Key Points
MDSCs are increased in patients with MM and have bidirectional interaction with tumors in the MM microenvironment.
MM-MDSCs promote MM growth and induce immune suppression; conversely, MM cells induce MDSC development.
Abstract
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous, immature myeloid cell population with the ability to suppress immune responses. MDSCs have been characterized in infections, inflammatory diseases, and solid tumors; however, their presence and role in the tumor-promoting, immune-suppressive microenvironment in hematologic malignancies remains unclear. We assessed the presence, frequency, and functional characteristics of MDSCs in patients with newly diagnosed, relapsed, and relapsed/refractory multiple myeloma (MM) compared with healthy donors. Additionally, we evaluated the immunomodulatory effects of lenalidomide and bortezomib on MDSCs in MM. CD11b+CD14-HLA-DR-/lowCD33+CD15+ MDSCs were significantly increased in both the peripheral blood and the bone marrow of patients with active MM compared with healthy donors. Furthermore, MDSCs induced MM growth while suppressing T-cell–mediated immune responses. Conversely, MM cells induced the development of MDSCs from healthy donor peripheral blood mononuclear cells, confirming a bidirectional interaction between MDSCs and MM cells and immune effector cells. Our results further suggest that MDSCs may be associated with the activity of disease in MM. Importantly, our studies suggest that inhibition of the tumor-promoting and immune-suppressive functions of MDSCs in MM may represent a promising novel immune-based therapeutic strategy.
Introduction
Recent studies have both defined the role of the bone marrow (BM) microenvironment in the pathophysiology of multiple myeloma (MM) and provided the framework for novel therapies targeting the interaction of malignant plasma cells and their surrounding stromal cells in the BM milieu. Importantly, the interaction of MM cells with BM accessory cells and with the extracellular matrix induces autocrine and paracrine signaling, mediating tumor growth, progression, and cell adhesion mediated–drug resistance, as well as immune suppression.1 Thalidomide, lenalidomide, and bortezomib are 3 novel agents that target the tumor cell in its microenvironment and can overcome cell adhesion mediated–drug resistance; they have been rapidly integrated into MM treatment, resulting in at least a doubling of patient median survival.2-4 Moreover, genomic and molecular changes induced by tumor cells in the surrounding stroma and immune cells have provided the framework for novel immunomodulatory approaches, including epigenetic strategies targeting histone modification via acetylation or methylation.5 For example, small molecule inhibitors of histone deacetylases have effects both against the tumor and the tumor microenvironment.6,7 Nevertheless, minimal residual disease commonly persists due to drug resistance and escape from immune surveillance, and novel therapies are urgently needed.
As in other cancers, the bidirectional interaction between MM cells and surrounding cells regulates tumor development on the one hand, while transforming the BM microenvironment into a tumor-promoting and immune-suppressive milieu on the other.8 Developments in targeted therapies have indicated that the generation of the most-effective therapeutic strategies requires not only targeting tumor or stroma cells, but also using methods to overcome the blockade of antitumor immune responses.9,10 In addition to lymphoid immune suppressor cells such as regulatory T cells (Tregs) and T helper (Th17) cells, distinct populations of myeloid cells such as myeloid-derived suppressor cells (MDSCs) can effectively block antitumor immune responses, thereby representing an important obstacle for immunotherapy.11-14 Specifically, myeloid lineage cells including macrophages, neutrophils, eosinophils, mast cells, and dendritic cells are fundamental elements of BM stroma.1 Myeloid cells can modulate both pro- and anti-inflammatory responses in cancer and regulate antigen presentation, as well as induce growth factor and cytokine secretion–mediating defense against pathogens and cancer cells. Conversely, suppressor myeloid cells promote tumor development, growth, immune escape, and metastasis by suppressing antitumor immune responses.12-15 Studies performed since 200111,16 have in particular focused on MDSCs with tumor-promoting and immune-suppressing activity in the stroma of solid tumors. MDSCs are heterogeneous, immature, myeloid progenitor cells, which can suppress effector T, natural killer T (NKT), and natural killer (NK) cell–mediated antitumor immune responses.15 While MDSCs are rare or absent in healthy individuals, increased numbers of MDSCs have been identified in tumor sites and the peripheral circulation.16-20 In mice, MDSCs have been identified, based upon low expression of major histocompatibility complex class II and CD80,21 to be neutrophillike CD11b+Gr1high or monocytelike CD11b+Gr1low cells.21-23 However, MDSCs in humans are highly heterogeneous and characterized by the expression of additional phenotypic surface antigens: high CD11b, CD33, and IL-4Rα; low or no CD14 and Lin expression; and variable expression of CD15 and CD66b.16,17,24,25 MDSCs can directly suppress effector T cells by producing arginases (ARG1), reactive species of oxygen (ROS), cyclooxygenase-2 (COX2), inducible nitric oxide synthase (iNOS), and immunosuppressive cytokines (IL-6, IL-10), as well as by depleting metabolic factors from the microenvironment required for T-cell activation.12,26-33 MDSCs can also inhibit effector T-cell responses by promoting Treg cell development and by disrupting naive T-cell homing to lymph nodes.33,34
Even though we and others have characterized the role of interactions of tumor cells with immune effector T and NK cells in the modulation of tumor growth and drug resistance,2,35 to date the myeloid compartment, particularly tumor-promoting and immune suppressive MDSCs and their bidirectional interaction with MM cells, has not been fully characterized. In this study, we assessed the presence and the frequency, as well as the phenotypic and functional characteristics, of MDSCs in the peripheral blood (PB) and BM of patients with MM. We identified a distinct MDSC population (CD11b+CD14-HLA-DR-/lowCD33+CD15+) with tumor-promoting and immune-suppressive activity in both the PB and the BM of patients with MM. Moreover, we determined the effects of lenalidomide and bortezomib on the induction of MDSCs by MM cells, as well as on MDSC function. These studies both define new mechanisms of action of these novel agents and validate MDSCs as a novel therapeutic target in MM.
Materials and methods
Cells and cell isolation
Dexamethasone-sensitive MM1.S and dexamethasone-resistant MM1.R MM cells were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL). Doxorubicin-resistant RPMI8226 (RPMI-DOX40) was obtained from Dr William Dalton (H. Lee Moffitt Cancer Center, Tampa, FL). RPMI8226 MM cells were purchased from American Type Culture Collection; plasma cell leukemia cells OPM1 and OPM2 were provided by Dr Edward Thompson (University of Texas Medical Branch, Galveston, TX); and IL-6–dependent plasma cell leukemia cells INA-6 were obtained from Dr Renate Burger (University of Kiel, Germany). All cell lines were maintained in RPMI1640 (BioWhittaker) containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (Life Technologies, Woburn, MA). Heparinized venous blood samples and/or aspirates of BM from patients with newly diagnosed (n = 4), relapsed (n = 4) or relapsed/refractory (n = 9) MM, and healthy donors (n = 5) were obtained after written informed consent per the Declaration of Helsinki and approval by the Institutional Review Board of the Dana-Farber Cancer Institute. Mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation. CD3+ T cells and MDSCs were selected from peripheral blood mononuclear cells (PBMCs) or bone marrow mononuclear cells (BMMCs) to obtain CD33-positive and CD33 and CD15 coexpressing MDSCs. Selection was done by either using magnetically labeled, positive-selection monoclonal antibodies (MAbs) against CD3 for T cells, as well as CD11b, CD14, and HLA-DR (Miltenyi Biotech, Auburn, CA) for MDSCs, or by sorting with a FACSAria IIu sorter using CD3 phycoerythrin (PE), CD11b APC-Cy7, CD14 Pacific Blue, CD15 APC, CD33 PE-Cy5, and HLA-DR PE-Cy7 MAbs (Becton Dickinson Biosciences, San Jose, CA) for MDSCs and CD11b+CD14+HLA-DR+ for antigen-presenting cells (APCs). The purity of the isolated cells was confirmed using anti-CD3Ab or anti-CD11b, CD14, CD33, CD15, and HLA-DR MAbs.
Reagents and compounds
The proteasome inhibitor bortezomib (1 mM) and the immunomodulatory drug lenalidomide (10 mM) were dissolved in dimethylsulfoxide and stored at −20°C. Anti-CD3 and anti-CD28 MAbs (10 μg/mL) (Becton Dickinson Biosciences); human interleukin-2 (rhIL-2) (50 IU) and interleukin-6 (2.5 ng/mL) (R&D Systems, Minneapolis, MN); and lipopolysaccharide (LPS) (1 μg/mL) (Sigma-Aldrich, Saint Louis, MO) were used to stimulate cells. Specific inhibitors were used to suppress MDSC-derived inhibitory factors in MDSC and autologous T-cell cocultures at the concentrations of 500 μM of Nω-Hydroxy-nor-l-arginine (nor-NOHA) (EMD Millipore, Billerica, MA) for ARG1; 500 μM of NG-Monomethyl-l-arginine (NMMA) (Sigma) for iNOS; 100 μM of apocynin (Sigma) for ROS; and 15 μm of celecoxib (Sigma) for COX2.
Cell culture and treatment
Bone marrow stroma cells (BMSCs) were generated from BM aspirates of patients with MM by culturing BMMCs, with or without depletion of MDSCs, for 4 to 6 weeks in Dulbecco’s modified Eagle medium-1α supplemented with 20% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (Life Technologies). PBMCs or BMMCs from patients with MM or from healthy donors were cultured in the absence or presence of lenalidomide (1 μM) and bortezomib (5 nM) for 16 hours. PBMCs from healthy donors were cocultured with MM cell lines MM1.S, RPMI8226, INA-6, and DOX40 for 4 to 8 days.
