Key Points
MZ B cells play a critical role in the production of PF4/heparin-specific antibodies.
Abstract
Heparin-induced thrombocytopenia (HIT) is an immune-mediated disorder that can cause fatal arterial or venous thrombosis/thromboembolism. Immune complexes consisting of platelet factor 4 (PF4), heparin, and PF4/heparin-reactive antibodies are central to the pathogenesis of HIT. However, the B-cell origin of HIT antibody production is not known. Here, we show that anti-PF4/heparin antibodies are readily generated in wild-type mice on challenge with PF4/heparin complexes, and that antibody production is severely impaired in B-cell–specific Notch2-deficient mice that lack marginal zone (MZ) B cells. As expected, Notch2-deficient mice responded normally to challenge with T-cell–dependent antigen nitrophenyl-chicken γ globulin but not to the T-cell–independent antigen trinitrophenyl-Ficoll. In addition, wild-type, but not Notch2-deficient, B cells plus B-cell–depleted wild-type splenocytes adoptively transferred into B-cell–deficient μMT mice responded to PF4/heparin complex challenge. PF4/heparin-specific antibodies produced by wild-type mice were IgG2b and IgG3 isotypes. An in vitro class-switching assay showed that MZ B cells were capable of producing antibodies of IgG2b and IgG3 isotypes. Lastly, MZ, but not follicular, B cells adoptively transferred into B-cell–deficient μMT mice responded to PF4/heparin complex challenge by producing PF4/heparin-specific antibodies of IgG2b and IgG3 isotypes. Taken together, these data demonstrate that MZ B cells are critical for PF4/heparin-specific antibody production.
Introduction
Heparin-induced thrombocytopenia (HIT) is the most common drug-induced, immune-mediated thrombocytopenia,1 usually occurring after 3 to 6 days of heparin treatment.2 A significant number of patients with HIT experience serious arterial and/or venous thrombosis and thromboembolism.3 Identification of antibodies that recognize PF4/heparin complexes has established HIT as an immune-mediated syndrome.1,4 These antibodies are predominantly polyclonal IgG1 isotype with some IgG2.5 The IgG antibodies that react with platelet factor 4 (PF4) and heparin to form IgG/PF4/heparin immune complexes are central to the pathogenesis of HIT.2 These immune complexes bind FcγRIIa on the platelet surface and induce platelet activation, resulting in thrombocytopenia and a high risk for thrombosis.6 Thrombocytopenia or thrombosis develops in a proportion (5%-30%) of patients who have PF4/heparin-specific antibodies.6
B-cell–derived plasma cells are responsible for the production of autoantibodies and are critical for the promotion of autoimmunity.7 Patients with HIT have features of a T-cell–independent immune response, characterized by rapid onset and decline of antibodies and no immunologic memory.1,8 Patients with HIT both quickly undergo development and then loss of anti-PF4/heparin antibodies, which often results in failure to regenerate the antibodies rapidly and robustly on second exposure to heparin.1,9 Occasionally when patients have 2 distinct episodes of HIT, the onset of the second HIT episode occurs no sooner after heparin exposure than that of the first one.10,11 Finally, T-cell–independent immune responses are normally triggered by antigens with repetitive epitopes,12 and the high-molecular-weight PF4/heparin complexes have such repetitive epitopes.13
However, patients with HIT also have some aspects of a T-cell–dependent immune response in that they rapidly produce PF4/heparin-reactive antibodies of the IgG isotype, indicating previous contact with PF4/heparin antigens and the involvement of helper T cells.14,15 Consistently, neonates, who have had no contact with foreign antigens before birth, do not generate anti-PF4/heparin antibodies on exposure to heparin.16 After undergoing cardiac surgery, neonates and infants have a much lower rate of HIT compared with older children receiving the same surgery.17 In addition, patients with severe HIT have T cells that are responsive to PF4/heparin and possess a T-cell receptor with highly restricted CDR3 regions.18 Thus, patients with HIT have an unusual immune response in that they exhibit having both T-cell–independent and T-cell–dependent immune responses.
The atypical immune response of these patients indicates possible involvement of a complex mixture of mature B-cell subsets during HIT pathogenesis. There are 3 subsets of long-lived mature B cells: marginal zone (MZ), B1, and follicular (FO) B cells.19,20 Nonrecirculating MZ B cells reside primarily in the MZs of the splenic lymphoid nodules,20 and their development specifically requires Notch2 signaling.21 Although Notch2 plays an important role in the development of CD4 and CD8 T cells22,23 and intraepithelial localization of intestinal mast cells,24 inactivation of the Notch2 pathway in the B-cell lineage leads to a specific reduction of MZ B cells without affecting B1 and FO B cells or other types of immune cells.21 Self-renewing B1 B cells are enriched in the peritoneal and pleural cavities and are derived from fetal liver B-cell progenitors.25 Recirculating FO B cells localize to the B-lymphoid follicles of the spleen and lymph node.26 MZ and B1 B cells contribute significantly to the initial rapid T-cell–independent IgM antibody response.27,28 MZ B cells can also produce high levels of IgG2 and IgG3 antibodies.29 FO B cells participate later in the T-cell–dependent antibody responses.28
Recently, a mouse model for PF4/heparin-induced antibody production has been established.30,31 Importantly, mouse anti-PF4/heparin antibodies share important serologic and functional characteristics with human HIT antibodies, including IgG isotypes, production kinetics, binding to mouse PF4/heparin complexes but not PF4 or heparin alone, and the ability to activate platelets in the presence of low-dose heparin.32 Here, we use this mouse model in combination with Notch2-deficient mice and adoptive transfer of B-cell subsets to dissect the immune response to PF4/heparin. We show that MZ B cells are critical for PF4/heparin-specific antibody production.
