Key Points
The secreted lymphangiogenic protein CCBE1 is essential for fetal but not postnatal erythropoiesis.
Loss of CCBE1 impairs erythroblastic island formation and function.
Abstract
The secreted protein CCBE1 is required for lymphatic vessel growth in fish and mice, and mutations in the CCBE1 gene cause Hennekam syndrome, a primary human lymphedema. Here we show that loss of CCBE1 also confers severe anemia in midgestation mouse embryos due to defective definitive erythropoiesis. Fetal liver erythroid precursors of Ccbe1 null mice exhibit reduced proliferation and increased apoptosis. Colony-forming assays and hematopoietic reconstitution studies suggest that CCBE1 promotes fetal liver erythropoiesis cell nonautonomously. Consistent with these findings, Ccbe1lacZ reporter expression is not detected in hematopoietic cells and conditional deletion of Ccbe1 in hematopoietic cells does not confer anemia. The expression of the erythropoietic factors erythropoietin and stem cell factor is preserved in CCBE1 null embryos, but erythroblastic island (EBI) formation is reduced due to abnormal macrophage function. In contrast to the profound effects on fetal liver erythropoiesis, postnatal deletion of Ccbe1 does not confer anemia, even under conditions of erythropoietic stress, and EBI formation is normal in the bone marrow of adult CCBE1 knockout mice. Our findings reveal that CCBE1 plays an essential role in regulating the fetal liver erythropoietic environment and suggest that EBI formation is regulated differently in the fetal liver and bone marrow.
Introduction
Regulation of erythropoiesis is essential throughout life, and loss of such regulation causes anemia associated with chronic disease states such as renal failure, cancer, and inflammation.1 Numerous proteins are identified to regulate the differentiation and proliferation of hematopoietic stem cells to mature erythrocytes in a cell-intrinsic fashion (reviewed in Hattangadi et al2). Two secreted factors, erythropoietin (EPO) and stem cell factor (SCF), have been demonstrated to stimulate erythropoiesis through actions on hematopoietic precursors.3,4
Extrinsic stimulation of erythroid precursors by EPO and SCF is achieved through binding and activation of cell surface receptors that generate intracellular signals to promote survival, differentiation, and proliferation. A second, less-understood mechanism to foster erythroblast survival and maturation is erythroblastic island (EBI) formation (reviewed in Chasis and Mohandas5 ). In EBIs, erythroid precursors are in direct contact with a central macrophage that is believed to function as a “nurse cell” to promote erythroid maturation in the fetal liver, bone marrow, and spleen.6,7 The precise functions of the EBI remain incompletely defined, but genetic studies support roles for the central macrophage in phagocytosing extruded erythroblast nuclei,8,9 delivering iron for hemoglobin synthesis, and supplying cytokines that support cell survival and growth.6,10,11 Tight adhesion between nascent erythroblasts and the central macrophage is thought to be critical for EBI function, and numerous genetically modified mice with fetal anemia have been shown to have EBIs with reduced erythroblast-macrophage adhesion. Examples include animals lacking the erythroblast-macrophage protein EMP,10 the intracellular protein palladin,12 and the transcription factor c-maf.13 Thus, factors required to establish EBIs within erythroblastic niches define an important but still poorly understood mechanism of extrinsic erythropoietic regulation.
Collagen and calcium-binding epidermal growth factor domain-containing protein 1 (CCBE1) is a highly conserved, secreted protein that has recently been identified as a critical lymphangiogenic factor. Zebrafish with mutations in ccbe1 fail to form lymphatic vessels,14 and human mutations in CCBE1 cause Hennekam syndrome,15 a rare disorder characterized by lymphatic vessel malformations (reviewed in Van Balkom et al16). Mice lacking CCBE1 fail to form any lymphatic vessels (Figure 1 and Bos et al17 ), demonstrating a conserved essential role for this secreted factor in regulating lymphatic vessel growth. In contrast to patients with Milroy’s disease, a primary lymphedema associated with mutations in VEGFR3,18,19 patients with Hennekam syndrome also exhibit mental retardation, suggesting that additional roles for CCBE1 exist outside the lymphatic vasculature.
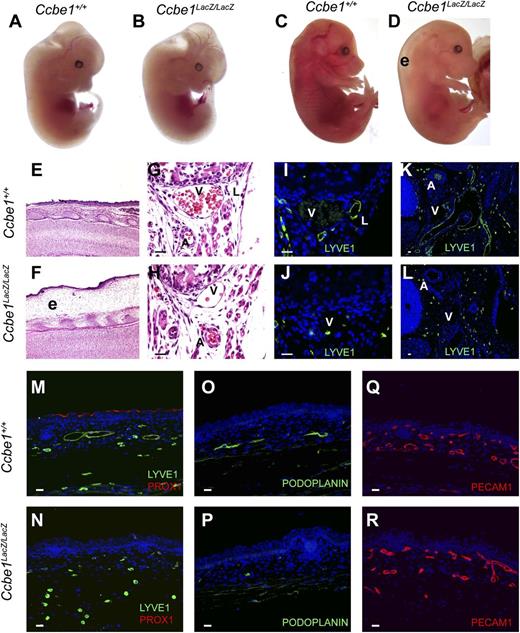
Ccbe1lacZ/lacZ embryos develop severe edema associated with a complete lack of lymphatic vessels. (A,B) E12.5 Ccbe1lacZ/lacZ embryos appear indistinguishable from wild-type littermates. (C,D) Severe cutaneous edema (“e”) is present in E16.5 Ccbe1lacZ/lacZ embryos. (E,F) Edema is revealed by H&E staining of skin from the E16.5 Ccbe1lacZ/lacZ and control embryos shown in panels C and D. (G-P) Ccbe1lacZ/lacZ embryos lack lymphatic vessels. (G,H) H&E staining of sagittal sections reveals a typical cluster of subcostal vessels including an artery (“A”), vein (“V”), and lymphatic (“L”) in an E16.5 wild-type embryo but the subcostal lymphatic is absent in a Ccbe1lacZ/lacZ littermate. (I,J) Anti-LYVE1 immunostaining confirms the absence of subcostal lymphatic vessels in an E16.5 Ccbe1lacZ/lacZ embryo. (K,L) Anti-LYVE1 immunostaining reveals a well-developed network of large lymphatic vessels adjacent to the carotid artery (“A”) and cardinal vein (“V”) in a transverse section from an E15.5 wild-type embryo, but none are present in a Ccbe1lacZ/lacZ littermate. (M-P) Immunostaining for LYVE1, PROX1, and PODOPLANIN identifies lymphatic vessels in the skin of an E16.5 wild-type embryo but not in a Ccbe1lacZ/lacZ littermate. Note the presence of LYVE1+;PROX1− cells that are likely to be tissue macrophages. (Q,R) CCBE1-deficient embryos have normal blood vessel growth. Platelet endothelial cell adhesion molecule staining of E16.5 wild-type and Ccbe1lacZ/lacZ littermate embryo skin is shown. The reduced number of total vessels in the Ccbe1lacZ/lacZ skin sample reflects the loss of lymphatic vessels. Scale bars indicate 20 μm. PECAM1, platelet endothelial cell adhesion molecule.
