj~ukPkrKQ4aQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. BEZ235-induced apoptosis correlates poorly with chemosensitivity and occurs independently of p53 and the p53-regulated BH3-only proteins PUMA and NOXA. (A) Five independently derived Eμ-Myc lymphomas were cultured for 24 hours with 20 nM etoposide and analyzed for annexin-V/PI positivity. (To facilitate comparison with Figure 2, the lymphomas have been stratified from most to least BEZ235 sensitive [at 125 nM] from left to right). The unique identifier for each lymphoma is provided above the bar graph. “R” denotes primary etoposide resistance (defined as AC50 [50% apoptosis concentration] < 20 nM at 24 hours). (B) Correlation of the percentage of cells undergoing apoptosis at 24 hours for an extended panel of lymphomas (n = 11) treated with 250 nM BEZ235 or 20 nM of etoposide (r, Pearson’s correlation coefficient). (C) Eμ-Myc lymphomas derived on a p53 null background (p53 null) or with previously characterized inactivating p53 mutations (p53 mutated [mut.]) were treated for 24 hours with BEZ235 prior to analysis for annexin-V/PI positivity. (D) The same p53-incompetent lymphomas were treated with etoposide (20 nM) for 24 hours prior to analysis for annexin-V/PI positivity. (E) Lymphomas derived on a Puma- (Puma −/−) or Noxa- (Noxa −/−) deficient background and a Puma/Noxa-competent (#4242) control were treated with BEZ235 for 24 hours prior to analysis for annexin-V/PI positivity. All graphs represent the mean (± SEM) for at least 3 independent experiments. WT, wild-type.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/15/10.1182_blood-2012-08-446096/4/m_2964f3.jpeg?Expires=1769155704&Signature=E9IXlXzJt2IUB7J1CPiBvZNJ8TNUp4L3c0zB1G2nr16etvQUF0HEON-AuILCK4fj7QBI0lcNNwBzvXonGUCpw2sNReEpQX1p~6im4GKA1~94o-dKdWEGwysZDcXEI5FAd4zXBN11CyVB5qxtUq8qV7MERYCHiuFnzSDZ7zWZvLLrj2OiHqFaJPP2JTTzUkAfBVaIAsNQlu5FRZcuD7f60Z40n3b~Ih1BnDE9yPk67rtSGVU3QRj~el2moFcwiMwSj0OgWMVn5DIvrGwkxiwfM41NG-GJNfX56VEjU2atqaL-g~JF3iom582L4Ts-lmyIZTEYcDiKQ3~lXREF2b5QWQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. BEZ235 induces apoptotic regressions and prolongs survival in Eμ-Myc lymphoma. Cohorts of mice (n = 9 per treatment condition) were injected intravenously with Eμ-Myc cells and allowed to develop disseminated lymphoma prior to initiation of daily treatment with BEZ235 (50 mg/kg by mouth) or vehicle (0.5% methylcellulose [MC]). (A) Peripheral white blood cell (WBC) counts for BEZ235 and vehicle-treated mice. (B) Subgroups of mice (n = 3 per treatment group) were monitored by 18F-FDG-PET scanning, with PET avidity quantified as a tumor:background ratio for axillary and inguinal lymph nodes. (C) Lymph node apoptosis was assessed by using fluorescent-activated cell sorting for sub G1 fraction on nuclear PI staining for cell suspensions prepared from 3 individual mice per treatment group at each time point. (D) Lymph nodes collected from mice on day 4 of treatment were harvested for protein lysates, separated by SDS-PAGE, and probed for phosphorylated proteins as indicated in figure legends. Lysate obtained from an untreated lymphoma transduced with a constitutively activated (myristoylated [MYR]) AKT is provided as a control. Each lane represents a lymph node lysate from an individual mouse. (E) Lymph nodes obtained from mice on day 4 of treatment with BEZ235 or vehicle were immunoblotted for BMF expression. Each lane represents a lymph node lysate from an individual mouse. (F) Kaplan-Meier survival curves for cohorts of mice (n = 10 per treatment condition) bearing p53-null (#3239) lymphoma and treated daily with BEZ235 or vehicle from day 3 after receiving transplanted cells (period of dosing indicated in gray). (G) Survival curves for cohorts of mice (n = 10 per treatment condition) bearing p53-competent (#102) lymphoma treated daily with BEZ235 from day 3 after receiving transplanted cells. In each case, a statistically significant prolongation of survival was observed (log-rank P < .05,). *P < .05 (Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/15/10.1182_blood-2012-08-446096/4/m_2964f7.jpeg?Expires=1769155704&Signature=qJuDgQs1Ld0t3NJPJRaJ2EuntNZX8CEiT9fEsDBPjqBMg0Rqz9rMXryhFptTv3AtF0vFnNg-Ax7r3PTVMoc59GETig-3YsZ10LgC9uUMluU-Y06Wrhb1pYBxxK2as02YMhfK72IVbY2TDTpURXqNZdA3lIV~jTfHwojW~Qq-ZVV3X~ife6gWFJtu5ALbFmEKrneR4QXo0y61tEaI4KAxb7eOQ7DaxFJKu6zkAxe2jF1A3MGKN2mMGXrk5KUEUbJeO94SIgYW61jWJFjnnOwHvF7fA4prk48Dyee5dR6NAXWbVFcan~dPRRFfR5ICCrk37pNz5MQ2bfswVlb03WvJtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)