Methods
Mice
Notch2 “flox” mice (Notch2fl/fl) on a C57BL/6 genetic background were provided by Dr. Shigeru Chiba at Tokyo University in Tokyo, Japan, and Dr. Maeda Takahiro at Beckman Research Institute of City of Hope in Duarte, California.21 CD19Cre C57BL/6 (B6.129P2(C)-Cd19tm1(cre)Cgn/J), wild-type C57BL/6, and μMT (B6.129S2-Igh-6tm1Cgn/J) mice were purchased from the Jackson Laboratory. Experimental and control mice were 8 to 10 weeks old. Mice were maintained in the Biological Resource Center at the Medical College of Wisconsin (MCW). All animal protocols were approved by the MCW Institutional Animal Care and Use Committee.
Flow cytometry
Single-cell suspensions of splenocytes, bone marrow, or peritoneal cells were treated with Gey solution to remove red blood cells and were resuspended in phosphate-buffered saline (PBS) supplemented with 2% fetal bovine serum (FBS). The cells were then stained with a combination of fluorescence-conjugated antibodies. Allophycocyanin (APC)-conjugated anti-B220, anti-IgM, anti-c-Kit and anti-F4/80, phycoerythrin (PE)-conjugated anti-CD4, anti-FcεR, anti-CD11b and anti-Ter119, PE–Cy7-conjugated anti-B220, anti-CD8, anti-NK1.1 and anti-CD23, and Biotin-conjugated anti-CD11c were purchased from eBioscience. APC-conjugated anti-Thy1.2 and streptavidin, and PE-conjugated anti-CD5 and anti-CD21 were purchased from BD Biosciences. PE-conjugated anti-IgD were purchased from Southern Biotech. APC-Cy7–conjugated anti-B220 and anti-Ly6G were purchased from BioLegend. Samples were applied to a flow cytometer (LSRII, Becton Dickinson). Data were collected and analyzed using FACSDiva software (Becton Dickinson).
PF4/heparin immunization
Mouse PF4 was expressed in a prokaryotic expression system and were isolated as described previously.30 Optimal PF4/heparin complex immunization of mice with a C57BL/6 genetic background has been described previously.30,31 Briefly, mouse PF4 and heparin were mixed in 1 × Hanks balanced salt solution (Invitrogen) at final concentrations of 200 μg/mL of PF4 and 4 U/mL of heparin, a previously optimized molar ratio (2.6:1) for PF4/heparin complex formation and immunogenicity,30 followed by 1-hour incubation at room temperature. Mice anesthetized with isoflurane were injected retro-orbitally, the most efficient route for production of PF4/heparin-specific antibodies,31 with 100 μL of the mouse PF4/heparin solution once daily for 5 days. Sera were collected before and at 7-day intervals after immunization. PF4/heparin-specific antibodies were measured by the enzyme-linked immunosorbent assay (ELISA). The specificity of the assay has been confirmed by experiments showing that the antibodies display greater binding to murine PF4 (mPF4)/heparin than to mPF4 alone and that antibody binding is inhibited by an excess amount of heparin.32 In addition, our studies have demonstrated that wild-type mice injected with mPF4/heparin complexes, but not mPF4 alone or heparin alone, develop marked levels of anti-mPF4/heparin antibodies.30,32
T-cell–dependent and T-cell–independent antigen immunizations
T-cell–dependent and T-cell–independent antigen immunizations were performed as described previously.33 Briefly, 8- to 10-week-old mice were immunized intraperitoneally with 100 μg of the T-cell–dependent antigen nitrophenyl-chicken γ globulin (NP-CGG; Sigma-Aldrich) mixed 1:1 with Alum adjuvant (Pierce) (200 μL/mouse) or 50 μg of the T-cell–independent antigen trinitrophenyl-Ficoll (TNP-Ficoll; Sigma-Aldrich) mixed 1:1 with Alum adjuvant (200 μL/mouse). Sera were collected before and at 8 and 15 days after immunization. Antigen-specific antibodies were measured by ELISA.
In vitro class-switching assay
An in vitro class-switching assay was performed as described previously.34 Briefly, FO (B220+CD21+CD23hi) or MZ (B220+CD21hiCD23lo) B cells were sorted by the fluorescence-activated cell sorter (FACS) from splenocytes of 8-week-old wild-type C57BL/6 mice. Then, the cells (2 × 105/mL) were cultured in 24-well flat-bottom plates in 1 mL of RPMI 1640 containing 10% FBS, 1% sodium pyruvate, 1% nonessential amino acids, 0.5% L-glutamine, 50 μM of 2-mercaptoethanol, 100 U/mL of penicillin, and 100 μg/mL of streptomycin at 37°C with 5% CO2 in a humidified cell culture incubator. To induce specific immunoglobulin isotype switching, the cells were stimulated with anti-CD40 (2 μg/mL) plus IL-4 (10 ng/mL) for the IgG1 switch,34 lipopolysaccharide (LPS; 15 μg/mL) plus interferon γ (IFNγ; 10 ng/mL) for the IgG2c switch,35 or LPS alone (15 μg/mL) for the IgG2b and IgG3 switch.34 After 4 days of culture, supernatants were collected for quantitation of immunoglobulins by ELISA.