Ccbe1lacZ/lacZ embryos develop severe edema associated with a complete lack of lymphatic vessels. (A,B) E12.5 Ccbe1lacZ/lacZ embryos appear indistinguishable from wild-type littermates. (C,D) Severe cutaneous edema (“e”) is present in E16.5 Ccbe1lacZ/lacZ embryos. (E,F) Edema is revealed by H&E staining of skin from the E16.5 Ccbe1lacZ/lacZ and control embryos shown in panels C and D. (G-P) Ccbe1lacZ/lacZ embryos lack lymphatic vessels. (G,H) H&E staining of sagittal sections reveals a typical cluster of subcostal vessels including an artery (“A”), vein (“V”), and lymphatic (“L”) in an E16.5 wild-type embryo but the subcostal lymphatic is absent in a Ccbe1lacZ/lacZ littermate. (I,J) Anti-LYVE1 immunostaining confirms the absence of subcostal lymphatic vessels in an E16.5 Ccbe1lacZ/lacZ embryo. (K,L) Anti-LYVE1 immunostaining reveals a well-developed network of large lymphatic vessels adjacent to the carotid artery (“A”) and cardinal vein (“V”) in a transverse section from an E15.5 wild-type embryo, but none are present in a Ccbe1lacZ/lacZ littermate. (M-P) Immunostaining for LYVE1, PROX1, and PODOPLANIN identifies lymphatic vessels in the skin of an E16.5 wild-type embryo but not in a Ccbe1lacZ/lacZ littermate. Note the presence of LYVE1+;PROX1− cells that are likely to be tissue macrophages. (Q,R) CCBE1-deficient embryos have normal blood vessel growth. Platelet endothelial cell adhesion molecule staining of E16.5 wild-type and Ccbe1lacZ/lacZ littermate embryo skin is shown. The reduced number of total vessels in the Ccbe1lacZ/lacZ skin sample reflects the loss of lymphatic vessels. Scale bars indicate 20 μm. PECAM1, platelet endothelial cell adhesion molecule.
In this study, we demonstrate that CCBE1 is required for definitive erythropoiesis during fetal life in mice. We find that loss of CCBE1 does not affect the maturation of nonerythroid hematopoietic cells or the development of other fetal liver cell types. Colony-forming assays, reconstitution of irradiated mice with fetal liver cells, and conditional deletion studies demonstrate that the requirement for CCBE1 during fetal erythropoiesis is hematopoietic cell nonautonomous. The fetal anemia phenotype observed in CCBE1-deficient mice closely resembles that of animals lacking palladin or c-maf that exhibit defective EBI formation, and we detect a role for CCBE1 in establishing normal macrophage function within the EBI. Unexpectedly, CCBE1 is not required for EBI formation in postnatal bone marrow and postnatal CCBE1-deficient mice are not anemic, even after acute blood loss. Thus, secreted CCBE1 is required to maintain the erythropoietic environment and support EBI formation specifically within the fetal liver.
Materials and methods
Mice
The Ccbe1 gene was disrupted in SV129 embryonic stem (ES) cells by replacing the coding portion of exon 3 with a gene-trap cassette containing a splice acceptor IRES-β polyA and an FRT-flanked neomycin resistance cassette (L1L2_Bact_P). A conditional allele was generated by replacing exon 3 with a loxP-flanked exon 3 cassette. Correctly targeted ES cells were injected into blastocysts and chimeric offspring were crossed with SV129 and C57Bl/6 mice. Vav-Cre and Ub-CreERT2 animals were generously provided by Drs Thomas Graf and Eric Brown, respectively.20,21 A total of 0.1 mL of 20 mg/mL tamoxifen in sunflower oil was injected intraperitoneally daily for 10 to 14 days to induce deletion in adult Ub-CreERT2;Ccbe1flox/lacZ mice. The University of Pennsylvania Institutional Animal Care and Use Committee approved all animal protocols.
Histology and immunohistochemistry
Embryos and tissues were fixed in 4% paraformaldehyde for 24 to 48 hours, dehydrated in 100% ethanol, and embedded in paraffin. Serial 8-μm-thick sections were subjected to hematoxylin and eosin (H&E) and/or immunohistochemical staining as detailed on the University of Pennsylvania Molecular Cardiology Research Center Histology and Gene Expression Core website (http://www.med.upenn.edu/mcrc/histology_core/). Antibodies used can be found in supplemental Methods.
Flow cytometry studies
Single cells were harvested from fetal livers of different ages and were resuspended in phosphate-buffered saline 2% fetal bovine serum for fluorescence-activated cell sorting. Antibodies used can be found in supplemental Methods.
Blood collection and analysis
Embryonic peripheral blood was collected from the carotid artery using heparinized capillary tubes. Adult peripheral blood was collected retro-orbitally. Hematocrit and complete blood count were analyzed with an automated hemocytometer (Hemavet; Drew Scientific Group). Blood smears were prepared using the wedge technique and were stained with Giemsa stain (Sigma GS500 and MG1L) and then photographed with a Nikon DS-Ri1.
Colony-forming assays
A total of 2 × 104 single total fetal liver cells from embryonic day (E) 14.5 embryos were mixed with methocult M3334 or M3434 medium (Stem Cell Technologies), plated onto 35-mm dishes, and cultured at 37°C in a 5% CO2 humidified atmosphere. The CFU-E colonies were scored at day 3 and the BFU-E, CFU-GM, CFU-GEMM colonies were scored at days 7 to 10. Three different cultures were derived independently from each fetal liver sample and total of 3 animals from each genotype were tested.
Fetal liver reconstitution experiments
Two million E14.5 fetal liver cells from Ccbe1+/+ or Ccbe1lacZ/lacZ mice were injected into the retro-orbital venous plexus of 8- to 10-week-old C57BL mice following exposure to 11 Gy γ-irradiation using a split dose. Peripheral blood profiles were analyzed 8 to 10 weeks after transplantation. Erythroblast island assays were performed using a protocol modified from that of the Chasis laboratory that is described in supplemental Methods.
Statistics
P values were calculated using an unpaired 2-tailed Student t test or χ2 analysis as indicated.
Results
Ccbe1lacZ/lacZ mice exhibit complete loss of lymphatic growth and prenatal death
To generate a null Ccbe1 allele and a reporter for Ccbe1 gene expression, we used gene targeting in ES cells to replace exon 3 of the Ccbe1 gene with a splice acceptor-IRES-lacZ cassette and an FRT-flanked neomycin resistance cassette (supplemental Figure 1). E9.5 to E12.5 Ccbe1lacZ/lacZ embryos were visually indistinguishable from wild-type littermates (Figure 1A-B). In contrast, Ccbe1lacZ/lacZ embryos at and beyond E14.5 appeared edematous (Figure 1C-D), consistent with a defect in lymphatic vascular function. Histologic analysis revealed normal lymphatic vessel growth in Ccbe1lacZ/+ and Ccbe1+/+ control embryos, but none were found in Ccbe1lacZ/lacZ embryos (Figure 1E-P). In contrast, platelet endothelial cell adhesion molecule staining revealed normal blood vessel patterning and growth in Ccbe1lacZ/lacZ embryos (Figure 1Q-R). These findings are consistent with those recently reported by Bos et al17 and demonstrate that CCBE1 is required for lymphatic but not blood vascular development in mice.
CCBE1-deficient embryos exhibit severe pallor and anemia after E12.5
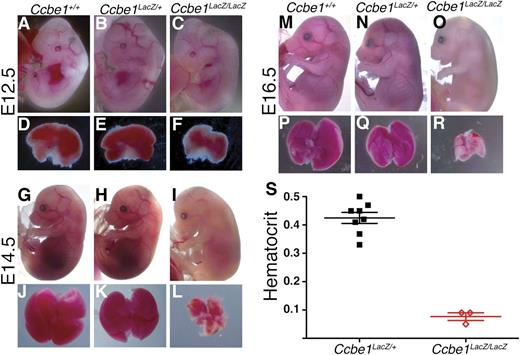
In addition to edema associated with loss of lymphatic vessels, Ccbe1lacZ/lacZ embryos were noted to have progressive pallor and a reduced fetal liver size after E12.5 (Figure 2A-R). This phenotype was present in approximately half of midgestation embryos on a mixed 129SV:C57Bl/6 background, but was completely penetrant in mice that had been backcrossed 6 generations onto a C57Bl/6 background. Fetal livers were normal or mildly reduced in size in Ccbe1lacZ/lacZ embryos at E12.5 (Figure 2D-F) and markedly smaller and pale by E14.5 and E16.5 (Figure 2J-L,P-R). Embryo pallor was not detectable at E12.5, but was evident by E14.5 and severe by E16.5 (Figure 2A-R). Consistent with these findings, the mean hematocrit in C57Bl/6 E15.5 Ccbe1lacZ/lacZ embryos was severely reduced compared with that of heterozygous littermates (Figure 2S). Extravascular blood indicative of hemorrhage was not visualized in the mutant embryos. These studies reveal that loss of CCBE1 in mice results in severe midgestation anemia, likely due to reduced fetal liver erythropoiesis.
Ccbe1lacZ/lacZ embryos exhibit midgestation anemia associated with reduced fetal liver size. (A-F) E12.5 Ccbe1lacZ/lacZ embryos do not appear paler than littermates and have normal or slightly reduced fetal liver sizes compared with littermate controls. (G-L) E14.5 Ccbe1lacZ/lacZ embryos appear paler than littermates and have markedly smaller fetal livers that are also pale when compared with littermate controls. (M-R) By E16.5, Ccbe1lacZ/lacZ embryos are severely pale and have markedly small and pale fetal livers. (S) Midgestation Ccbe1lacZ/lacZ embryos exhibit severely reduced hematocrits compared with heterozygous littermates on a C57Bl/6 background. Bars indicate mean and standard error of the mean (SEM).
Ccbe1lacZ/lacZ embryos exhibit midgestation anemia associated with reduced fetal liver size. (A-F) E12.5 Ccbe1lacZ/lacZ embryos do not appear paler than littermates and have normal or slightly reduced fetal liver sizes compared with littermate controls. (G-L) E14.5 Ccbe1lacZ/lacZ embryos appear paler than littermates and have markedly smaller fetal livers that are also pale when compared with littermate controls. (M-R) By E16.5, Ccbe1lacZ/lacZ embryos are severely pale and have markedly small and pale fetal livers. (S) Midgestation Ccbe1lacZ/lacZ embryos exhibit severely reduced hematocrits compared with heterozygous littermates on a C57Bl/6 background. Bars indicate mean and standard error of the mean (SEM).
Definitive erythropoiesis is impaired in the CCBE1-deficient fetal liver
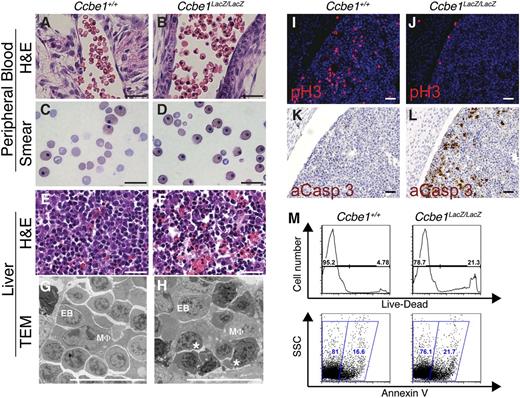
To better define the anemia in midgestation Ccbe1lacZ/lacZ embryos, we examined peripheral blood in histologic sections stained with H&E (Figure 3A-B) and Wright-Giemsa–stained peripheral blood smears (Figure 3C-D). Peripheral blood in E15.5 Ccbe1lacZ/lacZ embryos exhibited increased proportion of nucleated erythrocytes compared with littermate controls (51% ± 5% in Ccbe1lacZ/lacZ embryos compared with 22% ± 2% in control littermates at E15.5, n = 3), consistent with a defect in definitive erythropoiesis.22,23 Analysis of fetal liver sections by H&E staining revealed reduced numbers of hemoglobin-rich mature erythrocytes and pyknotic nuclei suggestive of apoptotic cells (Figure 3E-F). Apoptotic-appearing erythroblast nuclei were also evident in Ccbe1lacZ/lacZ fetal liver sections analyzed by transmission electron microscopy (Figure 3G-H). Staining for phospho-histone H3 revealed reduced numbers of proliferating cells in Ccbe1lacZ/lacZ fetal liver (109 ± 20 cells per high-power field in wild-type versus 54 ± 4 cells per high-power field in Ccbe1lacZ/lacZ fetal liver at E14.5, Figure 3I-J), and staining for activated caspase 3 confirmed an increased number of apoptotic cells (Figure 3K-L). Reduced fetal liver cell viability and increased apoptosis in E14.5 Ccbe1lacZ/lacZ embryos were also evident by flow cytometry (Figure 3M), consistent with the detection of increased numbers of caspase-3–positive cells in fetal liver sections (Figure 3L).
Ccbe1lacZ/lacZ embryos have reduced numbers of anuclear erythrocytes in the peripheral blood and reduced cell proliferation and increased cell death in the fetal liver. (A-D) Peripheral blood in Ccbe1lacZ/lacZ embryos reveals a large fraction of nucleated erythrocytes when examined in H&E-stained sections (A,B) or peripheral blood smears (C,D). (E-H) Ccbe1lacZ/lacZ fetal livers have large numbers of cells with pyknotic nuclei when examined in H&E-stained sections (E,F) and nuclear fragmentation consistent with apoptosis when examined using transmission electron microscopy (G,H). (I,J) Ccbe1lacZ/lacZ fetal livers exhibit reduced cell proliferation as measured by immunostaining for phospho-histone H3. (K,L) Ccbe1lacZ/lacZ fetal livers exhibit increased apoptosis as measured by immunostaining for activated caspase 3. (M) Flow cytometric analysis of Ccbe1lacZ/lacZ fetal livers reveals large numbers of dead (top) and apoptotic (bottom) cells compared with wild-type littermate controls. * indicates fragmented, apoptotic nucleus. Scale bars represent 25 μm. aCasp 3, activated caspase 3; EB, erythroblast; MΦ, macrophage; pH3, phospho-histone H3; TEM, transmission electron microscopy.
Ccbe1lacZ/lacZ embryos have reduced numbers of anuclear erythrocytes in the peripheral blood and reduced cell proliferation and increased cell death in the fetal liver. (A-D) Peripheral blood in Ccbe1lacZ/lacZ embryos reveals a large fraction of nucleated erythrocytes when examined in H&E-stained sections (A,B) or peripheral blood smears (C,D). (E-H) Ccbe1lacZ/lacZ fetal livers have large numbers of cells with pyknotic nuclei when examined in H&E-stained sections (E,F) and nuclear fragmentation consistent with apoptosis when examined using transmission electron microscopy (G,H). (I,J) Ccbe1lacZ/lacZ fetal livers exhibit reduced cell proliferation as measured by immunostaining for phospho-histone H3. (K,L) Ccbe1lacZ/lacZ fetal livers exhibit increased apoptosis as measured by immunostaining for activated caspase 3. (M) Flow cytometric analysis of Ccbe1lacZ/lacZ fetal livers reveals large numbers of dead (top) and apoptotic (bottom) cells compared with wild-type littermate controls. * indicates fragmented, apoptotic nucleus. Scale bars represent 25 μm. aCasp 3, activated caspase 3; EB, erythroblast; MΦ, macrophage; pH3, phospho-histone H3; TEM, transmission electron microscopy.
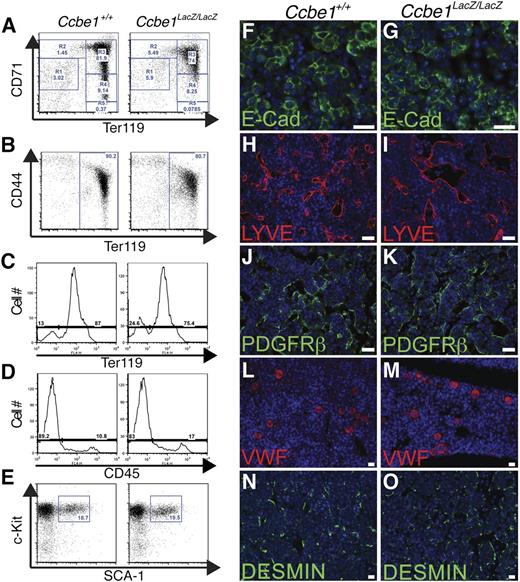
To further assess fetal liver erythropoiesis, flow cytometry of fetal liver cells was performed to evaluate erythroblast maturation. Analysis of erythroid cells and maturation state using staining for the Ter119 and CD7124 or Ter119 and CD44 surface markers25 revealed reduced frequencies of erythroblasts at all stages in Ccbe1lacZ/lacZ fetal livers, with no specific point of block in the erythrocyte maturation process (Figure 4A-B). The proportion of erythroid Ter119+ cells was slightly reduced in Ccbe1lacZ/lacZ fetal livers by E13.5 (Figure 4C), but the fraction of nonerythroid hematopoietic cells (CD45+) was unchanged (Figure 4D). Analysis of Lin-Sca+c-Kit+ stem cells at E12.5 revealed no reduction in the fraction of this population in Ccbe1lacZ/lacZ fetal livers (Figure 4E). The numbers of E-cadherin+ hepatocytes, LYVE1+ sinusoidal endothelial cells, PDGFRb+ pericytes, vWF+ megakaryocytes, and DESMIN+ stellate cells were unchanged in midgestation Ccbe1lacZ/lacZ fetal livers when assessed by immunostaining of fetal liver sections (Figure 4F-O). These findings identify a primary and specific defect in definitive erythropoiesis within the Ccbe1lacZ/lacZ fetal liver as the cause of anemia.
Ccbe1lacZ/lacZ fetal livers exhibit a specific defect in erythropoiesis. (A) Erythroblast maturation in Ccbe1lacZ/lacZ and control fetal livers was assessed by staining for CD71 and Ter119 at E13.5. Note that the number of erythroblasts in the Ccbe1lacZ/lacZ fetal liver was reduced at all stages of maturation. (B) Erythroblast maturation in Ccbe1lacZ/lacZ and control fetal livers was also assessed by staining for CD44 and Ter119 at E13.5. (C) The fraction of Ter119+ erythroblasts in Ccbe1lacZ/lacZ fetal livers is reduced at E13.5. (D) The fraction of CD45+Ter119− cells in Ccbe1lacZ/lacZ fetal livers is unchanged at E13.5. (E) Ccbe1lacZ/lacZ fetal livers have normal numbers of Lin-cKit+Sca1+ hematopoietic stem cells at E13.5. (E-N) Ccbe1lacZ/lacZ fetal livers have normal numbers of E-cadherin+ hepatocytes (F,G), LYVE1+ sinusoidal endothelial cells (H,I), PDGFRb+ vascular pericytes (J,K), vWF+ megakaryocytes (L,M), and Desmin+ stellate cells (N,O). Scale bars represent 25 μm.
Ccbe1lacZ/lacZ fetal livers exhibit a specific defect in erythropoiesis. (A) Erythroblast maturation in Ccbe1lacZ/lacZ and control fetal livers was assessed by staining for CD71 and Ter119 at E13.5. Note that the number of erythroblasts in the Ccbe1lacZ/lacZ fetal liver was reduced at all stages of maturation. (B) Erythroblast maturation in Ccbe1lacZ/lacZ and control fetal livers was also assessed by staining for CD44 and Ter119 at E13.5. (C) The fraction of Ter119+ erythroblasts in Ccbe1lacZ/lacZ fetal livers is reduced at E13.5. (D) The fraction of CD45+Ter119− cells in Ccbe1lacZ/lacZ fetal livers is unchanged at E13.5. (E) Ccbe1lacZ/lacZ fetal livers have normal numbers of Lin-cKit+Sca1+ hematopoietic stem cells at E13.5. (E-N) Ccbe1lacZ/lacZ fetal livers have normal numbers of E-cadherin+ hepatocytes (F,G), LYVE1+ sinusoidal endothelial cells (H,I), PDGFRb+ vascular pericytes (J,K), vWF+ megakaryocytes (L,M), and Desmin+ stellate cells (N,O). Scale bars represent 25 μm.
CCBE1 function supports the fetal erythropoiesis cell nonautonomously
No information presently exists regarding the molecular action of CCBE1 in lymphangiogenesis that might explain its role in erythropoiesis. To test for cell-autonomous CCBE1 requirements in erythropoiesis, we harvested fetal liver cells from E14.5 Ccbe1lacZ/lacZ and control littermate embryos and performed methylcellulose colony assays in the presence of EPO and SCF. Ccbe1lacZ/lacZ progenitors gave rise to normal numbers of CFU-E, BFU-E, and myeloid colonies (supplemental Table 1), indicating either that CCBE1 production is not required in clonogenic erythroid precursors themselves or that the in vitro conditions used for these assays overcome any such requirement. To distinguish these possibilities and test a cell-autonomous role for CCBE1 in vivo, we transplanted equal numbers of viable fetal liver cells from E14.5 Ccbe1lacZ/lacZ and control littermate embryos into lethally irradiated wild-type hosts. Analysis of genomic DNA from circulating blood cells of recipient mice after 8 weeks revealed 100% reconstitution (not shown). The numbers of circulating leukocytes, erythrocytes, and platelets in animals reconstituted with CCBE1-deficient hematopoietic cells were indistinguishable from those of littermate controls (wild-type vs Ccbe1lacZ/lacZ: leukocytes (K/μL), 10.76 ± 2.2 vs 11.73 ± 4.5; erythrocytes (M/μL), 10.75 ± 0.21 vs 10.34 ± 0.26; platelets (K/μL), 648 ± 80 vs 629 ± 30; n = 7 each group; P > .10). These findings reveal that CCBE1 regulates erythropoiesis in the fetal liver cell nonautonomously.
Loss of CCBE1 impairs EBI formation in the fetal liver
Genetic studies in mice have revealed 2 major classes of extrinsic or cell-nonautonomous regulators of fetal erythropoiesis: the known secreted erythropoietic factors EPO and SCF (kit ligand), and factors required for the formation and function of EBIs. The levels of Epo and Scf messenger RNA in both the fetal liver and total embryo of Ccbe1lacZ/lacZ animals were not significantly reduced (supplemental Figure 2), suggesting that CCBE1 does not act by reducing the production of SCF or EPO.
Analysis of knockout mice has identified a diverse set of proteins that promote the fetal liver erythropoiesis cell nonautonomously through effects on EBI formation and function. Immunostaining of fetal liver from Ccbe1lacZ/lacZ embryos revealed reduced numbers of F4/80+ macrophages relative to wild-type littermate controls (supplemental Figure 3A-B). Transmission electron microscopy of Ccbe1lacZ/lacZ fetal liver detected macrophages with cellular extensions that surrounded erythroblasts, but, as noted above, the erythroblasts frequently exhibited fragmented nuclei consistent with cellular apoptosis (Figure 3H). Thus, EBIs form in Ccbe1lacZ/lacZ fetal livers, but the numbers and function of the central macrophages in these islands may be reduced.
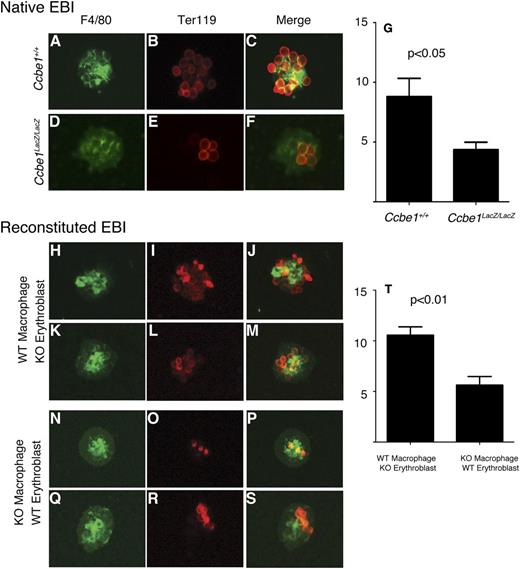
To test the integrity and function of the EBIs in Ccbe1lacZ/lacZ fetal livers, we next isolated intact islands from the fetal livers of control and Ccbe1lacZ/lacZ embryos. Intact EBIs isolated from E14.5 Ccbe1lacZ/lacZ fetal livers had smaller numbers of Ter119+ erythroblasts adherent to individual F4/80+ macrophages than those harvested from the fetal livers of littermate controls (Figure 5A-G), consistent with a defect in EBI function and/or formation. To determine whether reduced EBI formation in Ccbe1lacZ/lacZ fetal livers reflected a defect in erythroblast function and/or a defect in macrophage function, we performed reconstitution assays in which the 2 cell types were isolated and EBIs allowed to form after mixing CCBE1-deficient or wild-type erythroblasts with CCBE1-deficient or wild-type macrophages.26 CCBE1-deficient erythroblasts associated normally with wild-type macrophages (Figure 5H-M,T), suggesting that CCBE1 does not regulate erythroblast function in the EBI. In contrast, CCBE1-deficient macrophages exhibited reduced association with control erythroblasts (Figure 5N-T), suggesting that CCBE1 is required for central macrophage function within the EBI. These findings support a mechanism in which CCBE1 controls EBI formation within the fetal liver erythropoietic environment through the regulation of the central macrophage.
Ccbe1lacZ/lacZ fetal livers exhibit defective EBIs. (A-G) Native EBIs isolated from C57Bl/6 Ccbe1lacZ/lacZ fetal livers have reduced numbers of Ter119+ erythroblasts adherent to F4/80+ macrophages compared with those from wild-type littermates. (H-T) Reconstituted EBIs reveal normal adhesion of Ccbe1lacZ/lacZ erythroblasts to wild-type F4/80+ macrophages and reduced adhesion of wild-type erythroblasts to Ccbe1lacZ/lacZ F4/80+ macrophages. n = 30 to 40 islands per group; error bars indicate SEM. KO, Ccbe1lacZ/lacZ; WT, wild-type.
Ccbe1lacZ/lacZ fetal livers exhibit defective EBIs. (A-G) Native EBIs isolated from C57Bl/6 Ccbe1lacZ/lacZ fetal livers have reduced numbers of Ter119+ erythroblasts adherent to F4/80+ macrophages compared with those from wild-type littermates. (H-T) Reconstituted EBIs reveal normal adhesion of Ccbe1lacZ/lacZ erythroblasts to wild-type F4/80+ macrophages and reduced adhesion of wild-type erythroblasts to Ccbe1lacZ/lacZ F4/80+ macrophages. n = 30 to 40 islands per group; error bars indicate SEM. KO, Ccbe1lacZ/lacZ; WT, wild-type.
CCBE1 expression by nonhematopoietic cells supports fetal liver erythropoiesis
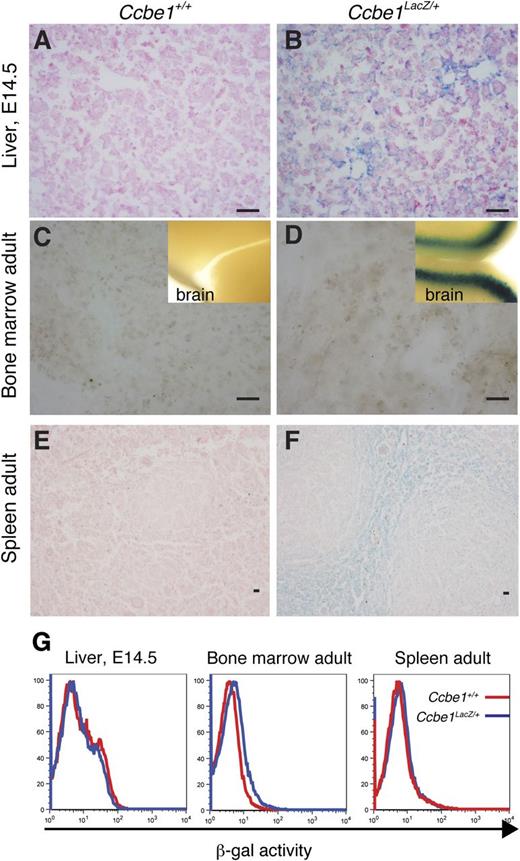
The finding that CCBE1 functions cell nonautonomously to support macrophage function in the EBIs of the fetal liver may be explained by expression of CCBE1 from macrophages or erythroblasts within the EBI itself or by CCBE1 expression from nonhematopoietic cells within the erythroid niche of the fetal liver. To better define the source of CCBE1 during fetal erythropoiesis, we performed X-gal staining of fetal liver derived from E14.5 Ccbe1lacZ embryos. Diffuse X-gal staining was detected throughout the fetal liver, with the strongest staining observed in cells adjacent to the portal veins (Figure 6A-B). X-gal staining of postnatal Ccbe1lacZ bone marrow stromal cells revealed no evidence of Ccbe1 expression, although brain tissue stained using the same technique was strongly positive (Figure 6C-D). Weak X-gal staining was detected in the stromal region of Ccbe1lacZ spleens (Figure 6E-F), but flow cytometric analysis of β-galactosidase activity using a fluorogenic substrate (FACS-Gal) revealed no evidence of Ccbe1 expression in hematopoietic cells (Figure 6G).
Ccbe1lacZ expression in prenatal and postnatal sites of erythropoiesis. (A,B) X-gal staining of E14.5 Ccbe1lacZ and littermate control fetal liver is shown. (C,D) X-gal staining of bone marrow stroma from Ccbe1lacZ and littermate control animals. Brain tissue from each animal was used as a control for the staining process (insets). (E,F) X-gal staining of Ccbe1lacZ and littermate control spleen. (G) FACS-Gal analysis of hematopoietic cells from the fetal liver, bone marrow, and spleen of Ccbe1lacZ and littermate control animals is shown.
Ccbe1lacZ expression in prenatal and postnatal sites of erythropoiesis. (A,B) X-gal staining of E14.5 Ccbe1lacZ and littermate control fetal liver is shown. (C,D) X-gal staining of bone marrow stroma from Ccbe1lacZ and littermate control animals. Brain tissue from each animal was used as a control for the staining process (insets). (E,F) X-gal staining of Ccbe1lacZ and littermate control spleen. (G) FACS-Gal analysis of hematopoietic cells from the fetal liver, bone marrow, and spleen of Ccbe1lacZ and littermate control animals is shown.
To definitively address the requirement for CCBE1 expression by hematopoietic cells, we used mice carrying a conditional Ccbe1 allele to generate Vav-Cre;Ccbe1flox/flox animals in which Cre deletes Ccbe1 in all hematopoietic cells prior to E12.520 (supplemental Figure 4). Vav-Cre;Ccbe1flox/flox mice were born in normal numbers, grew to maturity without overt phenotypes, and exhibited normal hematologic profiles postnatally (wild-type vs Vav-Cre;Ccbe1flox/flox: leukocytes (K/μL), 11.21 ± 1.61 vs 11.55 ± 4.5; erythrocytes (M/μL), 9.65 ± 0.60 vs 10.61 ± 0.58; platelets (K/μL), 607 ± 82 vs 597 ± 110; N = 5 each group; P > .10). Analysis of E14.5 Vav-Cre;Ccbe1flox/flox embryos revealed no evidence of anemia or changes in fetal liver size or cell proliferation or apoptosis (supplemental Figure 5). These findings reveal that CCBE1 is not required in hematopoietic cells, including both erythroblasts and macrophages of the EBI, to support fetal erythropoiesis. Instead CCBE1 secretion by nonhematopoietic cells acts on macrophages to support EBI formation within the fetal liver erythroid niche.
CCBE1 is not required for postnatal erythropoiesis under basal or stress conditions
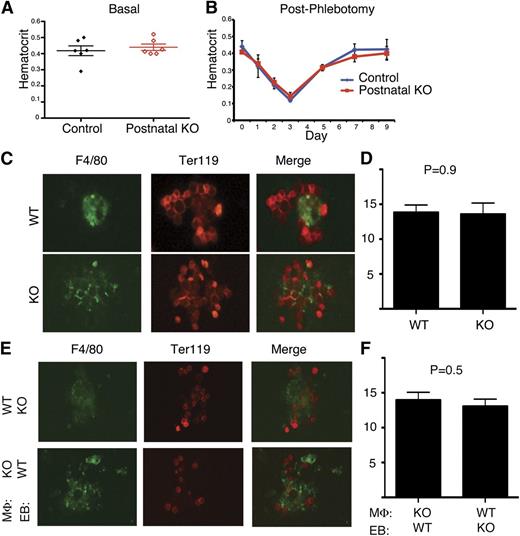
CCBE1 null murine embryos exhibit severe anemia whereas patients with Hennekam syndrome have not been reported to be anemic. This discrepancy may reflect differences in the degree of loss of CCBE1 function between gene-targeted mice and human mutants, because patients with Hennekam syndrome are believed to have hypomorphic CCBE1 alleles.15 To investigate the role of CCBE1 in postnatal erythropoiesis, we generated Ub-CreERT2;Ccbe1flox/lacZ mice in which Ccbe1 was inducibly and efficiently deleted postnatally by administration of tamoxifen (supplemental Figure 6, henceforth termed “postnatal CCBE1 KO mice”). Postnatal CCBE1 KO mice had hematocrits that were indistinguishable from those of control Ub-CreERT2;Ccbe1flox/+ animals (Figure 7A). Thus, CCBE1 is required for erythropoiesis in the fetal liver but not the adult bone marrow under basal conditions.
CCBE1 is not required for postnatal erythropoiesis or bone marrow EBI formation. (A) Loss of Ccbe1 was induced by tamoxifen administration to Ub-CreERT2; Ccbe1fl/lacZ (postnatal KO) and Ub-CreERT2; Ccbe1fl/+ (control) mice and hematocrits measured 12 weeks later. (B) Ub-CreERT2; Ccbe1fl/lacZ (postnatal KO) and control Ub-CreERT2; Ccbe1fl/+ (control) mice were phlebotomized 12 weeks after tamoxifen-induced deletion of Ccbe1 and hematocrits measured at the indicated time points after phlebotomy. n = 5 in each group. (C,D) Native EBIs isolated from postnatal KO (KO) bone marrow have similar numbers of Ter119+ erythroblasts adherent to F4/80+ macrophages as those from wild-type littermates. (E,F) Reconstituted EBIs reveal normal adhesion of CCBE1-deficient (KO) erythroblasts to wild-type F4/80+ macrophages, and vice versa. n = 12 to 45 islands per group; error bars indicate SEM. EB, erythroblast; MΦ, macrophage; WT, wild-type.
CCBE1 is not required for postnatal erythropoiesis or bone marrow EBI formation. (A) Loss of Ccbe1 was induced by tamoxifen administration to Ub-CreERT2; Ccbe1fl/lacZ (postnatal KO) and Ub-CreERT2; Ccbe1fl/+ (control) mice and hematocrits measured 12 weeks later. (B) Ub-CreERT2; Ccbe1fl/lacZ (postnatal KO) and control Ub-CreERT2; Ccbe1fl/+ (control) mice were phlebotomized 12 weeks after tamoxifen-induced deletion of Ccbe1 and hematocrits measured at the indicated time points after phlebotomy. n = 5 in each group. (C,D) Native EBIs isolated from postnatal KO (KO) bone marrow have similar numbers of Ter119+ erythroblasts adherent to F4/80+ macrophages as those from wild-type littermates. (E,F) Reconstituted EBIs reveal normal adhesion of CCBE1-deficient (KO) erythroblasts to wild-type F4/80+ macrophages, and vice versa. n = 12 to 45 islands per group; error bars indicate SEM. EB, erythroblast; MΦ, macrophage; WT, wild-type.
The observed difference in CCBE1 requirement between prenatal and postnatal animals may reflect important differences in the hematopoietic environments of the fetal liver and bone marrow. Alternatively, the requirement for CCBE1 during fetal life may be explained by the greater erythropoietic demand in the growing fetus compared with that of postnatal animals under basal conditions, as observed in other mutant mice such as flexed tail.27 To distinguish between these 2 possibilities, we tested the requirement for CCBE1 during stress erythropoiesis induced by phlebotomy in mature animals. Phlebotomy of both postnatal CCBE1 KO and control animals reduced the hematocrit to approximately 15% (Figure 7B). Postnatal Ccbe1 KO and control animals recovered from severe anemia with virtually identical kinetics of hematocrit rise (Figure 7B). These findings reveal that CCBE1 is has essential, nonredundant functions during fetal erythropoiesis but not postnatally, even under conditions of high erythropoietic stress and extramedullary hematopoiesis.
CCBE1 is not required for EBI formation in the postnatal bone marrow
Because embryonic anemia is associated with defective EBI formation in the CCBE1-deficient fetal liver, we next asked whether postnatal CCBE1 KO animals exhibit normal or defective EBI formation in the bone marrow. Intact EBIs isolated from postnatal CCBE1 KO bone marrow had numbers of Ter119+ erythroblasts adherent to individual F4/80+ macrophages similar to those observed in controls (Figure 7C-D). To further probe the function of postnatal CCBE1 KO macrophages, we performed reconstitution assays like those shown in Figure 5 for fetal liver cells. In contrast to our findings using cells harvested from fetal liver, CCBE1-deficient erythroblasts and macrophages harvested from postnatal CCBE1 KO animals associated normally with their wild-type counterparts (Figure 7E-F). These findings reveal that CCBE1 regulates EBI formation within the fetal liver but not the postnatal bone marrow erythropoietic environment.
Discussion
We have identified CCBE1 as a novel secreted factor that plays an unexpected, critical role in fetal erythropoiesis. Like the well-characterized secreted factors EPO and SCF, CCBE1 functions cell extrinsically to promote erythroid survival, maturation, and proliferation. In contrast to these factors, however, CCBE1 is not required for erythroid development ex vivo, and its critical role in definitive erythropoiesis is restricted to the fetal liver and not present in bone marrow or spleen. Although the precise molecular mechanism by which CCBE1 regulates erythropoiesis remains unknown, our studies identify the EBI macrophage as the likely target cell and highlight distinctly different requirements for fetal liver erythropoiesis vs postnatal bone marrow erythropoiesis.
Previous studies and our findings (Figure 1) establish CCBE1 as an essential lymphangiogenic factor in both fish and mice.14,17 Mutations in CCBE1 cause Hennekam syndrome,15,28 a familial recessive disease characterized by severely reduced lymphatic vessel growth and a variable degree of cognitive impairment, but with no reported hematologic abnormalities.16 Our experimental results provide several explanations for why CCBE1-deficient embryos are anemic but Hennekam patients are not. Ccbe1lacZ/lacZ mice are predicted to be absolutely deficient in CCBE1 activity. In contrast, patients with Hennekam syndrome are likely to be hypomorphic with some residual CCBE1 function because they have some lymphatic vessels and have not been found to have homozygous CCBE1 nonsense mutations predicted to confer a complete loss of activity.15,28 Because anemia is a less completely penetrant phenotype than loss of lymphatic vessels in mixed-strain mice, relatively normal erythropoiesis is more likely to occur in hypomorphic Hennekam patients than the more penetrant loss of lymphatic growth. In addition, we find that postnatal loss of CCBE1 does not confer anemia in mice, even under severe stress conditions. Thus, Hennekam patients most likely do not exhibit anemia because they have incomplete loss of CCBE1 function and because CCBE1 is specifically required for fetal but not postnatal erythropoiesis. It is also possible that Hennekam patients experience mild anemia in utero with no clinical consequences and rapid recovery after birth as erythropoiesis transitions from fetal liver to bone marrow.
Our observation that CCBE1 is required for both erythropoiesis and lymphatic growth suggests that these processes might be linked molecularly or biologically. Loss of VEGF-C29 or the activity of its receptor VEGFR330 results in a complete loss of lymphatic growth, identical to that observed in CCBE1-deficient mice, but neither confers midgestation anemia or fetal liver defects. Thus, there is no requirement for lymphatic vessels during fetal liver erythropoiesis and the connection between these phenotypes is not likely to reflect a biological role for lymphatic vessels in erythropoiesis. Instead, it seems more likely that CCBE1 participates in these two distinct biological processes by facilitating one or more shared signaling pathways.
Several lines of evidence support a mechanism in which CCBE1 promotes the erythropoietic microenvironment of the fetal liver to enhance EBI formation. The timing of onset of anemia, the cellular and molecular phenotype of the CCBE1-deficient fetal liver, and the impaired proliferation and survival observed in the erythroblasts of the CCBE1-deficient fetal liver closely phenocopy those in mice lacking the intracellular protein palladin12 or the transcription factor c-maf.13 Similar to these mutant embryos, EBIs are present in CCBE1-deficient fetal livers, but F4/80+ macrophages are reduced in number and their capacity to bind erythroblasts is markedly diminished. However, analysis of gene expression in the CCBE1-deficient fetal did not reveal a significant reduction in these genes or others previously associated with defective fetal liver EBI formation (supplemental Figure 7). These findings suggest either that CCBE1 regulates these genes posttranscriptionally or that additional, currently unidentified CCBE1-regulated genes promote fetal liver EBIs.
These studies reveal an unexpected molecular mechanism by which definitive hematopoiesis in the fetal liver differs from that in the bone marrow. We find that loss of CCBE1 does not impair postnatal erythropoiesis, and analysis of EBI formation in the bone marrow of postnatal CCBE1-deficient mice reveals no defect (Figure 7). These findings and our genetic studies demonstrating that CCBE1 expression is not required in hematopoietic cells support a unique and specific role for CCBE1 in establishing the fetal liver erythropoietic environment. Definitive erythropoiesis in the fetal liver is associated with differences in gene expression when compared with the same process in the bone marrow, most notably in the expression of fetal versus adult globin genes.31 A recent study has identified significant epigenetic differences in erythroblasts derived from the 2 sources, consistent with an environment-specific impact on gene expression,32 but the molecular factors responsible for these changes are not yet known. CCBE1 appears to one such factor, and future studies addressing CCBE1 function in the fetal liver are likely to provide new insight into the role of the environment in definitive erythropoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the Kahn and Weiss labs for their thoughtful comments and insights during the course of these studies and Dr Joel Chasis for advice regarding erythroblast island studies.
This work was supported by a grant from the National Institutes of Health (K08 AR101659) (D.E.).
Authorship
Contribution: Z.Z., D.R.E., H.B., E.K., V.K., Z.J., C.T., Y.Y., V.D., M.C., and M.L. designed and performed experiments. Z.Z., D.E., H.B., E.K., Z.J., M.J.W., and M.L.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark L. Kahn, Department of Medicine and Cardiovascular Institute, University of Pennsylvania, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: markkahn@mail.med.upenn.edu.
References
Author notes
Z.Z. and D.R.E. contributed equally to this study.