Flow cytometric analysis
PBMCs or BMMCs from patients with MM or from healthy donors were stimulated with LPS (1 μg/mL) or anti-CD3 (10 μg/mL) and anti-CD28 (20 μg/mL) MAbs, and then incubated with or without lenalidomide (1 μM) and bortezomib (5 nM) for 4 to16 hours. For intracellular cytokine determination assays, 5 μg/mL of brefeldin A solution (eBioscience, San Diego, CA) was added during the last 2 hours of incubation. Cells were costained with CD11b-APC-Cy7, CD14-Pacific blue, HLA-DR-PE-Cy7, CD33-PE-Cy5, and CD15-APC conjugated MAbs (BD Biosciences, San Jose, CA) to determine MDSC phenotype (gating within the CD11b+ on CD14-HLA-DR-/low cells expressing both CD33 and CD15 antigens). Cells were then fixed in 4% paraformaldehyde–phosphate-buffered saline and stained with PE-conjugated anti–IL-6, IL-10, interferon IFN-γ, granulocyte macrophage–CSF (GM-CSF), and COX2 MAbs, as well as COX1–fluorescein isothiocyanate antibodies (Abs); ARG1-CFS and surface antigen were detected by GM-CSFR-Alexa-488 MAb (Becton Dickinson Biosciences) in permeabilization buffer (0.5% saponin–phosphate-buffered saline). Intracytoplasmic and surface expression of molecules in MDSCs were detected by flow cytometry using BD LSRFortessa (Becton Dickinson Biosciences) and analyzed using Flowjo software (TreeStar, Ashland, OR).
Statistics
All in vitro experiments were performed in triplicate and repeated 3 times; a representative experiment is demonstrated in the figures. Statistical significance was determined by nonparametric Student t test, 2-tailed distribution, with minimal significance level P < .05. (See supplemental “Methods.”)
Results
Despite treatment strategies using novel therapeutic agents such as bortezomib and immunomodulatory drugs, patients still relapse due to minimal residual disease. To test the hypothesis that the bidirectional interaction of MM cells with immune cells induces a tumor-promoting, immunosuppressive microenvironment in MM, we assessed the presence and the frequency of MDSCs, as well as MDSC-mediated tumor promotion and immune suppression, in the BM and the PB of patients with MM.
Increased frequency of MDSCs in patients with MM
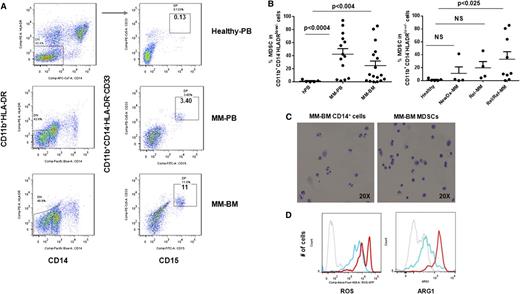
Because increased numbers of MDSCs have been found in patients with solid tumors,11,12,16,17,27,33,34,36-40 we first tested the hypothesis that the bidirectional interaction of MM cells with surrounding immune cells in tumor stroma may induce the development of MDSCs, which in turn support tumor development, growth, escape from immune surveillance, and metastasis in the MM microenvironment. Specifically, we analyzed the phenotype and the frequency of MDSCs in the BM (n = 17) and the PB (n = 14) of patients with newly diagnosed (n = 4), relapsed (n = 4), or relapsed/refractory (n = 9) MM, as well as in healthy donors (n = 5) (Table 1). Based on the phenotypic characteristics of MDSCs in solid tumors, we identified MDSCs as CD11b+/CD14-HLA-DR-/low/CD33+ and/or CD11b+/CD14-HLA-DR-/low/CD33+CD15+ phenotype by multiparameter flow cytometry analysis (Figure 1A). Representative flow cytometry dot plots demonstrate the gating strategy and expression of MDSCs in the PB and the BM from healthy donors and patients with MM. There was a significant increase in the number of MDSCs in both PBMCs (n = 14, mean = 42.3, SEM = 8.5, P < .0004) and BMMCs (n = 17, mean = 24.9, SEM = 7, P < .004) from patients with MM compared with healthy donors (n = 5, mean = 1.2, SEM = 0.7) (Figure 1B, left panel). Further analysis demonstrated that the frequency of MDSCs increased with disease progression with statistically significant (P < .025) increases in relapsed/refractory MM, compared with healthy donors (Figure 1B, right panel). In addition to phenotypic characterization of MDSCs, we also identified MDSCs morphologically in MM-BM (Figure 1C). However, Wright-Giemsa staining of MDSCs and CD14+HLA-DR+ myeloid cells isolated from MM-BM demonstrated no significant morphological differences between CD11b+CD14-HLA-DR-/low/CD33+CD15+ MDSCs (Figure 1C, right panel) and CD11b+CD14+HLA-DR+ myeloid cells (APCs) (Figure 1C, left panel). We next assessed intracellular expression of inhibitory molecules including ROS and ARG1 in MDSCs and CD11b+CD14+HLA-DR+ myeloid cells in MM (Figure 1D). There was a significant increase in both ROS (Figure 1D, left panel) and ARG1 (Figure 1D, right panel) expression in MDSCs compared with APCs in the BM from patients with relapsed MM. Therefore, as observed in solid tumors, CD11b+/CD14-HLA-DR-/low/CD33+CD15+ MDSCs were increased in both the PB and the BM of patients with active MM, have similar morphological characteristics to CD11b+CD14+HLA-DR+ APCs, and express increased ROS and ARG1.
Clinical disease characteristics of patients
| Patients . | Gender . | Age* . | Diagnosis . | Stage† . | Treatment status . | Treatment . | Cytogenetics . | Lymphocyte (K/μL) . | Myelocyte (K/μL) . | Neutrophil (K/μL) . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 62 (65) | MM IgAλ | I | Relapsed/refractory | 1. Revlimid, Velcade, dexamethasone | Normal | 0.8 | 0.32 | 2.84 |
| 2. Tanespimycin, Velcade | ||||||||||
| 2 | M | 46 (52) | MM IgAκ | I | Relapsed/refractory | 1. Revlimid, Velcade, dexamethasone → SCT → Revlimid, dexamethasone | Normal | 0.11 | 0.09 | 1.28 |
| 2. Revlimid, Velcade, dexamethasone | ||||||||||
| 3 | F | 62 (64) | MM IgAκ | I | Relapsed/refractory | 1. Revlimid, dexamethasone → SCT → Revlimid, dexamethasone | Abnormal: 14q32, 11q13, 4p16, 13q14.3, 13q34 | 0.74 | 0.28 | 1.24 |
| 4 | M | 66 (69) | MM IgGκ | III | Relapsed/refractory | 1. Thalidomide, Velcade, dexamethasone | Abnormal: 13delq | 0.61 | 0.09 | 8 |
| 2. Revlimid, melphalan, prednisone | ||||||||||
| 3. Cytoxan, etoposide, dexamethasone → SCT → Revlimid | ||||||||||
| 4. Thalidomide, Velcade, Doxil, dexamethasone → thalidomide, Velcade, dexamethasone | ||||||||||
| 5 | M | 56 (57) | MM IgGκ | I | Relapsed/refractory | 1. Velcade, dexamethasone → thalidomide, Velcade, dexamethasone + cisplatin, Doxil, Cytoxan, etoposide → SCT → Revlimid | Normal | 0.83 | 0.26 | 1.43 |
| 6 | F | 59 (63) | MM IgGκ | I | Relapsed/refractory | 1. Revlimid, Velcade, dexamethasone → SCT → Revlimid | Abnormal: +7, +16, 13del, 17delp, t(4:14) | 0.82 | 0.34 | 3.1 |
| 2. Perifosine/placebo, Velcade, dexamethasone | ||||||||||
| 7 | F | 51 (57) | MM IgAκ | II | Relapsed/refractory | 1. Thalidomide, dexamethasone → SCT → thalidomide | Abnormal: Trisomy 3, 5, 7, 9, 17, 19, t(4:14) | 1.15 | 0.23 | 3.17 |
| 2. Revlimid, dexamethasone | ||||||||||
| 3. Thalidomide, Velcade, Doxil, dexamethasone | ||||||||||
| 4. Panobinostat, dexamethasone | ||||||||||
| 8 | F | 60 (67) | MM IgGλ | I | Relapsed/refractory | 1. Thalidomide, dexamethasone | Abnormal: 17delp, trisomy 3, 7, 9, 11, 15 | 1.09 | 0.68 | 3.12 |
| 2. Velcade | ||||||||||
| 3. Ixazomib | ||||||||||
| 9 | M | 56 (60) | MM IgGκ | II | Relapsed/refractory | 1. Velcade, dexamethasone → Revlimid, Velcade, dexamethasone → SCT → Revlimid → Velcade, dexamethasone | Abnormal: Trisomy 3, 7, 9, 11, 15 | 0.53 | 0.18 | 3.56 |
| 2. Panobinostat/placebo, Velcade, dexamethasone | ||||||||||
| 10 | M | 54 (55) | MM IgGκ | I | Relapsed | 1. Revlimid, Velcade, Doxil, dexamethasone | Normal | 0.97 | 0.54 | 3.79 |
| 11 | F | 47 (55) | MM nonsecretory | I | Relapsed | 1. Thalidomide, Cytoxan, dexamethasone → SCT → thalidomide | Normal | 1.36 | 0.53 | 3.83 |
| 2. Marizomib | ||||||||||
| 12 | M | 49 (63) | MM IgGλ | I | Relapsed | 1. Vincristine, Doxil, dexamethasone → SCT | Abnormal: Trisomy 3, 7, 9, tetrasomy 11, 15 | 1.3 | 0.52 | 1.81 |
| 2. Velcade dexamethasone | ||||||||||
| 3. Revlimid, dexamethasone | ||||||||||
| 13 | M | 65 (70) | MM IgAλ | I | Relapsed | 1. Thalidomide, dexamethasone → SCT → thalidomide, dexamethasone | Abnormal: 14q32, 11q13, 4p16 | 0.62 | 0.65 | 3.28 |
| 2. Revlimid → Velcade, dexamethasone → SCT → Revlimid | ||||||||||
| 14 | F | 59 (59) | MM IgGλ | I | Newly diagnosed | 1. Revlimid, Velcade, dexamethasone, vorinostat | Normal | 3.36 | 0.72 | 3.68 |
| 15 | M | 61 (61) | MM IgGκ | I | Newly diagnosed | 1. Revlimid, Velcade, dexamethasone | Abnormal: Trisomy 3, 9, 11 | 2.09 | 0.31 | 2.55 |
| 16 | M | 46 (49) | MM IgGλ | II | Newly diagnosed | 1. Thalidomide, Velcade, dexamethasone → SCT → Revlimid | 13delq, 5delq, t(4:14) at diagnosis | 1.12 | 0.26 | 1.69 |
| 17 | M | 62 (67) | MM IgGκ | I | Newly diagnosed | 1. Thalidomide, dexamethasone → SCT → Revlimid | Normal | 0.54 | 0.05 | 0.99 |
| Patients . | Gender . | Age* . | Diagnosis . | Stage† . | Treatment status . | Treatment . | Cytogenetics . | Lymphocyte (K/μL) . | Myelocyte (K/μL) . | Neutrophil (K/μL) . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 62 (65) | MM IgAλ | I | Relapsed/refractory | 1. Revlimid, Velcade, dexamethasone | Normal | 0.8 | 0.32 | 2.84 |
| 2. Tanespimycin, Velcade | ||||||||||
| 2 | M | 46 (52) | MM IgAκ | I | Relapsed/refractory | 1. Revlimid, Velcade, dexamethasone → SCT → Revlimid, dexamethasone | Normal | 0.11 | 0.09 | 1.28 |
| 2. Revlimid, Velcade, dexamethasone | ||||||||||
| 3 | F | 62 (64) | MM IgAκ | I | Relapsed/refractory | 1. Revlimid, dexamethasone → SCT → Revlimid, dexamethasone | Abnormal: 14q32, 11q13, 4p16, 13q14.3, 13q34 | 0.74 | 0.28 | 1.24 |
| 4 | M | 66 (69) | MM IgGκ | III | Relapsed/refractory | 1. Thalidomide, Velcade, dexamethasone | Abnormal: 13delq | 0.61 | 0.09 | 8 |
| 2. Revlimid, melphalan, prednisone | ||||||||||
| 3. Cytoxan, etoposide, dexamethasone → SCT → Revlimid | ||||||||||
| 4. Thalidomide, Velcade, Doxil, dexamethasone → thalidomide, Velcade, dexamethasone | ||||||||||
| 5 | M | 56 (57) | MM IgGκ | I | Relapsed/refractory | 1. Velcade, dexamethasone → thalidomide, Velcade, dexamethasone + cisplatin, Doxil, Cytoxan, etoposide → SCT → Revlimid | Normal | 0.83 | 0.26 | 1.43 |
| 6 | F | 59 (63) | MM IgGκ | I | Relapsed/refractory | 1. Revlimid, Velcade, dexamethasone → SCT → Revlimid | Abnormal: +7, +16, 13del, 17delp, t(4:14) | 0.82 | 0.34 | 3.1 |
| 2. Perifosine/placebo, Velcade, dexamethasone | ||||||||||
| 7 | F | 51 (57) | MM IgAκ | II | Relapsed/refractory | 1. Thalidomide, dexamethasone → SCT → thalidomide | Abnormal: Trisomy 3, 5, 7, 9, 17, 19, t(4:14) | 1.15 | 0.23 | 3.17 |
| 2. Revlimid, dexamethasone | ||||||||||
| 3. Thalidomide, Velcade, Doxil, dexamethasone | ||||||||||
| 4. Panobinostat, dexamethasone | ||||||||||
| 8 | F | 60 (67) | MM IgGλ | I | Relapsed/refractory | 1. Thalidomide, dexamethasone | Abnormal: 17delp, trisomy 3, 7, 9, 11, 15 | 1.09 | 0.68 | 3.12 |
| 2. Velcade | ||||||||||
| 3. Ixazomib | ||||||||||
| 9 | M | 56 (60) | MM IgGκ | II | Relapsed/refractory | 1. Velcade, dexamethasone → Revlimid, Velcade, dexamethasone → SCT → Revlimid → Velcade, dexamethasone | Abnormal: Trisomy 3, 7, 9, 11, 15 | 0.53 | 0.18 | 3.56 |
| 2. Panobinostat/placebo, Velcade, dexamethasone | ||||||||||
| 10 | M | 54 (55) | MM IgGκ | I | Relapsed | 1. Revlimid, Velcade, Doxil, dexamethasone | Normal | 0.97 | 0.54 | 3.79 |
| 11 | F | 47 (55) | MM nonsecretory | I | Relapsed | 1. Thalidomide, Cytoxan, dexamethasone → SCT → thalidomide | Normal | 1.36 | 0.53 | 3.83 |
| 2. Marizomib | ||||||||||
| 12 | M | 49 (63) | MM IgGλ | I | Relapsed | 1. Vincristine, Doxil, dexamethasone → SCT | Abnormal: Trisomy 3, 7, 9, tetrasomy 11, 15 | 1.3 | 0.52 | 1.81 |
| 2. Velcade dexamethasone | ||||||||||
| 3. Revlimid, dexamethasone | ||||||||||
| 13 | M | 65 (70) | MM IgAλ | I | Relapsed | 1. Thalidomide, dexamethasone → SCT → thalidomide, dexamethasone | Abnormal: 14q32, 11q13, 4p16 | 0.62 | 0.65 | 3.28 |
| 2. Revlimid → Velcade, dexamethasone → SCT → Revlimid | ||||||||||
| 14 | F | 59 (59) | MM IgGλ | I | Newly diagnosed | 1. Revlimid, Velcade, dexamethasone, vorinostat | Normal | 3.36 | 0.72 | 3.68 |
| 15 | M | 61 (61) | MM IgGκ | I | Newly diagnosed | 1. Revlimid, Velcade, dexamethasone | Abnormal: Trisomy 3, 9, 11 | 2.09 | 0.31 | 2.55 |
| 16 | M | 46 (49) | MM IgGλ | II | Newly diagnosed | 1. Thalidomide, Velcade, dexamethasone → SCT → Revlimid | 13delq, 5delq, t(4:14) at diagnosis | 1.12 | 0.26 | 1.69 |
| 17 | M | 62 (67) | MM IgGκ | I | Newly diagnosed | 1. Thalidomide, dexamethasone → SCT → Revlimid | Normal | 0.54 | 0.05 | 0.99 |
The frequency and the functional characteristics of MDSCs were analyzed in the BMMCs and/or PBMCs obtained from patients with MM. The patient samples were chosen to represent the heterogeneity in this disease. Healthy donors were age matched. The counts of immune cells (lymphocytes, myelocytes, and neutrophils) are indicated as K/μL.
F, female; M, male; SCT, stem cell transplantation.
Age is shown as age at the time of diagnosis and (age at the time of sample collection).
Staging was defined using the International Staging System.
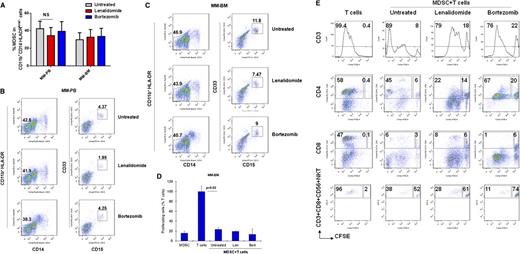
Increased frequency of MDSCs in patients with MM. The presence and the frequency of MDSCs were quantitated in the PB and the BM of patients with MM (Table 1) and in healthy donors by multiparameter flow cytometry. PBMCs and BMMCs were obtained from patients with newly diagnosed (n = 4), relapsed (n = 4), and relapsed/refractory (n = 9) MM, as well as from healthy donors (n = 5), and then stained for MDSCs using fluorochrome-labeled antibodies against CD11b, CD14, HLA-DR, CD33, CD15, and isotype controls. MDSCs were immunophenotyped as the CD11b+CD14-HLA-DR-/lowCD33+CD15+ population and quantitated as a percentage of gated cells by acquiring a minimum of 10 000 live events per sample. (A) Representative multiparameter dot plots of MDSCs within the CD11b+CD14-HLA-DR-/low population (gated, left) are shown as CD11b+CD14-HLA-DR-/lowCD33+CD15+ population (gated, right) in PBMCs from healthy donors (top row), as well as PBMCs (middle row) and BMMCs (bottom row) from patients with relapsed MM. (B) Shown is the frequency of CD11b+CD14-HLA-DR-/lowCD33+CD15+ MDSCs in PBMCs from healthy donors compared with PBMCs and BMMCs from patients with MM (left). The frequency of MDSCs in PBMCs from healthy donors compared with BMMCs from patients with newly diagnosed, relapsed, or relapsed/refractory MM are also shown (right). The data represent the percentage of MDSCs in PBMCs or BMMCs. Statistical significance is indicated (Student t test, 1-tailed distribution, P < .05). (C) Shown are representative cytospin images of CD11b+CD14+ myeloid cells (left) and CD11b+CD14-HLA-DR-/lowCD33+CD15+ MDSCs (right) with Wright-Giemsa staining in BMMCs from patients with relapsed MM. Photomicrographs show myeloid cells, identified by the mononuclear or polymorphonuclear cell nuclear staining (blue) using light microscopy (20 × 0.30 objective magnification) (Leica DM IL; Bannockburn, IL) and analyzed using a Leica DFC490 camera and Leica Application Suite version 2.8 software. (D) Intracellular expression of the inhibitory molecules ROS and ARG1 in MDSCs within MM-BMMCs are demonstrated by histogram plots. The x-axis represents ROS or ARG1, and the y-axis represents the number of positive cells within the MM-BMMCs. The negative control is shown (gray dotted line), as are the intracellular expression of ROS or ARG1 in CD11b+CD14+HLA-DR+ myeloid cells (blue line) and in CD11b+CD14-HLA-DR-/lowCD33+CD15+ MDSCs (red line) within BMMCs of patients with relapsed MM disease. The data shown are representative of 3 different experiments. hPB, PB from healthy donors; NewDx-MM, newly diagnosed patients; Rel-MM, relapsed patients; Rel/Ref-MM, relapsed/refractory patients.
Increased frequency of MDSCs in patients with MM. The presence and the frequency of MDSCs were quantitated in the PB and the BM of patients with MM (Table 1) and in healthy donors by multiparameter flow cytometry. PBMCs and BMMCs were obtained from patients with newly diagnosed (n = 4), relapsed (n = 4), and relapsed/refractory (n = 9) MM, as well as from healthy donors (n = 5), and then stained for MDSCs using fluorochrome-labeled antibodies against CD11b, CD14, HLA-DR, CD33, CD15, and isotype controls. MDSCs were immunophenotyped as the CD11b+CD14-HLA-DR-/lowCD33+CD15+ population and quantitated as a percentage of gated cells by acquiring a minimum of 10 000 live events per sample. (A) Representative multiparameter dot plots of MDSCs within the CD11b+CD14-HLA-DR-/low population (gated, left) are shown as CD11b+CD14-HLA-DR-/lowCD33+CD15+ population (gated, right) in PBMCs from healthy donors (top row), as well as PBMCs (middle row) and BMMCs (bottom row) from patients with relapsed MM. (B) Shown is the frequency of CD11b+CD14-HLA-DR-/lowCD33+CD15+ MDSCs in PBMCs from healthy donors compared with PBMCs and BMMCs from patients with MM (left). The frequency of MDSCs in PBMCs from healthy donors compared with BMMCs from patients with newly diagnosed, relapsed, or relapsed/refractory MM are also shown (right). The data represent the percentage of MDSCs in PBMCs or BMMCs. Statistical significance is indicated (Student t test, 1-tailed distribution, P < .05). (C) Shown are representative cytospin images of CD11b+CD14+ myeloid cells (left) and CD11b+CD14-HLA-DR-/lowCD33+CD15+ MDSCs (right) with Wright-Giemsa staining in BMMCs from patients with relapsed MM. Photomicrographs show myeloid cells, identified by the mononuclear or polymorphonuclear cell nuclear staining (blue) using light microscopy (20 × 0.30 objective magnification) (Leica DM IL; Bannockburn, IL) and analyzed using a Leica DFC490 camera and Leica Application Suite version 2.8 software. (D) Intracellular expression of the inhibitory molecules ROS and ARG1 in MDSCs within MM-BMMCs are demonstrated by histogram plots. The x-axis represents ROS or ARG1, and the y-axis represents the number of positive cells within the MM-BMMCs. The negative control is shown (gray dotted line), as are the intracellular expression of ROS or ARG1 in CD11b+CD14+HLA-DR+ myeloid cells (blue line) and in CD11b+CD14-HLA-DR-/lowCD33+CD15+ MDSCs (red line) within BMMCs of patients with relapsed MM disease. The data shown are representative of 3 different experiments. hPB, PB from healthy donors; NewDx-MM, newly diagnosed patients; Rel-MM, relapsed patients; Rel/Ref-MM, relapsed/refractory patients.
MDSCs promote tumor growth in the MM microenvironment
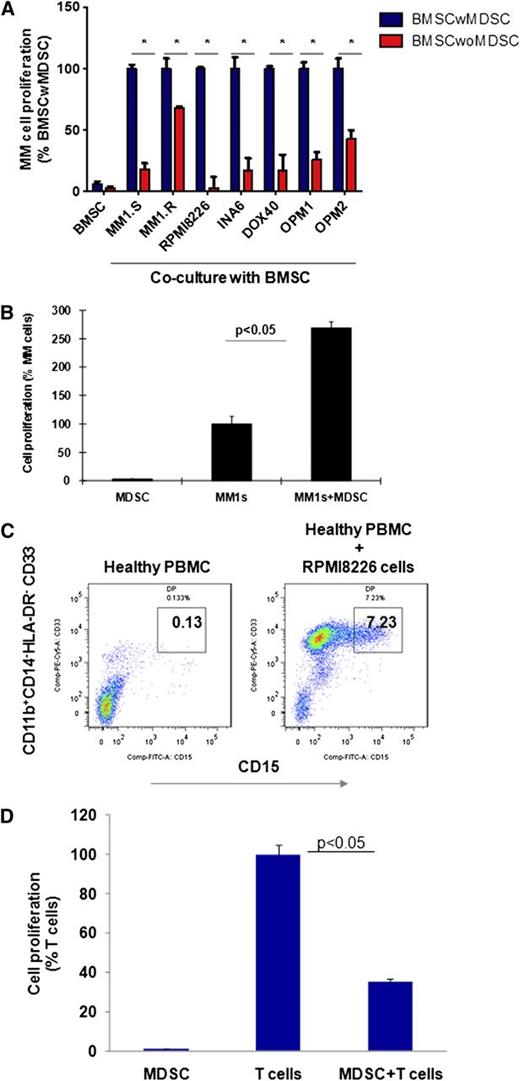
Because MDSCs in solid tumors induce tumor development and growth by providing a tumor-supportive and immune-suppressive microenvironment, we next assessed whether MDSCs within primary MM stroma play a similar role in promoting MM cell growth (Figure 2). 3H-Thymidine proliferation assay demonstrates that depletion of MDSCs from BMSCs significantly reduces the induction of MM cell proliferation by BMSCs (Figure 2A). Importantly, the MDSCs of patients with MM directly induce MM cell proliferation, confirming their significant tumor-promoting role in MM (Figure 2B). To determine the bidirectional interaction of MM cells and MDSCs in MM, we next assessed whether MM cells can induce MDSCs. Healthy donor PBMCs were cultured with MM cell lines for 4 to 6 days, and MDSCs were then quantitated by flow cytometric analysis. As shown in Figure 2C, MM cells significantly induced (50-fold) MDSCs from healthy donor PBMCs in vitro. Moreover, these MM cell-induced MDSCs from normal donors demonstrated significant suppressive activity against autologous T cells, confirming both the phenotypic and functional characteristics of MDSCs (Figure 2D). Our results therefore demonstrate a bidirectional interaction between MM cells and MDSCs, because MDSCs induce MM cell growth directly or within the BM stroma; conversely, MM cells trigger the development of MDSCs.
MDSCs promote tumor growth in the MM microenvironment. Bidirectional interaction between MM cells and MDSCs is demonstrated as MDSC-mediated MM growth; conversely, MM cells induced the development of MDSCs in vitro. (A) MDSC-mediated MM growth within the MM stroma is demonstrated by 3H-thymidine proliferation assay. BMSCs were generated in vitro from patients with MM BMMCs, with or without MDSC depletion, and then cocultured for 24 hours with a panel of BMSC-responsive MM cell lines. MM cell proliferation was measured by 3H-thymidine incorporation assay. Shown is the percentage of MM cell proliferation in cocultures of MM cell-BMSC without MDSC relative to MM cell–BMSC with MDSC. *The data represent the percentage of MM cell proliferation in cultures with BMSCs that have been generated from BM mononuclear cells with or without MDSC depletion (mean ± SD of triplicate cultures with statistical significance P < .05 by Student t test, 2-tailed distribution). Blue columns represent MM cells cultured with BMSCs, and red columns represent MM cells cultured with BMSCs without MDSCs. (B) The direct effect of MDSCs on MM growth was demonstrated in MM cell line–MDSC cocultures by 3H-thymidine incorporation assay. MDSCs were isolated from MM-BMMCs and cultured with MM cell lines for 48 hours. MM cell proliferation is shown relative to MM cells alone. The data represent mean ± SD of triplicate cultures and are representative of 3 different experiments. Statistical significance is indicated (Student t test, 1-tailed distribution, P < .05). (C) MM-induced MDSC development in healthy PBMCs is shown. PBMCs from healthy donors were cultured with a panel of MM cell lines for 6 days, and MDSCs were determined by multiparametric flow cytometry analysis. Representative dot plots are shown: CD33+CD15+ MDSCs (gated box) within the CD11b+CD14-HLA-DR-/low gated cells in healthy PBMCs (left), and healthy PBMCs cultured with MM cell line RPMI8226 (right). The data shown are representative of 3 different experiments. (D) MM cell–induced MDSCs were further characterized by their immune-suppressive activity against autologous healthy T cells. MDSCs were isolated from MM cell (RPMI8226) and healthy PBMC cocultures, and autologous healthy T cells were isolated from healthy PBMCs by FACS sorting. Then MDSCs were cultured with T cells in the presence of CD3/CD28/IL-2 stimulatory factors for 4 days, and T-cell proliferation was measured by 3H-thymidine incorporation. T-cell proliferation in cultures with or without MDSCs is shown. The data represent mean ± SD of triplicate cultures with statistical significance P < .05 (Student t test, 2-tailed distribution).
MDSCs promote tumor growth in the MM microenvironment. Bidirectional interaction between MM cells and MDSCs is demonstrated as MDSC-mediated MM growth; conversely, MM cells induced the development of MDSCs in vitro. (A) MDSC-mediated MM growth within the MM stroma is demonstrated by 3H-thymidine proliferation assay. BMSCs were generated in vitro from patients with MM BMMCs, with or without MDSC depletion, and then cocultured for 24 hours with a panel of BMSC-responsive MM cell lines. MM cell proliferation was measured by 3H-thymidine incorporation assay. Shown is the percentage of MM cell proliferation in cocultures of MM cell-BMSC without MDSC relative to MM cell–BMSC with MDSC. *The data represent the percentage of MM cell proliferation in cultures with BMSCs that have been generated from BM mononuclear cells with or without MDSC depletion (mean ± SD of triplicate cultures with statistical significance P < .05 by Student t test, 2-tailed distribution). Blue columns represent MM cells cultured with BMSCs, and red columns represent MM cells cultured with BMSCs without MDSCs. (B) The direct effect of MDSCs on MM growth was demonstrated in MM cell line–MDSC cocultures by 3H-thymidine incorporation assay. MDSCs were isolated from MM-BMMCs and cultured with MM cell lines for 48 hours. MM cell proliferation is shown relative to MM cells alone. The data represent mean ± SD of triplicate cultures and are representative of 3 different experiments. Statistical significance is indicated (Student t test, 1-tailed distribution, P < .05). (C) MM-induced MDSC development in healthy PBMCs is shown. PBMCs from healthy donors were cultured with a panel of MM cell lines for 6 days, and MDSCs were determined by multiparametric flow cytometry analysis. Representative dot plots are shown: CD33+CD15+ MDSCs (gated box) within the CD11b+CD14-HLA-DR-/low gated cells in healthy PBMCs (left), and healthy PBMCs cultured with MM cell line RPMI8226 (right). The data shown are representative of 3 different experiments. (D) MM cell–induced MDSCs were further characterized by their immune-suppressive activity against autologous healthy T cells. MDSCs were isolated from MM cell (RPMI8226) and healthy PBMC cocultures, and autologous healthy T cells were isolated from healthy PBMCs by FACS sorting. Then MDSCs were cultured with T cells in the presence of CD3/CD28/IL-2 stimulatory factors for 4 days, and T-cell proliferation was measured by 3H-thymidine incorporation. T-cell proliferation in cultures with or without MDSCs is shown. The data represent mean ± SD of triplicate cultures with statistical significance P < .05 (Student t test, 2-tailed distribution).
Immune-suppressive activity of MDSCs in MM
Due to the phenotypic heterogeneity of MDSCs in humans, it is essential to characterize MDSCs not only by their distinct phenotype, but also by their immune-suppressive activity in patients. Therefore, we next analyzed whether the CD11b+CD14-HLA-DR-/lowCD33+CD15+ MDSC population has immune-suppressive function in the MM-BM and PB microenvironment (Figure 3). MDSCs and autologous CD3+ T cells were isolated from the BM or the PB of patients with MM by using FACS sorting. MDSCs were then cocultured for 4 days with autologous T cells in the presence of T-cell stimulators (IL-2 and anti-CD3/CD28 Abs). 3H-Thymidine cell proliferation assay demonstrate that MDSCs significantly inhibit autologous T-cell proliferation in both MM-PB (Figure 3A) and MM-BM (Figure 3B). Next, the MDSC-mediated suppressive effect on each immune effector cell subpopulation, including CD4+ T cells, CD8+ T cells, and NKT cells in the MM-BM milieu was analyzed using carboxyfluorescein diacetate succinimidyl ester (CFSE)–flow cytometric analysis (Figure 3C). MDSCs, CD11b+CD14+HLA-DR+ APCs, and CD3+ T cells were sorted from MM-BM. CFSE-labeled T cells were then cultured in the presence of IL-2 and aCD3/CD28 MAbs for 6 to 8 days with either autologous MDSCs, autologous APCs, or healthy allogeneic PBMCs. Flow cytometric analysis demonstrated that autologous MM-BM CD11b+CD14+HLA-DR+ APCs were able to induce activated CD3+ T-cell proliferation comparable to healthy allogeneic PBMCs, whereas MDSCs significantly suppressed activated T-cell proliferation in MM-BM (Figure 3C, top row). CFSE labeling enabled us to define proliferating (dividing) and suppressed (nondividing) cells within each effector T-cell population. Specifically, CFSEhigh nondividing CD3+ T cells were significantly increased in the coculture of MDSCs and autologous T cells (8%) compared with cultures of either autologous APCs or allogeneic PBMCs with T cells (0.4%) (Figure 3C, top row). Within this CD3+ T-cell population, CD4+ T-cell proliferation was highly stimulated by both allogeneic PBMCs (dividing cells: 74%, nondividing cells: 0.3%) and autologous APCs (dividing cells: 58%, nondividing cells: 0.4%), but it was significantly suppressed by autologous MDSCs (dividing cells: 45%, nondividing cells: 6%) (Figure 3C, second row). Additionally, MDSCs demonstrated strong suppressive activity against both CD8+ T cells (dividing cells: 6%, nondividing cells: 3%) (Figure 3C, third row) and NKT cells (dividing cells: 38%, nondividing cells: 52%) (Figure 3C, bottom row). These results therefore show that CD11b+CD14-HLA-DR-/lowCD33+CD15+ MDSCs can induce immune suppression in the MM-BM microenvironment, which in turn downregulate CD4+ T-cell, CD8+ T-cell, and NKT cell–mediated antitumor immune responses. Of note, to identify MDSC subpopulations by their functional characteristics in the MM-BM microenvironment, CD14+HLA-DR+ APCs, CD11b+CD14+HLA-DR-/low monocytelike MDSCs, and CD11b+CD14-HLA-DR-/lowCD33+CD15+ neutrophillike MDSCs were isolated from MM-BM and cultured with autologous T cells for 4 days in the presence of T-cell stimulator factors. Proliferation assay by 3H-thymidine incorporation demonstrated that CD11b+CD14-HLA-DR-/lowCD33+CD15+ neutrophillike MDSCs have significantly increased immune-suppressive activity compared with CD11b+CD14+HLA-DR-/low monocytelike MDSCs in MM-BM (supplementary Figure 1).
Immune-suppressive activity of MDSCs in MM. MM-MDSC–mediated T-cell suppression was assessed in autologous MDSC–T-cell cultures by proliferation assays. MDSCs and autologous CD3+ T cells were isolated from (A) PBMCs or (B) BMMCs from patients with relapsed MM disease and were cultured in the presence of T-cell stimulators (CD3/CD28 MAbs and IL-2) for 4 days; then T-cell proliferation was measured by 3H-thymidine incorporation assay. The data represent mean ± SD of triplicate cultures. The data shown are representative of 3 different experiments. Statistical significance is indicated (Student t test, 1-tailed distribution, P < .05). (C) MDSC-mediated immune suppression was determined using CFSE flow cytometry analysis within autologous T-cell subpopulations including total CD3+ T cells, CD4+ T cells, CD8+ T cells, and CD3+CD8+CD56+ NKT cells in BMMCs from patients with relapsed MM disease. CD11b+CD14+HLA-DR+ APCs, CD11b+CD14-HLA-DR-/lowCD33+CD15+ MDSCs, and autologous CD3+ T cells were sorted from BMMCs of patients with relapsed MM disease. Allogeneic healthy donor PBMCs were used as control stimulator cells. T cells labeled with CFSE were cultured in the presence of aCD3/CD28 MAbs and IL-2 for 6 to8 days alone or with healthy allogeneic PBMCs, autologous APCs, or autologous MDSCs. Proliferating T-cell subpopulations were determined using CFSE costaining with MAbs against CD3, CD4, CD8, and CD56. CFSElow dividing cells represent proliferating total T cells, and CFSEhigh nondividing cells represent the suppressed total T-cell population (top, histogram plots). CFSElow proliferating (large gated box) and CFSEhigh nonproliferating (small gated box) T-cell subpopulations within CD4+ T cells (second row), CD8+ T cells (third row), and NKT cells (bottom row) plots are shown. (D) Mechanisms of MDSC-mediated T-cell suppression were investigated in MDSC-autologous T-cell cocultures using specific inhibitors of MDSC-associated suppressive factors ARG1 (nor-NOHA), iNOS (NMMA), ROS (apocynin), and COX2 (celecoxib). MDSCs and autologous CD3+ T cells were isolated from patients with relapsed MM disease, and then cocultured for 4 days in the presence of T-cell stimulators (aCD3/CD28 MAbs, IL-2), with or without inhibitors. T-cell proliferation was measured by 3[H]-thymidine incorporation assay. The percentage of proliferating T cells is demonstrated as T-cell+MDSC with inhibitor relative to T-cell+APC with inhibitor. The blue column indicates APC–autologous T-cell culture, and the red columns indicate MDSC–autologous T-cell culture. The data represent mean ± SD of triplicate cultures and are representative of 3 different experiments. Statistical significance is indicated (Student t test, 1-tailed distribution, P < .05).
Immune-suppressive activity of MDSCs in MM. MM-MDSC–mediated T-cell suppression was assessed in autologous MDSC–T-cell cultures by proliferation assays. MDSCs and autologous CD3+ T cells were isolated from (A) PBMCs or (B) BMMCs from patients with relapsed MM disease and were cultured in the presence of T-cell stimulators (CD3/CD28 MAbs and IL-2) for 4 days; then T-cell proliferation was measured by 3H-thymidine incorporation assay. The data represent mean ± SD of triplicate cultures. The data shown are representative of 3 different experiments. Statistical significance is indicated (Student t test, 1-tailed distribution, P < .05). (C) MDSC-mediated immune suppression was determined using CFSE flow cytometry analysis within autologous T-cell subpopulations including total CD3+ T cells, CD4+ T cells, CD8+ T cells, and CD3+CD8+CD56+ NKT cells in BMMCs from patients with relapsed MM disease. CD11b+CD14+HLA-DR+ APCs, CD11b+CD14-HLA-DR-/lowCD33+CD15+ MDSCs, and autologous CD3+ T cells were sorted from BMMCs of patients with relapsed MM disease. Allogeneic healthy donor PBMCs were used as control stimulator cells. T cells labeled with CFSE were cultured in the presence of aCD3/CD28 MAbs and IL-2 for 6 to8 days alone or with healthy allogeneic PBMCs, autologous APCs, or autologous MDSCs. Proliferating T-cell subpopulations were determined using CFSE costaining with MAbs against CD3, CD4, CD8, and CD56. CFSElow dividing cells represent proliferating total T cells, and CFSEhigh nondividing cells represent the suppressed total T-cell population (top, histogram plots). CFSElow proliferating (large gated box) and CFSEhigh nonproliferating (small gated box) T-cell subpopulations within CD4+ T cells (second row), CD8+ T cells (third row), and NKT cells (bottom row) plots are shown. (D) Mechanisms of MDSC-mediated T-cell suppression were investigated in MDSC-autologous T-cell cocultures using specific inhibitors of MDSC-associated suppressive factors ARG1 (nor-NOHA), iNOS (NMMA), ROS (apocynin), and COX2 (celecoxib). MDSCs and autologous CD3+ T cells were isolated from patients with relapsed MM disease, and then cocultured for 4 days in the presence of T-cell stimulators (aCD3/CD28 MAbs, IL-2), with or without inhibitors. T-cell proliferation was measured by 3[H]-thymidine incorporation assay. The percentage of proliferating T cells is demonstrated as T-cell+MDSC with inhibitor relative to T-cell+APC with inhibitor. The blue column indicates APC–autologous T-cell culture, and the red columns indicate MDSC–autologous T-cell culture. The data represent mean ± SD of triplicate cultures and are representative of 3 different experiments. Statistical significance is indicated (Student t test, 1-tailed distribution, P < .05).
To determine whether the suppressive activity of MDSCs is mediated by MDSC-derived factors such as ARG1, iNOS, and COX2, suppression assays in cocultures of MDSCs with autologous T cells from MM-BM were next performed in the presence of specific inhibitors against ARG1 (nor-NOHA), iNOS (NMMA), ROS (apocynin), and COX2 (celecoxib) (Figure 3D). There was no complete reversal of MDSC-induced T-cell suppression by the addition of these inhibitors. However, inhibitors of ARG1 and iNOS partially abrogated the MDSC-related immunosuppressive effect (P < .02) (Figure 3D). Therefore, arginine and nitric oxide synthases mediate, at least in part, MDSC-induced T-cell suppression in the MM-BM microenvironment.
Effect of lenalidomide and bortezomib on MDSCs in the MM microenvironment
Because novel agents such as the immunomodulatory drug lenalidomide and proteasome inhibitor bortezomib target both tumor cells and the BM microenvironment, we next assessed whether lenalidomide and bortezomib modulate MDSC-mediated immune suppression in the MM microenvironment.
To define the immunomodulatory effects of lenalidomide and bortezomib, PBMCs or BMMCs from patients with MM were incubated with or without lenalidomide (1 μM) and bortezomib (5 nM) for 16 hours, and MDSCs were then quantitated by multiparametric flow cytometry analysis (Figure 4A). Although the number of MDSCs was slightly decreased in some MM-PBMC (Figure 4B) and MM-BMMC (Figure 4C) cultures with lenalidomide, there was no significant change in the average number of MDSCs in cultures with lenalidomide or bortezomib compared with untreated cells (Figure 4A). Importantly, 3H-thymidine proliferation assay in the coculture of autologous T cells with MDSCs (Figure 4D) in the presence or absence of these novel drugs demonstrated that neither lenalidomide nor bortezomib could overcome MDSC-mediated T-cell suppression. Further analysis of CFSE-labeled T cells cultured with autologous MM-BM MDSCs indicated that MDSCs dramatically suppressed T-cell proliferation, particularly in CD8+ T cells and NKT cells, and that neither lenalidomide nor bortezomib can alter this effect (Figure 4E). Therefore, MDSCs are increased in the MM microenvironment, where they induce both MM cell proliferation and immune suppression. Moreover, neither lenalidomide nor bortezomib alter this effect.
The effect of lenalidomide and bortezomib on MDSCs in the MM microenvironment. (A) Lenalidomide’s and bortezomib’s effects on MDSC frequency in both MM PBMCs (n = 14) and BMMCs (n = 17) are shown. MM-PBMCs and MM-BMMCs were cultured with or without lenalidomide (1 μM) and bortezomib (5 nM) for 16 hours, and MDSCs were determined by flow cytometric immunophenotyping. The data shown are mean ± SEM for the percentage of gated MDSCs in the minimum of 10 000 events per sample. NS indicates the lack of statistical significance (Student t test, 1-tailed distribution, P < .05). Representative 2-parameter dot plots demonstrate CD33+CD15+ MDSCs (gated small box, right) within the CD11b+CD14-HLA-DR-/low gated cell population (left) in (B) PBMCs and (C) BMMCs from patients with MM that were cultured in the absence (top) or presence of lenalidomide (middle) or bortezomib (bottom). (D) Lenalidomide’s and bortezomib’s effects on MM-MDSC–mediated T-cell suppression were assessed in autologous MDSC–T-cell cultures in MM-BMMCs by proliferation assays. MDSCs and autologous CD3+ T cells were isolated from patients with relapsed MM disease, and then cocultured for 4 days with T-cell stimulators (aCD3/CD28 MAbs, IL-2) in the presence and the absence of lenalidomide (1 μM) or bortezomib (5 nM). T-cell proliferation was measured by 3H-thymidine incorporation assay. The percentage of proliferating T cells is demonstrated relative to T cells alone. The data represent mean ± SD of triplicate cultures and are representative of 3 different experiments. Statistical significance is indicated (Student t test, 1-tailed distribution, P < .05). (E) Lenalidomide’s and bortezomib’s effects on MDSC-mediated immune suppression were determined in autologous T-cell–MDSC cocultures. MDSCs and autologous CD3+ T cells were sorted from the BMMCs of patients with MM. T cells were labeled with CFSE and stimulated with aCD3/CD28 Abs and IL-2, and then cultured for 6 to 8 days alone or with autologous MDSCs in the presence or the absence of lenalidomide (1 μM) and bortezomib (5 nM). Proliferating T-cell subpopulations, including total CD3+ T cells, CD4+ T cells, CD8+ T cells, and CD3+CD8+CD56+ NKT cells were determined using CFSE costaining with MAbs against CD3, CD4, CD8, and CD56. CFSElow dividing cells represent proliferating total T cells, and CFSEhigh nondividing cells represent the suppressed T-cell population (top, histogram plots). Shown are plots of CFSElow proliferating (large gated box) and CFSEhigh nonproliferating (small gated box) T-cell subpopulations within CD4+ T cells (second row), CD8+ T cells (third row), and NKT cells (bottom row). Len, lenalidomide; Bort, bortezomib.
The effect of lenalidomide and bortezomib on MDSCs in the MM microenvironment. (A) Lenalidomide’s and bortezomib’s effects on MDSC frequency in both MM PBMCs (n = 14) and BMMCs (n = 17) are shown. MM-PBMCs and MM-BMMCs were cultured with or without lenalidomide (1 μM) and bortezomib (5 nM) for 16 hours, and MDSCs were determined by flow cytometric immunophenotyping. The data shown are mean ± SEM for the percentage of gated MDSCs in the minimum of 10 000 events per sample. NS indicates the lack of statistical significance (Student t test, 1-tailed distribution, P < .05). Representative 2-parameter dot plots demonstrate CD33+CD15+ MDSCs (gated small box, right) within the CD11b+CD14-HLA-DR-/low gated cell population (left) in (B) PBMCs and (C) BMMCs from patients with MM that were cultured in the absence (top) or presence of lenalidomide (middle) or bortezomib (bottom). (D) Lenalidomide’s and bortezomib’s effects on MM-MDSC–mediated T-cell suppression were assessed in autologous MDSC–T-cell cultures in MM-BMMCs by proliferation assays. MDSCs and autologous CD3+ T cells were isolated from patients with relapsed MM disease, and then cocultured for 4 days with T-cell stimulators (aCD3/CD28 MAbs, IL-2) in the presence and the absence of lenalidomide (1 μM) or bortezomib (5 nM). T-cell proliferation was measured by 3H-thymidine incorporation assay. The percentage of proliferating T cells is demonstrated relative to T cells alone. The data represent mean ± SD of triplicate cultures and are representative of 3 different experiments. Statistical significance is indicated (Student t test, 1-tailed distribution, P < .05). (E) Lenalidomide’s and bortezomib’s effects on MDSC-mediated immune suppression were determined in autologous T-cell–MDSC cocultures. MDSCs and autologous CD3+ T cells were sorted from the BMMCs of patients with MM. T cells were labeled with CFSE and stimulated with aCD3/CD28 Abs and IL-2, and then cultured for 6 to 8 days alone or with autologous MDSCs in the presence or the absence of lenalidomide (1 μM) and bortezomib (5 nM). Proliferating T-cell subpopulations, including total CD3+ T cells, CD4+ T cells, CD8+ T cells, and CD3+CD8+CD56+ NKT cells were determined using CFSE costaining with MAbs against CD3, CD4, CD8, and CD56. CFSElow dividing cells represent proliferating total T cells, and CFSEhigh nondividing cells represent the suppressed T-cell population (top, histogram plots). Shown are plots of CFSElow proliferating (large gated box) and CFSEhigh nonproliferating (small gated box) T-cell subpopulations within CD4+ T cells (second row), CD8+ T cells (third row), and NKT cells (bottom row). Len, lenalidomide; Bort, bortezomib.
Molecular signature of MDSCs in MM
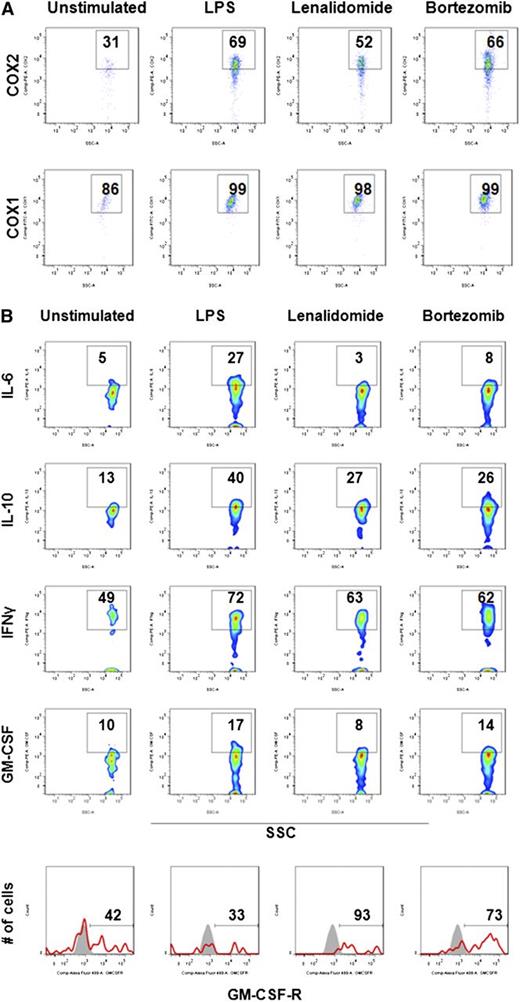
In addition to iNOS, ROS, and ARG1, MDSC-induced immune suppression in solid tumors can be mediated via COX2/prostoglandin E2 signaling and suppressive cytokines.27,29,30,41 To further characterize mechanisms of MDSC-associated immune suppression in MM, intracellular expression of suppressive factors was next assessed in MM-BM MDSCs using flow cytometry (Figure 5). MM-BMMCs were stimulated with LPS, and then cultured with or without lenalidomide and bortezomib. Intracellular flow cytometric analysis of the inflammatory suppressive molecules COX1/COX2 showed that lenalidomide decreased COX2 expression (Figure 5A, upper row) in activated MDSCs, whereas COX1 expression remained unchanged (Figure 5A, bottom row). Interestingly, intracellular expression of the suppressive cytokines IL-6 and IL-10, as well as the stimulatory cytokines IFN-γ and GM-CSF, was dramatically decreased in LPS-stimulated MDSCs cultured with lenalidomide or bortezomib; in contrast, cell surface expression of the GM-CSF receptor was significantly increased by lenalidomide and bortezomib treatment (Figure 5B).
Molecular signature of MDSCs in MM. Immunomodulatory effects of lenalidomide and bortezomib on the expression of MDSC-associated immune suppressive/inflammatory molecules were assessed in MM-BM using intracellular staining flow cytometry analysis. BMMCs from patients with relapsed MM disease were stimulated with LPS (1 μg/mL) and cultured for 16 hours with or without lenalidomide (1 μM) and bortezomib (5 nM). (A) Intracellular expression of immune-suppressive inflammatory molecules COX2 and COX1 were evaluated in MDSCs within BMMCs using anti-COX2 (PE) and anti-COX1 (fluorescein isothiocyanate ) MAbs. Representative 2-parameter dot plots indicate intracellular expression of COX2 (top row) and COX1 (bottom row) in MDSCs, gated as CD11b+CD14-HLA-DR-/lowCD33+CD15+. The data represent the percentage of positive cells per the minimum of 10 000 events per sample. The data shown are representative of 3 different experiments. (B) Intracellular expression of inhibitory cytokines (IL-6, IL10) and stimulatory cytokines (IFN-γ, GM-CSF), as well as surface expression of the GM-CSF receptor, were evaluated by flow cytometry in MM-BM MDSCs. MDSCs were gated as indicated above, and the percentage of expression of cytokines and receptors were demonstrated in representative 2-parameter dot plots. The data shown are representative of 3 different experiments. SSC, side-scattered light.
Molecular signature of MDSCs in MM. Immunomodulatory effects of lenalidomide and bortezomib on the expression of MDSC-associated immune suppressive/inflammatory molecules were assessed in MM-BM using intracellular staining flow cytometry analysis. BMMCs from patients with relapsed MM disease were stimulated with LPS (1 μg/mL) and cultured for 16 hours with or without lenalidomide (1 μM) and bortezomib (5 nM). (A) Intracellular expression of immune-suppressive inflammatory molecules COX2 and COX1 were evaluated in MDSCs within BMMCs using anti-COX2 (PE) and anti-COX1 (fluorescein isothiocyanate ) MAbs. Representative 2-parameter dot plots indicate intracellular expression of COX2 (top row) and COX1 (bottom row) in MDSCs, gated as CD11b+CD14-HLA-DR-/lowCD33+CD15+. The data represent the percentage of positive cells per the minimum of 10 000 events per sample. The data shown are representative of 3 different experiments. (B) Intracellular expression of inhibitory cytokines (IL-6, IL10) and stimulatory cytokines (IFN-γ, GM-CSF), as well as surface expression of the GM-CSF receptor, were evaluated by flow cytometry in MM-BM MDSCs. MDSCs were gated as indicated above, and the percentage of expression of cytokines and receptors were demonstrated in representative 2-parameter dot plots. The data shown are representative of 3 different experiments. SSC, side-scattered light.
Our results therefore demonstrate that MM-BM MDSCs induce immune suppression associated with increased expression of suppressor factors such as IL-6, IL-10, ARG1, iNOS, and ROS, which can be abrogated by lenalidomide and bortezomib. However, these drug-related effects could not overcome the functional immunosuppression conferred by MDSCs.
Discussion
Along with stroma cells, tumor-associated immune cells play an important role in tumor development and progression.1 In MM, characterizing the mechanisms whereby the BM milieu promotes immunosuppression may identify novel cellular/molecular therapeutic targets to modulate antitumor immune deficiency and thereby decrease minimal residual disease. Studies in solid tumors that were done since 2010 have demonstrated that tumor-associated myeloid immune cells, particularly MDSCs, play an important role in promoting tumor growth and suppressing antitumor immunity.12,15,42,43 However, their presence and function in tumor development and immune suppression have not been well characterized in hematologic malignancies, including MM.13,16,17,21,23,26,27,33,43 Specifically, we and others2,44,45 have focused on delineating the interaction between MM cells and immune effector T cells and NK cells, as well as lymphoid suppressor Tregs and Th17 cells and their response to novel therapeutic agents. But the mechanisms by which myeloid cells transform the MM-BM microenvironment into a tumor-promoting and immune-suppressive milieu in MM is not yet defined. We assessed MDSCs and MDSC-mediated tumor growth and T-cell suppression in MM, as well as their mechanism of action and response to lenalidomide and bortezomib.
MDSCs in patients with solid tumors have been characterized by distinct phenotypes, including Lin-HLA-DR-CD33+ or HLA-DR-CD14+ cells in melanoma, hepatocellular carcinoma, and prostate and renal cancer;25,36-40,46,47 Lin-HLA-DR-CD33+CD11b+ cells in breast cancer;17 and CD14-CD33+CD15+CD11b+ cells in non–small cell lung cancer.48 As in renal cancer, elevated levels of CD14+HLA-DR-/low MDSCs have recently been reported in patients with MM compared with healthy donors.49 However, due to this phenotypic heterogeneity of human MDSCs, they must also be characterized functionally for their ability to suppress T-cell activity. Based upon the previous phenotypic profile of MDSCs in solid tumors,24 we identified MDSCs with a distinct phenotype as CD11b+CD14-HLA-DR-/lowCD33+CD15+ in MM-BM and MM-PB. Of note, CD11b+CD14+HLA-DR-/low cells demonstrated either absent or less immune-suppressive activity compared with CD11b+CD14-HLA-DR-/lowCD33+CD15+ MDSCs in the MM microenvironment. This indicates that CD11b+CD14-HLA-DR-/lowCD33+CD15+ MDSCs modulate immunosuppression in the MM-BM. Interestingly, these MDSCs were dramatically increased in both the PB and the BM of patients with MM compared with those of healthy donors. Our data further suggest that MDSCs were differentially expressed in patients with different MM disease status and increase with disease progression, which is being prospectively studied in large randomized trials.
In addition to data from murine tumor models, recent studies have also demonstrated that solid tumor cell lines induce MDSC development in vitro.24 The interaction of MM cells with MDSCs also has important bidirectional biological sequelae, because our data show that MM cells induce development of MDSCs, which in turn provide a protective environment for tumor development and growth. In our study, human MM cell lines also induced MDSC development from healthy donor PBMCs, confirming bidirectional interaction between MDSCs and MM cells in the tumor-protective microenvironment. Moreover, MM-MDSCs were able to induce MM cell growth both directly or within the MM-BM stroma niche. These MDSCs were also able to suppress T cells, particularly CD8+ T cells and NKT cells, in the MM-BM. These studies identify targeting this interaction to both achieve clinical responses and relieve immunosuppression in MM.
We further characterized the mechanism of action of MM-BM MDSCs by demonstrating that the suppressive factors iNOS and ARG1 and the suppressive cytokines IL-6 and IL-10 are associated with their immune-suppressive effects. By using lenalidomide and bortezomib to target the tumor and its microenvironment, we and others2,4 have demonstrated that these agents induce T and NK cell–mediated antitumor response and modulate cytokine signaling. In this study, lenalidomide and bortezomib modulated IL-6 and IL-10 expression in MM-MDSCs, but they did not alter MDSC frequency or suppressive activity. Our ongoing efforts are attempting to identify novel agents or combinations that can not only positively affect the cytokine profile in this setting, but can also abrogate MDSC number and function.
In summary, inhibition of the tumor-promoting and immune-suppressing functions of stromal cells, particularly of MDSCs, represents a promising novel immune-based therapeutic strategy in MM and other cancers, with the potential for efficacy even in the context of advanced or high-risk disease. Specifically, immune suppressor cells (eg, Tregs, Th17 cells, and MDSCs) inhibit current therapies; conversely, enhancing effector immune cell activity and evading immune suppressor cell activity are critical to enhancing antitumor response. Importantly, our data demonstrate that MDSCs are increased in MM-BM, where they directly induce both MM cell proliferation and immunosuppression. Further understanding of the role of MDSCs in MM pathobiology and immunobiology, particularly delineating the molecular mechanisms mediating the tumor-promoting and immune-suppressive functions of MDSCs in the MM microenvironment, will provide potential new therapeutic strategies in MM. Moreover, elimination of MDSCs by novel agents may both enhance immune responses and improve patient outcome in MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Maureen French for her invaluable help in distribution of clinical samples.
This work was supported by the National Institutes of Health National Cancer Institute Specialized Program of Research Excellence in Myeloma (grant P50 CA100707) (K.C.A.); the National Institutes of Health National Cancer Institute Host-Tumor Cell Interactions in Myeloma: Therapeutic Applications (grant P01 CA78378) (K.C.A.); and the National Institutes of Health National Cancer Institute Molecular Sequelae of Myeloma–Bone Marrow Interactions: Therapeutic Applications (grant R01 CA50947) (K.C.A.).
Authorship
Contribution: G.T.G. made the hypothesis, designed and conducted the research, analyzed results, and made figures; G.T.G., G.W., and J.L.A. performed the experiments; T.H., C.M., J.L., N.R, N.C.M., P.G.R., and K.C.A. provided advice and clinical samples; and G.T.G. and K.C.A. wrote and edited the manuscript.
Conflict-of-interest disclosure: K.C.A. is an honoraria of Celgene, Millennium, Onyx; P.G.R. is an honoraria of Celgene, Millennium, Johnson &Johnson; N.C.M. is an honoraria of Celgene, Millennium, Novartis; and N.R. received research funding from, and is an honoraria of Amgen, Celgene, Novartis, AstraZeneca. The remaining authors declare no competing financial interests.
The current affiliation for K.C.A. is Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA.
Correspondence: Güllü Topal Görgün, Dana-Farber Cancer Institute, Department of Medical Oncology, 450 Brookline Ave, Boston, MA, 02215; e-mail: gullu_gorgun@dfci.harvard.edu.

![Figure 3. Immune-suppressive activity of MDSCs in MM. MM-MDSC–mediated T-cell suppression was assessed in autologous MDSC–T-cell cultures by proliferation assays. MDSCs and autologous CD3+ T cells were isolated from (A) PBMCs or (B) BMMCs from patients with relapsed MM disease and were cultured in the presence of T-cell stimulators (CD3/CD28 MAbs and IL-2) for 4 days; then T-cell proliferation was measured by 3H-thymidine incorporation assay. The data represent mean ± SD of triplicate cultures. The data shown are representative of 3 different experiments. Statistical significance is indicated (Student t test, 1-tailed distribution, P < .05). (C) MDSC-mediated immune suppression was determined using CFSE flow cytometry analysis within autologous T-cell subpopulations including total CD3+ T cells, CD4+ T cells, CD8+ T cells, and CD3+CD8+CD56+ NKT cells in BMMCs from patients with relapsed MM disease. CD11b+CD14+HLA-DR+ APCs, CD11b+CD14-HLA-DR-/lowCD33+CD15+ MDSCs, and autologous CD3+ T cells were sorted from BMMCs of patients with relapsed MM disease. Allogeneic healthy donor PBMCs were used as control stimulator cells. T cells labeled with CFSE were cultured in the presence of aCD3/CD28 MAbs and IL-2 for 6 to8 days alone or with healthy allogeneic PBMCs, autologous APCs, or autologous MDSCs. Proliferating T-cell subpopulations were determined using CFSE costaining with MAbs against CD3, CD4, CD8, and CD56. CFSElow dividing cells represent proliferating total T cells, and CFSEhigh nondividing cells represent the suppressed total T-cell population (top, histogram plots). CFSElow proliferating (large gated box) and CFSEhigh nonproliferating (small gated box) T-cell subpopulations within CD4+ T cells (second row), CD8+ T cells (third row), and NKT cells (bottom row) plots are shown. (D) Mechanisms of MDSC-mediated T-cell suppression were investigated in MDSC-autologous T-cell cocultures using specific inhibitors of MDSC-associated suppressive factors ARG1 (nor-NOHA), iNOS (NMMA), ROS (apocynin), and COX2 (celecoxib). MDSCs and autologous CD3+ T cells were isolated from patients with relapsed MM disease, and then cocultured for 4 days in the presence of T-cell stimulators (aCD3/CD28 MAbs, IL-2), with or without inhibitors. T-cell proliferation was measured by 3[H]-thymidine incorporation assay. The percentage of proliferating T cells is demonstrated as T-cell+MDSC with inhibitor relative to T-cell+APC with inhibitor. The blue column indicates APC–autologous T-cell culture, and the red columns indicate MDSC–autologous T-cell culture. The data represent mean ± SD of triplicate cultures and are representative of 3 different experiments. Statistical significance is indicated (Student t test, 1-tailed distribution, P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/15/10.1182_blood-2012-08-448548/4/m_2975f3.jpeg?Expires=1769085764&Signature=yZFqDFo~u~gEVXKMavkgGHXZPyG6wAcAh1gj3Z8qfE8FVeyK~or8ZSecfFdgb1bnIxKCH0WGwXH7yOwZmmtyce-iqCBgttdQTfmFZUzC0-m9DnwYWjzNSXvXsCy90nyiTM-40~yuKv6vKqwnUELyVds1fCMnYTbM5Bw37rG8mtq8pD1ystEfpzzExS2BtJbbb-YpfjDc-GVvJk7o1jBbPkXb--ga41VTqjVJd3w6F1Cw0pSOBu3LOpKplGlNhf-~Wgu2XfT-fvVprUCI6C~OhNRMNBg8sC5slGvveeAgY-LwhmARtkiME-yW-RKzZu2YT2OpezWu3zgzH1pSbT~x6w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)