Adoptive transfer experiment
For adoptive transfer of mixed cell populations, total B220+ splenic B cells were isolated from CD19CreNotch+/+ and CD19CreNotchfl/fl mice by magnetic cell sorting using anti-B220–coated MACS magnetic microbeads (Miltenyi Biotec). B-cell–depleted splenocytes were obtained from wild-type C57BL/6 mice by negative magnetic cell sorting using anti-B220–coated MACS microbeads. The isolated Notch2-sufficient or Notch2-deficient B cells were mixed 1:1 with B-cell–depleted wild-type splenocytes in PBS supplemented with 2% FBS; the cells were then transplanted into a partially irradiated (300 rads) 8- to 10-week-old μMT mouse by intravenous injection (8 × 106 cells/recipient). At 1 hour after adoptive transfer of the mixed cell populations, the recipients were immunized with mouse PF4/heparin. Sera were collected at indicated time points, and antigen-specific antibodies were measured by ELISA. For adoptive transfer of B cells, FO (B220+CD21+CD23hi) or MZ (B220+CD21hiCD23lo) B cells were sorted by FACS from splenocytes of 8-week-old wild-type C57BL/6 mice. The cells were suspended in PBS supplemented with 2% FBS and were then transplanted into irradiated (300 rads) 8- to 10-week-old μMT mice (1.5 × 106 cells/mouse) by intravenous injection. One hour after adoptive transfer of B cells, the recipients were immunized with mouse PF4/heparin or NP-CGG. Sera were collected at indicated time points, and antigen-specific antibodies were measured by ELISA.
Statistical analysis
All statistical analysis was performed with the 2-tailed unpaired Student t test.
Results
PF4/heparin-specific antibody production is inhibited in the absence of MZ B cells
The features of both T-cell–independent and T-cell–dependent immune responses in patients with HIT implicate involvement of a complex mixture of mature B-cell subsets. The presence of T-cell–independent features of immune response features in such patients prompted us to investigate the role of MZ B cells in HIT immune responses with an established mouse model, in which anti-PF4/heparin antibodies are generated on PF4/heparin complex challenge.30,31 As reported previously,21 our B-cell–specific Notch2 knockout mice (CD19CreNotch2fl/fl) on a C57BL/6 background displayed a marked reduction of MZ B cells but had normal populations of FO and B1 B cells (Figure 1A-B). In addition, B-cell–specific Notch2-deficient mice exhibited normal populations of other hematopoietic and immune cells compared with control wild-type mice (Figure 1C-D). B-cell–specific Notch2-deficient and control wild-type mice were immunized with the mouse PF4/heparin complex. Anti-PF4/heparin antibodies were detected by mouse PF4/heparin ELISA analysis. Anti-PF4/heparin antibodies appeared at day 8, peaked at day 15, and then declined thereafter in Notch2-sufficient (CD19CreNotch2+/+) mice after PF4/heparin complex challenge (Figure 2). In contrast, production of anti-PF4/heparin antibodies was barely detectable in Notch2-deficient mice at all of the time points examined. A slight increase in the level of PF4/heparin-specific antibodies was observed in Notch2-deficient mice at day 15; however, this difference was not significant (Figure 2).
Marked reduction of MZ B cells, but not FO or B1 B cells, or other immune cells in B-cell–specific Notch2-deficient mice. (A) Marked reduction of MZ B cells but normal FO and B1 B populations in B-cell–specific Notch2-deficient mice. Upper panels: Splenocytes from CD19CreNotch2+/+ and CD19CreNotch2fl/fl mice were stained with antibodies to B220, CD21, and CD23. In B220+-gated cells, FO B cells (CD21+CD23hi) and MZ B cells (CD21hi CD23lo/−) are shown. The percentages indicate cells in the gated B220+ population. Lower panels: Peritoneal cells from CD19CreNotch2+/+ and CD19CreNotch2fl/fl mice were stained with antibodies to IgM and CD5. In the gated live population, B1 B cells (IgM+CD5+) are shown. The percentages indicate cells in the gated live population. (B) Bar graphs show the percentages of MZ and FO B cells in the gated splenic B220+ population, and B1 B cells in the gated live peritoneal population from the indicated mice. (C) Bar graphs show the percentages of total (Thy1.2+), CD4, and CD8 T cells in the gated splenic lymphoid population from the indicated mice. (D) Bar graphs show the percentages of NK (NK1.1+), monocytes (CD11b+F4/80+), neutrophils (CD11b+Ly6G+), dendritic cells (DC) (CD11b+CD11c+), and erythrocytes (Ter119+) in the gated live splenocytes and of mast cells (c-Kit+FcεR+) in the gated live peritoneal cells from the indicated mice. Data are obtained from 3 CD19CreNotch2+/+ and 4 CD19CreNotch2fl/fl mice.
Marked reduction of MZ B cells, but not FO or B1 B cells, or other immune cells in B-cell–specific Notch2-deficient mice. (A) Marked reduction of MZ B cells but normal FO and B1 B populations in B-cell–specific Notch2-deficient mice. Upper panels: Splenocytes from CD19CreNotch2+/+ and CD19CreNotch2fl/fl mice were stained with antibodies to B220, CD21, and CD23. In B220+-gated cells, FO B cells (CD21+CD23hi) and MZ B cells (CD21hi CD23lo/−) are shown. The percentages indicate cells in the gated B220+ population. Lower panels: Peritoneal cells from CD19CreNotch2+/+ and CD19CreNotch2fl/fl mice were stained with antibodies to IgM and CD5. In the gated live population, B1 B cells (IgM+CD5+) are shown. The percentages indicate cells in the gated live population. (B) Bar graphs show the percentages of MZ and FO B cells in the gated splenic B220+ population, and B1 B cells in the gated live peritoneal population from the indicated mice. (C) Bar graphs show the percentages of total (Thy1.2+), CD4, and CD8 T cells in the gated splenic lymphoid population from the indicated mice. (D) Bar graphs show the percentages of NK (NK1.1+), monocytes (CD11b+F4/80+), neutrophils (CD11b+Ly6G+), dendritic cells (DC) (CD11b+CD11c+), and erythrocytes (Ter119+) in the gated live splenocytes and of mast cells (c-Kit+FcεR+) in the gated live peritoneal cells from the indicated mice. Data are obtained from 3 CD19CreNotch2+/+ and 4 CD19CreNotch2fl/fl mice.
Markedly impaired production of PF4/heparin-specific antibody in B-cell–specific Notch2-deficient mice. A marked impairment of PF4/heparin-specific IgG production in B-cell–specific Notch2-deficient mice. CD19CreNotch2+/+ and CD19Cre Notch2fl/fl mice were immunized with mouse PF4/heparin complexes. Sera were collected at the indicated time points after immunization, and mouse PF4/heparin-specific total IgG level was measured by ELISA. The horizontal lines indicate the mean values. Data shown are obtained from 9 mice of each genotype.
Markedly impaired production of PF4/heparin-specific antibody in B-cell–specific Notch2-deficient mice. A marked impairment of PF4/heparin-specific IgG production in B-cell–specific Notch2-deficient mice. CD19CreNotch2+/+ and CD19Cre Notch2fl/fl mice were immunized with mouse PF4/heparin complexes. Sera were collected at the indicated time points after immunization, and mouse PF4/heparin-specific total IgG level was measured by ELISA. The horizontal lines indicate the mean values. Data shown are obtained from 9 mice of each genotype.
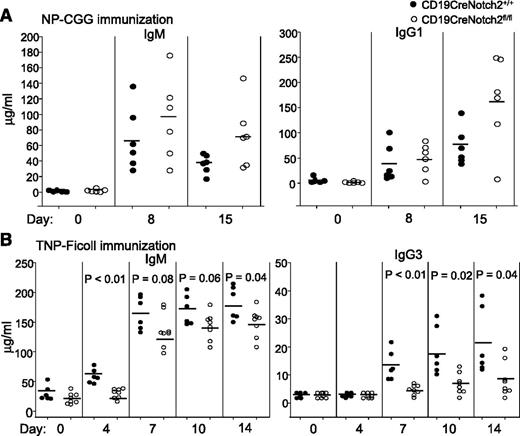
To confirm that our CD19CreNotch2fl/fl mice lacked only MZ B cells but retained normal populations and functions of other subsets of B cells, such as FO B cells, we performed challenge with the T-cell–dependent antigen NP-CGG or with the T-cell–independent antigen TNP-Ficoll on the mutant mice. Production of NP-specific IgM and IgG1 induced by the T-cell–dependent antigen NP-CGG was comparable between Notch2-deficient and Notch2-sufficient mice (Figure 3A), consistent with the presence of normal population and function of FO B cells in the mutant mice. In contrast, production of TNP-specific IgM and IgG3 was slightly and markedly reduced, respectively, in response to the T-cell–independent antigen TNP-Ficoll in Notch2-deficient relative to Notch2-sufficient mice (Figure 3B), in agreement with the lack of MZ B cells in the mutant mice. The presence of B1 B cells probably accounted for TNP-Ficoll–induced production of TNP-specific IgM in Notch2-deficient mice. These findings show that PF4/heparin-specific antibody production after PF4/heparin complex challenge is markedly deficient in the absence of MZ B cells.
T-cell–dependent and T-cell–independent immune responses in B-cell–specific Notch2-deficient mice. (A) Normal T-cell–dependent immune responses in B-cell–specific Notch2-deficient mice. CD19Cre Notch2+/+ (n = 6) and CD19CreNotch2fl/fl (n = 6) mice were immunized with the T-cell-dependent antigen NP-CGG. Sera were collected at the indicated time points after immunization, and NP-specific IgM (left) and IgG1 (right) levels were measured by ELISA. The horizontal lines indicate the mean values. (B) Reduced T-cell–independent immune responses in B-cell–specific Notch2-deficient mice. CD19CreNotch2+/+ (n = 6) and CD19CreNotch2fl/fl (n = 8) mice were immunized with the T-cell–independent antigen TNP-Ficoll. Sera were collected at the indicated time points after immunization, and TNP-specific IgM (left) and IgG3 (right) levels were measured by ELISA. The horizontal lines indicate the mean values.
T-cell–dependent and T-cell–independent immune responses in B-cell–specific Notch2-deficient mice. (A) Normal T-cell–dependent immune responses in B-cell–specific Notch2-deficient mice. CD19Cre Notch2+/+ (n = 6) and CD19CreNotch2fl/fl (n = 6) mice were immunized with the T-cell-dependent antigen NP-CGG. Sera were collected at the indicated time points after immunization, and NP-specific IgM (left) and IgG1 (right) levels were measured by ELISA. The horizontal lines indicate the mean values. (B) Reduced T-cell–independent immune responses in B-cell–specific Notch2-deficient mice. CD19CreNotch2+/+ (n = 6) and CD19CreNotch2fl/fl (n = 8) mice were immunized with the T-cell–independent antigen TNP-Ficoll. Sera were collected at the indicated time points after immunization, and TNP-specific IgM (left) and IgG3 (right) levels were measured by ELISA. The horizontal lines indicate the mean values.
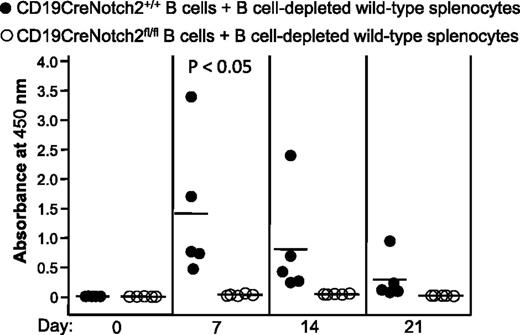
Notch2 also plays an important role in the development or localization of other immune cells, such as CD4 and CD8 T cells22,23 and mast cells.24 Specific deletion of Notch2 in the B-cell lineage leads to reduction of MZ B cells without detectable defects on other subsets of B cells or other immune cells21 (Figure 1); nonetheless, a non-MZ B-cell defect in the immune system of CD19CreNotch2fl/fl mice cannot be completely excluded. To confirm that the reduction of mPF4/heparin-specific antibody production in CD19CreNotch2fl/fl mice was the result of the marked reduction of MZ B cells but not the impairment of other immune cells, we performed mixed-cell adoptive transfer experiments. Total B220+ splenic B cells isolated from CD19CreNotch2+/+ or CD19CreNotch2fl/fl mice were mixed with B-cell–depleted splenocytes obtained from wild-type C57BL/6 mice and were then adoptively transferred into B-cell–deficient μMT mice, followed by immunization with mouse PF4/heparin complexes. PF4/heparin-specific antibodies were produced at days 7 and 14 postimmunization in μMT mice that received Notch2-sufficient, but not Notch2-deficient, B cells mixed with B-cell–depleted wild-type splenocytes (Figure 4). These data clearly demonstrate that defective B cells, but not other immune cells, are responsible for failure of PF4/heparin-specific antibody production in CD19CreNotch2fl/fl mice.
Defective B cells are responsible for the failure to produce PF4/heparin-specific antibodies in B-cell–specific Notch2-deficient mice. Splenic B cells were isolated from CD19CreNotch2+/+ or CD19CreNotch2fl/fl mice, mixed with B-cell–depleted wild-type splenocytes at a 1:1 ratio, and then adoptively transferred into partially irradiated μMT mice. The recipients were immunized with mouse PF4/heparin complexes. Sera were collected at the indicated time points after immunization. Total PF4/heparin-specific IgG level was measured by ELISA. The horizontal lines indicate the mean values. Data shown are obtained from 5 recipients in each group.
Defective B cells are responsible for the failure to produce PF4/heparin-specific antibodies in B-cell–specific Notch2-deficient mice. Splenic B cells were isolated from CD19CreNotch2+/+ or CD19CreNotch2fl/fl mice, mixed with B-cell–depleted wild-type splenocytes at a 1:1 ratio, and then adoptively transferred into partially irradiated μMT mice. The recipients were immunized with mouse PF4/heparin complexes. Sera were collected at the indicated time points after immunization. Total PF4/heparin-specific IgG level was measured by ELISA. The horizontal lines indicate the mean values. Data shown are obtained from 5 recipients in each group.
PF4/heparin-specific antibodies are IgG2b and IgG3 isotypes in mice
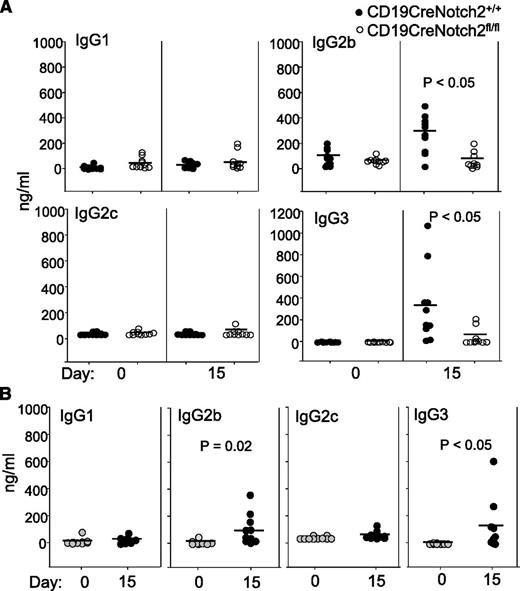
In patients with HIT, PF4/heparin-specific antibodies are predominantly of the IgG1 subclass with some IgG2.5 PF4/heparin-reactive IgM and IgA antibodies are also found in these patients.36 Previous studies have shown that anti-PF4/heparin antibodies generated in wild-type BALB/c mice, such as those generated in patients with HIT, are also predominantly of the IgG1 subclass.32 We examined the isotypes of anti-PF4/heparin antibodies in Notch2-sufficient and Notch2-deficient mice from the same breeding colony on a C57BL/6 background. After PF4/heparin complex challenge, Notch2-sufficient mice generated IgG2b and IgG3, but not IgG1 or IgG2c, isotypes of anti-PF4/heparin antibodies (Figure 5A). In contrast, Notch2-deficient mice did not generate anti-PF4/heparin antibodies of any isotype examined (Figure 5A). To confirm that wild-type C57BL/6 mice can generate IgG2b and IgG3 isotypes of anti-PF4/heparin antibodies, we immunized wild-type C57BL/6 mice from Jackson Laboratory with the mouse PF4/heparin complexes. Again, wild-type C57BL/6 mice generated IgG2b and IgG3 isotypes of anti-PF4/heparin antibodies (Figure 5B). Thus, PF4/heparin-specific antibodies generated in wild-type mice on a C57BL/6 background are of the IgG2b and IgG3 isotypes.
Production of anti-PF4/heparin IgG2b and IgG3 antibodies in wild-type mice with C57BL/6 background. (A) Production of PF4/heparin-specific IgG2b and IgG3 antibodies in Notch2-sufficient mice. CD19CreNotch2+/+ and CD19CreNotch2fl/fl mice on a C57 BL/6 background were immunized with mouse PF4/heparin. Sera were collected at the indicated time points after immunization. The different isotypes of PF4/heparin-specific antibodies were measured by ELISA. (B) Production of PF4/heparin-specific IgG2b and IgG3 antibodies in C57BL/6 mice. Wild-type C57BL/6 mice were immunized with mouse PF4/heparin. Sera were collected at the indicated time points after immunization. The different isotypes of PF4/heparin-specific antibodies in the sera were measured by ELISA. The horizontal lines indicate the mean values. Data shown are obtained from 10 mice of each genotype in both (A) and (B).
Production of anti-PF4/heparin IgG2b and IgG3 antibodies in wild-type mice with C57BL/6 background. (A) Production of PF4/heparin-specific IgG2b and IgG3 antibodies in Notch2-sufficient mice. CD19CreNotch2+/+ and CD19CreNotch2fl/fl mice on a C57 BL/6 background were immunized with mouse PF4/heparin. Sera were collected at the indicated time points after immunization. The different isotypes of PF4/heparin-specific antibodies were measured by ELISA. (B) Production of PF4/heparin-specific IgG2b and IgG3 antibodies in C57BL/6 mice. Wild-type C57BL/6 mice were immunized with mouse PF4/heparin. Sera were collected at the indicated time points after immunization. The different isotypes of PF4/heparin-specific antibodies in the sera were measured by ELISA. The horizontal lines indicate the mean values. Data shown are obtained from 10 mice of each genotype in both (A) and (B).
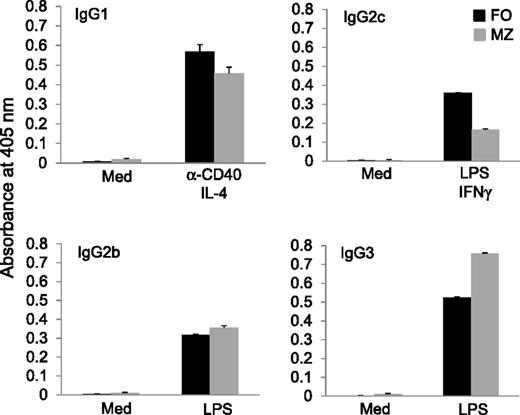
Although MZ B cells generate IgM antibodies in the initial T-cell–independent immune response,28 this subset of B cells is capable of producing high levels of IgG2 and IgG3 antibodies.29 To confirm the ability of MZ B cells to produce different isotypes of antibodies in vitro, MZ and FO B cells were sorted by FACS from wild-type C57BL/6 mice and were cultured under conditions favoring production of different isotypes of antibodies.34 In the presence of anti-CD40 and IL-4, cultured MZ B cells produced IgG1 antibodies at levels similar to those produced by FO B cells (Figure 6). Addition of LPS and IFNγ caused MZ B cells to produce IgG2c antibodies, although at lower levels than those of FO B cells (Figure 6). Lastly, LPS alone drove both MZ and FO B cells to produce comparably high levels of IgG2b and IgG3 (Figure 6). Thus, MZ B cells can produce not only IgM antibodies but also other isotypes of antibodies, including IgG2b and IgG3.
Production of IgG2b and IgG3 antibodies by MZ B cells in vitro. FO and MZ B cells were sorted by FACS from wild-type C57BL/6 mice. The cells were then cultured with anti-CD40 and IL-4 to induce IgG1 class switching, LPS and IFNγ to induce IgG2c class switching, or LPS to induce IgG2b and IgG3 class switching. The culture supernatants were collected after 4 days of culture, and different isotypes of antibodies were measured by ELISA. Results are representative of at least 3 independent experiments.
Production of IgG2b and IgG3 antibodies by MZ B cells in vitro. FO and MZ B cells were sorted by FACS from wild-type C57BL/6 mice. The cells were then cultured with anti-CD40 and IL-4 to induce IgG1 class switching, LPS and IFNγ to induce IgG2c class switching, or LPS to induce IgG2b and IgG3 class switching. The culture supernatants were collected after 4 days of culture, and different isotypes of antibodies were measured by ELISA. Results are representative of at least 3 independent experiments.
MZ B cells adoptively transferred into μMT mice produce anti-PF4/heparin IgG2b and IgG3 on antigen challenge
To further demonstrate that MZ B cells, but not FO B cells, are responsible for the production of PF4/heparin-specific antibodies, we examined whether wild-type MZ B cells adoptively transferred into B-cell–deficient μMT mice could respond to PF4/heparin challenge. MZ or FO B cells were sorted by FACS from wild-type C57BL/6 mice and were then adoptively transferred into μMT mice, followed by immunization with mouse PF4/heparin complexes. PF4/heparin-specific antibodies were produced on days 8 and 15 postimmunization in μMT mice that received MZ B cells, but not FO B cells (Figure 7A). Of note, no PF4/heparin-specific antibodies were produced on day 8 or 15 postimmunization in μMT mice that did not receive any B cells (Figure 7A). Consistent with the results shown in Figure 5, adoptively transferred MZ B cells derived from C57BL/6 mice generated IgG2b and IgG3, but not IgG1 or IgG2c, isotypes of anti-PF4/heparin antibodies (Figure 7C). To confirm that adoptively transferred FO B cells were functional, μMT mice receiving these B cells were immunized with the T-cell–dependent antigen NP-CGG. Most of the μMT mice that received FO B cells produced NP-specific IgG1 by day 15 postimmunization (Figure 7B). In contrast, all of the μMT mice that did not receive B cells did not generate any NP-specific IgG1 at any of the time points examined (Figure 7B). Thus, MZ B cells, but not FO B cells, adoptively transferred into μMT mice produce PF4/heparin-specific IgG2b and IgG3 on PF4/heparin challenge. Taken together, these data demonstrate that MZ B cells play a critical role in the production of PF4/heparin-specific HIT antibodies.
Production of PF4/heparin-specific antibodies by MZ B cells adoptively transferred into μMT mice. (A) Production of PF4/heparin-specific antibodies by MZ B cells, but not FO B cells, adoptively transferred into μMT mice. Partially irradiated μMT mice were adoptively transferred with FACS-sorted wild-type MZ B cells (MZ + μMT) (n = 6), FO B cells (FO + μMT) (n = 6), or no B cells (μMT) (n = 4), and were then immunized with mouse PF/heparin complexes. Sera were collected at the indicated time points after immunization. Total PF4/heparin-specific IgG level was measured by ELISA. The horizontal lines indicate the mean values. Data shown are obtained from 2 independent experiments. (B) Production of NP-specific antibodies by FO B cells adoptively transferred into μMT mice. Partially irradiated μMT mice were adoptively transferred with FACS-sorted wild-type FO B cells (FO + μMT) (n = 4) or no B cells (μMT) (n = 4), and were then immunized with the T-cell-dependent-antigen NP-CGG. Sera were collected at the indicated time points after immunization. NP-specific IgG1 levels were measured by ELISA. The horizontal lines indicate the mean values. (C) PF4/heparin-specific IgG2b and IgG3 production by MZ B cells adoptively transferred into μMT mice. The different isotypes of PF4/heparinspecific antibodies in the sera from (A) were measured by ELISA. The horizontal lines indicate the mean values.
Production of PF4/heparin-specific antibodies by MZ B cells adoptively transferred into μMT mice. (A) Production of PF4/heparin-specific antibodies by MZ B cells, but not FO B cells, adoptively transferred into μMT mice. Partially irradiated μMT mice were adoptively transferred with FACS-sorted wild-type MZ B cells (MZ + μMT) (n = 6), FO B cells (FO + μMT) (n = 6), or no B cells (μMT) (n = 4), and were then immunized with mouse PF/heparin complexes. Sera were collected at the indicated time points after immunization. Total PF4/heparin-specific IgG level was measured by ELISA. The horizontal lines indicate the mean values. Data shown are obtained from 2 independent experiments. (B) Production of NP-specific antibodies by FO B cells adoptively transferred into μMT mice. Partially irradiated μMT mice were adoptively transferred with FACS-sorted wild-type FO B cells (FO + μMT) (n = 4) or no B cells (μMT) (n = 4), and were then immunized with the T-cell-dependent-antigen NP-CGG. Sera were collected at the indicated time points after immunization. NP-specific IgG1 levels were measured by ELISA. The horizontal lines indicate the mean values. (C) PF4/heparin-specific IgG2b and IgG3 production by MZ B cells adoptively transferred into μMT mice. The different isotypes of PF4/heparinspecific antibodies in the sera from (A) were measured by ELISA. The horizontal lines indicate the mean values.
Discussion
The atypical immunologic characteristics of HIT have confounded our understanding of the mechanism by which B cells contribute to the immune pathogenesis of the disease. In our current study using a mouse model for production of anti-PF4/heparin antibodies, genetically modified mice, and adoptive transfer, we demonstrate that MZ B cells are responsible for production of anti-PF4/heparin antibodies of the IgG2b and IgG3 isotypes. These findings address an important and unsolved issue in the pathogenesis of HIT.
Notch2 not only is critical for MZ B-cell development but also plays an important role in the development of other immune cells. Notch2, along with Notch1, is required for T-helper 2 differentiation of CD4 T cells, and T-cell–specific deletion of Notch1 and Notch2 blocks T-helper 2 differentiation of CD4 T cells.22 Notch2 also plays an important role in the development of CD8 T cells. CD8 T-cell–specific deletion of Notch2 impairs the differentiation of T cells into CD8 cytotoxic T cells.23 In addition, Notch2 is required for proper localization of intestinal mast cells, and inducible deletion of Notch2 results in abnormal localization of mast cells in the lamina propria from the epithelial layer.24 Although Notch2 is required for the development of subsets of T cells or localization of mast cells, inactivation of Notch2 in the B-cell lineage in CD19CreNotch2fl/fl mice results in a specific reduction of MZ B cells without affecting other subsets of B cells or other immune cells (Figure 1)21 and causes a dramatic decrease in the production of PF4/heparin-specific antibodies. The low levels of production of anti-PF4/heparin antibody in CD19CreNotch2fl/fl mice (Figure 2) could be caused by insufficient deletion of Notch2 and the consequent presence of residual MZ B cells in some of these mice. Nonetheless, concern always arises that defects in immune cells other than MZ B cells are responsible for impaired production of PF4/heparin-specific antibodies in CD19CreNotch2fl/fl mice. Our finding that adoptive transfer of Notch2-deficient B cells mixed with wild-type B-cell–depleted splenocytes into B-cell–deficient μMT recipients failed to support a response to PF4/heparin complex challenge clearly demonstrates that Notch2-deficient B cells, but not other immune cells, are responsible for failure to produce PF4/heparin-specific antibodies in CD19CreNotch2fl/fl mice (Figure 4). Moreover, adoptive transfer of MZ B cells, but not FO B cells, into μMT recipients resulted in a normal response to PF4/heparin complex challenge, directly demonstrating a critical role of MZ B cells in producing PF4/heparin-specific antibodies (Figure 7).
MZ B cells are principal players in the rapid T-cell–independent immune response that is normally triggered by bacterial antigens with repetitive structures.27,37 Similarly, high-molecular-weight PF4/heparin complexes have repetitive epitopes.13 PF4 binds not only to heparin but also to negatively charged molecules (polyanions) present on the surface of gram-negative and gram-positive bacteria.38 The repetitive structures of PF4/heparin complexes are consistent with the important involvement of MZ B cells in HIT antibody production, as this subset of MZ B cells responds rapidly to blood-borne bacterial antigens with repetitive structures to generate antigen-specific antibodies that quickly decline, similar to the kinetics of HIT antibody production in humans.1,27,37 It is conceivable that, similar to PF4/polyanion complexes, PF4/heparin complexes are trapped in the MZ where the complexes encounter MZ B cells.
Although MZ B cells largely produce IgM antibodies,27,37 studies have clearly shown that MZ B cells can also produce antibodies of the IgG subclass independently of T-cell assistance.29,39 Of note, B1 B cells are also major players in the rapid T-cell–independent immune response and in the production of IgM antibodies19,27 ; however, it is not clear whether B1 B cells are able to generate IgG antibodies. Nonetheless, the possibility that B1 B cells contribute to HIT antibody production cannot be fully excluded. Further study of the potential involvement of B1 B cells in the production of PF4/heparin-specific HIT antibodies may be warranted.
We recognize that our findings of T-cell–independent features of PF4/heparin-specific antibody production in this mouse model vary with findings in human HIT and in previously published models of mouse HIT. Specifically, PF4/heparin-reactive antibodies in HIT in humans5,15,36 and in BALB/c mice challenged with PF4/heparin complexes32 are predominantly of the IgG1 isotype, with some IgG2. IgM and IgA antibodies are also produced in humans. Here, PF4/heparin-specific antibodies produced in wild-type C57BL/6 mice or in μMT mice that received wild-type MZ B cells of C57BL/6 origin are of IgG2b and IgG3 isotypes. PF4/heparin-specific antibodies of the IgA isotype were not observed in our model, whereas those of the IgM isotype were detected but were present in both immunized and nonimmunized mice (data not shown). The heterogeneity of the isotypes of PF4/heparin-reactive antibodies observed in patients with HIT may reflect the highly diverse immunogenetic background in these patients, which is not represented in inbred strains of mice (either C57BL/6 or BALB/c). The isotype discrepancy observed between C57BL/6 and BALB/c mice may be attributable to the well-recognized tendency of mice of different genetic backgrounds to produce antibodies of different isotypes.40 Interestingly, however, the isotypes of the PF4/heparin-specific antibodies that are produced in C57BL/6 mice are actually more functionally equivalent to those produced with HIT in humans than are those produced in BALB/c mice, in that mouse IgG2b and human IgG1 antibodies share the ability to fix complement, whereas mouse IgG3 and human IgG2 are similar in that they both recognize predominantly carbohydrate epitopes.41 Thus, our results suggest that C57BL/6 mice may serve as a more relevant model of human HIT antibody production than do BALB/c mice.
PF4/heparin immunization induces PF4/heparin-specific antibody production but fails to trigger thrombocytopenia or thrombosis in wild-type C57BL/6 mice.31 One potential reason for the failure of PF4/heparin immunization to trigger thrombocytopenia or thrombosis in wild-type mice is that the retro-orbital immunization route used in mice does not resemble the route by which humans are sensitized to heparin. However, the failure to recapitulate the thrombocytopenia and thrombosis associated with human HIT in mice exposed to PF4/heparin immunization is most likely attributable to species differences between humans and mice and, specifically, to the presence of FcγRIIA receptors on human platelets vs their absence from the surfaces of wild-type mouse platelets. To support this reasoning, previous studies have demonstrated that injection of anti-hPF4/heparin mouse monoclonal antibody (KKO) and heparin can induce thrombocytopenia and thrombosis in hFcγRIIA/hPF4 transgenic, but not wild-type, nontransgenic mice.42 Similarly, immunization-induced mouse anti-PF4/heparin antibodies can activate mouse platelets expressing human FcγRIIA in a heparin-dependent manner in vitro, but not wild-type platelets lacking a similar Fc receptor.32 Therefore, further study of the potential development of thrombocytopenia and thrombosis in hFcγRIIA transgenic mice after PF4/heparin immunization is warranted.
The rapid time course with which anti-PF4/heparin IgG antibodies are produced on exposure to heparin, followed by rapid decline on cessation of heparin therapy1,9 and together with the findings that major surgery is a significant risk factor for HIT,2,43-45 also raises the possibility that inflammation-induced breakdown of B-cell peripheral tolerance contributes to HIT pathogenesis. Anergy is a major physiological mechanism for the establishment of immune tolerance, especially in the periphery,46 and its breakdown can lead to severe autoimmune disease.47,48 Of note, both heparin, a glycosaminoglycan, and PF4 are normal body constituents, and it is likely that humans are exposed to the PF4/heparin antigen complex throughout their lives. Weak PF4/heparin HIT antibodies are present in 2% to 5% of healthy individuals,49,50 suggesting that some people may already be sensitized and have a breakdown of tolerance. Further study of the potential involvement of B-cell anergy in the production of PF4/heparin-specific HIT antibodies is required.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Richard H. Aster and Peter J. Newman for critically reviewing the manuscript.
This work is supported in part by National Institutes of Health grants PO1HL44612 (D.W. and D.K.N.) and R01AI079087 (D.W.) and by a Scholar Award from the Leukemia & Lymphoma Society (D.W.).
Authorship
Contribution: Y.Z. contributed to research design, performed the research, analyzed the results, and wrote the first draft of the manuscript. M.Y. contributed to research design and performed important initial experiments. A.P. performed some experiments. L.Y. provided intellectual input. D.K.N. provided intellectual input and critically reviewed the manuscript. R.W. provided intellectual input, supervised the study, and critically reviewed the manuscript. G.A. provided intellectual input and critical reagents. D.W. conceived and supervised the study, analyzed the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Demin Wang, Blood Research Institute, BloodCenter of Wisconsin, 8727 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: demin.wang@bcw.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal