Key Points
Our data from the mouse model and patients indicate that inflammatory neovascularization during GvHD is targetable via αv integrin.
We identify a negative regulation of GvHD-related neovascularization by miR-100.
Abstract
Acute graft-versus-host disease (GvHD) is a complex process involving endothelial damage and neovascularization. Better understanding of the pathophysiology of neovascularization during GvHD could help to target this process while leaving T-cell function intact. Under ischemic conditions, neovascularization is regulated by different micro RNAs (miRs), which potentially play a role in inflamed hypoxic GvHD target organs. We observed strong neovascularization in the murine inflamed intestinal tract (IT) during GvHD. Positron emission tomography imaging demonstrated abundant αvβ3 integrin expression within intestinal neovascularization areas. To interfere with neovascularization, we targeted αv integrin–expressing endothelial cells, which blocked their accumulation in the IT and reduced GvHD severity independent of immune reconstitution and graft-versus-tumor effects. Additionally, enhanced neovascularization and αv integrin expression correlated with GvHD severity in humans. Expression analysis of miRs in the inflamed IT of mice developing GvHD identified miR-100 as significantly downregulated. Inactivation of miR-100 enhanced GvHD indicating a protective role for miR-100 via blocking inflammatory neovascularization. Our data from the mouse model and patients indicate that inflammatory neovascularization is a central event during intestinal GvHD that can be inhibited by targeting αv integrin. We identify negative regulation of GvHD-related neovascularization by miR-100, which indicates common pathomechanistic features of GvHD and ischemia.
Introduction
The connection between inflammation and neovascularization has been demonstrated for different diseases1,2 including graft-versus-host disease (GvHD).3,4 However, the molecular and cellular mechanisms mediating neovascularization and the contribution to inflammation following allogeneic hematopoietic cell transplantation (allo-HCT) remain unresolved.
A central event in the early phase of GvHD following allo-HCT is the toxic damage of different recipient tissues.5 We have recently demonstrated a GvHD promoting role for the release of intracellular adenosine triphosphate,6 which is converted by ecto-5′-nucleotidase7 to metabolites such as adenosine diphosphate with known proangiogenic function.8 During this early phase, endothelial cells (ECs) are injured by chemotherapy,9 irradiation,10 and immunosuppressive drugs such as mammalian target of rapamycin (mTOR) inhibitors11 and calcineurin inhibitors.12 Toxic damage is followed by inflammatory neovascularization that contributes to GvHD3 by a yet unknown mechanism. In a variety of disease models, the process of neovascularization was shown to be determined by different micro RNAs (miRs)13,14 that have been identified as important regulators of gene expression in a wide range of organisms and biological systems.15 miRs are short, noncoding, single-strand RNA molecules that are transcribed as precursor molecules and are later processed into mature miRs. They control the expression of genes predominantly by translational repression and can negatively13,16 and positively17 regulate neovascularization by impacting signaling in ECs. miRs are therefore potential targets for therapies that aim to enhance or suppress neovascularization.
Another regulatory checkpoint of neovascularization is the interaction of ECs with extracellular matrix molecules (ECMs) via integrins.18 Basement membrane deposition and mechanical cues from the ECMs are transmitted via integrins to coordinate vessel sprouting and remodeling.19 Because of their transmembrane structure and their ability to bind to extracellular ligands, integrins function as central mediators during the neoangiogenic process.20
By using positron emission tomography (PET) imaging of αvβ3 integrin activity as a novel technique to localize and quantify ECs during murine GvHD, we could visualize neovascularization kinetics in the intestinal tract (IT) following allo-HCT. We show that inflammatory neovascularization can be inhibited by targeting αv integrin, which reduced GvHD severity. By using expression analysis of candidate miRs in the inflamed IT of mice developing GvHD, we identified miR-100 downregulation and demonstrated that inactivation of miR-100 enhanced GvHD. Therefore, we provide the first evidence that intestinal neovascularization during GvHD shares pathomechanistic features with adaptive blood vessel growth as it occurs in response to ischemia after arterial occlusion, where miR-100 functions as an endogenous repressor.
Methods
Human subjects
We collected all samples after approval by the ethics committee of the Albert-Ludwigs-University, Freiburg, Germany (protocol number: 267/11), and after written informed consent in accordance with the Declaration of Helsinki. Intestinal tissue biopsies were collected in a prospective manner from individuals undergoing allo-HCT with or without GvHD (supplemental Table 1; see the Blood Web site). GvHD grading was performed on the basis of histopathology. Human intestinal biopsies were stained for CD31 (clone: 1A10; Leica Microsystems) and αv integrin (polyclonal; Sigma-Aldrich).
Mice
C57BL/6 (H-2b, Thy-1.2) and BALB/c (H-2d, Thy-1.2) mice were purchased either from Charles River Laboratory (Sulzburg, Germany) or from the local stock of the animal facility at Freiburg University. Recipients between 6 and 12 weeks of age and only gender-matched combinations were used for transplant experiments. The luciferase (luc+) transgenic C57BL/6 mice have been previously described.21 The animal protocols (G-10/62, X-10/13H) were approved by the University Committee on the Use and Care of Laboratory Animals at Albert-Ludwigs-University, Freiburg, Germany.
Bone marrow transplantation (BMT) model and GvHD histopathology
BMT experiments were performed as previously described.22 Briefly, recipients were injected intravenously with 5 × 106 bone marrow (BM) cells after lethal irradiation with 900 centi Gray (cGy). To induce acute GvHD (aGvHD), 3 × 105 CD4+/CD8+ T cells (Tc’s) were given (C57BL/6→BALB/c) intravenously on day 0. Slides of small- and large-intestine samples collected on day 7 were stained with hematoxylin/eosin and scored by experienced pathologists (U.V.G., A.S.G.) blinded to the treatment groups on the basis of a published histopathology scoring system.23
Generation of BM-derived dendritic cells (DCs)
BM-derived DCs were prepared as described,24 except that interleukin (IL) 4 was not included in the culture.
Tc proliferation assay
Tc proliferation in vitro and in vivo was performed as previously described.22
B-cell lymphoma model
To investigate graft-versus-leukemia (GvL) activity of transferred donor Tc’s, we employed A20luc B-cell leukemia.25 Animals were injected with A20luc cells (BALB/c background, 5 × 105) 2 days prior to administration of the Tc’s from C57BL/6 donors.
In vivo cilengitide treatment
Cilengitide (EMD 121974) [cyclo Arg-Gly-Asp-D-Phe-(N-methyl)-Val; EMD 121974] is an antagonist selective for αvβ3 and αvβ5 integrins, with 50% inhibitory concentration values in the low nanomolar range for isolated αvβ3 integrins and was provided by Merck KGaA. The dosage administered intraperitoneally was 75 mg/kg twice daily from day –1 to day +10 after allo-HCT according to previous studies.26 Phosphate-buffered saline (PBS) was administered to the animals as control group.
In vivo bioluminescence imaging (BLI)
In vivo BLI was performed as previously described.22 Imaging data were analyzed and quantified with Living Image 3.0 Software (Calipers).
PET imaging
[68Ga]NODAGA-cyclo(Arg-Gly-Asp-D-Phe-Lys) was synthesized and labeled in the Department of Nuclear Medicine, Freiburg University Medical Center, as previously described.27 This peptide is structurally similar to cilengitide and has a comparable affinity to αv integrins as cilengitide.28
Six to 12 MBq/0.6 nmol per 0.1 mL of [68Ga]NODAGA-c(RGDfK) was injected via lateral tail vein. One hour after injection, mice were scanned for 30 minutes using the small animal PET scanner microPET Focus 120 (Concorde Microsystems). PET images were reconstructed with the 2D Ordered Subset Expectation Maximization algorithm provided by the scanner software. The resolution of the images ranges between 1.1 and 2.5 mm depending on the distance from the center of the field of view. Image counts per pixel per second were calibrated to activity concentrations (Bq/mL) by measuring a 68Ga cylindrical phantom filled with a known concentration of radioactivity.
Image analysis
To determine tracer concentration in various tissues, cylindrical regions of interest were placed in the abdominal region that exhibited the highest radioactivity using amide Software 0.9.2. Tracer uptake in the investigated mice is expressed as percent of the decay-corrected injected dose per gram of tissue. Previous studies have shown that uptake of [68Ga]NODAGA-c(RGDfK) correlates closely with the expression of activated αvβ3 integrins.27
In vitro cytotoxicity assay
Effector Tc’s were isolated from BALB/c recipients treated with cilengitide or PBS as control on day 14 after allo-HCT by depletion of non-CD3+ Tc’s (Miltenyi Biotec) and cocultured for 6 hours at 37°C with L1210 target cells at the indicated ratio of target:effector cells. Afterward cells were stained with annexin V–fluorescein isothiocyanate/propidium iodide (PI), and cytotoxicity was analyzed by flow cytometry.
Microarray analysis
RNA was isolated from intestines of untreated mice on day 14 after allo-HCT. A more detailed description can be found in the supplemental data (ArrayExpress accession number: E-MEXP-3807).
In vivo miR-100 antagomir treatment
Antagomirs were designed as previously described13 and custom synthesized (Biozym). Antagomir sequences were as follows: antagomir-100 (antag-100), 5′-cacaaguucggaucuacggguu-3′; antagomir control 2 (antag-cont2), 5′-caccaguuaggcucuacggauu-3′. Antagomirs were administered via tail vein injection of 8 mg/kg antagomir on days 0, 3, and 9 after allo-HCT.
Quantitative real-time PCR (qRT-PCR) for miR-100 expression
Total RNA was isolated from the small intestine of BALB/c recipients on days 0, 2, 6, and 12 after allo-HCT using miRNeasy MiniKit (50) (Qiagen). MiR-100 expression was measured by quantitative stem-loop PCR technology (TaqMan MicroRNA Assays; Applied Biosystems) as previously described.13
Intestinal tissue dissection
We adapted the protocol as previously described29 with modifications. After surgically removing and flushing the intestine, the tissue was next incubated in 1 mM dithiothreitol (Sigma-Aldrich). After washing with PBS, pieces were incubated 4 times in 5 mM EDTA (Merck) prior to a digestion step in 0.1% collagenase (Invitrogen), 2.5 U/mL dispase (Invitrogen), and 1 mg/mL DNaseI (Sigma-Aldrich) for 1 hour at 37°C.
Flow cytometry
Flow cytometry analysis was performed with a CyanADP (Beckman Coulter) and analyzed with FlowJo 7/8 software (Tree Star).
The following monoclonal antibodies purchased from BD Bioscience, BioLegend, eBiosciences, or AbD Serotec were used: B220 (RA3-6B2), CD4 (GK 1.5/RM4-5), CD8 (53-6.7), CD11c (HL3/N418), CD11b (M1/70), CD19 (6D5), CD45 (30-F11), CD31 (390), CD34 (MEC14.7) (HM34), CD40 (3_23), CD44 (IM7), CD51 (RMV7), CD62L (MEL-14), CD80 (16-10A1), CD86 (GL-1), CD107a (1D4B), epithelial cell adhesion molecule (EPCAM) (G8.8), F4/80 (CI:A3-1), Foxp3 (FJK-16s), Gr-1 (RB6-8C5), H-2Kb (AF6-88.5), H-2Kd (SF1-1.1), I-Ad (SF1-1.1), interferon γ (IFN-γ) (XMG1.2), IL-4 (11B11), IL-17A (TC11-18H10), and NK1.1 (PK136). The intracellular staining for Foxp3 and cytokines and for effector memory Tc’s (TEM’s), central memory Tc’s (TCM’s), and naive Tc’s (TN’s) was performed as previously described.22
Histology and vessel density measurement
The murine tissues were stained with anti-CD34 (clone: MEC14.7; BioLegend). Streptavidin-conjugated horseradish peroxidase and the diaminobenzidine-system (Dako) were used for visualization. For quantification of vessel density, murine small intestines were stained with biotin rat anti-mouse antibody CD31 (clone: MEC13.3; BD Biosciences) and visualized with streptavidin-conjugated AlexaFluor488 (Invitrogen). Ten sections per sample were investigated, and vessel density was determined by quantification of CD31+ area/total area using a Zeiss LSM 710 laser scanning microscope and Adobe Photoshop CS5 software.
Detection of tissue hypoxia
Sixty milligrams per kilogram of pimonidazole HCl (Hypoxyprobe Inc.), which selectively binds to oxygen-starved cells,30 was administered orally to mice. After 1 hour, animals were euthanized and intestine was isolated and fixed with 4% formaldehyde (Riedel-de Haen). Immunohistochemistry of paraffin sections was performed according to the manufacturer’s protocol.
Cytokine measurements
The serum of BALB/c recipients was collected on day 7 after allo-HCT, and the levels of the indicated cytokines were analyzed with the Cytometric Bead Array (CBA) Inflammation kit (BD Bioscience).
Statistical analysis
Data are reported as mean values ± standard deviation (SD). We compared pairs by the Student t test. Differences in animal survival were analyzed by log-rank test. A P value < .05 was considered statistically significant.
Results
Development of neovascularization in the early phase of GvHD
We first aimed to determine the kinetics and anatomical localization of neovascularization at serial time points following allo-HCT. Flow cytometry–based evaluation of the inflamed IT revealed that allo-HCT itself caused increased numbers of CD34+ and CD31+ ECs, which was further enhanced when donor Tc’s were added to the graft and GvHD evolved (Figure 1A). We found no significant increase of CD31+ cells in the IT of syngeneic BMT recipients compared with untreated mice or animals undergoing allo-HCT without Tc’s (Figure 1A). Compatible with the observation that neovascularization took place in the IT following allo-HCT, we detected increased RNA transcript levels of vascular endothelial growth factor and fibroblast growth factor in the IT of mice with GvHD as compared with BM controls (Figure 1B). [68Ga]NODAGA-c(RGDfK)–based PET imaging demonstrated a significantly higher signal in mice that had undergone allo-HCT with or without Tc’s (BM or BM/Tc) as compared with untreated animals (Figure 1C), indicating increased expression of activated αv integrin. Regions of the higher PET signal projected over the abdominal area, which indicated EC accumulation in the IT (Figure 1C). Collectively, these data indicate that the IT is a central location for neovascularization that occurs during the development of GvHD.
Neovascularization is increased in the IT of mice after allo-HCT. Allo-HCT was performed as described in the “Methods” section. (A) Intestines of mice from the indicated groups were digested and analyzed on day 14 after allo-HCT for the frequency of ECs using flow cytometry. Allo-HCT increased the number of CD34+ and CD31+ ECs in the total IT, as shown for representative flow cytometry images (left) and when quantified for each individual animal (right). The experiment was performed 3 times with at least 3 mice in each group, and the pooled results of the 3 independent experiments are shown. (B) RNA was isolated from IT of the indicated groups and used for comparative microarray analysis. Vascular endothelial growth factor (VEGF) (P = .003) and fibroblast growth factor (FGF) (P = .005) levels were significantly increased in the GvHD group as compared with BM controls. The experiment was performed once with 3 animals in each group. (C) Left panel: in vivo PET imaging of mice from the indicated groups after allo-HCT and application of [68Ga]NODAGA-c(RGDfK) is shown for 4 representative time points (days 0, 2, 7, and 21 after allo-HCT). Right panel: [68Ga]NODAGA-c(RGDfK) uptake over time is quantified in the abdominal area of mice, demonstrating a higher uptake in transplanted as compared with untreated animals, shown for 1 representative of 3 independent experiments, each performed with at least 3 animals per group. c, coronal; s, sagittal.
Neovascularization is increased in the IT of mice after allo-HCT. Allo-HCT was performed as described in the “Methods” section. (A) Intestines of mice from the indicated groups were digested and analyzed on day 14 after allo-HCT for the frequency of ECs using flow cytometry. Allo-HCT increased the number of CD34+ and CD31+ ECs in the total IT, as shown for representative flow cytometry images (left) and when quantified for each individual animal (right). The experiment was performed 3 times with at least 3 mice in each group, and the pooled results of the 3 independent experiments are shown. (B) RNA was isolated from IT of the indicated groups and used for comparative microarray analysis. Vascular endothelial growth factor (VEGF) (P = .003) and fibroblast growth factor (FGF) (P = .005) levels were significantly increased in the GvHD group as compared with BM controls. The experiment was performed once with 3 animals in each group. (C) Left panel: in vivo PET imaging of mice from the indicated groups after allo-HCT and application of [68Ga]NODAGA-c(RGDfK) is shown for 4 representative time points (days 0, 2, 7, and 21 after allo-HCT). Right panel: [68Ga]NODAGA-c(RGDfK) uptake over time is quantified in the abdominal area of mice, demonstrating a higher uptake in transplanted as compared with untreated animals, shown for 1 representative of 3 independent experiments, each performed with at least 3 animals per group. c, coronal; s, sagittal.
αv Integrin is found on ECs after allo-HCT and can be targeted by cilengitide
The interaction of ECs with ECMs can be targeted by cilengitide, a cyclic arginine–glycine–aspartic acid pentapeptide that selectively binds the cell surface receptors αvβ3 and αvβ5 integrin28 expressed on activated ECs during angiogenesis. The drug induces programmed cell death in angiogenic blood vessels by preventing interaction of their cell surface αv integrins with specific matrix ligands, such as vitronectin, tenascin, and fibronectin.31 To evaluate if intestinal ECs represent a target for cilengitide, we quantified αv integrin expression on ECs isolated from the IT of mice developing GvHD. The αv integrin subunit was expressed by a significant number of ECs (Figure 2A), whereas enterocytes were largely negative (supplemental Figure 1A). We found αv integrin expression on Tc’s, B cells, natural killer cells, and DCs (supplemental Figure 1B). The αv integrin expression on ECs was not different in untreated animals as compared with mice developing GvHD (supplemental Figure 1C).
αv Integrin is expressed on intestinal ECs after allo-HCT and can be targeted by cilengitide. Allo-HCT was performed as described in the “Methods” section. (A) Intestinal CD34+ ECs of mice euthanized on day 14 after allo-HCT express high levels of αv integrin as shown for 1 representative fluorescence-activated cell sorter histogram (left panel). The experiment was repeated 3 times with at least 3 animals resulting in a total of 11 analyzed mice. (B) PET imaging of mice on day 22 after allo-HCT with previous cilengitide or PBS treatment. The signal intensity of [68Ga]NODAGA-c(RGDfK) was significantly reduced in animals pretreated with cilengitide (P = .001) as shown for 1 representative mouse (left panel) and when quantified (right panel). The experiment was performed once with 3 animals per group. (C) Small and large bowels were isolated on day 14 after allo-HCT from the indicated groups and analyzed for the number of CD34+ ECs by histology. Cilengitide treatment significantly reduced the number of CD34+ ECs in the small (P = .0001) and large intestines (P = .001) as compared with PBS-treated animals. The experiment was performed 3 times with 3 mice in each group (animals were pooled: each data point represents an individual animal). (D) The complete ITs of transplanted mice were digested and analyzed on day 14 after allo-HCT for the number of CD34+ ECs using flow cytometry. Cilengitide treatment reduced the number of CD34+ ECs in the total IT. Three independent experiments (at least 3 animals per group/experiment) were pooled; each data point represents an individual animal. (E) Small bowel was isolated on day 14 after allo-HCT from the indicated groups and analyzed for the number of CD31+ ECs by immunofluorescence staining. Ten sections per sample were analyzed, and vessel density was assessed by quantification of CD31+ area/total area ± SD (n = 3 per group). One representative picture from each group is shown. (F) Transplanted BALB/c recipients were treated with pimonidazole HCl on day 7 after allo-HCT for 1 hour. Small and large bowels were isolated and analyzed for the abundance of hypoxia by peroxidase staining. Three sections per sample were analyzed, and hypoxia was assessed by quantification of pimonidazole adducts+ cells/per high power field ± SD (n = 7 per group). One representative picture from each group is shown. Two independent experiments (at least 3 animals per group per experiment) were pooled.
αv Integrin is expressed on intestinal ECs after allo-HCT and can be targeted by cilengitide. Allo-HCT was performed as described in the “Methods” section. (A) Intestinal CD34+ ECs of mice euthanized on day 14 after allo-HCT express high levels of αv integrin as shown for 1 representative fluorescence-activated cell sorter histogram (left panel). The experiment was repeated 3 times with at least 3 animals resulting in a total of 11 analyzed mice. (B) PET imaging of mice on day 22 after allo-HCT with previous cilengitide or PBS treatment. The signal intensity of [68Ga]NODAGA-c(RGDfK) was significantly reduced in animals pretreated with cilengitide (P = .001) as shown for 1 representative mouse (left panel) and when quantified (right panel). The experiment was performed once with 3 animals per group. (C) Small and large bowels were isolated on day 14 after allo-HCT from the indicated groups and analyzed for the number of CD34+ ECs by histology. Cilengitide treatment significantly reduced the number of CD34+ ECs in the small (P = .0001) and large intestines (P = .001) as compared with PBS-treated animals. The experiment was performed 3 times with 3 mice in each group (animals were pooled: each data point represents an individual animal). (D) The complete ITs of transplanted mice were digested and analyzed on day 14 after allo-HCT for the number of CD34+ ECs using flow cytometry. Cilengitide treatment reduced the number of CD34+ ECs in the total IT. Three independent experiments (at least 3 animals per group/experiment) were pooled; each data point represents an individual animal. (E) Small bowel was isolated on day 14 after allo-HCT from the indicated groups and analyzed for the number of CD31+ ECs by immunofluorescence staining. Ten sections per sample were analyzed, and vessel density was assessed by quantification of CD31+ area/total area ± SD (n = 3 per group). One representative picture from each group is shown. (F) Transplanted BALB/c recipients were treated with pimonidazole HCl on day 7 after allo-HCT for 1 hour. Small and large bowels were isolated and analyzed for the abundance of hypoxia by peroxidase staining. Three sections per sample were analyzed, and hypoxia was assessed by quantification of pimonidazole adducts+ cells/per high power field ± SD (n = 7 per group). One representative picture from each group is shown. Two independent experiments (at least 3 animals per group per experiment) were pooled.
In a next step, we studied if the PET-based αv integrin signal could be saturated by cilengitide treatment. We observed significantly reduced signal intensity, indicating effective saturation of αv integrin in the whole body by cilengitide (Figure 2B). Cilengitide also reduced the numbers of ECs in the small and large intestines as assessed by immunohistochemistry of the small and large intestines separately (Figure 2C), flow cytometry of the total IT (Figure 2D), and immunofluorescence-based vessel density measurement (Figure 2E). Because hypoxia was shown to trigger neovascularization,32 we next determined the levels of hypoxia in the IT via pimonidazole hydrochloride, a small-molecule hypoxia marker that selectively binds to oxygen-starved cells.30 The levels of hypoxia in the small bowel were higher as compared with the large bowel (Figure 2F), which may explain why the inhibition of neovascularization via cilengitide was more pronounced at this site. These data indicate that cilengitide reduced intestinal neovascularization during the development of GvHD. Comparable inhibitory effects on the vasculature were also seen in vivo in the solid tumor setting with reduced relative blood flow in tumor tissue after cilengitide therapy.33
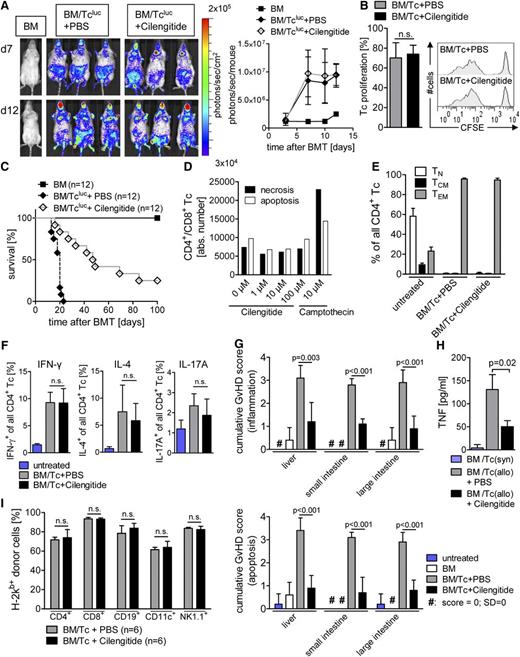
Cilengitide inhibits GvHD severity without blocking Tc function
Because Tc’s are the major effector cells that cause GvHD, we next aimed to determine the impact of cilengitide on Tcluc expansion in vivo. Allogeneic Tcluc’s migrated to secondary lymphoid organs and expanded without a significant inhibition by cilengitide treatment as shown for representative time points (Figure 3A, left panel) or as quantified over time (Figure 3A, right panel). Compatible with the BLI data, cilengitide had no impact on the expansion of carboxyfluorescein diacetate succinimidyl ester (CFSE)–positive Tc’s in vivo (Figure 3B) and in vitro (supplemental Figure 2A). Survival studies in a major histocompatibility complex disparate GvHD model indicated that cilengitide prolonged survival following allo-HCT as compared with PBS treatment (Figure 3C) without an impact on Tc viability (Figure 3D; supplemental Figure 2B). Cilengitide did not affect the frequency of CD4+ TN’s, TCM’s, or TEM’s (Figure 3E). There was also no difference in the abundance of T helper (Th)1, Th2, or Th17 Tc’s (Figure 3F); Foxp3+ Tc’s (supplemental Figure 2C); or intestinal granulocytes, macrophages, or monocytes (supplemental Figure 2D) between cilengitide- and PBS-treated groups. The absolute numbers of Tc’s in the spleen were not different in vehicle-treated compared with cilengitide-treated recipients (supplemental Figure 2E). In addition, the phenotype of Tc’s exposed to allogeneic DCs in vitro was independent of αv integrin inhibition by cilengitide (supplemental Figure 2F). There was no impact of cilengitide on the expression of costimulatory molecules on DCs in vitro (supplemental Figure 2G) or in vivo (supplemental Figure 2H) nor the ability of DCs to stimulate proliferation of allogeneic Tc’s (supplemental Figure 2I).
Cilengitide treatment improves survival after allo-HCT without affecting Tc proliferation. (A-C), (E-I) Allo-HCT was performed as described in the “Methods” section. (A) Distribution of donor-derived Tcluc’s is displayed in representative BLI images on days 7 and 21 after allo-HCT with coadministration of cilengitide or PBS as control (left panel) and quantified over time measuring the emitted photons from expanding Tcluc’s (n = 3 per group; right panel). Cilengitide administration had no significant impact on the expansion of Tcluc’s in comparison with the PBS treatment. The experiment was performed 3 times with at least 3 animals per group and similar results. (B) Splenocytes were isolated 24 hours after allo-HCT from BALB/c recipients and analyzed for the percentage of proliferated CFSE+/H-2Kb+/CD3+ donor Tc’s. Mean value for the amount of expanded Tc’s ± SD (left panel) and a representative histogram depicting CFSE dilution for each group is shown (right panel). The experiment was performed twice with at least 3 animals per group. (C) Survival of animals receiving BM alone ( ), BM/Tcluc+PBS (
), BM/Tcluc+PBS ( ), or BM/Tcluc+cilengitide (
), or BM/Tcluc+cilengitide ( ) (n = 12 in each group). Survival is improved in the cilengitide group as compared with the PBS-treated group (P < .0001). (D) CD4+/CD8+ Tc’s were exposed to cilengitide or camptothecin (10 µM) for 6 hours at the indicated concentrations, and viability was measured by flow cytometry for PI/annexin V. Annexin V and PI were used to differentiate between viable, necrotic, and apoptotic cells as follows: PI+/annexin V– (necrotic cells), PI+/annexin V+ and PI–/annexin V+ (apoptotic cells), and PI–/annexin V– (viable cells). In all, 100 000 Tc’s were analyzed for each sample, shown as absolute numbers ± SD. (E) Splenocytes from BALB/c recipients were isolated on day 14 after allo-HCT and stained for CD4, CD44, and CD62L. The quantification of TN, TEM, and TCM among CD4+ Tc’s is shown as a percentage ± SD from 1 representative of 2 independent experiments with at least 4 animals per group and comparable results. (F) Splenocytes from BALB/c recipients were isolated on day 14 after allo-HCT and stained for CD4, IL-4, IFN-γ, and IL-17A. The quantification of IL-4, IFN-γ, and IL-17A among the CD4+ Tc’s population is shown as a percentage ± SD. The experiment was performed twice each time with 5 animals per group and comparable results. (G) Histopathology of the intestines and liver was quantified as indicated in the “Methods” section. The mean score ± SD for inflammation (upper panel) and apoptosis (lower panel) is shown for the liver and small and large intestines separately (n = 5 in each group) from 1 of 2 independent experiments, each performed with at least 3 animals per group. (H) Animals were euthanized on day 7 after allo-HCT, and serum was isolated from the indicated groups to measure TNF. The mean value ± SD is shown representing 3 individual animals per group. The experiment was performed once with 3 animals per group. (I) Mice were euthanized on day 30 after allo-HCT, and donor engraftment was analyzed by flow cytometry for the indicated immune cell types. The mean value ± SD is shown for 1 representative of 2 independent experiments (n = 6 per group).
) (n = 12 in each group). Survival is improved in the cilengitide group as compared with the PBS-treated group (P < .0001). (D) CD4+/CD8+ Tc’s were exposed to cilengitide or camptothecin (10 µM) for 6 hours at the indicated concentrations, and viability was measured by flow cytometry for PI/annexin V. Annexin V and PI were used to differentiate between viable, necrotic, and apoptotic cells as follows: PI+/annexin V– (necrotic cells), PI+/annexin V+ and PI–/annexin V+ (apoptotic cells), and PI–/annexin V– (viable cells). In all, 100 000 Tc’s were analyzed for each sample, shown as absolute numbers ± SD. (E) Splenocytes from BALB/c recipients were isolated on day 14 after allo-HCT and stained for CD4, CD44, and CD62L. The quantification of TN, TEM, and TCM among CD4+ Tc’s is shown as a percentage ± SD from 1 representative of 2 independent experiments with at least 4 animals per group and comparable results. (F) Splenocytes from BALB/c recipients were isolated on day 14 after allo-HCT and stained for CD4, IL-4, IFN-γ, and IL-17A. The quantification of IL-4, IFN-γ, and IL-17A among the CD4+ Tc’s population is shown as a percentage ± SD. The experiment was performed twice each time with 5 animals per group and comparable results. (G) Histopathology of the intestines and liver was quantified as indicated in the “Methods” section. The mean score ± SD for inflammation (upper panel) and apoptosis (lower panel) is shown for the liver and small and large intestines separately (n = 5 in each group) from 1 of 2 independent experiments, each performed with at least 3 animals per group. (H) Animals were euthanized on day 7 after allo-HCT, and serum was isolated from the indicated groups to measure TNF. The mean value ± SD is shown representing 3 individual animals per group. The experiment was performed once with 3 animals per group. (I) Mice were euthanized on day 30 after allo-HCT, and donor engraftment was analyzed by flow cytometry for the indicated immune cell types. The mean value ± SD is shown for 1 representative of 2 independent experiments (n = 6 per group).
Cilengitide treatment improves survival after allo-HCT without affecting Tc proliferation. (A-C), (E-I) Allo-HCT was performed as described in the “Methods” section. (A) Distribution of donor-derived Tcluc’s is displayed in representative BLI images on days 7 and 21 after allo-HCT with coadministration of cilengitide or PBS as control (left panel) and quantified over time measuring the emitted photons from expanding Tcluc’s (n = 3 per group; right panel). Cilengitide administration had no significant impact on the expansion of Tcluc’s in comparison with the PBS treatment. The experiment was performed 3 times with at least 3 animals per group and similar results. (B) Splenocytes were isolated 24 hours after allo-HCT from BALB/c recipients and analyzed for the percentage of proliferated CFSE+/H-2Kb+/CD3+ donor Tc’s. Mean value for the amount of expanded Tc’s ± SD (left panel) and a representative histogram depicting CFSE dilution for each group is shown (right panel). The experiment was performed twice with at least 3 animals per group. (C) Survival of animals receiving BM alone ( ), BM/Tcluc+PBS (
), BM/Tcluc+PBS ( ), or BM/Tcluc+cilengitide (
), or BM/Tcluc+cilengitide ( ) (n = 12 in each group). Survival is improved in the cilengitide group as compared with the PBS-treated group (P < .0001). (D) CD4+/CD8+ Tc’s were exposed to cilengitide or camptothecin (10 µM) for 6 hours at the indicated concentrations, and viability was measured by flow cytometry for PI/annexin V. Annexin V and PI were used to differentiate between viable, necrotic, and apoptotic cells as follows: PI+/annexin V– (necrotic cells), PI+/annexin V+ and PI–/annexin V+ (apoptotic cells), and PI–/annexin V– (viable cells). In all, 100 000 Tc’s were analyzed for each sample, shown as absolute numbers ± SD. (E) Splenocytes from BALB/c recipients were isolated on day 14 after allo-HCT and stained for CD4, CD44, and CD62L. The quantification of TN, TEM, and TCM among CD4+ Tc’s is shown as a percentage ± SD from 1 representative of 2 independent experiments with at least 4 animals per group and comparable results. (F) Splenocytes from BALB/c recipients were isolated on day 14 after allo-HCT and stained for CD4, IL-4, IFN-γ, and IL-17A. The quantification of IL-4, IFN-γ, and IL-17A among the CD4+ Tc’s population is shown as a percentage ± SD. The experiment was performed twice each time with 5 animals per group and comparable results. (G) Histopathology of the intestines and liver was quantified as indicated in the “Methods” section. The mean score ± SD for inflammation (upper panel) and apoptosis (lower panel) is shown for the liver and small and large intestines separately (n = 5 in each group) from 1 of 2 independent experiments, each performed with at least 3 animals per group. (H) Animals were euthanized on day 7 after allo-HCT, and serum was isolated from the indicated groups to measure TNF. The mean value ± SD is shown representing 3 individual animals per group. The experiment was performed once with 3 animals per group. (I) Mice were euthanized on day 30 after allo-HCT, and donor engraftment was analyzed by flow cytometry for the indicated immune cell types. The mean value ± SD is shown for 1 representative of 2 independent experiments (n = 6 per group).
) (n = 12 in each group). Survival is improved in the cilengitide group as compared with the PBS-treated group (P < .0001). (D) CD4+/CD8+ Tc’s were exposed to cilengitide or camptothecin (10 µM) for 6 hours at the indicated concentrations, and viability was measured by flow cytometry for PI/annexin V. Annexin V and PI were used to differentiate between viable, necrotic, and apoptotic cells as follows: PI+/annexin V– (necrotic cells), PI+/annexin V+ and PI–/annexin V+ (apoptotic cells), and PI–/annexin V– (viable cells). In all, 100 000 Tc’s were analyzed for each sample, shown as absolute numbers ± SD. (E) Splenocytes from BALB/c recipients were isolated on day 14 after allo-HCT and stained for CD4, CD44, and CD62L. The quantification of TN, TEM, and TCM among CD4+ Tc’s is shown as a percentage ± SD from 1 representative of 2 independent experiments with at least 4 animals per group and comparable results. (F) Splenocytes from BALB/c recipients were isolated on day 14 after allo-HCT and stained for CD4, IL-4, IFN-γ, and IL-17A. The quantification of IL-4, IFN-γ, and IL-17A among the CD4+ Tc’s population is shown as a percentage ± SD. The experiment was performed twice each time with 5 animals per group and comparable results. (G) Histopathology of the intestines and liver was quantified as indicated in the “Methods” section. The mean score ± SD for inflammation (upper panel) and apoptosis (lower panel) is shown for the liver and small and large intestines separately (n = 5 in each group) from 1 of 2 independent experiments, each performed with at least 3 animals per group. (H) Animals were euthanized on day 7 after allo-HCT, and serum was isolated from the indicated groups to measure TNF. The mean value ± SD is shown representing 3 individual animals per group. The experiment was performed once with 3 animals per group. (I) Mice were euthanized on day 30 after allo-HCT, and donor engraftment was analyzed by flow cytometry for the indicated immune cell types. The mean value ± SD is shown for 1 representative of 2 independent experiments (n = 6 per group).
Consistent with its impact on survival, cilengitide treatment led to reduced GvHD severity by decreasing the apoptosis and inflammation-based GvHD histopathology score for the small and large intestines and liver (Figure 3G) and the production of proinflammatory tumor necrosis factor (TNF) (Figure 3H). Because previous studies had shown that inhibition of angiogenesis by anti–vascular endothelial growth factor receptor (VEGFR) 1/anti-VEGFR2 antibodies impaired hematopoietic reconstitution,3 we next studied engraftment in mice that received cilengitide, which was independent of cilengitide treatment of different lymphoid and myeloid lineages (Figure 3I).
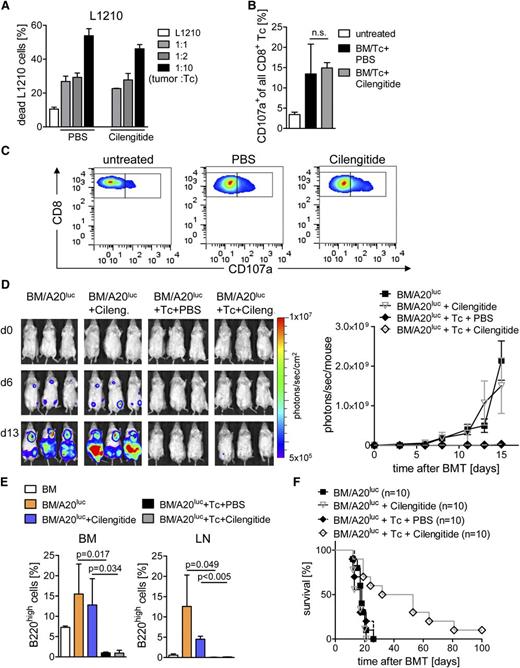
Effector function of cytotoxic T lymphocytes is required to reject tumor cells following allo-HCT. To probe in vitro cytolytic activity of Tc’s against L1210 target cells, Tc’s were isolated from PBS- or cilengitide-treated BALB/c recipients on day 14 after allo-HCT. Cytolytic activity of Tc’s was found to be independent of cilengitide treatment (Figure 4A). Expression of CD107a in CD8+ Tc’s isolated after allo-HCT was comparable in recipients treated with cilengitide vs PBS (Figure 4B-C). In a further step, the A20luc lymphoma rejection model34 was used to study if in vivo antitumor activity was maintained during cilengitide treatment. Where indicated, continuous PBS or cilengitide treatment was given. Serial BLI images demonstrated leukemic BM and organ infiltration at different time points after transplantation in the BM/A20luc and BM/A20luc+cilengitide group. Animals that received BM/A20luc+Tc, independent of treatment with PBS or cilengitide, achieved tumor control as determined by loss of tumor signal (Figure 4D) and quantified by flow cytometry for B220high A20 leukemic cells in BM and lymph nodes (LNs) (Figure 4E), which is indicative of an intact in vivo effector function of CD8+ Tc’s. The survival of A20 lymphoma-bearing mice was significantly prolonged when cilengitide treatment was given (Figure 4F). The improved survival was due to the impact on GvHD, whereas there was no evidence that GvL effects were enhanced by cilengitide. Cilengitide treatment had no direct effect on the survival of animals receiving BM/A20luc without Tc’s (Figure 4E). These data indicate a significant impact of cilengitide on GvHD severity without impacting Tc expansion, antitumor immunity, and immune reconstitution after allo-HCT.
Cilengitide administration allows for CD8-mediated cytotoxicity. Allo-HCT was performed as described. (A) CD4+/CD8+ Tc’s were isolated from BALB/c recipients on day 14 after allo-HCT and cilengitide or PBS treatment and used as effector cells against L1210 cells in the indicated ratios of target:effector. Values are the percentage of annexin V+/PI+ cells ± SD from 1 representative experiment. Experiments were repeated 3 times in triplicates with similar results. (B-C) Splenocytes were isolated on day 14 after allo-HCT from BALB/c recipients and stained for CD8 and CD107a. (B) Quantification of CD107a+ population as percentage ± SD of all CD8+ Tc’s. One representative of 2 independent experiments is shown, each performed with at least 4 animals per group. (C) Representative flow cytometry data for the indicated groups described in (B) are shown. (D) Expansion and rejection of A20luc cells in BALB/c recipients receiving BM alone, with Tc’s and PBS or cilengitide. Days 0, 6, and 13 as representative time points are shown (left panel). Photons emitted from the A20luc cells in vivo are shown over time for the indicated groups (n = 3 per group). The experiment was performed 3 times with at least 3 animals per group and showed similar results (right panel). (E) Mice from the indicated groups were euthanized on day 13 after allo-HCT. LN (axillary, mesenteric, inguinal) and BM from the femur was isolated and analyzed for the presence of B220high A20luc cells by flow cytometry. The percentage (mean ± SD) of B220high A20luc cells in the BM (left) and LN (right panel) is shown. The experiment was performed once with at least 3 mice in each group. (F) Survival of BALB/c recipients after allo-HCT receiving additional A20luc and PBS or cilengitide. Survival is improved in the BM/A20luc+Tc+cilengitide-treated group ( ) as compared with BM/A20luc+Tc+PBS (
) as compared with BM/A20luc+Tc+PBS ( ; P = .005), BM/A20luc+cilengitide (
; P = .005), BM/A20luc+cilengitide ( ; P = .025), and BM/A20luc alone (
; P = .025), and BM/A20luc alone ( ; P = .003). The experiment was performed twice, and the number of mice per group is indicated in the survival graph.
; P = .003). The experiment was performed twice, and the number of mice per group is indicated in the survival graph.
Cilengitide administration allows for CD8-mediated cytotoxicity. Allo-HCT was performed as described. (A) CD4+/CD8+ Tc’s were isolated from BALB/c recipients on day 14 after allo-HCT and cilengitide or PBS treatment and used as effector cells against L1210 cells in the indicated ratios of target:effector. Values are the percentage of annexin V+/PI+ cells ± SD from 1 representative experiment. Experiments were repeated 3 times in triplicates with similar results. (B-C) Splenocytes were isolated on day 14 after allo-HCT from BALB/c recipients and stained for CD8 and CD107a. (B) Quantification of CD107a+ population as percentage ± SD of all CD8+ Tc’s. One representative of 2 independent experiments is shown, each performed with at least 4 animals per group. (C) Representative flow cytometry data for the indicated groups described in (B) are shown. (D) Expansion and rejection of A20luc cells in BALB/c recipients receiving BM alone, with Tc’s and PBS or cilengitide. Days 0, 6, and 13 as representative time points are shown (left panel). Photons emitted from the A20luc cells in vivo are shown over time for the indicated groups (n = 3 per group). The experiment was performed 3 times with at least 3 animals per group and showed similar results (right panel). (E) Mice from the indicated groups were euthanized on day 13 after allo-HCT. LN (axillary, mesenteric, inguinal) and BM from the femur was isolated and analyzed for the presence of B220high A20luc cells by flow cytometry. The percentage (mean ± SD) of B220high A20luc cells in the BM (left) and LN (right panel) is shown. The experiment was performed once with at least 3 mice in each group. (F) Survival of BALB/c recipients after allo-HCT receiving additional A20luc and PBS or cilengitide. Survival is improved in the BM/A20luc+Tc+cilengitide-treated group ( ) as compared with BM/A20luc+Tc+PBS (
) as compared with BM/A20luc+Tc+PBS ( ; P = .005), BM/A20luc+cilengitide (
; P = .005), BM/A20luc+cilengitide ( ; P = .025), and BM/A20luc alone (
; P = .025), and BM/A20luc alone ( ; P = .003). The experiment was performed twice, and the number of mice per group is indicated in the survival graph.
; P = .003). The experiment was performed twice, and the number of mice per group is indicated in the survival graph.
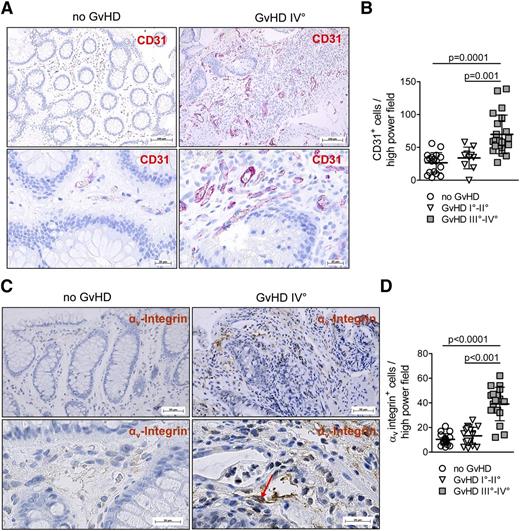
Neovascularization and αv integrin are increased in human GvHD lesions
To clarify if the observed neovascularization in mice developing GvHD could also be detected in human GvHD tissues, we investigated patient samples with or without GvHD of individuals having undergone allo-HCT and receiving diagnostic colonoscopy (supplemental Table 1). Conditioning and GvHD prophylaxis were restricted to 2 major protocols previously reported.35,36 The endothelial marker CD31 was used to determine the level of neovascularization. We found that the severity of intestinal GvHD determined on the basis of histology was strongly correlated with the number of ECs in GvHD lesions as shown for representative cases (Figure 5A) and when all patients were analyzed (Figure 5B). Importantly, the intestinal human ECs displayed high αv integrin expression (Figure 5C), and αv integrin expression was significantly correlated with the severity of GvHD (Figure 5D). These data indicate that neovascularization in inflamed lesions is a feature of human acute intestinal GvHD and based on their αv integrin expression could be targeted by cilengitide, which is already in use for the treatment of glioblastoma in humans.37
Neovascularization and αv integrin expression are features of human GvHD. (A) Representative sections of the IT are shown for patients with or without GvHD. The panels show red immunohistochemical staining for CD31 in the microvasculature. (B) The immunohistochemical analysis for CD31 in the IT of healthy and GvHD patients is shown. CD31 expression is increased in patients with GvHD III°-IV° compared with GvHD I°-II° (P = .001) or patients without GvHD (P = .0001). (C) Histopathological analysis for αv integrin in the IT of patients with or without GvHD. αv Integrin expression is preferentially found in ECs of patients with GvHD with brown immunohistochemical staining for αv integrin in the microvasculature. Representative sections of the IT are shown for healthy and GvHD patients. (D) The immunohistochemical analysis for αv integrin in the IT of healthy and GvHD patients is shown with an increase of αv integrin expression in patients with GvHD III°-IV° compared with GvHD I°-II° (P < .001) or patients without GvHD (P < .0001).
Neovascularization and αv integrin expression are features of human GvHD. (A) Representative sections of the IT are shown for patients with or without GvHD. The panels show red immunohistochemical staining for CD31 in the microvasculature. (B) The immunohistochemical analysis for CD31 in the IT of healthy and GvHD patients is shown. CD31 expression is increased in patients with GvHD III°-IV° compared with GvHD I°-II° (P = .001) or patients without GvHD (P = .0001). (C) Histopathological analysis for αv integrin in the IT of patients with or without GvHD. αv Integrin expression is preferentially found in ECs of patients with GvHD with brown immunohistochemical staining for αv integrin in the microvasculature. Representative sections of the IT are shown for healthy and GvHD patients. (D) The immunohistochemical analysis for αv integrin in the IT of healthy and GvHD patients is shown with an increase of αv integrin expression in patients with GvHD III°-IV° compared with GvHD I°-II° (P < .001) or patients without GvHD (P < .0001).
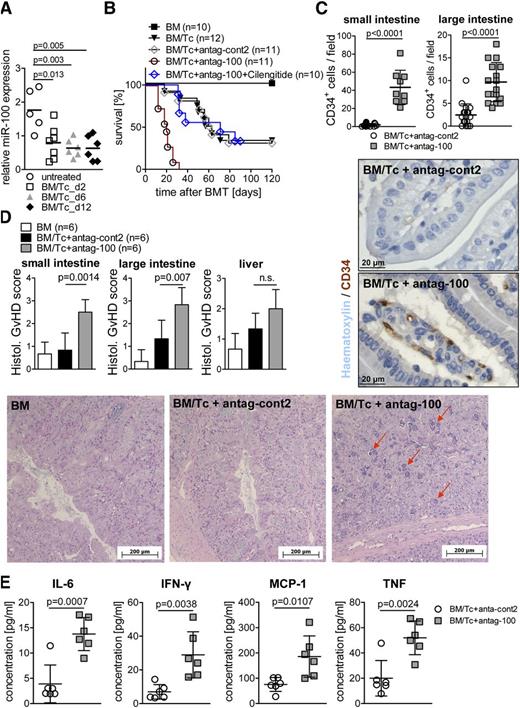
MiR-100 antagonism enhances GvHD
Because tissue ischemia due to atherosclerotic arterial occlusion or narrowing, which is regulated by miRs,13 and a hypoxic microenvironment of the inflamed IT may share common features, we next studied the role of different miRs in the small bowel by miR array to identify candidates for further analysis (data not shown). MiRs isolated from the small bowel of untreated mice vs mice developing GvHD showed that miR-100 was downregulated on days 2, 6, and 12 after allo-HCT as quantified by qRT-PCR (Figure 6A), compatible with reduced suppression of neovascularization by miR-100 when GvHD evolved. MiR-100 is a previously reported negative regulator of neovascularization in murine vascular ligation–induced ischemia models.13 By using miR-100–specific antagomir treatment in a model of delayed GvHD, we observed that blocking the function of miR-100 enhanced GvHD with reduced survival (Figure 6B) and increased neovacularization in the small and large intestines (Figure 6C). The group that received miR-100 antagomir and cilengitide had a significantly improved survival as compared with the group that received miR-100 antagomir only (P < .001) (Figure 6B). In line with reduced survival, the blockade of miR-100 led to higher GvHD histopathology scores (Figure 6D) and increased levels of proinflammatory IL-6, IFN-γ, monocyte chemoattractant protein 1, and TNF (Figure 6E) as compared with the group that received the nonfunctional antag-cont2. These data support the concept that neovascularization is not only a bystander effect of intestinal inflammation but significantly contributes to the pathophysiology of the disease and that antagonizing its negative regulator miR-100 enhances disease severity.
MiR-100 antagonism enhances GvHD. (A) Allo-HCT was performed as described in the “Methods” section. MiR-100 expression on days 2, 6, and 12 after allo-HCT is displayed for the small bowel using qRT-PCR. MiR-100 expression is downregulated on day 2 (P = .013), day 6 (P = .003), and day 12 (P = .005) after allo-HCT as compared with untreated animals. The experiment was performed 3 times with at least 3 mice in each group. (B-E) Mice were transplanted with 5 million BM cells and 100 000 CD4+/CD8+ Tc’s to induce a delayed GvHD phenotype. The number of mice is indicated for the respective groups. (B) Survival was reduced in the group treated with antag-100 compared with antag-cont2–treated animals (P < .0001), untreated mice (P < .0001), BM controls (P < .0001), or antag-100+cilengitide treatment (P < .0001). The experiment was performed 3 times, and the resulting data were pooled. (C) The number of CD34+ ECs was analyzed by immunohistochemistry in small and large bowels from the indicated groups, isolated on day 14 after allo-HCT. Antag-100 treatment significantly increased the number of CD34+ ECs in the small (P < .0001) and large intestines (P < .0001) as compared with antag-cont2–treated animals. The experiment was performed once with 6 individual animals per group. Representative pictures from the small bowel stained for CD34 (peroxidase staining) are shown. (D) Histopathology of the intestines was quantified as indicated in the “Methods” section. The mean score ± SD for cumulative histopathology (apoptosis and inflammation) is shown for 1 of 2 independent experiments, each performed with 6 individual animals per group. (E) Animals were euthanized on day 13 after allo-HCT, and serum was isolated from the indicated groups. The mean values for IL-6, IFN-γ, monocyte chemoattractant protein 1, and TNF ± SD are displayed for 1 of 2 independent experiments, representing 6 individual animals per group.
MiR-100 antagonism enhances GvHD. (A) Allo-HCT was performed as described in the “Methods” section. MiR-100 expression on days 2, 6, and 12 after allo-HCT is displayed for the small bowel using qRT-PCR. MiR-100 expression is downregulated on day 2 (P = .013), day 6 (P = .003), and day 12 (P = .005) after allo-HCT as compared with untreated animals. The experiment was performed 3 times with at least 3 mice in each group. (B-E) Mice were transplanted with 5 million BM cells and 100 000 CD4+/CD8+ Tc’s to induce a delayed GvHD phenotype. The number of mice is indicated for the respective groups. (B) Survival was reduced in the group treated with antag-100 compared with antag-cont2–treated animals (P < .0001), untreated mice (P < .0001), BM controls (P < .0001), or antag-100+cilengitide treatment (P < .0001). The experiment was performed 3 times, and the resulting data were pooled. (C) The number of CD34+ ECs was analyzed by immunohistochemistry in small and large bowels from the indicated groups, isolated on day 14 after allo-HCT. Antag-100 treatment significantly increased the number of CD34+ ECs in the small (P < .0001) and large intestines (P < .0001) as compared with antag-cont2–treated animals. The experiment was performed once with 6 individual animals per group. Representative pictures from the small bowel stained for CD34 (peroxidase staining) are shown. (D) Histopathology of the intestines was quantified as indicated in the “Methods” section. The mean score ± SD for cumulative histopathology (apoptosis and inflammation) is shown for 1 of 2 independent experiments, each performed with 6 individual animals per group. (E) Animals were euthanized on day 13 after allo-HCT, and serum was isolated from the indicated groups. The mean values for IL-6, IFN-γ, monocyte chemoattractant protein 1, and TNF ± SD are displayed for 1 of 2 independent experiments, representing 6 individual animals per group.
Discussion
aGvHD is a major complication of allo-HCT and significantly contributes to mortality and morbidity associated with this treatment option. A better understanding of the pathomechanistic features of GvHD is critical to improve the outcome of allo-HCT. Recent studies had identified neovascularization to occur during aGvHD.3 Here we report that inflammatory neovascularization observed during GvHD is regulated by αv integrin and miR-100 and can be targeted by αv integrin blocking or enhanced by miR-100 antagonism.
We first clarified when and where neovascularization takes place during GvHD. To monitor this process under real-time in vivo conditions, we used PET-based whole body imaging and observed that following transplantation, neovascularization was most abundant in the IT, occurred already on day 2 after allo-HCT, and continued for the following weeks until the death of the animals. The results were verified by more conventional methods using immunohistochemistry and flow cytometry. As the interaction of ECs with ECMs via integrins is crucial during neovascularization,18-20 we used cilengitide, an inhibitor already in clinical use for the inhibition of angiogenesis in anticancer therapy.37 Our studies showed that development of aGvHD required αν integrin function because the survival of mice developing GvHD was significantly improved by cilengitide treatment independent of Tc expansion. The advantage of this preventive approach over conventional immunosuppressive therapy may be that Tc function is not targeted. Compatible with this concept, we observed intact GvL effects and immune reconstitution when αν integrin was inhibited. Previous work has identified different integrins such as β238 and β7 integrin39 as critical mediators in the ability of Tc’s to induce GvHD. Conversely, αν integrin was never found to be involved in allogeneic Tc function despite a well-described role in neovascularization.18
To apply the concept of αν integrin inhibition to patients development aGvHD, it was important to study neovascularization in the IT of humans undergoing allo-HCT. We observed a strong correlation between the number of CD31+ ECs in the intestines of individuals that had undergone allo-HCT and the extent of aGvHD. We found that comparable to the mouse, human ECs located in intestinal GvHD lesions displayed high αv integrin expression, indicating that these cells could be targeted by cilengitide.
To better understand the regulation of neovascularization during GvHD, we tried to identify pathomechanistic parallels with other conditions causing neovascularization. Reactive neovascularization is a key feature of ischemia found during coronary artery disease and peripheral arterial disease. Recent studies have shown that miRs negatively13,16 or positively17 regulate ischemia-induced neovascularization by impacting signaling in ECs. We found that miR-100 was downregulated when aGvHD evolved, suggesting its role as a negative regulator that is degraded after transplantation and then permits the development of GvHD. Functional inactivation of miR-100 with antagomir treatment enhanced GvHD severity, indicating a protective role for miR-100 by blocking inflammatory neovascularization during GvHD. The role of miR-100 had only been previously reported for adaptive neovascularization following arterial occlusion13 but not for inflammatory neovascularization. This common feature may be explained by hypoxia, which occurs during ischemia and which is also a key feature of the inflamed microenvironment in different inflammatory diseases.40,41 The presence of hypoxia in the human synovium of patients with active rheumatoid arthritis (RA) was shown using microelectrodes,42 and a comparable finding was made in inflamed joints when using a rat model of RA.43 Another inflammatory process connected to hypoxia is wound healing. Wounds exhibit areas of marked hypoxia due to lack of perfusion caused by vascular damage and the intense metabolic activity of cells infiltrating the wound.41 Oxygen tension measurements in a rodent model showed that immediately postinjury, wound oxygen tension was significantly reduced,44 indicating that hypoxia in inflamed areas and production of hypoxia-regulated factors promote angiogenesis during inflammatory wound healing.45 These studies support our concept for common regulatory mechanisms of neovascularization by hypoxia during ischemia and inflammation related to GvHD. Importantly, the inflammatory neovascularization had a negative impact on GvHD-related mortality and proinflammatory cytokine production, which proves its functional contribution to the disease.
Our findings on the occurrence of neovascularization during GvHD in conjunction with data derived from a variety of inflammatory diseases including asthma,46 RA,47-49 skin inflammation,50 and inflammatory bowel disease51 suggest that inflammation and neovascularization are functionally connected. Another study identified αν integrin to be required in DCs for Th17 cell differentiation in experimental autoimmune encephalomyelitis,52 whereas we observed no effect of αν integrin inhibition on Tc’s and DCs in the GvHD model. This observation could be due to model-specific differences in particular alloreactivity vs autoimmunity and the fact that the other group used a genetic model,52 whereas we applied a pharmacologic αν integrin inhibitor. We could expand on the previous findings by demonstrating that neovascularization can be targeted via ανβ3 integrin antagonism, and we identified miR-100 as a central negative regulator of this process.
In summary, we provide the first in vivo evidence that αv integrin is upregulated on ECs participating in intestinal neovascularization during GvHD in the mouse and corresponding expression in human GvHD lesions. Our findings indicate that inflammatory neovascularization is a central event during GvHD and can be inhibited by targeting αv integrin–expressing ECs. Mechanistically, the process of neovascularization is negatively regulated by miR-100, which indicates pathophysiological similarities between ischemic and inflammatory neovascularization.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sophie Krüger and Volker Schmidt for excellent technical assistance, and Merck Serono AG for valuable suggestions and for providing cilengitide.
Merck Serono has reviewed the publication, and the views and opinions described in the publication do not necessarily reflect those of Merck Serono.
This work was supported by the Deutsche Forschungsgemeinschaft, Germany (SFB850 [R.Z. and W.A.W.], Z2 project; Heisenberg Fellowship, DFG ZE 872/2-1 [R.Z.]; and Gr3459/2-1 [S.G.]), and by a Comprehensive Cancer Center Freiburg seeding grant (R.A.D., R.Z., W.A.W.).
Authorship
Contribution: F.L. helped to design the experiments, performed experiments, and helped to write the manuscript; S.G. and F.B. helped to design and perform miR-100–related experiments and helped with the manuscript; M.B., R.A.D., and F.B. planned and performed PET imaging experiments; M.F. synthesized and labeled [68Ga]NODAGA-c(RGDfK); G.P. and A.K.H. helped with mouse experiments and flow cytometry; K.R. and O.P. analyzed the vessel density in the IT of mice by microscopy; H.D. helped with immunohistochemistry; U.V.G. and A.S.G. performed histologic analysis; J.F. and W.A.W. helped to design experiments and discussed data; and R.Z. developed the overall concept, designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests. A patent for the application of cilengitide for GvHD prophylaxis or treatment is pending (Merck AG).
Correspondence: Robert Zeiser, Department of Hematology and Oncology, Freiburg University Medical Center, Albert-Ludwigs-University, Hugstetterstr. 55, 79106 Freiburg, Germany; e-mail: robert.zeiser@uniklinik-freiburg.de.

![Figure 1. Neovascularization is increased in the IT of mice after allo-HCT. Allo-HCT was performed as described in the “Methods” section. (A) Intestines of mice from the indicated groups were digested and analyzed on day 14 after allo-HCT for the frequency of ECs using flow cytometry. Allo-HCT increased the number of CD34+ and CD31+ ECs in the total IT, as shown for representative flow cytometry images (left) and when quantified for each individual animal (right). The experiment was performed 3 times with at least 3 mice in each group, and the pooled results of the 3 independent experiments are shown. (B) RNA was isolated from IT of the indicated groups and used for comparative microarray analysis. Vascular endothelial growth factor (VEGF) (P = .003) and fibroblast growth factor (FGF) (P = .005) levels were significantly increased in the GvHD group as compared with BM controls. The experiment was performed once with 3 animals in each group. (C) Left panel: in vivo PET imaging of mice from the indicated groups after allo-HCT and application of [68Ga]NODAGA-c(RGDfK) is shown for 4 representative time points (days 0, 2, 7, and 21 after allo-HCT). Right panel: [68Ga]NODAGA-c(RGDfK) uptake over time is quantified in the abdominal area of mice, demonstrating a higher uptake in transplanted as compared with untreated animals, shown for 1 representative of 3 independent experiments, each performed with at least 3 animals per group. c, coronal; s, sagittal.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/17/10.1182_blood-2012-07-442665/4/m_3307f1.jpeg?Expires=1765890190&Signature=mQbVramHKBrYroMamh3emEaJEYgQkl60riunvsi6wRXoNvOggd9l3eV2jqwpx8mCdEdMvjmVJvO3im9SCdeNJlrA61lB7Oq-f2dd8LmrsEDa8OlF0AZXV0OMAqIEmMN8rGbv8zHO4X~9BVZQvTH90DduIsmEHUanHdYUTXOPy1xw5hoNkCOhgicDktIgYZ~jI2xNbh8D~aHYugPfGe1KBBrO~FbgACPw4-F7CiRTzzhzSrUIlSjJL5YOq3Bq1H3JojCheL4RDekDKRxrzmwn3oml9b7SWDNmQoJ3w6hrMqftCfhsOtgrvXAzCb5goM9BTrVLeuC56Vnty5b39v~1wA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. αv Integrin is expressed on intestinal ECs after allo-HCT and can be targeted by cilengitide. Allo-HCT was performed as described in the “Methods” section. (A) Intestinal CD34+ ECs of mice euthanized on day 14 after allo-HCT express high levels of αv integrin as shown for 1 representative fluorescence-activated cell sorter histogram (left panel). The experiment was repeated 3 times with at least 3 animals resulting in a total of 11 analyzed mice. (B) PET imaging of mice on day 22 after allo-HCT with previous cilengitide or PBS treatment. The signal intensity of [68Ga]NODAGA-c(RGDfK) was significantly reduced in animals pretreated with cilengitide (P = .001) as shown for 1 representative mouse (left panel) and when quantified (right panel). The experiment was performed once with 3 animals per group. (C) Small and large bowels were isolated on day 14 after allo-HCT from the indicated groups and analyzed for the number of CD34+ ECs by histology. Cilengitide treatment significantly reduced the number of CD34+ ECs in the small (P = .0001) and large intestines (P = .001) as compared with PBS-treated animals. The experiment was performed 3 times with 3 mice in each group (animals were pooled: each data point represents an individual animal). (D) The complete ITs of transplanted mice were digested and analyzed on day 14 after allo-HCT for the number of CD34+ ECs using flow cytometry. Cilengitide treatment reduced the number of CD34+ ECs in the total IT. Three independent experiments (at least 3 animals per group/experiment) were pooled; each data point represents an individual animal. (E) Small bowel was isolated on day 14 after allo-HCT from the indicated groups and analyzed for the number of CD31+ ECs by immunofluorescence staining. Ten sections per sample were analyzed, and vessel density was assessed by quantification of CD31+ area/total area ± SD (n = 3 per group). One representative picture from each group is shown. (F) Transplanted BALB/c recipients were treated with pimonidazole HCl on day 7 after allo-HCT for 1 hour. Small and large bowels were isolated and analyzed for the abundance of hypoxia by peroxidase staining. Three sections per sample were analyzed, and hypoxia was assessed by quantification of pimonidazole adducts+ cells/per high power field ± SD (n = 7 per group). One representative picture from each group is shown. Two independent experiments (at least 3 animals per group per experiment) were pooled.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/17/10.1182_blood-2012-07-442665/4/m_3307f2.jpeg?Expires=1765890190&Signature=Ta~7SrF3TBB4en8thQgQ4FUYXV4cjksWXiPdHZKZrUsQ1BnLfGKj6VkBo~QpuRpJftfT0M4KWVaRPkmgwdiER99MgR9uOUjJ8fBhkoSNGK~8mud47cvMFb4PMmwdueJKD6l1djVrZGkZl1q0MVHpurCX-svtpCbsG9WTEl89Op3g2XhNfHR1K-00qDpoqySU~2Dge7j5Y9ztJGeuqTky5laM5uuoiRuLeU9otC1JzPwxhNAFF3vALxPWBpVkIWnMvR4i9AF0KjqkKn7QKxLMHeb-FWSHOzIxzwTf5lAnRiyUG34wslcB~mSszF5xuqJEwOLuxbnp7NWHfAi0T0ac4Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal