Key Points
Promoter DNA methylation, an epigenetic process, is functionally relevant for regulating the expression of endothelial cell–enriched genes.
Abstract
Proximal promoter DNA methylation has been shown to be important for regulating gene expression. However, its relative contribution to the cell-specific expression of endothelial cell (EC)-enriched genes has not been defined. We used methyl-DNA immunoprecipitation and bisulfite conversion to analyze the DNA methylation profile of EC-enriched genes in ECs vs nonexpressing cell types, both in vitro and in vivo. We show that prototypic EC-enriched genes exhibit functional differential patterns of DNA methylation in proximal promoter regions of most (eg, CD31, von Willebrand factor [vWF], VE-cadherin, and intercellular adhesion molecule-2), but not all (eg, VEGFR-1 and VEGFR-2), EC-enriched genes. Comparable findings were evident in cultured ECs, human blood origin ECs, and murine aortic ECs. Promoter-reporter episomal transfection assays for endothelial nitric oxide synthase, VE-cadherin, and vWF indicated functional promoter activity in cell types where the native gene was not active. Inhibition of DNA methyltransferase activity indicated important functional relevance. Importantly, profiling DNA replication timing patterns indicated that EC-enriched gene promoters with differentially methylated regions replicate early in S-phase in both expressing and nonexpressing cell types. Collectively, these studies highlight the functional importance of promoter DNA methylation in controlling vascular EC gene expression.
Introduction
The functional identity of an endothelial cell (EC) is dictated, in part, by its unique gene expression profile. The application of microarray profiling has reinforced the view that the concept of EC-enriched genes is valid and functionally relevant with respect to cellular phenotype.1-3 In this regard, epigenetic processes are now appreciated to play a key role in regulating gene transcription. However, the relative contribution in ECs is not well understood.4,5
Decreased expression of constitutively active genes in ECs is a key component of EC dysfunction, such as is observed with endothelial nitric oxide synthase (eNOS) in atherosclerosis.6 Defining whether perturbations of the DNA methylation status of key EC-enriched genes contributes to changes in gene expression and cellular phenotype requires a firm understanding of DNA methylation profiles of these genes under normal conditions. We previously identified a differentially methylated region (DMR) at the proximal promoter of the eNOS/NOS3 gene.7 Although non-EC types showed high levels of methylation, a repressive mark associated with transcriptional silencing, ECs lacked DNA methylation in this region. These and other studies have suggested that epigenetics plays an important role in the regulation of gene expression in vascular ECs.8,9 DNA methylation also needs to be transmitted faithfully to nascent DNA subsequent to the replication of the genetic code. Generally, early timing of DNA replication in the cell cycle correlates with global gene expression,10 although less is known about whether this paradigm applies to cell-restricted genes, especially within the vascular endothelium.
The contribution of DNA methylation to EC gene regulation remains to be fully explored. We therefore wished to determine whether the epigenetic mechanisms first characterized for eNOS in ECs is a unique feature for eNOS expression or is also applicable to a repertoire of EC-enriched genes. Discerning the epigenetic state of unique cell types is a key goal of the International Human Epigenomic Consortium. Similarly, the Encyclopedia of DNA Elements (ENCODE) project also aims to delineate functional elements and chromatin signatures for specific cell types.11 These genome-based approaches are, using currently existing methodologies, examining these changes in low resolution. High-resolution data are especially needed. In this study, we describe both in vitro and in vivo studies of differential DNA methylation in the proximal promoter regions of the EC-enriched genes CD31/PECAM1, Endoglin/ENG, ICAM-2, P-selectin/SELP, Tie-2/TEK, VE-cadherin/CDH5, and von Willebrand factor (vWF) in terminally differentiated ECs vs non-ECs, in both humans and mice, and provide evidence that epigenetic modifications are functionally important for EC gene expression. Furthermore, DNA replication timing studies show that EC-enriched genes with DMRs replicate in early S-phase in both ECs and non-ECs. This was an exciting yet unexpected finding. The aim of the study was to determine the functional relevance of promoter DNA methylation for EC gene expression. Here we describe important features regulating EC-enriched gene expression involving both chromatin-based and cell cycle pathways.
Methods
All animal studies were performed in accordance with the guidelines of the Canadian Council on Animal Care and were approved by the University of Toronto Animal Care Committee. This study was conducted in accordance with the Declaration of Helsinki.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were isolated from fresh umbilical cords as previously described.12,13 Cryopreserved human aortic vascular smooth muscle cells (HuAoVSMCs; ScienCell), human neonatal dermal microvascular endothelial cells (HMVECs), human epidermal keratinocytes, and human hepatocytes (Lonza) were maintained as recommended. Blood outgrowth endothelial cells (BOECs) were obtained from Dr Hebbel.14 Isolation and culturing of BOECs was conducted as previously reported and assayed at passages 3 to 5 after 6 to 8 weeks in culture.
Cell treatments
HUVECs and HuAoVSMCs were treated at passages 3 to 5 with 5-azacytidine (Sigma) at 5 μmol/L every 48 hours for 7 days or a combined treatment of 5-azacytidine and Trichostatin A (TSA; Sigma), where cells were treated with 5-azacytidine for 7 days, and TSA was added (1 μmol/L) for the final 24 hours. For cell cycle analyses, HuAoVSMCs were treated with 5-azacytidine (Sigma) at 5 μmol/L for 24, 48, 72, or 120 hours.
Generation of methyl 450K bead array and chromatin immunoprecipitation-seq data
DNA methylation 450K Bead Array data were generated by the R. Myers and D. Absher laboratories at the HudsonAlpha Institute for Biotechnology (Huntsville, AL), and HUVEC Pol2 chromatin immunoprecipitation-seq data were generated by the M. Snyder laboratory at Stanford University (Stanford, CA). These data were collected as part of the ENCODE consortium (http://www.genome.gov/10005107)11 and are available on the University of California, Santa Cruz Genome Browser. RepeatMasker data were generated by Arian Smit’s RepeatMasker program (http://www.repeatmasker.org).
5-Bromo-2′-deoxyuridine labeling and flow activated cell sorting
Asynchronous populations of HUVECs and HuAoVSMCs were pulse labeled with 5-bromo-2′-deoxyuridine (BrdU) as previously described.15 Briefly, BrdU (Sigma) was added to cells (50 μM) and incubated for 1 hour at 37°C. Equal numbers of cells (50 000) were sorted into cell cycle fractions based on DNA content (G1, S1, S2, S3, S4, and G2). Sorted cells were collected directly into lysis buffer, and DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen). Salmon sperm DNA (20 μg; Invitrogen) and 100 000 copies of BrdU-labeled Escherichia coli DNA was added to each fraction prior to extraction to control for DNA recovery and immunoprecipitation efficiency. DNA was eluted in 500 μL Tris-EDTA. BrdU-labeled cells and extracted DNA were manipulated in the dark to prevent photolysis of BrdU-incorporated DNA.
Sodium bisulfite genomic sequencing and pyrosequencing
Genomic DNA from human cultured cells (5 μg) was subjected to sodium bisulfite treatment as described previously.7,16,17 Murine genomic DNA (500 ng) was bisulfite converted using the EZ DNA Methylation-Direct kit (Zymo Research, Irvine, CA) and subjected to nested polymerase chain reaction amplification (supplemental Table 3 on the Blood website). For pyrosequencing analysis, 10 pmol of primers per reaction and a biotinylated reverse primer were used (EpigenDx Inc; supplemental Tables 2 and 3).
Statistics
Unless otherwise stated, all experiments were performed a minimum of 3 times, and data represent the mean ± standard error of the mean. Statistical analyses were performed using a Student t test or analysis of variance, as appropriate. P < .05 was considered statistically significant.
Detailed methods are described in the supplemental Materials.
Results
EC-enriched gene promoters are differentially methylated in human ECs vs non-EC types: low-resolution and high-resolution mapping
We first analyzed promoter DNA methylation for key EC-enriched genes using data generated by the ENCODE consortium.11 We used Methyl 450K Bead Array data to define regions of methylation at proximal promoters of key EC-enriched genes in ECs and non-ECs. The region surrounding the start of transcription displayed no methylation in HUVEC, whereas the same region was partially methylated or methylated in AoSMCs or hepatocytes for the genes NOS3/eNOS and CDH5/VE-cadherin (Figure 1). Regions of differential methylation were not located within repetitive elements. Importantly, unmethylated regions corresponded to an enrichment in Pol2 binding in HUVECs and the transcriptional start site (TSS) of the gene (Figure 1). Analysis of downstream coding regions of eNOS and VE-cadherin did not reveal other DMRs in ECs vs non-ECs (data not shown).
DNA methylation profiles at EC-enriched gene promoters. Promoter DNA methylation profiles of (A) NOS3/eNOS and (B) CDH5/VE-Cadherin genes in HUVECs, AoSMCs, and hepatocytes. Levels of methylation are color coded, where blue represents unmethylation, purple represents partial methylation, and orange represents full methylation, as identified by the Illumina Infinium Human Methylation 450K Bead Array platform. Regions of repetitive elements are indicated in black, identified by the RepeatMasker program. Signal enrichment of Pol2 chromatin immunoprecipitation-seq data in HUVECs is shown in orange, with the maximum signal indicated on the y-axis. Black arrow denotes the TSS. The UCSC Genome Browser was used to generate the track displays.46
DNA methylation profiles at EC-enriched gene promoters. Promoter DNA methylation profiles of (A) NOS3/eNOS and (B) CDH5/VE-Cadherin genes in HUVECs, AoSMCs, and hepatocytes. Levels of methylation are color coded, where blue represents unmethylation, purple represents partial methylation, and orange represents full methylation, as identified by the Illumina Infinium Human Methylation 450K Bead Array platform. Regions of repetitive elements are indicated in black, identified by the RepeatMasker program. Signal enrichment of Pol2 chromatin immunoprecipitation-seq data in HUVECs is shown in orange, with the maximum signal indicated on the y-axis. Black arrow denotes the TSS. The UCSC Genome Browser was used to generate the track displays.46
We used high-resolution assays to examine DNA methylation of promoter regions around the TSS of EC-enriched genes using methyl-DNA immunoprecipitation (MeDIP), followed by quantitative polymerase chain reaction (qPCR), a robust method for assessing DNA methylation at specific loci.18 The genomic regions analyzed and citations used to determine the TSSs are summarized in supplemental Table 4.12 MeDIP analysis of several EC-enriched genes demonstrated clear DNA methylation differences between HUVECs and HuAoVSMCs. The EC-enriched genes eNOS, VE-cadherin, CD31, and vWF showed higher relative levels of promoter methylation in HuAoVSMCs compared with HUVECs (Figure 2A-D).
MeDIP analysis of EC and non-EC enriched genes. Relative methylation levels as determined by methyl-DNA IP (MeDIP) in HUVECs and AoVSMCs. EC-enriched genes (A) eNOS, (B) VE-cadherin, (C) CD31, and (D) vWF display greater levels of methylation in AoVSMCs relative to HUVECs. (E) VEGFR2 is hypomethylated in both HUVECs and AoVSMCs. (F) iNOS is known to be methylated in both cell types, which is observed here. (G) E-Cadherin serves as a positive control for promoter methylation, as it is not expressed in either cell type. (H) Cyclophilin A is expressed in both cell types, and hypomethylation is evident in both HUVECs and AoVSMCs. IP’d DNA was normalized to total input DNA for each cell type and gene and is expressed in arbitrary units (au). Data represent mean ± standard error of the mean (n = 3). *Statistical significance at P < .05 of HUVECs compared with AoVSMCs.
MeDIP analysis of EC and non-EC enriched genes. Relative methylation levels as determined by methyl-DNA IP (MeDIP) in HUVECs and AoVSMCs. EC-enriched genes (A) eNOS, (B) VE-cadherin, (C) CD31, and (D) vWF display greater levels of methylation in AoVSMCs relative to HUVECs. (E) VEGFR2 is hypomethylated in both HUVECs and AoVSMCs. (F) iNOS is known to be methylated in both cell types, which is observed here. (G) E-Cadherin serves as a positive control for promoter methylation, as it is not expressed in either cell type. (H) Cyclophilin A is expressed in both cell types, and hypomethylation is evident in both HUVECs and AoVSMCs. IP’d DNA was normalized to total input DNA for each cell type and gene and is expressed in arbitrary units (au). Data represent mean ± standard error of the mean (n = 3). *Statistical significance at P < .05 of HUVECs compared with AoVSMCs.
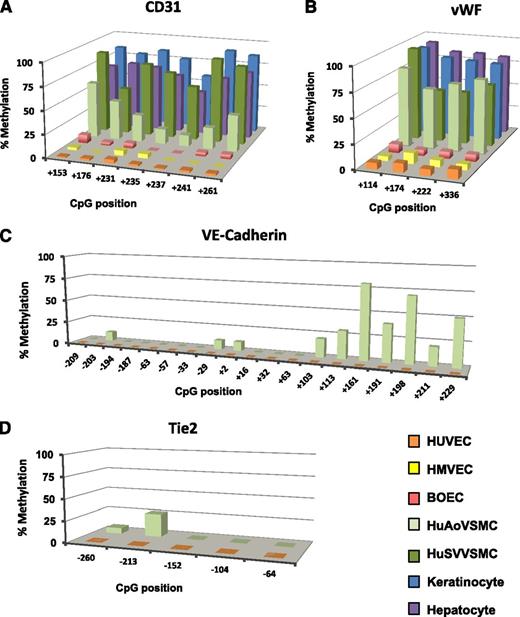
Using gold standard, high-resolution sodium bisulfite sequencing and single-strand DNA analysis or quantitative pyrosequencing in EC and non-EC types, we confirmed our screen of promoter methylation of EC-enriched genes. Consistent with MeDIP assays, bisulfite analyses identified robust differences in DNA methylation between HUVECs and HuAoVSMCs at proximal promoter regions of EC-enriched genes. Pyrosequencing analyses for CD31 in the EC types HUVECs, HMVECs, and BOECs and in non-EC types HuAoVSMCs, human saphenous vein vascular smooth muscle cells (HuSVVSMCs), keratinocytes, and hepatocytes was conducted. CD31 displayed very low levels of DNA methylation in all EC types, whereas high levels of methylation were observed in non-ECs (Figure 3A). The findings from the quantitative pyrosequencing assay were confirmed by single-strand analysis results (supplemental Figure 2).
EC-enriched genes are methylated in non-ECs. Promoter methylation of the EC-enriched genes (A) CD31, (B) vWF, (C) VE-cadherin, and (D) Tie-2. EC types HUVEC (orange), HMVEC (yellow), and BOEC (red) are compared with non-EC types AoVSMC (light green), SVVSMC (dark green), keratinocytes (blue), and hepatocytes (purple). BOECs exhibit a cobblestone shape, have high proliferative capacity, take up acetylated low-density lipoprotein, and are uniformly positive for several endothelial markers.14 BOECs are expanded from small numbers of cells after long-term culture and are distinct from the early outgrowth colonies obtained after 4 to 7 days in culture, as reviewed by others.47,48 (A and B) Quantitative pyrosequencing and (C and D) single-strand analysis (n ≥ 15) of bisulfite-converted DNA was used to assess methylation. Extensive mixing studies of in vitro methylated or mock-methylated templates revealed that pyrosequencing a polymerase chain reaction product cannot distinguish 0% from 5% methylation or 100% from 95% methylation. Single-strand plasmid clone analysis indicates that CpG sites are not methylated in ECs for these genes.
EC-enriched genes are methylated in non-ECs. Promoter methylation of the EC-enriched genes (A) CD31, (B) vWF, (C) VE-cadherin, and (D) Tie-2. EC types HUVEC (orange), HMVEC (yellow), and BOEC (red) are compared with non-EC types AoVSMC (light green), SVVSMC (dark green), keratinocytes (blue), and hepatocytes (purple). BOECs exhibit a cobblestone shape, have high proliferative capacity, take up acetylated low-density lipoprotein, and are uniformly positive for several endothelial markers.14 BOECs are expanded from small numbers of cells after long-term culture and are distinct from the early outgrowth colonies obtained after 4 to 7 days in culture, as reviewed by others.47,48 (A and B) Quantitative pyrosequencing and (C and D) single-strand analysis (n ≥ 15) of bisulfite-converted DNA was used to assess methylation. Extensive mixing studies of in vitro methylated or mock-methylated templates revealed that pyrosequencing a polymerase chain reaction product cannot distinguish 0% from 5% methylation or 100% from 95% methylation. Single-strand plasmid clone analysis indicates that CpG sites are not methylated in ECs for these genes.
Genomic regions corresponding to exon 1 of vWF are known to be functionally important. The 4 CpG sites downstream of the vWF TSS in exon 1 were assessed, and low levels of methylation in HUVECs and HMVECs were found in comparison with dense methylation in the non-EC HuAoVSMCs, HuSVVSMCs, keratinocytes, and hepatocytes (Figure 3B). The eNOS promoter also showed dense methylation in both hepatocytes and keratinocytes (supplemental Figure 3A). Methylation patterns in the ICAM2 promoter was similar to other EC-enriched genes, as HUVECs were 0% methylated and HuAoVSMCs were densely methylated, apart from two CpG sites at −24 and −12 (supplemental Figure 3C). The EC-enriched genes VE-cadherin and Tie2 showed no methylation in HUVECs, whereas high levels of DNA methylation were observed in HuAoVSMCs (Figure 3C-D). Differential methylation of VE-cadherin was restricted to a region downstream of the TSS, with methylation in HuAoVSMCs observed at positions +103 to +229 (Figure 3C). Although there were low levels of DNA methylation seen in the proximal promoter of the Tie2 gene, clear differences in DNA methylation across cell types was evident (Figure 3D).
In contrast to the other EC-enriched genes examined where genomic regions surrounding the TSS evidenced no DNA methylation, P-selectin displayed low but evident levels of methylation in HUVECs. In contrast, dense methylation was seen in HuAoVSMCs (supplemental Figure 3D).
Murine EC-enriched genes are differentially methylated in EC and non-EC types
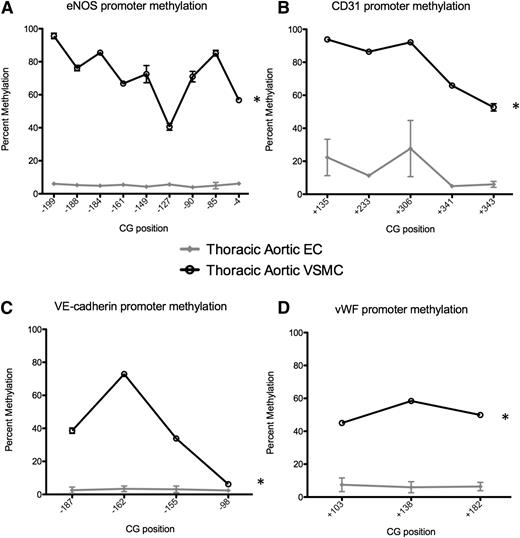
We examined murine promoters in vivo, namely the EC-enriched genes eNOS, CD31, VE-cadherin, and vWF, in descending thoracic aortic ECs and descending thoracic AoVSMCs. We used pyrosequencing analysis of bisulfite-converted DNA to assess the methylation status and found that eNOS, VE-cadherin, CD31, and vWF gene promoters were hypomethylated in ECs and heavily methylated in AoVSMCs (Figure 4), consistent with our findings in human ECs and non-ECs. Therefore, the DMRs of EC-enriched genes identified in cultured human cells are in agreement with our in vivo methylation analyses in the mouse.
Differential promoter DNA methylation of EC-enriched genes in mouse EC and VSMC. Promoter methylation was determined using pyrosequencing of bisulfite-converted DNA. The promoters of (A) eNOS, (B) CD31, (C) VE-cadherin, and (D) vWF are unmethylated in ECs (gray) and densely methylated in VSMCs (black). Data are presented as the mean ± standard error of the mean (n = 3-7 mice studied per gene). *Statistical significance between the 2 cell types (P < .001). Previous studies by us show that the 9 CpG sites in the murine eNOS promoter (−199/−4) are completely unmethylated in ECs, whereas complete methylation was observed in AoVSMCs.7
Differential promoter DNA methylation of EC-enriched genes in mouse EC and VSMC. Promoter methylation was determined using pyrosequencing of bisulfite-converted DNA. The promoters of (A) eNOS, (B) CD31, (C) VE-cadherin, and (D) vWF are unmethylated in ECs (gray) and densely methylated in VSMCs (black). Data are presented as the mean ± standard error of the mean (n = 3-7 mice studied per gene). *Statistical significance between the 2 cell types (P < .001). Previous studies by us show that the 9 CpG sites in the murine eNOS promoter (−199/−4) are completely unmethylated in ECs, whereas complete methylation was observed in AoVSMCs.7
EC-enriched gene promoters are not methylated in human blood outgrowth ECs
We wished to determine the methylation in a cell type capable of initiating stable outgrowth ECs. BOECs are an EC type, derived from cultured peripheral blood, and are capable of establishing mature outgrowth ECs.14 We used well-validated methods to isolate and characterize these cells, as previously described.14 Because eNOS, as well as the EC-enriched genes CD31 and vWF, is fundamental to the proper functioning of BOECs, we were interested in determining the DNA methylation status of these EC genes along this EC differentiation cascade. Using quantitative pyrosequencing of bisulfite-converted DNA from blood outgrowth ECs, the promoters of eNOS, vWF, and CD31 were analyzed. All EC-enriched genes displayed hypomethylation in BOECs (Figure 3A-B; supplemental Figure 3A,C-D), displaying a methylation pattern similar to mature ECs.
Promoter activity of EC-enriched genes in non-EC types
We have previously demonstrated robust expression of episomal eNOS promoter/reporter constructs in cell types in which eNOS is not normally expressed.7 Surprisingly, these same genomic regions exhibited exquisite cell specificity when stably integrated into the genome in insertional transgene murine promoter/reporter studies.19,20 This implied that eNOS episomal vectors or naked DNA did not faithfully recapitulate expression of the native gene, which highlighted functional relevance of chromatin-based mechanisms. We used episomal constructs containing the regions −487/+247 and −1912/+1 of human vWF and VE-cadherin, respectively, given that the same regions are very EC-enriched in insertional promoter transgenes.21,22 These episomal constructs were transiently transfected into ECs and non-EC types that do and do not express eNOS, vWF, or VE-cadherin, respectively (Table 1; supplemental Figure 1). These data indicate that VE-cadherin and vWF promoter reporter espisomal constructs are transcriptionally active even when the native chromatin-based genes are not, as we previously noted for eNOS.7
Promoter activity of EC-enriched genes in ECs and non-ECs
| Cell type . | Empty vector . | pGL3-VE-cadherin-1912/+1* . | pGL3-vWF −487/+247* . | pGL3-eNOS −1193/+109* . |
|---|---|---|---|---|
| HeLa | 1.00 ± 0.09 | 6.95 ± 0.56 | 1.49 ± 0.15 | 3.72 ± 0.06 |
| HEK293 | 1.00 ± 0.08 | 5.17 ± 0.45 | 2.72 ± 0.29 | 1.95 ± 0.11 |
| HuAoVSMCs | 1.00 ± 0.07 | 1.69 ± 0.10 | 1.02 ± 0.09 | 3.91 ± 0.45 |
| HPAECs | 1.00 ± 0.20 | 13.12 ± 1.07 | 3.66 ± 0.40 | 6.50 ± 0.10 |
| HCAECs | 1.00 ± 0.05 | 9.59 ± 0.63 | 4.00 ± 0.51 | 9.80 ± 0.92 |
| HUVECs | 1.00 ± 0.06 | 22.89 ± 1.05 | 11.55 ± 0.86 | 10.90 ± 0.77 |
| Cell type . | Empty vector . | pGL3-VE-cadherin-1912/+1* . | pGL3-vWF −487/+247* . | pGL3-eNOS −1193/+109* . |
|---|---|---|---|---|
| HeLa | 1.00 ± 0.09 | 6.95 ± 0.56 | 1.49 ± 0.15 | 3.72 ± 0.06 |
| HEK293 | 1.00 ± 0.08 | 5.17 ± 0.45 | 2.72 ± 0.29 | 1.95 ± 0.11 |
| HuAoVSMCs | 1.00 ± 0.07 | 1.69 ± 0.10 | 1.02 ± 0.09 | 3.91 ± 0.45 |
| HPAECs | 1.00 ± 0.20 | 13.12 ± 1.07 | 3.66 ± 0.40 | 6.50 ± 0.10 |
| HCAECs | 1.00 ± 0.05 | 9.59 ± 0.63 | 4.00 ± 0.51 | 9.80 ± 0.92 |
| HUVECs | 1.00 ± 0.06 | 22.89 ± 1.05 | 11.55 ± 0.86 | 10.90 ± 0.77 |
The episomal constructs eNOS pGL3 −1193/+109, vWF pGL3 −487/+247, and VE-cadherin pGL3 −1912/+1 were transiently transfected into the EC human coronary artery endothelial cells (HCAECs), human pulmonary artery endothelial cells (HPAECs), HUVECs, and non-EC types HeLa, HEK293, and HuAoVSMCs. Shown are the fold increases in luciferase expression of eNOS pGL3 −1193/+109, vWF pGL3 −487/+247, and VE-cadherin pGL3 −1912/+1 constructs. Studies are controlled for transfection efficiency across cell types, as previously demonstrated.7 Steady-state levels of eNOS, VE-cadherin, and vWF mRNAs were not detectable in HuAoVSMCs as determined by reverse transcriptase (RT)-qPCR (supplemental Figure 1), in contrast to the observed in vitro activity of episomal EC-enriched promoter/reporter constructs. The eNOS pGL3 −1193/+109 episomal construct was active in the non-EC types HeLa, HEK293, and HuAoVSMCs, as previously described.7 Similar to eNOS, the VE-cadherin pGL3 −1912/+1 construct demonstrated a 5.17 ± 0.45- and 6.95 ± 0.56-fold increase in expression when transiently transfected into HEK293 and HeLa cell types, respectively. Robust expression was also demonstrated in all EC types. Interestingly, the vWF pGL3 −487/+247 construct also demonstrated expression when transfected into the non-ECs HeLa, HEK293, and HuAoVSMCs (1.49 ± 0.15, 2.72 ± 0.29, and 1.02 ± 0.09, respectively), as well as in the 3 EC types assayed.
Data expressed as luciferase units, relative to empty vector for each cell type. Shown is the mean ± standard error of the mean (n = 3).
Functional role of methylation in repressing EC-enriched genes in non-EC types
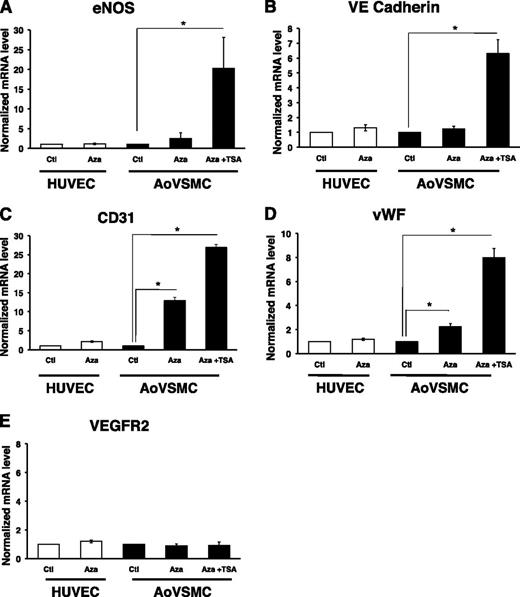
The presence of DNA methylation at proximal promoter regions of these EC-enriched genes suggested that this chromatin mark may silence the expression of these genes in nonexpressing cell types. Reexpression of epigenetically silenced genes can be achieved by inhibiting DNA methyltransferase or histone deacetylase (HDAC) activity. Treatment with 5-azacytidine, a DNA methyltransferase inhibitor, alone or in combination with TSA, an inhibitor of HDAC activity, can be sufficient to reactivate some genes.23 For other genes, combined treatment of 5-azacytidine and TSA results in synergistic activation compared with each treatment alone.24,25 To demonstrate a functional role for DNA methylation at the proximal promoter regions of EC-enriched genes in non-ECs, we treated both HUVECs and HuAoVSMCs with 5-azacytidine, alone or in combination with TSA. The EC-enriched genes eNOS and vWF showed modest upregulation in mRNA expression in HuAoVSMCs when treated with 5-azacytidine alone (Figure 5A,D). CD31 exhibited a robust response to long-term treatment with 5-azacytidine (Figure 5C). The greatest increase in expression was observed when cells were treated in combination with 5-azacytidine and TSA (Figure 5A-D). Manipulation of epigenetic processes is sufficient to induce expression of normally repressed EC-enriched genes in non-ECs. Collectively, these data indicate a functional role for promoter methylation in the repression of EC-enriched genes in a non-EC type like HuAoVSMCs.
Functional role of promoter DNA methylation of EC-enriched genes in AoVSMC. RT-qPCR analysis of mRNA levels in HUVECs and AoVSMCs treated with control (Ctl), 5-azacytidine (Aza; 5 μM, 7 days), or a combination of Aza and trichostatin A (TSA; 1 μM, 24 hours). Results are displayed relative to Ctl within each cell type. (A) eNOS, (B) VE-cadherin, (C) CD31, and (D) vWF display greater expression in AoVSMCs with combined Aza and TSA treatment. (E) VEGFR2 expression did not change with treatment. Addition of synthetic capped polyadenylated luciferase RNA was used for RNA recovery and first-strand cDNA synthesis efficiency. HUVEC/AoVSMC ratios of absolute RNA copy numbers for each gene are as follows: eNOS (750:1); CD31 (2500:1), VE-cadherin (5000:1); VEGFR2 (10:1); vWF (10 000:1). A representative experiment (mean ± standard error, n = 4) is shown. *Statistically significant difference between treated samples and control for each cell type (P < .05).
Functional role of promoter DNA methylation of EC-enriched genes in AoVSMC. RT-qPCR analysis of mRNA levels in HUVECs and AoVSMCs treated with control (Ctl), 5-azacytidine (Aza; 5 μM, 7 days), or a combination of Aza and trichostatin A (TSA; 1 μM, 24 hours). Results are displayed relative to Ctl within each cell type. (A) eNOS, (B) VE-cadherin, (C) CD31, and (D) vWF display greater expression in AoVSMCs with combined Aza and TSA treatment. (E) VEGFR2 expression did not change with treatment. Addition of synthetic capped polyadenylated luciferase RNA was used for RNA recovery and first-strand cDNA synthesis efficiency. HUVEC/AoVSMC ratios of absolute RNA copy numbers for each gene are as follows: eNOS (750:1); CD31 (2500:1), VE-cadherin (5000:1); VEGFR2 (10:1); vWF (10 000:1). A representative experiment (mean ± standard error, n = 4) is shown. *Statistically significant difference between treated samples and control for each cell type (P < .05).
VEGFR-1 and VEGFR-2 promoters are not differentially methylated in ECs vs non-EC types
Surprisingly, not all EC-enriched genes examined were differentially methylated between HUVECs and HuAoVSMCs. The promoter region of VEGFR2 did not display an enrichment of methylation in HuAoVSMCs, as determined by MeDIP analysis (Figure 2E). The absence of proximal promoter methylation was confirmed by examining Methyl 450K Bead Array data at the promoters of VEGFR1 and VEGFR2 in ECs and non-ECs, in addition to single-strand DNA analysis and pyrosequencing methods (supplemental Figure 4A-D). Bisulfite analysis demonstrated that the VEGFR2 promoter was also not methylated in keratinocytes or hepatocytes (supplemental Figure 4D). Importantly, VEGFR2 did not show any changes in expression when treated either with 5-azacytidine alone or in combination with TSA (Figure 5E). These findings suggest that DNA methylation is not important for the transcriptional regulation of VEGFR1 and VEGFR2.
eNOS is not hydroxymethylated in ECs and non-ECs
The recent discovery of 5-hydroxymethylcytosine (5hmC) as a modified base in mammalian DNA, catalyzed by the TET family of proteins, has led to newer insight into the epigenetic regulation of genes.26 The modified cytosine species 5hmC and 5-methylcytosine are indistinguishable upon sodium bisulfite conversion, and importantly, DNA containing 5hmC is not efficiently amplified by polymerase chain reaction following bisulfite conversion.27 As such, using an antibody directed against 5hmC in a technique similar to MeDIP, or hydroxymethyl-DNA immunoprecipitation, we analyzed the promoter region of the EC-enriched genes eNOS, CD31, VE-cadherin, and vWF in HUVECs (supplemental Figure 5). In contrast to robust levels of methylation at the promoter of eNOS in HuAoVSMCs, we failed to detect appreciable steady-state levels of hydroxymethylation at the eNOS promoter in this same cell type (supplemental Figure 5A). The same was true for the promoters of CD31, VE-cadherin, and vWF (supplemental Figure 5B-D). We can therefore be confident the observed bisulfite findings address 5-methylcytosine and not 5-hmC.
Differentially methylated EC-enriched genes replicate in early S-phase in ECs and non-ECs
Although DNMT1 is thought to be present at the replication fork during S-phase,28 how methylation patterns on newly replicated DNA at cell-specific genes is poorly understood. It is generally accepted that transcriptionally active genes replicate early, whereas inactive genes replicate late, during S-phase of the cell cycle. This principle was reaffirmed in recent whole genome analyses.29 For example, the β-globin gene replicates late in nonerythroid cells and early in erythroid cells,30 whereas the α-globin gene replicates early in both cell types.29 Because patterns of gene expression and alterations in the cell cycle are concomitant processes in vascular pathobiology, we argue that we need to learn more about these processes. It is surprising how little is known about DNA replication timing of tissue-specific genes, especially within the vascular endothelium.
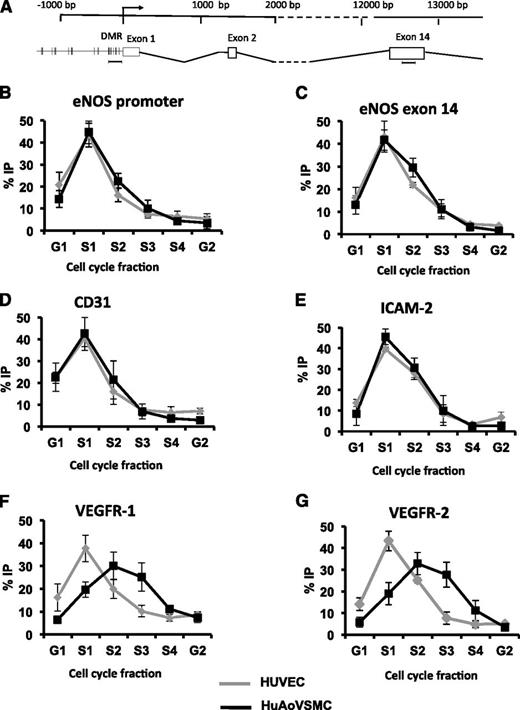
To investigate the DNA replicating timing patterns of EC-enriched genes, we used a BrdU-pulse labeling and qPCR-based approach.29 Cyclophilin A, a housekeeping gene expressed in both ECs and non-ECs, exhibited early S-phase replication, whereas β-globin, which is expressed specifically in erythrocytes and is not transcriptionally active in ECs and non-ECs, exhibited late S-phase replication (supplemental Figure 6C-D), as shown by others.30 We next determined the timing of DNA replication for eNOS in both expressing and nonexpressing cell types. Both the proximal promoter and exon 14 region (∼13 kb downstream of the TSS) of the NOS3 locus displayed early S-phase replication in HUVECs and HuAoVSMCs (Figure 6B-C). This surprising finding provides evidence that the entire eNOS gene replicates early in both ECs and non-ECs. This was not expected. Replication timing analyses determined that CD31 and ICAM2 both displayed early S-phase replication in both ECs and non-ECs (Figure 6D-E), as observed with eNOS. Again, these findings were unexpected, because generally, early DNA replication timing correlates with gene expression.10 Interestingly, the EC-enriched genes VEGFR-1 and VEGFR-2 displayed early S-phase (S1) replication in HUVECs and late S-phase (S2-S3) replication in a nonexpressing cell type (Figure 6F-G). As stated, these were the only EC-enriched genes that did not display differential DNA methylation between ECs and non-ECs (supplemental Figure 4).
Replication timing profile of eNOS and EC-enriched genes in ECs and non-ECs. (A) A schematic of the NOS3 locus, with primer locations used to detect eNOS. The relative abundance (percent IP) of (B) the proximal promoter of eNOS and (C) exon 14 of eNOS in each cell cycle fraction quantified by qPCR in HUVECs (gray) and HuAoVSMCs (black). Data represent mean ± standard error of the mean, n = 4. The relative abundance (percent IP) of EC-enriched genes in each cell cycle fraction is shown for (D) CD31, (E) ICAM-2, (F) VEGFR-1, and (G) VEGFR-2, quantified by qPCR. Data represent mean ± standard error of the mean, n = 3.
Replication timing profile of eNOS and EC-enriched genes in ECs and non-ECs. (A) A schematic of the NOS3 locus, with primer locations used to detect eNOS. The relative abundance (percent IP) of (B) the proximal promoter of eNOS and (C) exon 14 of eNOS in each cell cycle fraction quantified by qPCR in HUVECs (gray) and HuAoVSMCs (black). Data represent mean ± standard error of the mean, n = 4. The relative abundance (percent IP) of EC-enriched genes in each cell cycle fraction is shown for (D) CD31, (E) ICAM-2, (F) VEGFR-1, and (G) VEGFR-2, quantified by qPCR. Data represent mean ± standard error of the mean, n = 3.
Newly replicated eNOS promoter has low levels of DNA methylation in early S-phase
Since we determined that EC-enriched genes replicate in early S-phase in ECs and non-ECs, we were motivated to determine when in S-phase the promoter region of eNOS becomes methylated in HuAoVSMCs. Site-specific methylation analysis at the proximal promoter of eNOS displayed an average 40% methylation in G1 and S1 phases (supplemental Figure 7A). In S2, methylation levels were observed to be double that of S1. For the remainder of S-phase, methylation levels at eNOS were comparable to the methylation level of a whole dish of cells (supplemental Figure 7A). The levels of eNOS methylation that are observed in terminally differentiated cells (supplemental Figure 3A) do not occur until late S-phase. These results indicate that, although the replication timing of eNOS occurs in S1 phase, there is a measurable lag in remethylation of the nascent strand of the hemimethylated DNA duplex. To address a possible cause or effect relationship, we treated AoVSMCs with 5-azacytidine for 24 hours and profiled the timing of eNOS replication. It was evident that the timing of eNOS replication remained in early S-phase (supplemental Figure 7B). Thus, inhibition of DNA methylation at the eNOS promoter did not affect the replication timing of eNOS. Not all cells in S-phase are dynamically proceeding through DNA replication. We then asked whether AoVSMCs arrested in S-phase have a distinct DNA methylation profile. As shown in supplemental Figure 8B, the eNOS DNA methylation profile did not differ between cells proceeding or arrested in S-phase.
Discussion
This report demonstrated that most EC-enriched genes show evidence of differential DNA methylation of proximal promoter regions. These genomic regions, which encompass the start sites of transcription, are unmethylated in ECs that express these mRNAs and are methylated in nonexpressing cell types, such as HuAoVSMCs, keratinocytes, and hepatocytes. We identified DMRs in the EC-enriched genes PECAM1/CD31, vWF, VE-cadherin, ICAM-2, P-selectin, Endoglin, and Tie2. Importantly, our in vivo studies of murine EC-enriched gene promoters recapitulated our findings. Use of DNA methylation inhibitors indicated that DNA methylation is functionally relevant. Inhibition of global DNA methylation, either alone or combined with inhibitors of HDAC activity, led to increases in expression of select mRNAs in cell types that do not basally express these mRNAs. We infer that DNA methylation actively represses transcription of these transcripts in nonexpressing cell types. This is an important concept given that most models of cell-specific gene expression focus on what activates or enhances transcription in expressing cell types. This may help explain the promiscuous activity of episomal-based promoter reporter constructs for EC-enriched genes in non-EC types, which do not normally express the native gene in the context of chromatin.
We found that for the majority of EC-enriched genes that contain DMRs in their proximal promoter, DNA replication timing occurs in early S-phase in both ECs and non-ECs. This finding is not consistent with the general paradigm of replication timing patterns and gene expression. Importantly, most studies are based on global approaches of replication timing analyses and focus on developmentally controlled and tissue-specific genes that show differential DNA replication timing profiles between cell types.31,32 We examined publicly available data on replication timing in embryonic stem cells, neural progenitor cells, and lymphoblasts and noted early replication timing for eNOS33 (www.replicationdomain.org). We found that eNOS is replicated early in the cell cycle, and there is a small, but discernible, delay in DNA methylation. Early replication of a locus with a DMR may allow sufficient time for accurate methylation inheritance after each cell division, ensuring the faithful transmission of epigenetic signatures at tissue-specific genes. Our novel findings indicate that future studies in this area are now needed.
Our work highlights that for some genes (eNOS, vWF, CD31, ICAM2, and P-selectin), very high levels of DNA methylation were observed in nonexpressing cell types, whereas for other genes (VE-cadherin, tie2, and endoglin), lower levels of DNA methylation are associated with gene silencing. In general, the relationship between the absolute levels of DNA methylation and its functional effects are not well understood. Another important point relates to the basal presence of DNA methylation in expressing cell types. The promoters for eNOS, vWF, Endoglin, Tie2, ICAM2, and CD31 are nearly completely unmethylated in ECs. In contrast, P-selectin is partially methylated in ECs. The functional relevance of this basal methylation of P-selectin and whether it represents population heterogeneity remain to be defined.
We demonstrated that VEGFR1 and VEGFR2 did not contain DMRs in their proximal promoters. In disease settings, these genomic regions can be methylated. For instance, the VEGFR2 promoter and exon 1 were reported to be methylated in prostate cancer and upon ischemic injury.34,35 Interestingly, VEGFR1 and VEGFR2 are among the earliest EC-enriched genes to be expressed in vascular development. Expression of VEGFR1 and VEGFR2 is first detected at embryonic day (ED)6.536 and ED7.0,37 respectively. This contrasts with the later onset expression of other endothelial enriched genes: Tie2 (ED7.5),37 VE-cadherin (ED8.5),38 and vWF (ED8.5).39 The expression of eNOS occurs even later, at ED9.5, after the establishment of robust unidirectional flow.20 Therefore, an intriguing hypothesis is that the timing of onset expression during vascular development may be an important determinant of the presence of differential methylation in EC-enriched genes.
We found that VEGFR1 and VEGFR2 replicated early in expressing cell types and late in nonexpressing cell types (Figure 6). These two EC-enriched genes also did not contain DMRs in their proximal promoter regions. Studies have shown that genes with high absolute CpG density at the promoter replicate early in mammalian cells.40 High CpG density regions can also represent CpG islands if the observed/expected ratio is high. In this regard, the promoter regions of VEGFR1 and VEGFR2 represent CpG islands. Interestingly, when the areas around the TSSs of all EC-enriched genes were analyzed for the presence of CpG islands, only VEGFR1 and VEGFR2 showed an observed/expected ratio of a strong CpG island (>0.75), whereas the other EC-enriched genes were classified as poor CpG islands (supplemental Table 4). The relationship between the presence or absence of DNA methylation and timing of onset of gene expression in development, DNA replication timing in the mitotic cell cycle, and the presence of CpG islands requires further study.
We assayed the methylation status of EC-enriched gene promoters in blood outgrowth ECs and found comparable findings to results in HUVECs and HMVECs. This is an important finding, as BOECs have proved to be valuable for several therapeutic applications, including the ability to home to sites within the tumor neovasculature for gene delivery.41,42 Interestingly, recent studies by others have shown that in early proangiogenic cells, the eNOS promoter is epigenetically silenced.43 These early proangiogenic cells are distinguishable from BOECs, especially because the DNA methylation profile of EC-enriched genes in BOECs is similar to our findings in mature vessel wall ECs.
Taken together, our findings demonstrate the first studies of promoter DNA methylation and the regulation of EC-enriched gene expression in both endothelial outgrowth cells and mature ECs. We provided evidence for the role of 5-methylcytosine in regulating the expression of a number of EC-enriched genes within the vascular endothelium. Importantly, the novel concept introduced here is that EC-enriched genes are transcriptionally repressed in nonexpressing cell types by epigenetic mechanisms. These studies provide newer insight into the importance of epigenetic processes within the vascular endothelium. Given that chromatin-based pathways provide a newly appreciated mechanism by which environmental or exogenous stimuli can interact with the static DNA code, there may be translational implications for these findings. For example, hemodynamic stimuli influence EC gene expression in blood vessels.20,44,45 Whether hemodynamic forces modify gene expression in ECs, in part, via chromatin-based pathways needs further study. However, a role for changes in 5-methylcytosine content in diseases of the vascular endothelium remains to be defined.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the P.A.M. laboratory for critical review of this manuscript.
This work was supported by Canadian Institutes of Health grant MOP 79475 and National Heart, Lung, and Blood Institute grant PO1 HL076540-06A1 (P.A.M.). P.A.M. holds the Keenan Chair in Medical Research at St. Michael's Hospital and the University of Toronto. A.V.S. is a recipient of the Canadian Institutes of Health Research Collaborative Graduate Training Program in Molecular Medicine award.
Authorship
Contribution: A.V.S., R.S.B., A.G., A.K., B.J.K., M.S.-C.Y., H-S.J.M., and M.S. performed experiments and collected data; R.P.H., P.O., and W.C.A. provided reagents, performed select experiments, and gave critical intellectual input; and A.V.S. and P.A.M. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interest.
Correspondence: Philip A. Marsden, Li Ka Shing Knowledge Institute, St Michael's Hospital, 209 Victoria Street, Room 522, Toronto, Ontario, Canada M5B 1C6; e-mail: p.marsden@utoronto.ca.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal